A new species of Thecidellina (Brachiopoda) from the Kermadec Islands, southwest Pacific Ocean
Jeffrey H. Robinson A *
A *
A
Abstract
A new thecidellinine species, Thecidellina lueteri, is described and figured from the Kermadec Islands, southwest Pacific Ocean. This increases the number of extant species in this genus to 13, six of them occurring in the Pacific. The most distinctive morphological features of the new species are lateral expansions and paired posterior outgrowths on the calcitic pole. Ontogenetic series of images are provided for both T. lueteri n. sp. and for T. insolita Hoffmann et al. 2009 from Lord Howe Ridge.
Keywords: calcitic pole, Kermadec Islands, Lord Howe Rise, new species, ontogenetic series, southwest Pacific, Thecidellina.
Introduction
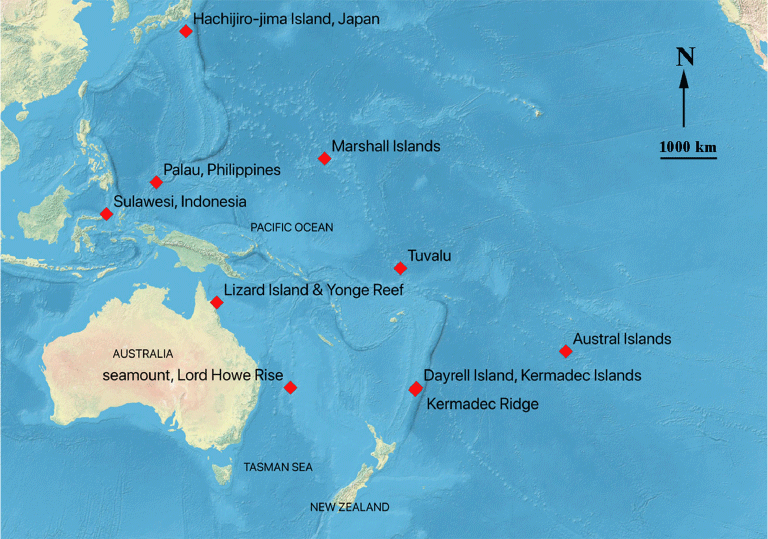
This paper describes and figures a new Recent species of the micromorphic brachiopod genus Thecidellina Thomson, 1915 from the Kermadec Islands and Kermadec Ridge, northeast of New Zealand (Fig. 1).
Lee and Robinson (2003) first identified this material as Thecidellina maxilla (Hedley, 1899) and suggested that all Recent Indo-Pacific species of Thecidellina might be variants of T. maxilla. Hoffmann et al. (2009) suggested that thecidellinine species diversity is highly underestimated and proposed that the widely reported Pacific and Indian Ocean species T. maxilla may represent a species-complex. Robinson (2021) figured newly designated lectotype material of T. maxilla from the type locality (Tuvalu), noted that the lectotype specimens did not closely resemble material identified as T. maxilla from other localities (Robinson 2021, fig. 1) and suggested that T. maxilla s.s. might be restricted to a relatively small geographical range. Simon et al. (2018, p. 222) noted that ‘the genus Thecidellina has a strong potential for allopatric speciation’. The new Thecidellina species described and named herein brings the number of extant Thecidellina species in the Pacific Ocean to six, and the total number of extant species worldwide to 13.
Lee and Robinson (2003, p. 356) also suggested that published ontogenetic series of thecideids were needed as the main points of difference (at that time) between described species ‘are based either on adult size, shape, and size of attachment scar, or on features which change during ontogeny’. This led to a series of publications that included ontogenetic development in thecideids (Logan 2004; Logan 2008; Hoffmann and Lüter 2009, 2010; Baker and Carlson 2010; Baker and Logan 2011; Simon and Hoffmann 2013; Simon et al. 2018). Ontogenetic studies of several species of Thecidellina by Hoffmann and Lüter (2010) led to the creation of the genus Minutella as the ‘pseudodeltidium’ was shown to always be curved (now termed the rugideltidium) in Minutella and always flat (now termed the planodeltidium) in Thecidellina, at all stages of shell development.
Additional taxonomic features in Thecidellina and Minutella have been recognised and new terminology for thecideids has been introduced and is used herein. These include: ‘calcitic connections’ for spicule growth between the brachial bridge and brachial ridge (Hoffmann et al. 2009); ‘rugideltidium’ and ‘planodeltidium’ (Logan and Baker 2013); ‘posterior outgrowths’ on the calcitic pole (Simon and Hoffmann 2013); ‘lateral expansions’ on the calcitic pole (Simon et al. 2018). However, the terms interbrachial bridge and intrabrachial ridge are cumbersome; the simpler terms brachial bridge and brachial ridge are preferred. Hoffmann and Lüter (2010) used the presence/absence of calcitic connections and the direction of their growth as diagnostic characters.
The ontogenetic development for six Thecidellina species has been described and figured previously (Logan 2008; Hoffmann and Lüter 2009, 2010; Baker and Logan 2011; Simon et al. 2018). Hoffmann et al. (2009) described a new species T. insolita from Lizard Island, the Great Barrier Reef, Australia (Fig. 1) and figured the interior of the holotype, interior morphological details of the shell of several other specimens and aspects of the biology of this species, but did not figure an ontogenetic series. This study extends the geographical range of T. insolita to the Lord Howe Rise and provides an ontogenetic series of images (from single-locality populations) for both T. insolita from the Lord Howe Rise and for T. lueteri n. sp. from Dayrell Island. Both ontogenetic series show significant late-stage morphological development in the dorsal valve.
Materials and methods
The material is held in the Department of Geology, University of Otago, Dunedin, in Te Papa Tongarewa, the National Museum of New Zealand, Wellington, and in the National Institute of Water and Atmospheric Research (NIWA) at Greta Point, Wellington, (Table 1). Material from Dayrell Island, in the Kermadec Islands, was collected by F. J. Brook and a block with specimens attached was gifted to D. E. Lee (~1990). Material from the Kermadec Ridge was collected by N. Mitchell on the FV Santa Maria in 1997. Material from a seamount north of Middleton Reef on the Lord Howe Rise was collected by NIWA and one block with specimens attached was gifted to J. H. Robinson by D. P. Gordon (~2010). Two more blocks (held by Te Papa and NIWA) from the same seamount with specimens attached were also examined.
| Species/specimens | Location | Depth | Latitude and Longitude Station | Catalogue number | |
|---|---|---|---|---|---|
| Thecidellina lueteri n. sp. | |||||
| 100+ articulated specimens and ventral valves attached to rock, some specimens detached | Dayrell Island, Kermadec Islands | 3 m | 29.246°S, 177.858°W | OU 39457, 43431–33, 47483 | |
| 44 articulated specimens removed from substrate (calcareous algae) | Kermadec Ridge | 120 m | 29.583°S, 178.083°W Station 1010/52 | BR.001721 | |
| Thecidellina insolita | |||||
| 20 articulated specimens and ventral valves attached to rock, some specimens detached | Seamount, Lord Howe Ridge | 292–330 m | 29.219°S, 159.008°E TAN0308/55 | OU 44461 | |
| 17 articulated specimens and ventral valves attached to rock, some specimens detached | Seamount, Lord Howe Ridge | 292–330 m | 29.219°S, 159.008°E TAN0308/55 | NIWA 158971 | |
| 13 articulated specimens and ventral valves attached to rock | Seamount, Lord Howe Ridge | 292–330 m | 29.219°S, 159.008°E TAN0308/55 | BR.002265 | |
BR, Te Papa Tongarewa, National Museum of New Zealand, NIWA, National Institute of Water and Atmospheric research, OU, University of Otago, Department of Geology.
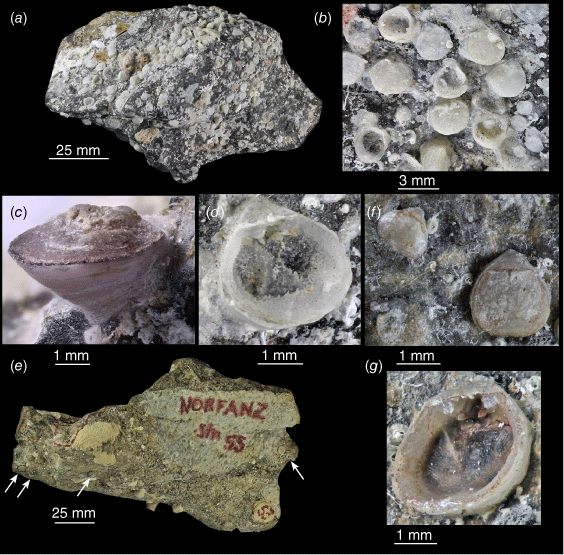
The material was collected live for both species described herein, and the shells are still attached to the rock substrate (Fig. 2a–g). In both species, the cicatrix (where the valve cements to the rock) may be at the posterior (Fig. 2c) but is often the ventral surface beneath the valve (Fig. 2d). In the latter case, the ventral valve floor is very thin and transparent, and it is very difficult to remove specimens from the rock without shattering the ventral valve. While most images of specimens (Figs 3–6) were taken with a Hitachi TM3000 SEM (at NIWA, Greta Point, Wellington), images of the ventral valve interior of specimens still attached to the rock (Fig. 2b, d, g) were taken with a Canon EOS 1200D SLR camera (and image-stacked) and are of lesser quality.
SLR images of Thecidellina specimens still attached to substrate. (a–d) OU 39457. (a) Block of stone from Dayrell Island, the Kermadec Islands, with Thecidellina lueteri n. sp. attached to it. (b) Close-up of attached Thecidellina specimens, note tiny juveniles. (c) Oblique view of T. lueteri n. sp. specimen attached to rock with cicatrix at posterior. (d) Oblique view of T. lueteri n. sp. ventral valve attached to rock with cicatrix underneath. (e–g) NIWA 158971. (e) Block of stone from a seamount on the Lord Howe Rise with T. insolita attached to it (specimens arrowed). (f) Close-up of adult and medium-sized specimens of T. insolita. (g) Oblique close-up of ventral valve interior and hemispondylium prongs of T. insolita.
Systematics
Genus Thecidellina Thomson, 1915
Endopunctate thecidellinine brachiopods. Shell roundish to drop-shaped. Ventral valve with planodeltidium; teeth commonly covered with secondary shell material. Dorsal valve with median septum; lophophore schizolophous; cardinal process sub-quadrate, its medio-posterior margin is prominent, straight, serving as muscle attachment site for diductor muscles; lateral adductor muscle scars kidney-shaped, reaching beyond insertion of lateral lobes of cardinal process; median adductor muscles attached to floor of visceral cavity forming two distinct median adductor muscle scars; calcitic pole usually completely fused to floor of cardinal process in adult specimens (after Hoffmann and Lüter 2010).
Thecidellina lueteri n. sp. (Figs 2a–d, 3a–m, 4a–m)
Thecidellina maxilla (Hedley, 1899) Lee 1990: 273; Dawson 1990: 435; Dawson 1992: 31; Brook 1998a: 239: appendix 2; Brook 1998b: 190; Lee and Robinson 2003: 350–352, fig. 28–35; MacFarlan et al. 2009: 262; Duffy and Ahyong 2015: 95.
Thecidellina sp. nov. 1 Robinson et al. 2023: 249.
OU 47483, a dorsal valve (the ventral valve remains attached to the substrate). Holotype held in the Department of Geology, University of Otago, Dunedin, New Zealand.
This species is named for Carsten Lüter (Museum für Naturkunde, Berlin) in recognition of his extensive work on the Thecideoidea. However, as accents cannot be used in latin names (Article 27 of the ICZN) the spelling ‘lueter’ is used.
The adult dorsal valves have a narrow, concave median septum, a calcitic pole with lateral expansions and paired posterior outgrowths. The extensive calcitic connections develop late in ontogeny, extending from the brachial bridge to the brachial ridge (but not fusing to it). The spicule canopy varies from absent to partially developed.
The shells are drop-shaped in outline, usually slightly longer than wide, with a planodeltidium flanked by very narrow interareas (Fig. 3a, b). The largest specimen observed was 4.2 mm long and 4.0 mm wide. Internally, the ventral valve floor is usually very thin and transparent (Fig. 2d), or, if the cicatrix is at the posterior, non-tuberculate and with deep muscle pits. A tiny juvenile ventral valve 0.8 mm long (Fig. 3c) has well-developed hemispondylium prongs. In adult specimens, the hemispondylium prongs extend from a thin base-plate attached to the posterior wall or the valve floor (Fig. 3d).
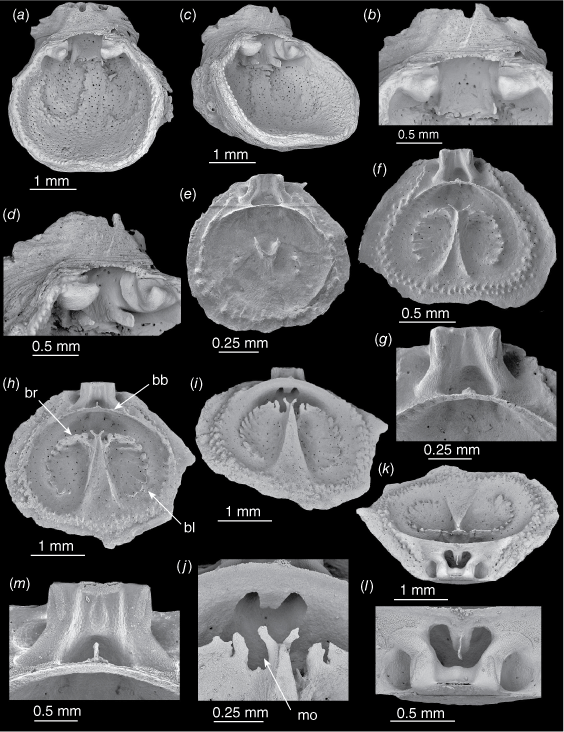
Thecidellina lueteri n. sp. from Dayrell Island. (a, b) OU 39457a. An articulated specimen. (a) Dorsal view. (b) Lateral view. (c) OU 39457b. Juvenile ventral valve. (d) OU 39457c. Partial adult ventral valve still articulated with dorsal valve, hemispondylium base-plate attached to valve floor. (e–g) OU 39457d. Juvenile dorsal valve. (e) Interior view. (f) Oblique interior view. (g) Close-up of spikes. (h–j) OU 39457e. Juvenile dorsal valve. (h) Interior view. (i) Oblique interior view. (j) Close-up of spikes and protoseptum. (k–m) OU 39457f. Medium-sized dorsal valve. (k) Oblique interior view. (l) Posterior view (m) Close-up of cardinal process and posterior outgrowths. Abbreviations: hbp, hemispondylium base-plate.
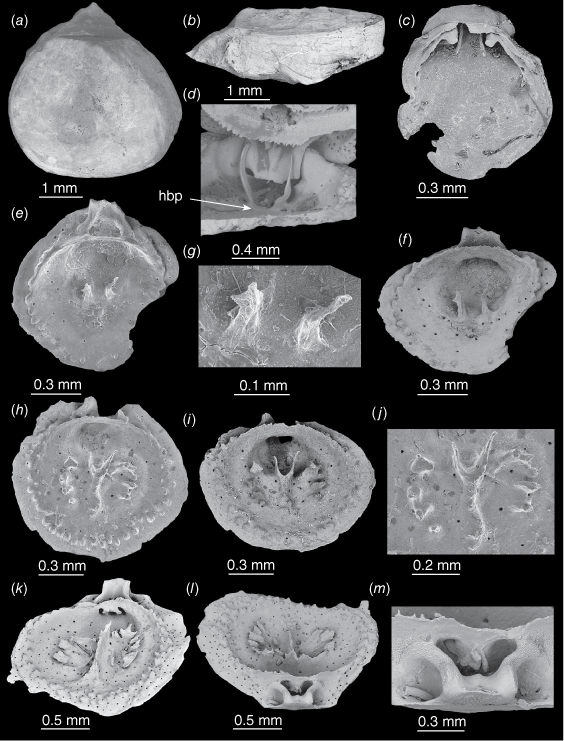
In a dorsal valve 0.8 mm long (Fig. 3e–g) there is a complete brachial bridge and two tiny spikes as the first part of the brachidium. A single row of tubercules has formed around the rim, but the cardinal process is not complete. A 1.2 mm long dorsal valve (Fig. 3h–J) shows the two tiny spikes have coalesced at the posterior tip of the protoseptum that runs anteriorly. The brachial ridge and brachial lobes have begun to form as radially-directed, individual plates.
In a dorsal valve 1.5 mm long (Fig. 3k–m) a slim median septum has formed, the radially-directed individual plates have increased in size, the cardinal process has a slim central lobe, and the tuberculate rim has developed a second row of tubercles. The calcitic pole has begun to develop beneath the brachial bridge but is not yet connected to the valve floor. Viewed from the anterior, the calcitic pole has two lateral expansions below the brachial bridge (Fig. 3k); viewed from the posterior the calcitic pole has two closely-parallel, posterior outgrowths (Fig. 3l–m).
In a dorsal valve 1.8 mm long (Fig. 4a–c) the median septum is still very narrow, the radially-directed plates of the brachial ridge and brachial lobes continue to extend and have coalesced at their bases, and the visceral gap is wide but spicules that form the calcitic connections are beginning to grow from the brachial bridge towards the brachial ridge (Fig. 4b, c). The lateral expansions and posterior outgrowths on the calcitic pole now extend well beyond the brachial bridge (Fig. 4c).
Thecidellina lueteri n. sp. from Dayrell Island. (a–c) OU 39457g. Medium-sized dorsal valve. (a) Interior view. (b) Posterior view. (c) Close-up of brachial bridge with the lateral expansions and posterior outgrowths of the calcitic pole extending from beneath. (d, e) OU 39457h. Adult dorsal valve. (d) Interior view. (e) Close-up of processes growing from the septum tip and adjacent brachial ridge. (f–j) OU 47483. Holotype. Adult dorsal valve. (f) Interior view. (g) Lateral view of cardinal process, posterior outgrowths and calcitic connections. (h) Close-up of marsupial orifices and ovarial notches. (i) Posterior view. (j) Close-up of posterior and posterior outgrowths. (k–m). OU 39457i. Adult dorsal valve articulated with partial ventral valve. (k) Interior view. (l) Lateral view. (m) Posterior view. Abbreviations: bb, brachial bridge; bc, brachial cavity; bhp, broken hemispondylium prong; bl, brachial lobe; br, brachial ridge; cc, calcitic connections; ccs, calcitic connection spicules; le, lateral expansions; mo, marsupial orifice; mou, mouth (dried tissue); on, ovarial notch; po, posterior outgrowth.
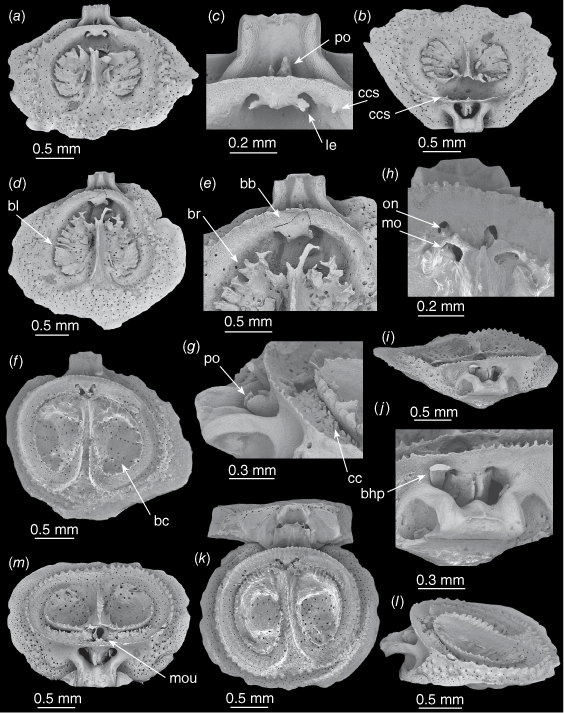
In a dorsal valve 2.3 mm long (Fig. 4d, e) the septum is concave at the anterior end, processes extend from the posterior tip of the septum and the tips of adjacent radial plates; these processes appear to coalesce to form the marsupial orifices. The posterior corners of the cardinal process are bevelled, and the median lobe has increased in size.
In a dorsal valve 2.8 mm long (Fig. 4f–j, the holotype) the dorsal septum is now concave along its entire length with small serrations along each edge; the radial plates have fully coalesced forming the complete brachial ridge, brachial lobes and brachial cavities; the visceral gap has been mostly closed by the growth of spicules (the calcitic connections) from the brachial bridge to the brachial ridge (Fig. 4f, g); the arches of the marsupial orifices are complete; ovarial notches have formed; and the calcitic connection spicules appear to extend into the ovarial notches (Fig. 4h). The lateral expansions appear to extend the full length of the calcitic pole and to be continuous with the paired posterior outgrowths; thus there is one lateral expansion/posterior outgrowth on either side of the calcitic pole (Fig. 4j) (note the broken hemispondylium prong partially obscuring the left lateral expansion in Fig. 4i, j).
In a second dorsal valve 2.8 mm long (Fig. 4k–m) the top of the dorsal septum twists to the side, presenting one side as a single, thin, strongly-serrated, ventrally-directed ridge (a feature unique to this specimen). A dorsal valve of T. lueteri n. sp. figured in Lee and Robinson (2003, fig. 31, as T. maxilla) had a very limited spicule canopy, this was not seen in any other specimens examined from Dayrell Island. However, specimens from the Kermadec Ridge have partial spicule canopies. The calcitic connection spicules appear to extend into the ovarial notches. The dried tissue of the mouth is visible in Fig. 4m.
The lectotypes of T. maxilla from the type locality (Tuvalu, Robinson 2021, figs 3–6) differ from T. lueteri n. sp. in having a strongly tuberculate ventral valve floor, the calcitic pole is an anteriorly-facing folded sheet without posterior outgrowths, there are usually extensive spicule canopies and calcitic connections are small or absent. Thecidellina lueteri n. sp. differs from all other material identified as T. maxilla (10 localities in the southwest Pacific and Indian Oceans, see Robinson 2021) in the calcitic pole; no other populations have lateral expansions and paired posterior outgrowths.
Thecidellina japonica Hayasaka, 1938 from off Hachijiro-jima Island, Japan (Hoffmann and Lüter 2010, pl. 5, figs 19–24) differs from T. lueteri n. sp. in the calcitic pole, with a single posterior outgrowth but no lateral expansions and the calcitic connections grow from the brachial ridge towards the brachial bridge (the opposite of T. lueteri n. sp.).
Thecidellina congregata Cooper, 1954 is known from the Marshall Islands (Cooper 1954, pl. 80, fig. 6–15; Zumwalt 1970, plates 1–4, 6–8) and Palau Island, the Philippines (Logan 2008, fig. 6.10–17). The studies of Cooper, Zumwalt and Logan all figure dorsal valve interiors with massive spiculation over the brachial cavities (unlike T. lueteri n. sp.); however, details of the calcitic pole were not described.
Thecidellina insolita Hoffmann et al. 2009 (figs 2a–m, 3a, b) from Lizard Island, Great Barrier Reef, Australia (and specimens from Lord Howe Rise described and figured herein) differs from T. lueteri n. sp. in the dorsal median septum, the calcitic pole and the lack of calcitic connections.
Thecidellina malawiana Simon et al. 2018 from Sulawesi, Indonesia, has triangular lateral expansions that extend the full length of the calcitic pole (Simon et al. 2018, pl. 3, fig. 1e, f), but no posterior outgrowths. The calcitic connections develop early in T. malawiana, remain rather narrow, and connect the brachial ridge and brachial bridge. Calcitic connections in T. lueteri n. sp. develop quite late and are wider, but don’t connect the brachial bridge to the brachial ridge. T. malawiana has well-defined spicule canopies over the brachial cavities, T. lueteri n. sp. does not.
Brook (1998b) noted that Thecidellina from the Kermadec Islands was found in subtidal caves, tunnels and overhangs with a diverse invertebrate fauna. The rock sample collected by Brook at a depth of 3 m (Fig. 2a) shows a dense population of T. lueteri n. sp. with a wide size range of individuals, from tiny juveniles to adults, and many old and worn ventral valves of dead individuals. Specimens from the Kermadec Ridge were collected at a depth of 120 m (Table 1).
Apparent growth of the calcitic connection spicules into the ovarial notches is visible in other published Thecidellina material; Bitner (2007, fig. 3f, from the Austral Islands) and Simon et al. (2018, figs 5a, 7, 8a, b, from Sulawesi, Indonesia).
Thecidellina insolita Hoffmann, Klann and Matz, 2009 (Figs 2e–g, 5a–m, 6a–l)
Thecidellina insolita Hoffmann, Klann and Matz, 2009, 341–349, figs 2a–l, 3a–h, table 1
Thecidellina maxilla (Hedley, 1899) MacFarlan et al. 2009: 262
Thecidellina sp. nov. 2: Robinson et al. 2023: 249
The calcitic pole is tongue-shaped and has a thin plate as a posterior outgrowth; in most specimens the calcitic pole does not fuse with the valve floor. The marsupial orifices may be incomplete; ovarial notches are absent. The median septum is triangular and concave in small to medium-sized specimens; in adults the posterior half of the median septum widens and becomes convex. The visceral gap is open; the brachial cavities lack a spicule canopy or may be partly covered by a single mass of canopying spicules. The lobes of the cardinal process may become swollen in adults with the middle lobe enveloping the calcitic pole (after Hoffmann et al. 2009).
The ventral valve is drop-shaped, adult specimens usually longer than wide (Figs 2f, 5a); the largest specimen observed was 5.2 mm long and 4.4 mm wide. The planodeltidium is striated and slightly raised above the interareas (Fig. 5a, b); the planodeltidium usually slopes posterioventrally from the hinge line (Fig. 2f, g) but may slope posteriodorsally (Fig. 5a–d).
Thecidellina insolita from the Lord Howe Rise. (a–d) OU 44461a, ventral valve. (a) Interior view. (b) Close-up of teeth and hemispondylium base-plate. (c) Oblique interior view. (d) Close-up showing broken hemispondylium prongs. (e) NIWA 158971a. Juvenile dorsal valve interior. (f, g). NIWA 158971b. Young specimen, dorsal valve. (f) Interior view. (g) Close-up of brachial bridge and cardinal process. (h–m) OU 44461b. Medium-sized dorsal valve. (h) Interior view. (i) Oblique interior view. (j) Close-up of marsupial orifice, processes on tip of septum and tongue-shaped calcitic pole. (k) Posterior view. (l) Close-up showing tongue-shaped calcitic pole with the thin posterior outgrowth. (m) Close-up of thin brachial bridge and the thin posterior outgrowth of the calcitic pole extending from beneath it. Abbreviations: bb, brachial bridge; bl, brachial lobe; br, brachial ridge; mo, marsupial orifice.
The ventral valve inner surface is punctate but not markedly tuberculate. A specimen with a thick ventral valve (and the cicatrix at the posterior) has large, sunken muscle pits (Fig. 5a, c). In specimens with a thin ventral valve floor, the muscle pits were not obvious (Fig. 2g). The base-plate of the hemispondylium is attached to the valve (not excavate). The prongs are parallel and slightly curved (although broken short in imaged specimen). The lateral and anterior margins are tuberculate where they meet the dorsal valve margins. (Fig. 5c).
In a dorsal valve 1.3 mm long (Fig. 5e) a protoseptum is just beginning to form and has two tiny ventrally-directed spikes posteriorly, the brachial bridge has formed and there is a single row of tubercles around the lateral and anterior margins.
In a dorsal valve 2 mm long (Fig. 5f, g) the median septum is wide anteriorly and narrows to a thin ridge posteriorly. The septum tip has two tiny processes arching over the marsupial orifices. The brachial ridge and brachial lobes have begun to form, two curving lines of individual plates directed ventro-radially. There is a double row of tubercles around the lateral and anterior margins (Fig. 5f). The cardinal process has a faint median lobe; the calcitic pole and posteriorly-directed outgrowth have begun to develop (Fig. 5g).
In a dorsal valve 2.8 mm long (Fig. 5h–m), the septum is wide anteriorly, reduced to a sharp point posteriorly and is concave its whole length. The ventro-radially directed individual plates have coalesced in the brachial ridge but are still separate on the brachial lobes (Fig. 5h, i). There are incomplete marsupial orifices on each side of the septum tip with small processes arching over them (Fig. 5j). The calcitic pole is a flattened tongue with a very thin posterior outgrowth (Fig. 5k, l). The cardinal process has wide lateral lobes and a small median lobe (Fig. 5l, m).
In a partial dorsal valve (estimated to be 2.5–2.7 mm long, Fig. 6a, b) a single row of tubercles is developing on the concave median septum, and the three lobes of the cardinal process are becoming thickened. In a dorsal valve 4.2 mm long (Fig. 6c) the posterior half of the median septum is beginning to widen. The processes on the tip of the septum don’t quite complete the marsupial orifices.
Thecidellina insolita from the Lord Howe Rise. (a, b) NIWA 158971c. (a) Partial medium-sized dorsal valve. (b) Close-up of marsupial orifice, brachial bridge and cardinal process. (c) NIWA 158971d. Medium-sized dorsal valve. (d, e) NIWA 158971e. (d) Adult dorsal valve. (e) Close-up of septum tip, complete marsupial orifice and spicules. (f–i) OU 44461c. (f) Adult dorsal valve with a complete marginal rim. (g) Close-up of septum tip, broken marsupial notch processes and tiny processes on brachial ridge, (h) Posterior view. (i) Close-up of calcitic pole and cardinal process with swelling middle lobe. (j–l) OU 44461d. Adult dorsal valve. (j) Interior view. (k) Close-up of marsupial orifices, septum tip, large cardinal process, swollen median lobe. (l) Close-up from posterior of large cardinal process, swollen median lobe and large diductor muscle scar. Abbreviations: ccs, calcitic connection spicules; cmo, complete marsupial orifice; imo, incomplete marsupial orifice.
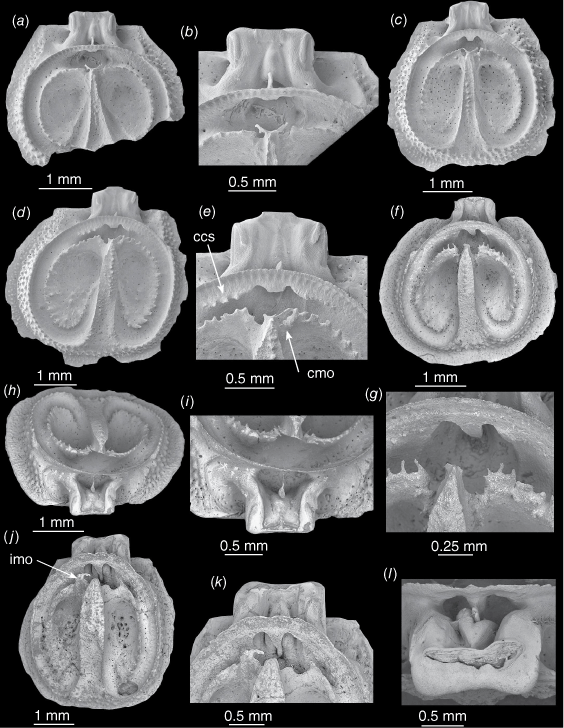
In a dorsal valve 4.6 mm long (Fig. 6d, e) the posterior half of the median septum has widened and become convex. Spicules that are probably the beginnings of calcitic connections are forming on each side of the brachial bridge (but no specimens have complete calcitic connections). The right marsupial orifice is complete.
In a dorsal valve 3.3 mm long (Fig. 6f–i) the posterior half of the median septum is wider than the anterior half, is concave and has a row of tubercles; the valve has a wide, complete, tuberculate marginal rim. The posterior part of the brachial ridge has tiny, ventrally-directed processes; these may represent limited development of a spicule canopy (Fig. 6g). The median lobe of the cardinal process is swollen and is in contact with the posterior outgrowth on the calcitic pole (Fig. 6h, i).
In a dorsal valve 4.4 mm long (Fig. 6j–l) the septum has become very broad. The cardinal process is very large and robust, both lateral lobes and the median lobe are very swollen, the median lobe partially envelops the calcitic pole and its posteriorly-directed plate, and the diductor muscle scar is prominent (Fig. 6j, l). There are no calcitic connections and the marsupial orifices do not appear to be complete (or have been completed and damaged).
Hoffmann et al. (2009, p. 343) listed in their diagnosis of T. insolita that this species may have their brachial cavities ‘partly covered by a single mass of canopying spicules’. This was not observed in any specimens from the Lord Howe Rise.
Clark and Roberts (2008, p. 11) described the NORFANZ Station TAN0308/55 where the Thecidellina material was collected from the Lord Howe Rise: ‘This was a single, relatively large and very rugged seamount, flat topped at 280–290 m, with steep sides’. There were relatively few specimens of Thecidellina insolita available on the small rocks collected (Fig. 2e) and very few juvenile specimens.
It is interesting to note that, while there is usually a lot of morphological variation between the southwest Pacific Thecidellina populations (Robinson 2021, fig. 1), specimens of T. insolita from locations approximately 2000 km apart (Fig. 1) are clearly conspecific (Lizard Island, Hoffmann et al. 2009, fig. 2; Lord Howe Rise, this study, Figs 5, 6).
Figs 4 and 6 show the value of ontogenetic series from a single locality. In both species figured herein, mid-sized and adult specimens might be identified as separate species if they were found individually and from different localities. For example, the dorsal valves in Fig. 4d, f and the dorsal valves in Fig. 6c, j.
Conclusions
A new species, Thecidellina lueteri, is described from the Kermadec Islands, raising the number of Thecidellina species in the Pacific to six. The ontogenetic series figures of both species show that the internal morphology of late stage adult dorsal valves may differ significantly from that of young adult dorsal valves from a single locality.
Acknowledgements
My thanks to Daniel Leduc of NIWA, Wellington, for help with the SEM; Dennis Gordon, NIWA Wellington, for the gift and loan of specimens; and reviewers Daphne Lee and Donald MacFarlan for their helpful comments on the manuscript.
References
Baker PG, Carlson SJ (2010) The early ontogeny of Jurassic thecideoid brachiopod and its contribution to the understanding of thecideoid ancestry. Palaeontology 53(3), 645-667.
| Crossref | Google Scholar |
Baker PG, Logan A (2011) Support from early juvenile Jurassic, Cretaceous and Holocene Thecideoid species for a postulated common early ontogenetic pattern in Thecideoid brachiopods. Palaeontology 54(1), 111-131.
| Crossref | Google Scholar |
Bitner MA (2007) Recent Brachiopods from the Austral Islands, French Polynesia, South-Central Pacific. Zoosystema 29(3), 491-502.
| Google Scholar |
Brook FJ (1998a) Stratigraphy and paleontology of Pleistocene submarine volcanic‐sedimentary sequences at the northern Kermadec Islands. Journal of the Royal Society of New Zealand 28(2), 235-257.
| Crossref | Google Scholar |
Brook FJ (1998b) The coastal molluscan fauna of the northern Kermadec Islands, Southwest Pacific Ocean. Journal of the Royal Society of New Zealand 28(2), 185-233.
| Crossref | Google Scholar |
Clark MR, Roberts CD (2008) Fish and invertebrate biodiversity on the Norfolk Ridge and Lord Howe Rise, Tasman Sea (NORFANZ voyage, 2003). New Zealand Aquatic Environment and Biodiversity Report No. 28. 131 pp. Available at https://docs.niwa.co.nz/library/public/NZAEBR28.pdf
Cooper GA (1954) Recent Brachiopods. In ‘Bikini and nearby atolls. Part 2. Oceanography (biologic)’. Geological Survey Professional Paper 260–E, F, G. pp. 315–318. (US Government Printing Office: Washington) Available at https://pubs.usgs.gov/pp/0260e/report.pdf
Duffy CA, Ahyong ST (2015) Annotated checklist of the marine flora and fauna of the Kermadec Islands Marine Reserve and northern Kermadec Ridge, New Zealand. Bulletin of the Auckland Museum 20, 19-124.
| Google Scholar |
Elliot GF (1953) The classification of the thecidean brachiopods. Journal of Natural History 6(69), 693-701.
| Crossref | Google Scholar |
Elliot GF (1958) Classification of the thecidean brachiopods. Journal of Paleontology 32(2), 373.
| Google Scholar |
Hayasaka I (1938) A new Neotreme genus of Brachiopoda from Japan. The Venus 8(1), 9-13.
| Google Scholar |
Hedley C (1899) Mollusca of Funafuti, part 2, Pelecypoda and Brachiopoda. Memoirs of the Australian Museum 3(80), 508-510.
| Google Scholar |
Hoffmann J, Lüter C (2009) Shell development, growth and sexual dimorphism in the Recent thecideide brachiopod Thecidellina meyeri sp. nov. from the Lesser Antilles, Caribbean. Journal of the Marine Biological Association of the United Kingdom 89, 469-479.
| Crossref | Google Scholar |
Hoffmann J, Klann M, Matz F (2009) Recent thecideide brachiopods from the northern Great Barrier Reef, Australia (SW Pacific Ocean). Zoosystematics and Evolution 85(2), 341-349.
| Crossref | Google Scholar |
Lee DE, Robinson JH (2003) Kakanuiella (gen. nov.) and Thecidellina: Cenozoic and Recent thecideide brachiopods from New Zealand. Journal of the Royal Society of New Zealand 33(1), 341-361.
| Crossref | Google Scholar |
Logan A (2004) Ecological, reproductive and ontogenetic features in Pajaudina atlantica Logan (Thecideidae, Brachiopoda, Recent) from the Canary Islands. Marine Ecology 25, 207-215.
| Crossref | Google Scholar |
Logan A (2008) Holocene thecideide brachiopods from the north-western Pacific Ocean: systematics, life habits and ontogeny. Systematics and Biodiversity 6(3), 405-413.
| Crossref | Google Scholar |
Logan A, Baker P (2013) The development and shell microstructure of the pseudodeltidium and interarea in thecideide brachiopods. Palaeontology 56(2), 433-455.
| Crossref | Google Scholar |
MacFarlan DAB, Bradshaw M, Campbell HJ, Cooper RA, Lee DE, MacKinnon D I, Waterhouse JB, Wright AJ, Robinson JH (2009) Phylum Brachiopoda, lampshells. In ‘New Zealand Inventory of Biodiversity. Volume One. Kingdom Animalia. Radiata, Lophotrochozoa, Deuterostomia’. (Ed. DP Gordon) pp. 255–267. (Canterbury University Press: Christchurch)
Robinson JH (2021) Description and figures of new lectotype and paralectotype material of Recent brachiopod Thecidellina maxilla (Hedley, 1899). Records of the Australian Museum 73(5), 137-146.
| Crossref | Google Scholar |
Robinson JH, MacFarlan DAB, Waterhouse B (2023) Chapter 15. Kingdom Animalia, phylum Brachiopoda (lamp shells). In ‘The Marine Biota of Aotearoa New Zealand. Updating our marine biodiversity inventory’. (Eds M Kelly, S Mills, M Terezow, C Sim-Smith, W Nelson) pp. 239–254. (NIWA Biodiversity Memoir 136) Available at https://docs.niwa.co.nz/library/public/NIWAbm136-ch15.zip
Simon E, Hoffmann J (2013) Discovery of Recent thecideide brachiopods (Order: Thecideida, Family: Thecideidae) in Sulawesi, Indonesian Archipelago, with implications for reproduction and shell size in the genus Ospreyella. Zootaxa 3694(5), 401-433.
| Crossref | Google Scholar | PubMed |
Simon E, Lüter C, Logan A, Mottequin B (2018) Recent thecideide brachiopods (Thecideida, Thecideoidea) from northern Sulawesi (Indonesia) with discovery of a new Thecidellina species (Thecidellinidae). Zootaxa 4526(4), 481-515.
| Crossref | Google Scholar | PubMed |