Grazing management of Australian native woody regeneration as an effective nature-based climate-change solution
C. M. Waters A B * , R. B. Hacker C , A. Sekaran B and A. R. Grant DA
B
C Present address:
D Present address:
Abstract
Rangelands are playing a major role in delivering ~50% of the Australian land-sector abatement across some 42 million ha. Carbon credits are an incentive for the regeneration of native woody vegetation through grazing management that remove past suppression activities preventing the establishment of forest cover.1 Although there are divergent views on the use of grazing management as a credible land management activity and carbon market mechanism, guidelines to support effective woody regeneration outcomes are lacking. We review the literature, adopting a case-study approach for the semi-arid rangelands in south-eastern Australia, asking ‘What is the capacity for grazing management to influence patterns of woody recruitment and growth’? The role of grazing in the context of woody plant encroachment and life stages of regeneration for mulga (Acacia aneura) were examined. We identify climate as the primary driver setting the potential for carbon accumulation and outline the capacity of grazing management to directly and indirectly affect temporal patterns of accretion, to influence initial site condition, germination and establishment, growth and mortality. Grazing management will determine the direction and pattern of carbon accumulation by influencing (i) the size of the seed pool available to commence the regeneration process and buffer the effects of preceding fire on seed production; (ii) the functionality of the landscape, suitability of seedbed conditions and resource retention; (iii) the successful establishment of woody seedlings through herbivory or trampling; (iv) the growth rate of young plants and time required to reach reproductive maturity or forest canopy height; and (v) fuel availability and the capacity to manage fire. On the basis of this information, we develop broad grazing management principles and guidelines. The question is therefore not about whether specific grazing management is essential to allow regeneration to occur, but the extent to which grazing management can allow the potential set by climate to be realised.
Keywords: carbon, grazing management, livestock impacts, mulga, nature-based solution, seedling survival, woody plant encroachment, woody regeneration.
Introduction
The 2019 IPCC Climate Change and Land report identified a critical reliance on afforestation and reforestation to achieve almost all mitigation pathways that would limit global warming to 1.5°C (IPCC 2019). Nature-based solutions (NbS) such as afforestation and reforestation are recognised as one of the most cost-effective climate mitigation approaches (Griscom et al. 2017). Although at a global scale, vegetation-based carbon sinks can provide an estimated 30% of the required CO2 mitigation to meet 2030 targets, the relative influence of climate and land management in achieving carbon sequestration has been less clearly identified. For example, the role of forest and grazing management in realising global biomass potential has been shown to be considerable, comparable in magnitude to emissions arising from deforestation (Erb et al. 2018).
Australian arid and semi-arid environments are characterised by ‘pulse’ growth events following periods of above-average rainfall (Kenny and Moxham 2022) and play an important role in the global carbon cycle through vegetation productivity (Wang et al. 2023) and carbon fluxes (Cleverly et al. 2016). Here, Australian climate variability can result in large fluxes in carbon sinks and sources, with arid and semi-arid environments contributing up to 60% of the interannual variability in the global carbon cycle (Poulter et al. 2014). Understanding the relative influence of ‘natural’ events and ‘human-induced’ land management activities is of central importance in the policy design of NbS to ensure credible land management activities are incentivised and climate mitigation is real.
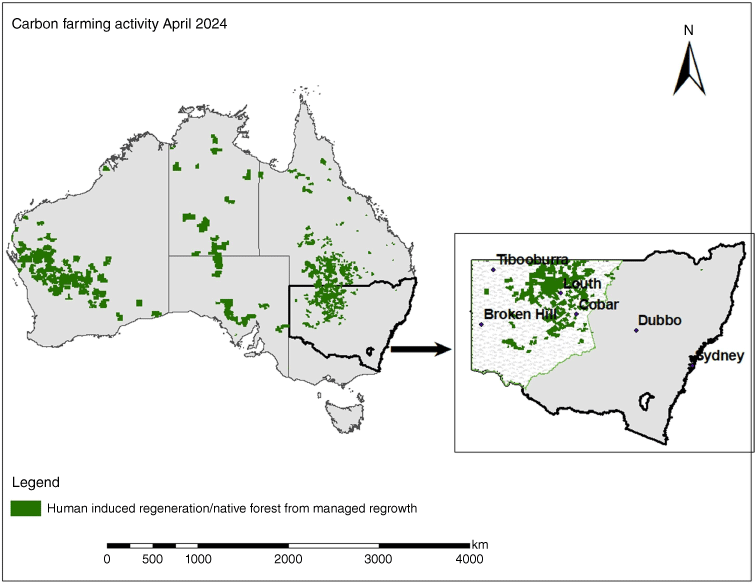
Australia’s commitment to reduce national greenhouse-gas emissions by 43% below 2005 levels by 2030 and achieve net zero emissions by 2050 was recently established through the Climate Change Act 2022. These commitments are currently being met, in part, by the Australian Carbon Credit Unit (ACCU) Scheme that provides incentives for a range of activities, including the management of vegetation-based forest growth. Eligible activities are defined by rules set out in ‘methods’, which ensure emissions reductions are real and in addition to business-as-usual operations. Approximately 50% of the Australian land-sector abatement has generated approximately 45.8 million ACCUs across >42 million ha almost exclusively within semi-arid or arid rangelands (Fig. 1). These carbon credits are being delivered using two methodologies that revolve around the regeneration of native forests, the ‘Human-Induced Regeneration of a Permanent Even-aged Native Forest’ (HIR) and ‘Native Forest from Managed Regrowth’ (NFfMR) (CER 2024). Both methods vary in eligibility criteria, but both essentially incentivise the regeneration of native woody vegetation through management changes that remove past suppression activities preventing the establishment of forest cover. Primarily, these activities will involve new grazing management activities (for domestic and/or feral animals) or the cessation of mechanical clearing (Baumber et al. 2020; CER 2024).
Spatial extent of registered carbon farming activities under the 'Human-induced Regeneration' and 'Native Forest from Managed Regrowth' methods. Areas represent property boundaries and thus overestimate the total of >42 million ha currently being managed for carbon sequestration as carbon estimation areas are often a subset of areas within each property.
The capacity of grazing management to influence woody regeneration in these arid and semi-arid environments has recently been challenged, sparking debate over the use of grazing management as a credible land management activity that can underpin carbon market mechanisms (Beare and Chambers 2021; Macintosh et al. 2022a, 2022b, 2024; Eldridge and Sala 2023). These divergent views have arisen from studies of the tree–grass balance in relation to woody plant encroachment. Here, overgrazing (persistent grazing pressure) has been implicated in the removal of competition between grasses and trees, creating opportunities for woody plants to establish and persist. This has led some to suggest that the removal or reduction of livestock grazing will not result in increased regeneration and growth of woody plants (Eldridge and Sala 2023). However, herbivory has been shown to suppress woody regeneration, with multiple studies showing an increase in woody plant cover with livestock exclusion (see Retallack et al. (2024) for Australia and Su et al. (2015), and references therein, for China). Hence, it remains unclear how grazing management may influence the woody regeneration outcomes.
Studies of the tree–grass balance have traditionally been undertaken through either competition-based or demographic models. The former (e.g. Walker and Noy-Meir 1982) explains tree–grass equilibria in terms of competition between woody plants and grasses where a range of equilibrium states is feasible, determined by the interaction between rainfall amount and seasonality, and soil properties, particularly depth and texture, modified by herbivory. The alternative demographic approach considers the impact of biotic and abiotic factors (e.g. herbivory, fire, climate) on the vital traits of woody species as they transition through life stages from seeds to mature trees. In this review, we provide an assessment of the capacity of grazing management to influence woody regeneration by using the demographic approach. This approach will allow an examination of how strategic or tactical grazing that recognises critical regeneration and growth traits of woody plants may serve to provide opportunities for carbon sequestration in vegetation as well as livestock production.
As a NbS, forest regeneration has been identified as providing the largest, long-term (2050), economic sequestration potential in Australia compared with other approaches in the technology pipeline (Fitch et al. 2022). Despite carbon farming being a significant new land-use option for Australian semi-arid rangelands with socio-economic co-benefits (Cowie et al. 2019; Baumber et al. 2020), there is a lack of locally relevant, evidence-based grazing management actions that may achieve native forest regeneration and an absence of clear guiding principles for grazing management to influence forest regeneration. Given the cost-effective advantages of forest regeneration, and the immediate reliance on this approach to meet Australia’s greenhouse-gas emissions reduction policy, these principles can support a more nuanced approach for grazing management to influence forest regeneration.
In this review, we aim to:
provide a comprehensive synthesis of the literature to understand the capacity for grazing management to influence patterns of woody recruitment and growth; and
develop a set of guiding principles that would result in effective grazing management that allows native forest regeneration.
We define grazing management as ‘the manipulation of the soil–plant–animal complex of the grazing land in pursuit of a desired result’ (Allen et al. 2011, p. 13). This is considered to encompass the manipulation of the frequency, timing and intensity of livestock grazing, management of the herbivory exerted by non-domestic herbivores, management of fire, and changes to infrastructure intended to allow these manipulations to achieve native forest regeneration.
Initially, we examine the role of grazing in the context of woody plant encroachment. We then use a case study of the semi-arid rangelands in south-eastern Australia and review the literature identifying the key biological characteristics at critical life stages of regeneration for a major palatable native plant, mulga (Acacia aneura F.Muell. ex Benth), a dominant or co-dominant species across much of the case study region. The evidence for grazing management impact at each stage is reviewed. On the basis of a synthesis of this information, we then develop broad grazing management principles and practical guidelines that may result in feasible and effective management of forest regeneration in semi-arid environments and provide a viable NbS to address climate change.
Woody plant encroachment and the role of grazing
Major drivers of woody plant encroachment
In this review, we define woody plant encroachment (WPE) as an increase in the abundance of indigenous woody vegetation (Van Auken 2009). WPE is a defining feature not only of arid and semi-arid environments in Australia (Harrington 1979; Noble 1997), but also elsewhere across the globe (Scholes and Archer 1997; Archer et al. 2017). Where it occurs, WPE is associated with ‘land degradation’ reflected in reduced pastoral productivity (Noble 1997) and declining landscape function (Archer 2009). Although WPE is widely associated with overgrazing by domestic livestock (Archer et al. 2017), it is recognised as one of several factors driving woody recruitment and successional processes. It is therefore important to understand the role of grazing land management in the context of other drivers.
Climate is recognised as a primary driver of WPE, but reduced fire frequency and climate change also play important roles (Harrington 1979; Noble 1997; Eldridge et al. 2011; Archer et al. 2017). Witt (2013), using local knowledge from land managers in south-western Queensland, identified climatic extremes that lead to episodic recruitment and mass mortality events, with total grazing pressure and the absence of fire as the most important factors in WPE. Whereas there may be some agreement between the science and practice in the major influencing factors of WPE, they can often be interacting (Scholes and Archer 1997; Throop and Archer 2007; Witt 2013), resulting in debate over the relative importance of each, including grazing.
Multi-decadal Australian climatic extremes have been shown to result in mass tree and shrub recruitment events during periods of high rainfall (Noble 1997; Watson et al. 1997; Witt 2013; Kenny and Moxham 2022) and mass mortality during protracted droughts (Fensham et al. 2019; Godfree et al. 2019). While episodic seedling recruitment and mortality driven by wet and dry cycles may appear spectacular, ongoing ‘background’ recruitment continually occurs for many woody species, with some recruits entering the population even in dry years, but most spectacularly following heavy rains at the appropriate time (Brown 1985; Gardiner 1986; Burrows et al. 1988; Booth et al. 1996; see also Brown and Archer 1999). This is likely a ‘hedge-betting’ adaptive strategy of woody species in Australian arid (Watson et al. 1997) and semi-arid environments (Kenny and Moxham 2022).
As future climate scenarios predict an increase in the frequency of Australian droughts by as much as −6 to +64% (Jenkins and Warren 2015), the requirement for grazing management to be adaptive, responding to recruitment opportunities during post-drought recovery periods and to minimise negative impacts during periods of drought, is emphasised.
Apart from an increased frequency and intensity of climatic extremes, climate change has also resulted in increased concentration of carbon dioxide in the atmosphere. The role of elevated CO2 (eCO2) in driving enhanced photosynthesis and ‘greening’ trends in semi-arid areas has been highlighted (Poulter et al. 2014; Rifai et al. 2022). However it is unclear whether these trends reflect changes in woody or grass cover (Rifai et al. 2022). Van Auken (2009) noted that the period of greatest WPE in North American grasslands (1850–1870) does not coincide with the higher concentrations of eCO2 found today and argued that eCO2 concentrations are therefore unlikely to play a major role in the stimulation of WPE. Free-air CO2 enrichment (FACE) studies have provided empirical evidence of the impacts of eCO2 on tree growth, with increases of 23–30% in net primary productivity being reported for temperate forests (Norby et al. 2005; McCarthy et al. 2010). More recent FACE experiments relevant to Australian environments have shown that the magnitude of this effect is dependent on nutrient availability and can be dampened when nitrogen (Norby et al. 2010) or phosphorus (Ellsworth et al. 2017) availability is limited, a situation which is typical of Australian soils. Therefore, the magnitude of eCO2 on tree growth in Australia remains uncertain, but is likely to have some enhancement effect.
Changes in fire regimes have fundamentally altered the structure and composition of arid and semi-arid rangelands (Harrington 1979; Noble 1997; Scholes and Archer 1997). Disturbance such as grazing-induced loss in perennial grass cover, reduces fuel loads and fire frequency, resulting in an altered competitive balance between tree and grass in favour of woody plants (Hodgkinson and Harrington 1985; Archer et al. 2017). In altering the tree–grass competition, grazing rather than being a primary cause of WPE, appears to play a modifying role (Van Auken 2009).
It is difficult to separate the effects of seasonal conditions from those resulting from grazing herbivores, either directly as a loss of perennial grass cover or indirectly through WPE (Silcock and Fensham 2013; Zhu et al. 2016; Archer et al. 2017). This has resulted in a general acceptance that processes and drivers of WPE are likely to be a result of multiple, interacting factors (Eldridge et al. 2011; Archer et al. 2017) where site condition, grazing management histories, soil, topography and landform may each modify these drivers at a local scale. For example, edaphic characteristics can control the effectiveness of rainfall where topography (slope) influences the lateral distribution of water by its influence on runoff and run-on (Wu and Archer 2005). Where grazing impacts have been assessed, this has often been undertaken without consideration of confounding effects of local abiotic and biotic factors (Milchunas and Lauenroth 1993; Maestre et al. 2022). This has led to a general acceptance that ‘robust generalisations’ around the causes of WPE are elusive (Archer et al. 2017; Mochi et al. 2022) with climate, elevated CO2 concentrations, fire regime as well as grazing being implicated and associated with positive, neutral and negative effects on WPE (Scholes and Archer 1997; Archer et al. 2017).
Although rates of woody recruitment, dynamics and patterns of successional processes may be dependent on interacting factors, location-specific factors related to climate, fire frequency, grazing intensity and edaphic site characteristics (Eldridge et al. 2011; Muňoz-Robles et al. 2011a), as well as plant functional traits, may also dictate species-specific, local responses to the major drivers of WPE (Brown and Archer 1999; Eggemeyer and Schwinning 2009; Archer et al. 2017). This has been illustrated in contrasting results from several studies. Kenny and Moxham (2022) highlighted the importance of mass recruitment in above-average rainfall seasons where it coincides with low grazing pressure, suggesting that it is a combination of both the ideal climate conditions for recruitment and a lack of grazing pressure that set the conditions for enhanced recruitment. Watson et al. (1997) reported that low rates of background, continuous recruitment were at least as important as larger episodic recruitment events in driving population dynamics for both Eremophila maitlandii and E. forestii in arid environments.
Grazing can reduce seedling densities of woody plants directly through herbivory, which may be significant enough to change the population dynamics of the woody plants (Travers et al. 2019). Contrasts of pastoral and conservation land use (where herbivores have been excluded) have shown a 1.1% increase in non-photosynthetic vegetation (woody component as well as senescent vegetation) under conservation, providing clear evidence of a management effect in arid rangelands (Retallack et al. 2024). In this latter study, vegetation community composition and condition as well as position in landscape were both identified as important factors in determining grazing impact. Recognising and accounting for the local factors influencing woody plant regeneration is thus of central importance in understanding how grazing may influence the trajectory of woody regeneration.
| Summary. WPE is primarily driven by climate, and while ongoing background woody seedling recruitment occurs, episodic recruitment and mortality is a response to extreme climatic events that produce wet and dry cycles. Seasonal conditions may therefore set the preconditions for the population dynamics of woody plants, but regeneration outcomes may be modified by other drivers. Processes and drivers of WPE are likely to be a result of multiple, interacting factors such as site condition, grazing management histories, soil, topography and landform, which may each modify vegetation responses at a local scale. |
| WPE can be both a response to, and a cause of, ‘land degradation’ and is viewed as a symptom of overgrazing only where persistent and intense grazing pressure removes perennial grasses and alters fire frequencies and the competitive balance between woody and herbaceous species. Contrasts between pastoral and conservation areas provide clear evidence that the removal of livestock can influence this balance and result in regeneration of woody vegetation. |
Herbivore dietary selectivity
The extent to which herbivores directly affect trees and shrubs in attempting to meet their daily intake requirements will depend on the dietary preference of the herbivore, which is largely driven by the palatability of species. However, herbivore impact needs to be considered in the context of the relative availability of alternative forage, which will change with vegetation community type, season and climate variability. Pahl (2020) illustrated this well, providing a comprehensive assessment of the sequential utilisation of forage for different herbivores under contrasting seasonal conditions for major vegetation communities in southern Australian rangelands.
Pahl (2020) emphasised that many past studies of herbivore dietary composition reported the abundance of plant species in herbivore diets without accounting for the relative abundance of alternative forage, making any dietary preference hierarchy qualitative rather than quantitative. An adaptation of dietary preference hierarchy under wet and dry seasonal conditions for popular box–mulga woodlands is provided in Supplementary Fig. S1. Here, the proportion of woody vegetation in the diets of sheep and cattle may increase under increasingly dry conditions. Where palatable woody species such as mulga (Acacia aneura) are a dominant component of the vegetation, cattle diets will include moderate levels of woody vegetation (Pahl 2020). A radiocarbon dating study by Witt et al. (2000), assessing sheep diet from the mid-1930s to mid-1990s by using deposits of sheep dung from shearing sheds, showed that sheep dung contained 10–30% of Acacia (likely A.aneura and A. cambagei) over all these years, suggesting a significant intake of mulga by sheep.
Goats will ordinarily consume a greater proportion and a wider range of tree and shrub species than do either sheep or cattle, with Acacia aneura and Dodonea viscosa contributing up to 30% and 83%, of goat diets respectively (Squires 1980). However, when pasture biomass falls below 180 kg ha–1, shrubs and trees may also contribute a large proportion of sheep (22%) and cattle (34%) diets (Squires 1980). A ~4-year grazing study in south-western Queensland also showed that during dry conditions sheep diets contained a higher proportion of mulga (35%), compared with <6% usually present in sheep diets (McMeniman et al. 1986). In the study of Kenny and Moxham (2022), arid woody species considered as ordinarily unpalatable were exposed to herbivory by rabbits when alternative forage was unavailable. The impact of rabbits and goats on woody vegetation can be exacerbated by selective grazing behaviours that suppress the seedling growth of some woody species in a targeted manner. For instance, Munro et al. (2009) found that even low densities of rabbits suppressed mulga establishment, a finding replicated in several other studies of arid-zone woody species. The selective browsing behaviour of goats in targeting the seedlings of palatable species such as mulga and cypress pine is evident in the field but undocumented; however, goat browsing of unpalatable shrubs during dry conditions has been reported (Harrington 1986). Kangaroos are likely to pose a direct threat only to woody plant regeneration in the seedling stage by trampling or herbivory (Travers et al. 2019) and only under extreme dry conditions when there is little alternative feed on offer (Pahl 2020). The consequence of selective grazing behaviour is that near total exclusion of goats and sheep (and potentially Dorper sheep, see below) may be necessary to achieve establishment of palatable woody species as even low numbers may suppress growth. The requirement for exclusion of cattle is less clear (also see below).
When there is an abundance of feed, herbivores may exercise their greatest dietary selectivity, although goats, sheep, cattle and macropods will overlap in ephemeral forbs and green perennial grasses (Pahl 2020). However, as seasonal conditions become dry, goats, sheep, rabbits and cattle will have an increasing proportion of browse in their diets (Dawson and Ellis 1994; Pahl 2020). This highlights the importance of accounting for the prevailing local seasonal conditions when considering the impacts of grazing on both woody regeneration and growth.
| Summary. Different herbivores will have different dietary preferences. Goats will consume a greater proportion and a wider range of tree and shrub species than do either sheep or cattle. However, what herbivores eat will depend on the relative abundance of alternative forage. Here, feed is consumed sequentially according to a preference hierarchy specific to the herbivore type. Dietary preferences are most obvious when feed is abundant. Under these conditions, the proportion of woody vegetation such as mulga may contribute up to 30% of the diets of goats and sheep. Under increasingly dry conditions when little alternative forage is available, the proportion of woody vegetation in the diets of both cattle and sheep will increase. The consequences are that under deteriorating seasonal conditions sheep, goats, rabbits, as well as cattle, will each have the potential to consume greater amounts of woody vegetation than usual. |
Herbivore grazing behaviour
Grazing impact will be determined by how far herbivores can travel to water and how often they need to water. This varies among types of herbivore, recognising that water demands are driven by temperature, animal physiology and body condition. However, the most important factor will be the availability of forage (James et al. 1999; Fukuda et al. 2009).
Waterpoints act as a focal point for herbivores. The piosphere effect reflects the radial grazing pattern created where grazing impact is greatest close to water and decreases with distance from water (James et al. 1999). Studies have shown high grazing intensity near water points results in vegetation compositional shifts from palatable perennial species to increased annual species (Friedel et al. 2003; Landsberg et al. 2003; Hendricks et al. 2005), accompanied by decreased species diversity (Hendricks et al. 2005; Todd 2006) and increased soil erosion (Tongway et al. 2003; Tabeni et al. 2014). However, results can be dependent on the size of the paddock relative to the number of water points as well as the number and type of herbivores and the distribution of land types.
The distance travelled by cattle to water can vary up to 20 km (James et al. 1999), but most time (90%) will be spent grazing within a 3 km radius (Hunt et al. 2007; Crowley 2015). Others have reported cattle grazing within a radius of ~5 km (NSW DPI (Department of Primary Industries) 2014) and up to 10 km, with 80% of the grazing occurring within 2 km of water (MLA 2024). This contrasts with sheep and goats that can forage 3 km from water (James et al. 1999; Letnic et al. 2015). Domestic livestock and feral goats are more dependent on water than are kangaroos. Red kangaroos and Euros have been reported to have a much larger range than domestic livestock and goats (James et al. 1999). Other factors will alter or distort the pattern of the piosphere effect such as the placement of fences, dietary preferences, and the amount of shade available to herbivores (James et al. 1999).
| Summary. The location and access to waterpoints is an important factor in determining grazing behaviour. Cattle may graze up to 5–10 km from water, compared with sheep and goats that will graze within 3 km from water. Consequently, within 3 km from water, total grazing pressure can be more concentrated and the impact on both pasture and woody vegetation may be amplified. This highlights the importance of water-point management and infrastructure in influencing the grazing behaviour of herbivores. |
Case-study region
The study area is situated in western New South Wales (NSW) in south-eastern Australia and supports extensive pastoral activities that include the management of domestic and feral animals. Much of the study region has been characterised by WPE over multiple decades and clearing of woody plants. Mulga (Acacia aneura) is a long-lived perennial tree species that grows between 5 and 10 m tall (Crisp 1978) and has an extensive distribution range across the study region. Mulga is also common across southern Australian rangelands where it is either a dominant or co-dominant species (Randell 1992). Across its geographic range, mulga exhibits a high degree of polymorphism that largely relates to differences in leaf morphology (Partridge 1996). Mulga is regarded as a valuable resource to the pastoral industry particularly in the provision of drought forage (Casburn and Atkinson 2016), but is also a woody species subject to WPE.
Total grazing pressure
A defining feature of the study region is that pastoralism, through grazing management practices that persistently remove ground cover, combined with changes in the density of water points, has increased total grazing pressure (TGP) from managed (domestic livestock) and unmanaged native and feral herbivores (such as feral goats, kangaroos and rabbits) substantially altering the competitive balance between trees and grass (discussed above). TGP is a defining issue in southern Australian rangelands where substantial numbers of native and feral animal populations co-exist with livestock (Fisher et al. 2004; Hacker et al 2019); at times, the grazing pressure of non-domestic herbivores can represent almost twice that exerted by livestock alone (Waters et al. 2019). The impact of total grazing pressure is not just confined to pastoral areas but also conservation areas where both introduced and native herbivores can negatively affect tree and shrub recruitment (Bond and Keeley 2005; Prowse et al. 2019). In western NSW, a dingo/wild dog barrier fence excludes this predator and, consequently, the region experiences some of the highest kangaroo and goat densities in southern Australian rangelands (Waters et al. 2019).
Recently introduced ‘exclusion’ fencing, designed primarily to provide protection from dingos, is also effective in restraining unmanaged goats and kangaroos. Such fencing is sometimes erected as a boundary around several adjacent properties forming a ‘cluster’, although in western NSW exclusion fencing is more usually erected on a property-by-property basis. Within properties, particularly those without boundary exclusion fencing, less expensive ‘TGP fencing’ is commonly erected to control the movement of unmanaged goats and, to a lesser degree, kangaroos.
Where adequate control of feral goats cannot be achieved by fencing infrastructure, traps at watering points, or self-mustering facilities established for livestock handling, are an economically attractive means of reducing total grazing pressure and are considered a socially acceptable form of control (Sinclair et al. 2019). However, the presence of kangaroos may increase TGP to the point where goats or sheep are more likely to browse establishing woody plants.
Unmanaged or feral goats, kangaroos and rabbits, all have the potential to directly affect the stages of woody plant regeneration. Effects can be expected in relation to landscape function, seedling survival, and growth of regenerating species. Grazing management aimed at increasing woody regeneration will therefore need to consider the TGP to which a site is exposed, the non-domestic component of which may be greater than the livestock component (Atkinson et al. 2019).
Hardier sheep breeds
While TGP management is a defining feature of southern Australian rangelands, the increased prevalence of hardier (sheep) breeds such as the Dorper (Alemseged and Hacker 2014), and managed goat enterprises in lieu of opportunistic harvesting of feral populations (NSW DPI (Department of Primary Industries) 2018), has resulted in relatively recent changes in the enterprise mix of these areas. Hardier sheep breeds will have dietary preferences and feed requirements different from the traditional sheep and cattle, potentially modifying grazing patterns and the ability to manipulate woody growth.
In a review of largely South African studies, Brand (2000) compared Dorper, Merino and goat grazing behaviour and dietary preferences and found that Dorpers have less selective diets, utilising a greater number of plants than do Merinos. However, Dorpers have been reported to select more shrubs in their diets (35.7%) than do Merinos (13.9%) whereas Merinos selected more grass (86.1%) than did Dorpers (63.7%) (Roux 1992, cited in Brand 2000).
In a more recent Australian study, scat analysis employing DNA barcoding found diets of Dorpers to be more diverse than those of Merinos, but reported no statistical differences in diet selectivity between the two species because of the large variation in diet selection among individual Dorpers (Lee et al. 2018). These authors also reported goat diets to be less diverse than Merino and Dorper diets; however, they noted that the study coincided with good seasonal conditions and the abundance of feed may have allowed Merinos to access a wide variety of preferred feed. Brand (2000) also reported that Dorpers utilise a wide variety of herbage and browse, which enables them to walk shorter distances to feed than do Merinos, along with consuming less herbage mass than do Merinos (on the basis of equivalent metabolic weight), which may have implications for the spatial patterns of grazing but is yet untested.
Key biological characteristics of mulga (Acacia aneura)
As discussed above, livestock grazing can interact with climate, environment, and other native and feral herbivores to influence the population dynamics of woody species. To more deeply understand the influence of these interactions the following sections focus on how climate–environment–herbivory influence different life stages of mulga. When possible, the literature reviewed prioritises studies relevant to mulga within the study region. However, literature for other regionally important woody species, or similar rainfall zones in Australian states (QLD, Queensland; NT, Northern Territory; WA, Western Australia; SA, South Australia), as well as some relevant international literature, has been cited where appropriate.
Flowering and seed set
Mulga produces mature seed pods if sufficient rainfalls occur at the right times, with peak flowering resulting from good summer or winter rain. In a 1-year irrigation experiment involving 30 mulga trees in western NSW, Preece (1971a) found that most mulga (63–100%) flowered when winter (November) or late summer (March) rains exceeded 100 mm irrespective of irrigation, although light flowering occurred at any time. Seed production and specifically maturation occurs only with summer rains. In the same experiment, Preece (1971a) found that seed pods mature only following summer (January–March) rains, provided winter rainfall (May–August) in the same year exceeds 25 mm (Davies and Kenny 2013). This flowering–seed maturation cycle may take up to 10 months (Preece 1971a; Simmons 1987) and examination of historical rainfall records since 1890 suggests that seed set could be expected once every 6 years in western NSW (Preece 1971a).
Thus, mulga appears to have relatively specific seasonal requirements for seed production. The strong climatic influence on mulga’s seed production is consistent with another study from NT, which concluded that the proportion of flowering or fruiting mulga trees is explained primarily by 6–7-month soil moisture (along with day length and temperature) (Friedel et al. 1994). Further, there is evidence of masting (profuse flowering and seeding) in mulga occurring from extremely high rainfall events (Wright and Zuur 2014). This dependence on rainfall contrasts with other regionally important woody species (Eremophila, Dodonaea and Senna), which have more seasonal flowering patterns and seed set in the winter–spring–summer period (Hodgkinson 1979; Cunningham et al. 1981).
Fire can have a profound effect on seed production and thus on the size of the seed pool available to initiate a regeneration process. Individuals that re-sprout after a fire may not produce seed for 5–10 years (Hodgkinson and Harrington 1985). Massive reductions in shrub seed production per hectare have been recorded immediately following a grass fire, with effects on seed production evident for at least 6 years (Harrington 1986). The fire history of an area being considered for a HIR project should be considered in relation to the likely size of the seed pool available to initiate the regeneration process.
Some evidence suggests that under heavy sheep stocking rates, fruit set can be affected directly through herbivory for unpalatable species, for example, Eremophila gilesii (Burrows 1973). However, the experimental conditions of this latter study are unlikely to be feasibly achieved in extensive pastoral systems and it is improbable that grazing could directly influence flowering and seed set of woody species, and thus the seed supply. It is likely that indirect effects of grazing could influence the size of the seed pool. Grazing of young regrowth may have the effect of producing smaller plants (Brown 1985), delaying the onset of flowering or the amount of seed set. However, we are not aware of published evidence that directly supports this. An indirect effect of grazing on the seed pool could also be exercised via an impact on seed predation. Mochi et al. (2022) found that grazing reduced predation on seed of the invasive woody species Vachellia caven by half in an Argentinian savanna, possibly owing to reduced refuge availability for granivores afforded by the lower biomass of grazed plots. In central Australia, seed predation by granivores reduced a massive seed pool pulse produced by a mass seeding event of slender mulga (Acacia aptaneura) in 2011, to very low pre-mast levels within 18 months (Wright and Zuur 2014). We are unaware of any published studies of the effect of grazing on seed predation, or seed pool size, for woody species in western NSW.
Germination and establishment
While rainfall can promote large seed pools in mulga, germination-ready seed pools can be considerably smaller. The initial quantity of mulga seeds depends on the amount of rainfall and is independent of tree size (diameter or height) (Wright and Fensham 2017). In a study site in the NT, a 651 mm annual rainfall (in an otherwise 280 mm MAR area) increased seed pools from 6.8 seeds per m2 to 1520 seeds per m2 (Wright and Zuur 2014). Seed pools may also be quickly depleted by horizontal and vertical seed movement and seed predation despite Hodgkinson (1979) suggesting it was unlikely that seed predation would have much effect on the size of future shrub populations.
A 7-year seed bank study in the NT showed that the high rainfall-mediated seed pools in the top 2 cm of soil persisted for only 18 months owing to these horizontal and vertical movements (Wright and Zuur 2014). In non-mast years, seeds collected in NSW had an average viability of 20% (Preece 1971a). Burrows et al. (1988) recommended a minimum of 40 seed trees per hectare to ensure that seed rain can cover the entire landscape, which suggests that past clearing patterns may be important in understanding the site regeneration potential.
Background germination rates in mulga average ~2%, even following a period of drought (Preece 1971b). However, any additional germination is strongly dependent on rainfall and temperatures. For example, during a drought year, <0.003 seedlings per shrub, compared with 0.8 seedlings per shrub following a wet year, have been reported (Wright and Fensham 2017; Wright et al. 2023). Mass germination events can occur following heavy rains (Hall et al. 1964). This dependence on rainfall is further supported by Anderson and Hodgkinson (1997) who found more mulga seedlings upslope (40–60%) than downslope (20–35%), reasoning that upslope areas retained more soil moisture. From observations of Preece (1971b), it is evident that follow-up rain is required within 3 months of seeding for successful mulga germination. Using historic climatic records from NSW and NT, Preece (1971b) concluded that successful germination may occur once in 8–9 years.
Fire can be a stimulant for mulga germination, but is not required for germination (Hodgkinson and Harrington 1985). In a fire-burn experiment in the NT, mulga germination rates were highest at temperatures of 70–100°C, irrespective of the heat duration (5–60 min) (Wright et al. 2016).
Average mulga establishment rates (proportion of surviving germinants) are low, ~50%; however, rates can be highly responsive to rainfall. Witt et al. (2011), in a series of old enclosure sites in south-western QLD, demonstrated that tree regeneration (<15 cm diameter) was reduced (4–7 individuals) at sites with lower mean rainfall (280–320 mm) than at those (2–182 individuals) with higher mean rainfall (420–480 mm). In a mulga shrubland in NSW, seedling densities increased from 1700 to 4200 per ha between March 1983 and September 1984, following a period of above-average rainfall (Hodgkinson and Harrington 1985). The establishment of woody seedlings (individuals <30 cm in height) in semi-arid and arid zones therefore appears to be a continuous process, with some recruits entering the population even in dry years, but is most spectacular following heavy rains at the appropriate time (Brown 1985; Gardiner 1986; Burrows et al. 1988; Booth et al. 1996; see also Brown and Archer 1999).
Although mulga establishment is primarily climate-driven, grazing can markedly reduce establishment rates. Livestock grazing may directly (herbivory or trampling) limit or prevent the recruitment and establishment of mulga and several other woody species in semi-arid areas. This has been documented in ‘several studies (Harrington 1979; Fensham et al. 2011; Witt et al. 2011). In another example, a 390-ha livestock and rabbit-proof reserve in SA showed no seedlings outside the reserve, whereas seedlings within the reserve survived and persisted for 30 years (Hall et al. 1964). Young mulga densities (<15 cm diameter) were much higher in ungrazed areas, averaging 1650 trees per hectare, than in macropod-only grazed areas with 196 trees per hectare and all herbivore grazed areas with 100 trees per hectare across three sites (Fensham et al. 2011). The effect of removal of all grazing pressure (full exclosure) on regeneration of mulga in south-western QLD was also demonstrated by Witt et al. (2011) at a series of old exclosure sites. Here, tree regeneration was reduced at sites with lower mean rainfall (290–310 mm) compared with those with higher mean rainfall (368–469 mm); however, the comparisons is complicated by the fact that two of the three lower-rainfall sites were fenced to exclude stock only rather than all herbivores.2 Moore et al. (2001) concluded on the basis of a modelling exercise that suppressing fire (which reduces biomass) and grazing can let mulga develop into dense stands.
Any direct impact of grazing on woody seedlings will depend on herbivore type. Sheep (Merinos) and goats are considered to be likely to affect woody seedlings severely. The effects of Dorper sheep are probably equally as severe as those of Merinos, perhaps more so, because their dietary habits are more akin to those of goats (Alemseged and Hacker 2014). Burrows et al. (1988) recommended heavy grazing by sheep in winter to control mulga seedlings in cleared areas, implying that sheep grazing directly affects mulga seedling cohorts. Pressland (1976) reported a much greater impact of sheep than of cattle on the density and height of young regenerating mulga. Cattle appear to be relatively benign in terms of their impact on woody seedlings (although Mochi et al. (2022) observed that their impact on woody seedlings in Argentina was sufficient to offset the benefit of grazing in reducing seed predation). Fensham et al. (2012) observed a marked fence-line contrast between dense mulga and open country in the Quilpie district in south-western QLD, which may have been attributed to differences in grazing histories. Here, currently dense mulga had been subjected to moderate grazing by cattle over the course of a recruitment event in the 1970s, whereas the now open country was continuously grazed by sheep. Grazing by cattle may therefore be an appropriate management practice, depending on seasonal conditions, to reduce competing growth and lower mortality of a woody seedling cohort; however, no local studies have specifically tested this hypothesis. Evidence of no impact from grazing has also been reported. In a study at Cobar, NSW, two 30.5 m2 plots were monitored four times between 1964 and 1972, one inside and one outside livestock exclosures (with sheep density of 1.7–2.8 per ha). Both plots were recorded with similar seedlings counts, two inside and one or two outside (Cunningham and Walker 1973).
Native and feral herbivores may also strongly influence establishment rates. The capacity of feral goats to destroy mulga seedlings, noted by Peeters and Butler (2014), probably extends to other woody species as well. Anecdotal evidence is available (Peeters and Butler 2014) to indicate that kangaroos may also destroy mulga seedlings, although their impact may be minimal (Travers et al. 2019), with little if any impact on larger plants (Griffiths and Baker 1966). Wilson et al. (1992) noted that rabbits ‘consume almost all seedlings of these [semi-arid zone tree] species’ (p. 64). Crisp (1978) observed that almost no regeneration of mulga occurs in the presence of rabbits. This high impact by rabbits is prevalent in other species. For example, Whipp et al. (2012) noted that in many regions of eastern Australia, Callitris recruited abundantly in the late 1800s before rabbit infestation, livestock grazing and drought, which disrupted recruitment until the 1950s when wetter conditions returned and control of rabbits by myxomatosis was achieved. Preece (1971b) concluded that germination of mulga should have occurred approximately once every 9 years in parts of western NSW in which mulga regeneration is absent. No explanation of this situation was provided but it would be reasonable to assume that herbivory by rabbits was at least partially responsible. Although rabbit abundance is currently low in the case study region due to biological control agents, vigilance against any increase in the population would need to be ongoing.
Peeters and Butler (2014) noted that plant establishment and growth may be retarded once soils have lost their A-horizon through erosion, and that restoration of mulga vegetation may be more problematic where there are large areas of bare ground. More generally, the issue concerns the extent to which landscape functionality limits the capacity of woody (or other) vegetation to germinate and successfully establish. This capacity will be limited in dysfunctional landscapes in which resources, particularly water and nutrients, are not retained within the local system. Grazing management has a central role to play in preserving functional landscapes or attempting to restore dysfunctional ones. For example, the maintenance or improvement of ground cover will be fundamental to the control of run-off and sediment production (Muňoz-Robles et al. 2011a, 2011b). Bean et al. (2015) demonstrated that landscape functionality was more important than (grass) seed availability in determining the regeneration of a degraded mulga landscape in western NSW.
Following germination, usually in the summer–autumn period, most woody seedlings can survive the first winter even if rainfall is not high. The fate of seedling cohorts, and recruitment into the established population, is primarily determined by the conditions experienced in the following summer (Booth 1986; Booth et al. 1996; Harrington 1991). If rainfall in the first summer is low, survival of seedlings may be dependent on the biomass of ground storey species competing for limited soil moisture. Alternatively, if the first summer is wet, woody seedlings may survive even in the presence of a competing ground storey. Competition from perennial grasses appears to be particularly important and was able to eliminate the entire seedling cohort of the woody narrow-leaf hopbush (Dodonaea attenuata) growing in relatively intact woollybutt (Eragrostis eriopoda) grassland (Harrington (1991), although the relative seedling drought tolerance of different woody species would probably influence this interaction. Burrows (1973), for example, demonstrated the greater drought tolerance of mulga seedlings than that of Eremophila gilesii (turkey bush). Harrington (1979) noted that removal of all herbaceous vegetation (equivalent to the destruction of perennial grasses by historic grazing as well as removal of ephemeral growth) markedly improved the survival of hopbush seedlings over summer, and regular trimming of the ground layer (equivalent to a high level of utilisation of both perennial grasses and ephemerals) significantly improved seedling survival for plots irrigated in late spring, although not for plots irrigated in early spring or autumn.
Although perennial grasses provide the strongest competition, growth of annuals in the winter following germination could also influence survival by reducing the amount of soil moisture available in the subsequent (dry) summer. In this situation, successful establishment of a woody seedling cohort may depend primarily on survival over the first summer and may be largely independent of the density of the established population. Moore et al. (2001), following Burrows (1973), did model mulga recruitment as a function of adult tree density, but noted that perennial grasses tend to out-compete mulga seedlings <0.3 m in height, so the relevance of competition from adult trees for establishment seems doubtful. This suggests that grazing can have indirect effects on germination and establishment by regulating the level of competition experienced by woody seedlings in the first summer, provided the seedlings themselves are not consumed by herbivores.
| Summary. Germination and establishment appear to be a critical growth stage where grazing can directly or indirectly influence establishment rates. It appears that the preservation of woody seedling cohorts must extend to protection from grazing by feral or native herbivores, including rabbits, goats and kangaroos, in addition to domestic livestock (total grazing pressure). |
Growth
Growth in mulga can be slow. Pot experiments have shown that seedlings grow at 59.5 mg g−1 day−1 (Atkin et al. 1999). In another SA study, the height of mulga seedlings inside a 390-ha livestock and rabbit-proof fence was found to increase at only 1.2 cm per year (Crisp 1978). Given these slow growth rates, reproductive maturity will be attained slowly. Although there is no consistency in the records, Beadle (1948; cited in Preece 1971a) reported that it takes 10 years for mulga to reach reproductive age, whereas Preece (1971a) noted that under glasshouse conditions, reproductive maturity occurred at 18–20 months. Munro et al. (2009) reported a 190 cm mulga flowering in a livestock-proof enclosure in SA.
Water availability can accelerate growth rates, in terms of height and diameter. In a study at Wanaaring, NSW, Cunningham and Walker (1973), monitoring mulga over 7.5 years, reported that 58% of height growth was explained by rainfall. In an artificial irrigation experiment with 30 trees in western NSW, Preece (1971a) found that control trees grew 15% of their original height, whereas irrigated trees grew at a mean of 35% of their original height, with this increase being directly proportional to the frequency of irrigation. In the NT, stem-elongation rates measured over 3 years at fortnightly intervals showed that when rainfall was <50 mm, stem-elongation rates were <0.1 mm per day, but when rainfall was >50 mm, the rates increased to 0.1–0.4 mm per day (Winkworth 1973). Contrary to the expectation that elevated CO2 concentration will increase growth rates, glasshouse experiments have shown no such response in mulga seedlings (Atkin et al. 1999; Evans et al. 2000).
Although growth is largely climate (rainfall)-driven, grazing can strikingly suppress growth rates in mulga. Within the study area, some studies have reported the effect of grazing on the growth of woody plants (Table 1). At Cobar, NSW, grazing reduced the height of mulga and pine to 53% and 39% respectively, of the growth of ungrazed plants, and maintained most of the initial population below the grazing height of sheep. For mulga, only 9 of 28 trees were able to grow beyond the ‘grazing height’ of 1 m after 7.5 years, whereas all 44 ungrazed mulgas were able to do so (Cunningham and Walker 1973). For pine, the corresponding figures were 5 of 11 for the grazed treatment and 15 of 18 for the ungrazed treatment. Sheep grazing had little impact on the unpalatable species, turpentine. In another study, ungrazed mulga at Arabella, south-western Queensland, had a growth rate comparable to that at Cobar, but the effect of grazing in suppressing height growth was even more marked; even at the lightest grazing rate (20% pasture utilisation), none of the seedlings grew in height, whereas ungrazed seedlings grew at 22 cm per year (Brown 1985). In the same study, stem diameters increased by approximately 300% in ungrazed plots but only by an average of approximately 25% under grazing. Over 90% of ungrazed trees had grown above sheep grazing height (considered to be 120 cm) after 4 years. Both goats and cattle have the capacity to access vegetative material beyond the reach of sheep and may therefore reduce growth, even if regenerating cohorts have grown beyond 120 cm. Cattle, in particular, may break down branches to access ‘leaf’ material.
| Species | Location/Period | Grazed | Ungrazed | |||
|---|---|---|---|---|---|---|
| Initial height (m) | Growth rate (m year−1) | Initial height (m) | Growth rate (m year−1) | |||
| (range, in parentheses) | (range, in parentheses) | |||||
| Mulga A (Acacia aneura) | Arabella (QLD) | <0.9 m | 0 C | <0.9 | 0.22 | |
| 1980–1984 | (~0 to 0.41) | |||||
| Mulga B (Acacia aneura) | Cobar (NSW) | Most <1 m | 0.12 | Most <1 | 0.23 | |
| 1964–1972 | (−0.008 to 0.43) | (0.09 to 0.51) | ||||
| White cypress pine B (Callitris glaucophylla) | Cobar (NSW) | Most <1 m | 0.09 | Most <1 | 0.22 | |
| 1964–1972 | (0.02 to 0.26) | (0.05 to 0.56) | ||||
| Turpentine B (Eremophila sturtii) | Cobar (NSW) | Most <1 m | 0.07 | Most <1 | 0.08 | |
| 1964–1972 | (−0.05 to 0.22) | (−0.008 to 0.17) | ||||
At a regional scale, Fensham et al. (2012) found that mean annual rainfall (MAR) was overwhelmingly important in determining above-ground biomass of mulga dry forest, with contributing effects from mean maximum temperature (negative) and soil depth (positive). Distance to the nearest watering point, used as an index of long-term grazing pressure, was not significant. The data set included several sites, at which grazing management had clearly affected above-ground woody biomass, and these probably accounted in part for the residual variability of the model. Although it is likely that mean annual rainfall would be found to be the dominant factor influencing above-ground biomass at regional scales even if a more sensitive measure of grazing management had been available, the result is not inconsistent with other published data (Table 1) indicating considerable potential for grazing management to influence growth and biomass accumulation at the site level. A feature of all these observations is the wide variation in growth rates among individual plants, with some individuals sometimes exhibiting a decrease in height when the average growth was positive.
| Summary. Rainfall has a strong positive effect on the growth of mulga, whereas grazing substantially reduces growth rates. Growth-rate reduction leads to both reduction of biomass as well as slowing the time taken to reach reproductive maturity, and, hence, grazing has the potential to substantially alter the population dynamics of this woody species. |
Mortality
Background mortality rates of mulga appear to be low, between ~0.3% and 0.58% (Crisp 1978; Davies and Kenny 2013), whereas drought-induced mortality may result in as much as ~50% mortality (Fensham et al. 2019; Godfree et al. 2019). Episodic, widespread drought-induced death of mulga and other woody species has been commonly reported for the semi-arid areas of eastern Australia (Condon 1949; Fensham and Holman 1999; Fensham et al. 2011, 2012, 2019; Godfree et al. 2019). Although climate may be a primary driver of mortality, local scale features such as position in the landscape can also modify mortality rates (Anderson and Hodgkinson 1997; Rossi et al. 2018). Here, downslope positions had greater mulga mortality rates than did upslope, particularly when grasses were present (Anderson and Hodgkinson 1997); however, both the orientation of the mulga vegetation patch and the relative proximity of bare patches appear to be important in modulating water availability (Rossi et al. 2018).
The role of grazing in adult mulga mortality is less clear. Fensham et al. (2012) found that recent tree mortality across the mulga lands of south-western QLD was related (positively or negatively) to measures of drought (3-yearly rainfall deficit), maximum temperature, soil depth and coarse sand content, but not to a grazing index represented by the distance of sites to the nearest watering point. Brown (1985), at Arabella, found no significant difference in mortality of non-seedling individuals between grazed and ungrazed populations across a wide range of grazing intensities.
Grazing can exacerbate drought-induced mortalities, especially of mulga saplings. In the study of Brown (1985), grazing had no significant direct effect on mortality but in one period of drought-related deaths, 22 of 23 deaths were recorded in grazed paddocks in which mulga growth had been strongly suppressed (Table 1). Brown considered that this suppression of growth may have contributed to mortality in dry periods by maintaining plants in a drought-susceptible state. Several studies have observed higher rates of mortality among smaller (juvenile) than larger (adult) plants. Cunningham and Walker (1973), in a study lasting 175 months at Wanaaring (within the study region), recorded higher mortality rates for plants <1 m (~2–44%) than for those >1 m (~0–17%).
Other factors that influence mortality are fire and patterns of clearing. It is repeatedly noted that mature mulga has a low fire tolerance (Cunningham et al. 1981; Latz et al. 1995) and reported rates of mortality are as high as 80–100% for adults in NSW (Hodgkinson and Harrington 1985). For susceptible species, intense fire is not required to produce tree death; less intense fires that result in scorching of all leaves are sufficient (Hodgkinson and Harrington 1985).
An indirect effect of grazing could arise through density-dependent mortality. Fensham et al. (2012) found no evidence of density-dependent effects at the regional scale in their analysis of drought-related tree deaths in south-western Queensland. However, they noted ‘widespread death’ in the dense mulga stand at Quilpie, described above. In western NSW, Cunningham and Walker (1973) found higher, drought-related mortality at their Wanaaring site than at Cobar, where tree density was much lower, and considered that ‘catastrophic death’ would appear to be a feature of more dense stands in dryer areas. Fensham et al. (2012) concluded that ‘while density-dependent mortality can occur in mulga it did not emerge as a dominant influence (at regional scales) and is probably less important than in eucalypt woodlands’ (p. 905). However, in local situations, grazing management could have the effect of allowing the development of dense stands that would be susceptible to subsequent drought.
| Summary. Mulga mortality rates are low. Large mortality events can occur from droughts and fires. On one hand, grazing could potentially intensify drought-induced mortality rates of the young size-class populations of mulga. On the other hand, grazing could aid in keeping fire frequencies low, which are otherwise detrimental to all size classes of mulga plants. However, it is imperative to note that dense stands as a result of WPE may have larger drought mortality than do less dense mulga stands; the threshold of which is unknown. |
Effect of fire on the demography of woody species
Seedlings of all woody species are susceptible to fire and the seasonal conditions that result in large germination events are likely also to produce the fuel loads capable of carrying an effective fire (900–1200 kg ha–1, Campbell and Hacker 2000) that could destroy most of the seedlings (Hodgkinson and Harrington 1985). Management of this fuel load in a way that reduces or eliminates the risk posed by fire to the initiation of a regeneration event, while preserving much of the seedling cohort, is an essential element in the management of regeneration. The potential for use of cattle for this purpose has been discussed previously.
Once past the seedling stage, the susceptibility of woody species to fire varies greatly and has been summarised in Nolan et al. (2019); however, as noted above, mature mulga has a low fire tolerance. For susceptible species, intense fire is not required to produce tree death. Death can be expected provided all leaves are scorched (Hodgkinson and Harrington 1985).
| Overall summary. Whereas the potential for regeneration of woody vegetation at a site will be determined by climate, grazing management has the potential to affect the temporal pattern of woody growth and, consequently, carbon accretion directly by influencing the dynamics of this process at critical stages of growth which include the following: and indirectly by influencing |
| Apart from the use of grazing in the long term to suppress fire, which is a current requirement under the HIR method for carbon accretion accounting, grazing management will primarily influence patterns of carbon accumulation in the early years of regeneration through its influence on the fate of woody seedling cohorts and the early growth of juvenile plants. Specific grazing management activities relevant to these stages include the following: |
Grazing management can influence the accumulation of carbon stocks at all stages of the regeneration process, particularly in the early years when regenerating woody plants are still within the grazing height of livestock or feral herbivores (Fig. 2). Grazing has the potential to influence the magnitude and pattern of carbon accumulation by the following:
|
Grazing management principles
On the basis of this review of the literature, we propose a series of principles, guidelines and applications for grazing management to support the promotion of vegetation-based carbon accumulation in HIR projects. These are modified from the principles and guidelines proposed by Hacker and McDonald (2021) for sustainable grazing management of the southern rangelands (Table 2). Although supported by current literature, these principles can be regarded as provisional and require verification by future research.
| Principle | Management guideline | Management application | |
|---|---|---|---|
| 1. Maintain landscape functionality to ensure suitable conditions for germination and establishment of woody seedlingsA | 1.1. Obtain the best available estimates of the DSE ratings for sheep (Merinos and Dorpers), goats, cattle and kangaroos and monitor total DSE over land units. | For all regeneration stages, adjust livestock grazing pressure tactically to maintain or improve ground cover. (H) | |
| For all regeneration stages, management of total grazing pressure, including control of non-domestic herbivores by appropriate fencing, water control or commercial harvesting of kangaroos. (H) | |||
| 1.2. Develop an estimate of the carrying capacity of the property for the current enterprise and current land condition, including the availability of browse, and express this as a benchmark value of DSE days ha–1 per 100 mm of (average) annual rainfall. | |||
| For all regeneration stages, utilise no more than 20%A of annual pasture production. (H) | |||
| 1.3. Estimate prospective short-term grazing capacity on the basis of seasonal climate forecasts and the carrying capacity benchmark (or some alternative approach such as a grazing chart). | |||
| 1.4. Develop land management objectives (considering biodiversity and production issues as well as resource condition) for each management unit and associated grazing management strategies defined in terms of variables that can be monitored as a basis for action, such as ground cover and utilisationA,B level of key species. | |||
| 1.5. Establish monitoring systems that will provide the input required for tactical grazing responses and for assessing progress towards the management objective (these will be different systems, based on different measurements or observations). | |||
| 1.6. Develop ‘trigger points’ (dates beyond which stocking decisions should not be delayed, Hacker et al. 2006) appropriate to the local environment to assist with management decision making. | |||
| 1.7. Manage non-domestic grazing pressure by appropriate and socially acceptable means (e.g. non-commercial culling of kangaroos by professional shooters, trapping and sale of unmanaged goats with no release of animals of no commercial value). | |||
| 2. Ensure the survival of woody seedling cohorts | 2.1. Use cattle to reduce ephemeral growth during the winter following germination, and competitive ephemeral or perennial grass growth during the first summer. | During germination and establishment, use cattle to reduce competing ground storey growth in the winter and first summer following germination. (L) | |
| 2.2. Exclude grazing by all other livestock, and by native or feral herbivores. | Following a germination and establishment event, exclude grazing by sheep and goats. (M) | ||
| 2.3. Establish fire breaks and/or use livestock to reduce fuel loads in carbon and adjacent areas to ensure that seedlings are protected from fire. | During germination and establishment, use fire breaks and/or grazing of surrounding areas to protect seedlings from fire. (H) | ||
| 3. Maximise early-stage growth to ensure woody species grow beyond grazing height as quickly as possible | 3.1. Exclude grazing by all livestock and feral herbivores until regenerating plants have grown beyond the maximum grazing height. | During germination and establishment, exclude grazing by all herbivores until regenerating cohorts exceeds grazing height of >1.3 m (sheep) or >1.5 m (goats and cattle). (H) | |
| Given mulga growth rates (approx. 22 cm per year) exclusion from grazing may be required for 6 years. (M) | |||
| 3.2. Exclude fire by establishing fire breaks and/or use of livestock to reduce fuel loads within areas being managed for carbon and adjacent areas. | Maintain the use of fire breaks and/or grazing of surrounding areas to protect seedlings from fire. (H) | ||
| 4. Develop infrastructure to allow best management of both domestic and non-domestic grazing pressureA | 4.1. Ensure that carbon project areas can be managed independently of areas retained for pastoral production. | At all stages of regeneration, fence carbon areas to allow management for carbon objectives. (H) | |
| 4.2. Use fencing specifications that provide the most cost-effective control of non-domestic grazing pressure.A | For all regeneration stages, use appropriate fencing and water-point infrastructure to allow strategic periods of pasture rest & woody regeneration by excluding all grazing, or manage total grazing pressure consistent with management objectives. (H) | ||
| 4.3. Fence according to land type as far as possible where new or replacement fencing is being erected.A | |||
| 4.4. Establish infrastructure at watering points that allows efficient mustering of livestock and humane control of access to water by non-domestic herbivores.A | |||
| 5. Maximise opportunities for long-term carbon accumulation | 5.1. Exclude fire by establishing fire breaks and/or use of livestock to reduce fuel load in carbon and adjacent areas (as for Guideline 3.2). | For all regeneration stages, use fire breaks and/or grazing of surrounding areas to protect carbon areas from fire (H) | |
| 5.2. Adjust grazing tactically to allow periodic recruitment of new woody plants to replace senescent individuals. | Facilitate background recruitment of woody cohorts by tactical adjustment of grazing that allows periodic recruitment of replacement plants. (M) | ||
| 5.3. Utilise no more than 20%A of the annual forage production for grazing (or ensure that utilisation of perennial grasses does not exceed 20%). | Graze with sheep or goats to reduce dense seedling cohorts that may result in high stem density stands (L) | ||
| 6. Ensure that long-term landscape integrity is maintained | 6.1. Ensure on-going monitoring of ground cover under forest canopy and adjust total grazing pressure as required to ensure that erosional cover thresholds are met. | For all regeneration stages, adjust livestock grazing pressure tactically to maintain or improve ground cover. (H) |
Effectiveness of grazing management
We have provided a set of principles for grazing management and specific guidelines for the management of vegetation specifically for carbon sequestration under the HIR method that are based on an examination of biophysical constraints and opportunities. It is important to recognise that the ability for pastoralists to implement these will be contingent on socio-economic factors, including land manager skills and capability as well as farm infrastructure.
Land manager skills and capability
The success of grazing management will depend on land manager skills, capacity and motivation to implement science-based strategies and can be expected to have a broad range of outcomes. For example, an evaluation of groundcover incentive programs conducted in the study area found wide variation in on-ground outcomes where land managers were financially supported to implement self-guided, improved grazing management through infrastructure upgrades and/or grazing management plans. Program evaluation that included quantification of outcomes through remotely sensed ground-cover estimates showed that of 170 projects where total grazing-pressure fencing was funded, 40% had distinctly positive outcomes (increasing trends in ground cover), 23% had mixed outcomes, 21% had neutral outcomes and 16% had poor outcomes (Local Land Services, unpubl. data, 2018). The positive outcomes demonstrated that infrastructure and grazing management could improve groundcover. The mixed or neutral outcomes potentially represented situations where management systems were inappropriate or inconsistently implemented. The negative outcomes possibly reflected situations where landholders failed to adopt management suitable for improving groundcover. This suggests that it is likely that relying on land managers to implement grazing management to facilitate woody regeneration may have uncertain outcomes. Better outcomes require ongoing support and relationship building from technical providers, flexibility in the implementation of plans and involvement of land manager in planning and evaluation processes (Swann and Richards 2016).
A regional benchmarking survey conducted in 2017 (Local Land Services, unpubl. data, 2017) showed that 60% of landholders had a ‘professional’ farming style, carefully considering alternative practices in their property management. Approximately, 50% of landholders recognised improved grazing management as a reason to make property operational changes; 35% indicated that they had undertaken recent (past 3 years) grazing/land management training. However, Ison and Russell (2000) broadly considered that land managers in the study region generally place a low value on knowledge derived off-farm. In relation to woody regeneration activities, this suggests that although some landholders remain open to grazing management training, and to changing their operations, there remains a large proportion of land managers that may be resistant to the adoption of new strategies.
Landholder capacity to adopt alternative grazing management strategies is determined by access to physical, human, psychological, financial, natural and social resources. Of these, the factors affecting an important component such as control of TGP include the availability of adequate finance for fencing, labour for construction and maintenance, the support of neighbours and contractors, good markets and income, as well as favourable seasonal conditions (Local Land Services, unpubl. data, 2017).
In reviewing the outcome of incentive funding processes, Swann and Richards (2016) considered that to achieve long-term outcomes in grazing management programs, specific emphasis must be placed on keeping landholders motivated beyond the initial ‘honeymoon’ period of project implementation. Landholders need to be involved from project planning to project evaluation stages, and be provided with relevant and engaging training, as well as ongoing advisory, information and extension support services.
Infrastructure factors
Because most parts of the study area are subject to the influence of unmanaged herbivores, investment in adequate infrastructure development is necessary to apply differential grazing management to carbon project areas. Traditional property infrastructure, such as plain wire fencing and open-access waterpoints, fails to provide control of grazing pressure from macropods, meat sheep and unmanaged goats, resulting in mediocre land condition and a susceptibility to drought, widespread in the region (Waters et al. 2019; Hacker and McDonald 2021). Achieving a suitable level of containment or exclusion to control grazing pressure according to vegetative growth requires the following:
Filling knowledge gaps
There is a lack of research that directly examines the relationship between grazing management and woody regeneration in the context of carbon sequestration. We identified the potential role of cattle in ensuring the survival of woody seedlings by reducing competition from ephemeral growth (particularly in winter) and perennial grasses (in summer), which is yet untested. However, insights compiled from studies of woody plant encroachment and the biological characteristics of woody species such as mulga provide a reasonably coherent account of management aimed at carbon sequestration in regenerating native forests. Although it ris not feasible to be prescriptive regarding specific guidelines for all species and major range types within the area being managed under the Human-induced regeneration methodology in western NSW, we consider that the six grazing principles identified here could be broadly applied.
Direct examination of our proposed principles of grazing management can be undertaken using several novel approaches to separate the impacts of grazing management from those of climate. For example, the use of remotely sensed photosynthetic/non-photosynthetic and ground cover to measure changes over temporal and spatial scales appropriate for arid and semi-arid environments has been shown to allow quantitative assessment of management impacts (Bastin et al. 2012; Hobbs et al. 2018; Retallack et al. 2023, 2024; McDonald et al. 2024). These approaches, as well as the application of LiDAR technologies (Moore et al. 2001), are providing unprecedented opportunities to precisely measure the outcome of grazing management at a local scale. These approaches will enable a more nuanced approach than, for example, global meta-analyses that mask the local-scale complexities and are insensitive to local interactions among vegetation communities, landscape and management context (see Eldridge et al. 2016; Maestre et al. 2022 for examples).
Conclusions
In the semi-arid rangelands of western NSW, grazing management does have substantial potential to influence the patterns of woody recruitment and growth, which are fundamentally determined by climate and seasonal patterns. The issue then is not about whether specific grazing management is essential to allow regeneration to occur but the extent to which grazing management can allow the potential set by climatic variation to be realised. It must be acknowledged that woody regeneration has occurred extensively in this region without management aimed at promoting it, especially in certain Bioregions. The increased prevalence of WPE is both a response to, and a cause of, ‘land degradation’ and a symptom of overgrazing only where persistent and intense grazing pressure removes perennial grasses and alters fire frequencies and the competitive balance between woody and herbaceous species. The sustainable grazing management principles we identify set out guidance around the management of native woody regeneration to achieve dual use of rangelands for pastoralism and vegetation-based sequestration.
We found that grazing management, including the management of both domestic livestock and native and feral herbivores, may influence the success of woody regeneration directly through herbivory and indirectly by regulating the level of herbaceous competition with woody seedlings, the incidence of fire, and landscape function. Direct consumption of seedlings by herbivores will be important for some species whereas competition from herbaceous species is particularly important in the first summer after germination. Seedlings of all woody species are destroyed by fire, which is likely to be promoted by the seasonal conditions that foster large germination events, but the susceptibility of mature plants to fire varies considerably among species. Landscape function and condition affects the retention of rainfall by soils. By determining the growth rate of young plants, grazing will have consequences for the rate of carbon accumulation, the time taken to achieve forest cover, and the susceptibility of regenerating stands to drought in the early establishment phase.
Although climate will determine the potential for carbon accumulation at a site, grazing management may directly affect the temporal pattern of accretion, influencing the dynamics of this process at three critical stages, which include (i) initial site condition, (ii) germination and establishment and (iii) growth and mortality. In addition, grazing management has the potential to indirectly influence the size of the regenerating seed pool, and the incidence of fire.
Apart from its use for long-term fire suppression, grazing management will be influential mainly in the early years of regeneration when management activities will revolve around
Data availability
Data source for Fig. 1 is publicly available https://cer.gov.au/markets/reports-and-data/accu-project-and-contract-register?view=Projects.
Conflicts of interest
Cathy Waters is an employee of GreenCollar, which invests in environmental markets and projects including partnerships with land managers and traditional owners to deliver HIR offset projects. Ron Hacker has undertaken consultancies for Climate Friendly Pty Ltd, which provides carbon project services to land managers that deliver HIR carbon projects. All other co-authors have no conflicts of interest.
Declaration of funding
Aaranya Sekaran is supported by Australian Research Council Laureate Fellowship FL190100003.
Acknowledgements
This paper extends an initial literature review jointly funded by NSW Department of Climate Change, Energy, the Environment and Water (previously NSW Treasury) and Western Local Land Services.
References
Alemseged Y, Hacker RB (2014) Introduction of Dorper sheep into Australian rangelands: implications for production and natural resource management. The Rangeland Journal 36, 85-90.
| Crossref | Google Scholar |
Allen VG, Batello C, Berretta EJ, Hodgson J, Kothmann M, Li X, McIvor J, Milne J, Morris C, Peeters A, Sanderson M, The Forage and Grazing Terminology Committee (2011) An international terminology for grazing lands and grazing animals. Grass and Forage Science 66, 2-28.
| Crossref | Google Scholar |
Anderson VJ, Hodgkinson KC (1997) Grass-mediated capture of resource flows and the maintenance of banded mulga in a semi-arid woodland. Australian Journal of Botany 45, 331-342.
| Crossref | Google Scholar |
Archer SR, Andersen EM, Predick KI, Schwinning S, Steidl RJ, Woods SR (2017) Woody Plant Encroachment: Causes and Consequences. In ‘Rangeland Systems’. (Ed. D Briske) pp. 25–84. (Springer: Cham, Switzerland) 10.1007/978-3-319-46709-2_2.
Atkin OK, Schortemeyer M, McFarlane N, Evans JR (1999) The response of fast- and slow-growing Acacia species to elevated atmospheric CO2: an analysis of the underlying components of relative growth rate. Oecologia 120, 544-554.
| Crossref | Google Scholar | PubMed |
Atkinson T, Hacker RB, Melville GJ, Reseigh J (2019) Land managers’ and service providers’ perspectives on the magnitude, impact and management of non-domestic grazing pressure in the southern rangelands of Australia. The Rangeland Journal 41, 461-476.
| Crossref | Google Scholar |
Australian Government (2024) Clean energy Regulator – Carbon Credits (Carbon Farming Initiative) (Human-Induced Regeneration of a Permanent Even-Aged Native Forest—1.1) Methodology Determination 2013. Available at https://cer.gov.au/schemes/australian-carbon-credit-unit-scheme/accu-scheme-methods/closed-methods/human-induced-regeneration-permanent-even-aged-native-forest-closed
Bastin G, Scarth P, Chewings V, Sparrow A, Denham R, Schmidt M, O’Reagain P, Shepherd R, Abbott B (2012) Separating grazing and rainfall effects at regional scale using remote sensing imagery: a dynamic reference-cover method. Remote Sensing of Environment 121, 443-457.
| Crossref | Google Scholar |
Baumber A, Waters C, Cross R, Metternicht G, Simpson M (2020) Carbon farming for resilient rangelands: people, paddocks and policy. The Rangeland Journal 42, 293-307.
| Crossref | Google Scholar |
Bean JM, Melville GJ, Hacker RB, Clipperton SP (2015) Seed availability, landscape suitability and the regeneration of perennial grasses in moderately-degraded rangelands in semi-arid Australia. The Rangeland Journal 37, 249-259.
| Crossref | Google Scholar |
Bond WJ, Keeley JE (2005) Fire as a global ‘herbivore’: the ecology and evolution of flammable ecosystems. Trends in Ecology & Evolution 20, 387-394.
| Crossref | Google Scholar | PubMed |
Booth C, King G, Sanchez-Bayo F (1996) Establishment of woody weeds in western New South Wales. 1. Seedling emergence and phenology. The Rangeland Journal 18, 58-79.
| Crossref | Google Scholar |
Brand TS (2000) Grazing behaviour and diet selection by Dorper sheep. Small Ruminant Research 36, 147-158.
| Crossref | Google Scholar | PubMed |
Brown R (1985) The growth and survival of young mulga (Acacia aneura F.Muell) trees under different levels of grazing. The Rangeland Journal 7, 143-148.
| Crossref | Google Scholar |
Brown JR, Archer S (1999) Shrub invasion of grassland: recruitment is continuous and not regulated by herbaceous biomass or density. Ecology 80, 2385-2396.
| Crossref | Google Scholar |
Burrows WH (1973) Studies in the dynamics and control of wood weeds in semi-arid Queensland. Queensland Journal of Agricultural and Animal Sciences 30, 57-64 Available at https://era.dpi.qld.gov.au/id/eprint/13156/ [accessed 8 November 2024].
| Google Scholar |
Burrows WH, Scanlan JC, Anderson ER (1988) Plant ecological relations in open forests, woodlands and shrublands. In ‘Native Pastures in Queensland. The Resources and their Management’. (Eds WH Burrows, JC Scanlan, MT Rutherford) pp. 72–90. (Department of Primary Industries, Queensland Government: Brisbane, QLD, Australia)
Casburn G, Atkinson T (2016) Using mulga as a forage supplement for livestock in droughts August 2016, Primefact 1487, 1st edn. Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0003/671421/using-mulga-as-a-forage-supplement-for-livestock-in-droughts.pdf [accessed 8 November 2024]
CER (2024) Clean Energy Regulator. Available at https://cer.gov.au/document/human-induced-regeneration-projects-and-how-they-affect-management-land-property-scale-guidance-0 [accessed 13 February 2025]
Cleverly J, Eamus D, Luo Q, Coupe NR, Kljun N, Ma X, Ewenz C, Li L, Yu Q, Huete A (2016) The importance of interacting climate modes on Australia’s contribution to global carbon cycle extremes. Scientific Reports 6, 23113.
| Crossref | Google Scholar | PubMed |
Condon RW (1949) Mulga death in the west Darling country. Journal of the Soil Conservation Service of New South Wales 5, 7-14.
| Google Scholar |
Cowie A, Waters C, Garland F, Orgill S, Baumber A, Cross R, O’Connell D, Metternicht G (2019) Assessing resilience to underpin implementation of Land Degradation Neutrality: a case study in the rangelands of western New South Wales, Australia. Environmental Science and Policy 100, 37-46.
| Crossref | Google Scholar |
Crisp MD (1978) Demography and survival under grazing of three Australian semi-desert shrubs. Oikos 30, 520-528.
| Crossref | Google Scholar |
Crowley GM (2015) ‘Trends in natural resource management in Australia’s Monsoonal North: the beef industry’. (The Cairns Institute, James Cook University: Cairns, QLD, Australia). Available at http://ndl.ethernet.edu.et/bitstream/123456789/1736/1/39%2C2015.pdf.pdf [accessed 8 November 2024]
Cunningham GM, Walker PJ (1973) Growth and survival of mulga (Acacia aneura F.Muell. Ex Benth) in western New South Wales. Tropical Grasslands 7, 69-77.
| Google Scholar |
Davies SJJF, Kenny SA (2013) The ages and fecundity of some arid-zone plants in Western Australia. The Rangeland Journal 35, 455-468.
| Crossref | Google Scholar |
Dawson TJ, Ellis BA (1994) Diets of mammalian herbivores in Australian arid shrublands: seasonal effects on overlap between red kangaroos, sheep and rabbits and on dietary niche breadths and electivities. Journal of Arid Environments 26, 257-271.
| Crossref | Google Scholar |
Eggemeyer KD, Schwinning S (2009) Biogeography of woody encroachment: why is mesquite excluded from shallow soils? Ecohydrology 2, 81-87.
| Crossref | Google Scholar |
Eldridge DJ, Sala O (2023) Australia’s carbon plan disregards evidence. Science 382, 894.
| Crossref | Google Scholar | PubMed |
Eldridge DJ, Bowker MA, Maestre FT, Roger E, Reynolds JF, Whitford WG (2011) Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecological Letters 14, 709-722.
| Crossref | Google Scholar | PubMed |
Eldridge DJ, Poore AGB, Ruiz‐Colmenero M, Letnic M, Soliveres S (2016) Ecosystem structure, function, and composition in rangelands are negatively affected by livestock grazing. Ecological Applications 26, 1273-1283.
| Crossref | Google Scholar | PubMed |
Ellsworth DS, Anderson IC, Crous KY, Cooke J, Drake JE, Gherlenda AN, Gimeno TE, Macdonald CA, Medlyn BE, Powell JR, Tjoelker MG, Reich PB (2017) Elevated CO2 does not increase eucalypt forest productivity on a low-phosphorus soil. Nature Climate Change 7, 279-282.
| Crossref | Google Scholar |
Erb K-H, Kastner T, Plutzar C, Bais ALS, Carvalhais N, Fetzel T, Gingrich S, Haberl H, Lauk C, Niedertscheider M, Pongratz J, Thurner M, Luyssaert S (2018) Unexpectedly large impact of forest management and grazing on global vegetation biomass. Nature 553, 73-76.
| Crossref | Google Scholar | PubMed |
Evans JR, Schortemeyer M, McFarlane N, Atkin OK (2000) Photosynthetic characteristics of 10 Acacia species grown under ambient and elevated atmospheric CO2. Australian Journal of Plant Physiology 27, 13-25.
| Google Scholar |
Fensham RJ, Holman JE (1999) Temporal and spatial patterns in drought-related tree dieback in Australian savanna. Journal of Applied Ecology 36, 1035-1050.
| Crossref | Google Scholar |
Fensham RJ, Silcock JL, Dwyer JM (2011) Plant species richness responses to grazing protection and degradation history in a low productivity landscape: plant species richness and grazing exclusion. Journal of Vegetation Science 22, 997-1008.
| Crossref | Google Scholar |
Fensham RJ, Fairfax RJ, Dwyer JM (2012) Potential aboveground biomass in drought-prone forest used for rangeland pastoralism. Ecological Applications 22, 894-908.
| Crossref | Google Scholar | PubMed |
Fensham RJ, Laffineur B, Allen CD (2019) To what extent is drought-induced tree mortality a natural phenomenon? Global Ecology and Biogeography 28, 365-373.
| Crossref | Google Scholar |
Fisher A, Hunt L, James C, Landsberg J, Phelps D, Smyth A, Watson, I (2004) ‘Review of Total Grazing Pressure Management Issues and Priorities for Biodiversity Conservation in the Rangelands.’ A Resource to Aid NRM Planning. Desert Knowledge CRC Project 3 (August 2004). (Commonwealth of Australia: Canberra, ACT, Australia)
Fitch P, Battaglia M, Lenton A, Feron P, Gao L, Mei Y, Hortle A, Macdonald L, Pearce M, Occhipinti S, Roxburgh S, Steven A (2022) ‘Australia’s sequestration potential.’ (CSIRO) Available at https://www.csiro.au/en/research/environmental-impacts/emissions/carbon-dioxide-removal/carbon-sequestration-potential
Friedel MH, Nelson DJ, Sparrow AD, Kinloch JE, Maconochie JR (1994) Flowering and fruiting of arid zone species of Acacia in central Australia. Journal of Arid Environments 27, 221-239.
| Crossref | Google Scholar |
Friedel MH, Sparrow AD, Kinloch JE, Tongway DJ (2003) Degradation and recovery processes in arid grazing lands of central Australia. Part 2: vegetation. Journal of Arid Environments 55, 327-348.
| Crossref | Google Scholar |
Fukuda Y, McCallum HI, Grigg GC, Pople AR (2009) Fencing artificial waterpoints failed to influence density and distribution of red kangaroos (Macropus rufus). Wildlife Research 36, 457-465.
| Crossref | Google Scholar |
Gardiner HG (1986) Dynamics of perennial plants in the mulga (Acacia aneura F.Muell) zone of Western Australia. I. Rates of population change. The Rangeland Journal 8, 18-27.
| Crossref | Google Scholar |
Godfree RC, Knerr N, Godfree D, Busby J, Robertson B, Encinas-Viso F (2019) Historical reconstruction unveils the risk of mass mortality and ecosystem collapse during pancontinental megadrought. Proceedings of the National Academy of Sciences 116, 15580-15589.
| Crossref | Google Scholar | PubMed |
Griffiths M, Barker RD (1966) The plants eaten by sheep and by kangaroos grazing together in a paddock in south-western Queensland. CSIRO Wildlife Research 11, 145-167.
| Crossref | Google Scholar |
Griscom BW, Adams J, Ellis PW, Houghton RA, Lomax G, Miteva DA, Schlesinger WH, Shoch D, Siikamäki JV, Smith P, Woodbury P, Zganjar C, Blackman A, Campari J, Conant RT, Delgado C, Elias P, Gopalakrishna T, Hamsik MR, Herrero M, Kiesecker J, Landis E, Laestadius L, Leavitt SM, Minnemeyer S, Polasky S, Potapov P, Putz FE, Sanderman J, Silvius M, Wollenberg E, Fargione J (2017) Natural climate solutions. Proceedings of the National Academy of Sciences 114, 11645-11650.
| Crossref | Google Scholar | PubMed |
Hacker RB, McDonald SE (2021) Prospects for sustainable use of the pastoral areas of Australia’s southern rangelands: a synthesis. The Rangeland Journal 43, 185-209.
| Crossref | Google Scholar |
Hacker RB, Sinclair K, Waters CM (2019) Total grazing pressure – a defining concept for extensive pastoral systems in the southern rangelands of Australia. The Rangeland Journal 41, 457.
| Crossref | Google Scholar |
Hall E, Specht R, Eardley C (1964) Regeneration of the vegetation on Koonamore vegetation reserve, 1926-1962. Australian Journal of Botany 12, 205-264.
| Crossref | Google Scholar |
Harrington G (1979) The effects of feral goats and sheep on the shrub populations in a semi-arid woodland. The Rangeland Journal 1, 334.
| Crossref | Google Scholar |
Harrington GN (1991) Effects of soil moisture on shrub seedling survival in semi-arid grassland. Ecology 72, 1138-1149.
| Crossref | Google Scholar |
Hendricks HH, Bond WJ, Midgley JJ, Novellie PA (2005) Plant species richness and composition a long livestock grazing intensity gradients in a Namaqualand (South Africa) protected area. Plant Ecology 176, 19-33.
| Crossref | Google Scholar |
Hobbs TJ, Wenham D, Herrmann T, Brandle R, Maconochie J, Baird G, Schutz A, Howell S, Spencer J, Owen J, Fitzgerald L, Bowen Z, Wood H (2018) Environmental values in Cooper-Eromanga Basin. In ‘DWE technical report 2018/04’. (Department for Environment and Water and Department for Energy and Mining). Available at https://data.environment.sa.gov.au/Content/Publications/DEW-TR-2018-04.pdf [accessed 8 November 2024]
Hodgkinson KC (1979) The shrubs of the poplar box (Eucalyptus populnea) lands and their biology. The Australian Rangeland Journal 1, 280-293.
| Crossref | Google Scholar |
Hodgkinson K, Harrington G (1985) The case for prescribed burning to control shrubs in eastern semi-arid woodlands. The Rangeland Journal 7, 64-74.
| Crossref | Google Scholar |
Hunt LP, Petty S, Crowley RA, Fischer A, Ash A, MacDonald N (2007) Factors affecting the management of cattle grazing distribution in northern Australia: preliminary observations of paddock size and water points. The Rangeland Journal 29, 169-179.
| Crossref | Google Scholar |
IPCC (2019) Summary for Policymakers. In ‘Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems’. (Eds PR Shukla, J Skea, E Calvo Buendia, V Masson-Delmotte, HO Pörtner, DC Roberts, P Zhai, R Slade, S Connors, R van Diemen, M Ferrat, E Haughey, S Luz, S Neogi, M Pathak, J Petzold, J Portugal Pereira, P Vyas, E Huntley, K Kissick, M Belkacemi, J Malley). Available at https://www.ipcc.ch/site/assets/uploads/sites/4/2022/11/SRCCL_SPM.pdf
Ison R, Russell DB (Eds) (2000) ‘Agricultural extension and rural development: breaking out of traditons; a second-order systems perspective.’ (Cambridge University Press: Cambridge UK). Available at https://catdir.loc.gov/catdir/samples/cam032/98051725.pdf [accessed 8 November 2024]
James CD, Landsberg J, Morton SR (1999) Provision of watering points in the Australian arid zone: a review of effects on biota. Journal of Arid Environments 41, 87-121.
| Crossref | Google Scholar |
Jenkins K, Warren R (2015) Quantifying the impact of climate change on drought regimes using the Standardised Precipitation Index. Theoretical and Applied Climatology 120, 41-54.
| Crossref | Google Scholar |
Kenny SA, Moxham C (2022) Does above‐average rainfall stimulate a recruitment pulse in semi‐arid woodlands of southeastern Australia? Journal of Vegetation Science 33, e13148.
| Crossref | Google Scholar |
Landsberg J, James CD, Morton SR, Muller WJ, Stol J (2003) Abundance and composition of plant species along grazing gradients in Australian rangelands. Journal of Applied Ecology 40, 1008-1024.
| Crossref | Google Scholar |
Lee TRC, Alemseged Y, Mitchell A (2018) Dropping hints: estimating the diets of livestock in rangelands using DNA metabarcoding of faeces. Metabarcoding and Metagenomics 2, e22467.
| Crossref | Google Scholar |
Letnic M, Laffan SW, Greenville AC, Russell BG, Mitchell B, Fleming PJS (2015) Artificial watering points are focal points for activity by an invasive herbivore but not native herbivores in conservation reserves in arid Australia. Biodiversity and Conservation 24, 1-16.
| Crossref | Google Scholar |
Macintosh A, Butler D, Larraondo P, Evans MC, Ansell D, Waschka M, Fensham R, Eldridge D, Lindenmayer D, Gibbons P, Summerfield P (2024) Australian human-induced native forest regeneration carbon offset projects have limited impact on changes in woody vegetation cover and carbon removals. Communications Earth & Environment 5, 149.
| Crossref | Google Scholar |
Maestre FT, Le Bagousse-Pinguet Y, Delgado-Baquerizo M, Eldridge DJ, Saiz H, Berdugo M, Gozalo B, Ochoa V, Guirado E, García-Gómez M, Valencia E, Gaitán JJ, Asensio S, Mendoza BJ, Plaza C, Díaz-Martínez P, Rey A, Hu H-W, He J-Z, Wang J-T, Lehmann A, Rillig MC, Cesarz S, Eisenhauer N, Martínez-Valderrama J, Moreno-Jiménez E, Sala O, Abedi M, Ahmadian N, Alados CL, Aramayo V, Amghar F, Arredondo T, Ahumada RJ, Bahalkeh K, Ben Salem F, Blaum N, Boldgiv B, Bowker MA, Bran D, Bu C, Canessa R, Castillo-Monroy AP, Castro H, Castro I, Castro-Quezada P, Chibani R, Conceição AA, Currier CM, Darrouzet-Nardi A, Deák B, Donoso DA, Dougill AJ, Durán J, Erdenetsetseg B, Espinosa CI, Fajardo A, Farzam M, Ferrante D, Frank ASK, Fraser LH, Gherardi LA, Greenville AC, Guerra CA, Gusmán-Montalvan E, Hernández-Hernández RM, Hölzel N, Huber-Sannwald E, Hughes FM, Jadán-Maza O, Jeltsch F, Jentsch A, Kaseke KF, Köbel M, Koopman JE, Leder CV, Linstädter A, le Roux PC, Li X, Liancourt P, Liu J, Louw MA, Maggs-Kölling G, Makhalanyane TP, Issa OM, Manzaneda AJ, Marais E, Mora JP, Moreno G, Munson SM, Nunes A, Oliva G, Oñatibia GR, Peter G, Pivari MOD, Pueyo Y, Quiroga RE, Rahmanian S, Reed SC, Rey PJ, Richard B, Rodríguez A, Rolo V, Rubalcaba JG, Ruppert JC, Salah A, Schuchardt MA, Spann S, Stavi I, Stephens CRA, Swemmer AM, Teixido AL, Thomas AD, Throop HL, Tielbörger K, Travers S, Val J, Valkó O, van den Brink L, Ayuso SV, Velbert F, Wamiti W, Wang D, Wang L, Wardle GM, Yahdjian L, Zaady E, Zhang Y, Zhou X, Singh BK, Gross N (2022) Grazing and ecosystem service delivery in global drylands. Science 378, 915-920.
| Crossref | Google Scholar | PubMed |
McCarthy HR, Oren R, Johnsen KH, Gallet-Budynek A, Pritchard SG, Cook CW, LaDeau SL, Jackson RB, Finzi AC (2010) Re-assessment of plant carbon dynamics at the Duke free-air CO2 enrichment site: interactions of atmospheric [CO2] with nitrogen and water availability over stand development. New Phytologist 185, 514-528.
| Crossref | Google Scholar | PubMed |
McDonald SE, Simmons AT, Harden S, Orgill SE, Guerschman J, Strong C (2024) Managing grazing to increase ground cover in rangelands: using remote sensing to detect change. The Rangeland Journal 46, RJ24021.
| Crossref | Google Scholar |
McMeniman NP, Beale IF, Murphy GM (1986) Nutritional evaluation of south-west Queensland pastures. I. Botanical and nutrient content of diets selected by sheep grazing on Mitchell grass and mulga/grassland Associations. Australian Journal of Agricultural Research 37, 289-302.
| Crossref | Google Scholar |
Milchunas DG, Lauenroth WK (1993) Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecological Monographs 63, 327-366.
| Crossref | Google Scholar |
MLA (2024) Grazing distribution | Meat & Livestock Australia MLA Corporate. Available at https://www.mla.com.au/research-and-development/Grazing-pasture-management/native-pasture/grazing-management/grazing-distribution/ [accessed 20 August 2024]
Mochi LS, Mazía N, Biganzoli F, Aguiar MR (2022) Contrasting effects of grazing on the early stages of woody encroachment in a Neotropical savanna. Basic and Applied Ecology 60, 13-24.
| Crossref | Google Scholar |
Moore JL, Howden SM, McKeon GM, Carter JO, Scanlan JC (2001) The dynamics of grazed woodlands in southwest Queensland, Australia, and their effect on greenhouse gas emissions. Environment International 27, 147-153.
| Crossref | Google Scholar | PubMed |
Muňoz-Robles C, Reid N, Tighe M, Briggs SV, Wilson B (2011a) Soil hydrological and erosional responses in patches and inter-patches in vegetation states in semi-arid Australia. Geoderma 160, 524-534.
| Crossref | Google Scholar |
Muñoz-Robles C, Reid N, Tighe M, Briggs SV, Wilson B (2011b) Soil hydrological and erosional responses in areas of woody encroachment, pasture and woodland in semi-arid Australia. Journal of Arid Environments 75(10), 936-945.
| Crossref | Google Scholar |
Munro NT, Moseby KE, Read JL, Munro NT, Moseby KE, Read JL (2009) The effects of browsing by feral and re-introduced native herbivores on seedling survivorship in the Australian rangelands. The Rangeland Journal 31, 417-426.
| Crossref | Google Scholar |
Nolan RH, Sinclair J, Waters C, Mitchell P, Eldridge DJ, Paul KI, Roxburgh S, Butler DW, Ramp D (2019) Risks to carbon dynamics in semi-arid woodlands of eastern Australia under current and future climates. Journal of Environmental Management 235, 500-510.
| Crossref | Google Scholar | PubMed |
Norby RJ, DeLucia EH, Gielen B, Calfapietra C, Giardina CP, King JS, Ledford J, McCarthy HR, Moore DJP, Ceulemans R, De Angelis P, Finzi AC, Karnosky DF, Kubiske ME, Lukac M, Pregitzer KS, Scarascia-Mugnozza GE, Schlesinger WH, Oren R (2005) Forest response to elevated CO2 is conserved across a broad range of productivity. Proceedings of the National Academy of Sciences 102, 18052-18056.
| Crossref | Google Scholar | PubMed |
Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proceedings of the National Academy of Science 107, 19368-19373.
| Crossref | Google Scholar | PubMed |
NSW DPI (Department of Primary Industries) (2014) Prime Fact. Water requirements for sheep and cattle. Available at https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0009/96273/Water-requirements-for-sheep-and-cattle.pdf [accessed 8 November 2024]
NSW DPI (Department of Primary Industries) (2018) General position paper Development of the goat industry in NSW. Available at https://www.dpi.nsw.gov.au/animals-and-livestock/goats/position-paper [accessed 8 November 2024]
Pahl L (2020) Macropods, feral goats, sheep and cattle. 2. Equivalency in what and where they eat. The Rangeland Journal 41, 519-533.
| Crossref | Google Scholar |
Poulter B, Frank D, Ciais P, Myneni R, Andela N, Bi J, Broquet G, Canadell J, Chevallier F, Liu Y, Running S, Sitch S, Werf G (2014) Contribution of semi-arid ecosystems to interannual variability of the global carbon cycle. Nature 509, 600-603.
| Crossref | Google Scholar | PubMed |
Preece P (1971a) Contributions to the biology of mulga. I. Flowering. Australian Journal of Botany 19, 21-38.
| Crossref | Google Scholar |
Preece P (1971b) Contributions to the biology of mulga. II. Germination. Australian Journal of Botany 19, 39-49.
| Crossref | Google Scholar |
Pressland AJ (1976) Possible effects of removal of mulga on rangeland stability in south western Queensland. The Rangeland Journal 1, 24-30.
| Crossref | Google Scholar |
Prowse TAA, O’Connor PJ, Collard SJ, Rogers DJ (2019) Eating away at protected areas: total grazing pressure is undermining public land conservation. Global Ecology and Conservation 20, e00754.
| Crossref | Google Scholar |
Randell BR (1992) Mulga. A revision of the major species. Journal of the Adelaide Botanic Garden 14, 105-132.
| Google Scholar |
Retallack A, Finlayson G, Ostendorf B, Clarke K, Lewis M (2023) Remote sensing for monitoring rangeland condition: current status and development of methods. Environmental and Sustainability Indicators 19, 100285.
| Crossref | Google Scholar |
Retallack A, Rifai S, Finlayson G, Ostendorf B, Lewis M (2024) Remote sensing for rangeland conservation monitoring: impacts of livestock removal after 15 years. Journal of Applied Ecology 61, 2111-2122.
| Crossref | Google Scholar |
Rifai SW, De Kauwe MG, Ukkola AM, Cernusak LA, Meir P, Medlyn BE, Pitman AJ (2022) Thirty-eight years of CO2 fertilization has outpaced growing aridity to drive greening of Australian woody ecosystems. Biogeosciences 19, 491-515.
| Crossref | Google Scholar |
Rossi MJ, Ares JO, Jobbágy EG, Vivoni ER, Vervoort RW, Schreiner-McGraw AP, Saco PM (2018) Vegetation and terrain drivers of infiltration depth along a semiarid hillslope. Science of The Total Environment 644, 1399-1408.
| Crossref | Google Scholar | PubMed |
Scholes RJ, Archer SR (1997) Tree–grass interactions in savannas. Annual Review of Ecology and Systematics 28, 517-544.
| Crossref | Google Scholar |
Silcock J, Fensham R (2013) Arid vegetation in disequilibrium with livestock grazing: evidence from long‐term exclosures. Austral Ecology 38, 57-65.
| Crossref | Google Scholar |
Sinclair K, Curtis AL, Hacker RB, Atkinson T (2019) Stakeholder judgements of the social acceptability of control practices for kangaroos, unmanaged goats and feral pigs in the south-eastern rangelands of Australia. The Rangeland Journal 41, 485-496.
| Crossref | Google Scholar |
Squires V (1980) Chemical and botanical composition of the diets of oesophageally fistulated sheep, cattle and goats in a semi-arid Eucalyptus populnea woodland community. The Rangeland Journal 2, 94-103.
| Crossref | Google Scholar |
Su H, Liu W, Xu H, Wang Z, Zhang H, Hu H, Li Y (2015) Long-term livestock exclusion facilitates native woody plant encroachment in a sandy semiarid rangeland. Ecology and Evolution 5, 2445-2456.
| Crossref | Google Scholar | PubMed |
Swann E, Richards R (2016) What factors influence the effectiveness of financial incentives on long-term natural resource management practice change? Evidence Base 2016, 1-32.
| Crossref | Google Scholar |
Tabeni S, Garibotti IA, Pissolito C, Aranibar JN (2014) Grazing effects on biological soil crusts and their interaction with shrubs and grasses in an arid rangeland. Journal of Vegetation Science 25, 1417-1425.
| Crossref | Google Scholar |
Throop HL, Archer SR (2007) Interrelationships among shrub encroachment, land management, and litter decomposition in a semidesert grassland. Ecological Applications: A Publication of the Ecological Society of America 17, 1809-1823.
| Crossref | Google Scholar | PubMed |
Todd SW (2006) Gradients in vegetation cover, structure and species richness of Nama‐Karoo shrublands in relation to distance from livestock watering points. Journal of Applied Ecology 43, 293-304.
| Crossref | Google Scholar |
Tongway DJ, Sparrow AD, Friedel MH (2003) Degradation and recovery processes in arid grazing lands of central Australia. Part 1: soil and land resources. Journal of Arid Environments 55, 301-326.
| Crossref | Google Scholar |
Travers SK, Eldridge DJ, Val J, Oliver I (2019) Rabbits and livestock grazing alter the structure and composition of mid-storey plants in a wooded dryland. Agriculture, Ecosystems & Environment 277, 53-60.
| Crossref | Google Scholar |
Van Auken OW (2009) Causes and consequences of woody plant encroachment into western North American grasslands. Journal of Environmental Management 90, 2931-2942.
| Crossref | Google Scholar | PubMed |
Walker BH, Noy-Meir I (1982) Aspects of the stability and resilience of savanna ecosystems. In ‘Ecology of Tropical Savannas’. Ecological Studies. (Eds BJ Huntley, BH Walker) pp. 556–590. (Springer: Berlin, Germany) 10.1007/978-3-642-68786-0_26
Wang S, Fu B, Wei F, Piao S, Maestre FT, Wang L, Jiao W, Liu Y, Li Y, Li C, Zhao W (2023) Drylands contribute disproportionately to observed global productivity increases. Science Bulletin 68, 224-232.
| Crossref | Google Scholar | PubMed |
Waters CM, McDonald SE, Reseigh J, Grant R, Burnside DG (2019) Insights on the relationship between total grazing pressure management and sustainable land management: key indicators to verify impacts. The Rangeland Journal 41, 535-556.
| Crossref | Google Scholar |
Watson IW, Westoby M, Holm AMcR (1997) Continuous and episodic components of demographic change in arid zone shrubs: models of two Eremophila species from Western Australia compared with published data on other species. Journal of Ecology 85, 833-846.
| Crossref | Google Scholar |
Whipp RK, Lunt ID, Spooner PG, Bradstock RA (2012) Changes in forest structure over 60 years: tree densities continue to increase in the Pilliga forests, New South Wales, Australia. Australian Journal of Botany 60, 1-8.
| Crossref | Google Scholar |
Winkworth RE (1973) Eco-physiology of mulga (Acacia aneura). Tropical Grasslands 7, 43-48.
| Google Scholar |
Witt GB (2013) Vegetation changes through the eyes of the locals: the ‘artificial wilderness’ in the mulga country of south-west Queensland. The Rangeland Journal 35, 299-314.
| Crossref | Google Scholar |
Witt GB, Berghammer LJ, Beeton RJS, Moll EJ (2000) Retrospective monitoring of rangeland vegetation change: ecohistory from deposits of sheep dung associated with shearing sheds. Austral Ecology 25, 260-267.
| Crossref | Google Scholar |
Witt GB, Noël MV, Bird MI, Beeton RJS (Bob), Menzies NW (2011) Carbon sequestration and biodiversity restoration potential of semi-arid mulga lands of Australia interpreted from long-term grazing exclosures. Agriculture, Ecosystems & Environment 141, 108-118.
| Crossref | Google Scholar |
Wright BR, Fensham RJ (2017) Fire after a mast year triggers mass recruitment of slender mulga (Acacia aptaneura), a desert shrub with heat-stimulated germination. American Journal of Botany 104, 1474-1483.
| Crossref | Google Scholar | PubMed |
Wright BR, Zuur AF (2014) Seedbank dynamics after masting in mulga (Acacia aptaneura): Implications for post-fire regeneration. Journal of Arid Environments 107, 10-17.
| Crossref | Google Scholar |
Wright BR, Latz PK, Zuur AF (2016) Fire severity mediates seedling recruitment patterns in slender mulga (Acacia aptaneura), a fire-sensitive Australian desert shrub with heat-stimulated germination. Plant Ecology 217, 789-800.
| Crossref | Google Scholar |
Wright BR, Nipper M, Nipper N, Merson SD, Guest T (2023) Mortality rates of desert vegetation during high‐intensity drought at Uluru–Kata Tjuta National Park, Central Australia. Austral Ecology 48, 699-718.
| Crossref | Google Scholar |
Wu XB, Archer S (2005) Scale-dependent influence of topography-based hydrologic features on vegetation patterns in savanna landscapes. Landscape Ecology 20, 733-742.
| Crossref | Google Scholar |
Zhu Z, Piao S, Myneni RB, Huang M, Zeng Z, Canadell JG, Ciais P, Sitch S, Friedlingstein P, Arneth A, Cao C, Cheng L, Kato E, Koven C, Li Y, Lian X, Liu Y, Liu R, Mao J, Pan Y, Peng S, Penuelas J, Poulter B, Pugh TAM, Stocker BD, Viovy N, Wang X, Wang Y, Xiao Z, Yang H, Zaehle S, Zen N (2016) Greening of the Earth and its drivers. Nature Climate Change 6, 699-718.
| Crossref | Google Scholar |
Footnotes
1 Forest cover is defined as an area of at least 0.2 ha, dominated by trees and shrubs that are at least 2 m tall and provide crown cover of at least 20% of the land area. Crown cover is the amount of land covered by the outer edges (diameter) of a tree/shrub or groups of trees/shrubs (Australian Government 2024).
2 Note that the comparison is further complicated by the fact that the exclosure type given by Witt et al. (2011) for at least one site appears to conflict with that given by Fensham et al. (2012).



