Impacts of bracteole removal and seeding rate on seedling emergence of halophyte shrubs: implications for rangeland rehabilitation in arid environments
Mounir Louhaichi A B H , Sawsan Hassan A , Ali Mekki Missaoui C , Serkan Ates B , Steven L. Petersen D , Abdoul Aziz Niane E , Slim Slim F and Azaiez Ouled Belgacem GA International Centre for Agricultural Research in the Dry Areas (ICARDA), PO Box 950764 – Amman, Jordan.
B Department of Animal and Rangeland Sciences, Oregon State University, Corvallis, OR 97330, USA.
C Centre for Applied Genetic Technologies, University of Georgia, 111 Riverbend Road, Athens, GA 30602, USA.
D Department of Plant and Wildlife Sciences, Brigham Young University, Provo, UT 84602, USA.
E International Centre for Agricultural Research in the Dry Areas (ICARDA), PO Box 114/5055 – Beirut, Lebanon.
F Higher School of Agriculture of Mateur, University of Carthage, Mateur, Tunisia.
G ICARDA, Arabian Peninsula Regional Program, PO Box 13979 – Dubai, UAE.
H Corresponding author. Email: m.louhaichi@cgiar.org
The Rangeland Journal 41(1) 33-41 https://doi.org/10.1071/RJ18064
Submitted: 4 December 2017 Accepted: 8 January 2019 Published: 12 February 2019
Journal compilation © Australian Rangeland Society 2019 Open Access CC BY-NC-ND
Abstract
Direct seeding techniques often result in unsatisfactory outcomes in rangeland rehabilitation, primarily because of low seedling emergence and poor establishment. Seed processing techniques aimed at improving seedling emergence have gained interest by pasture managers. The purpose of this study was to investigate the combined effects of bracteole removal and seeding rate on seedling emergence in seven halophytic species: Atriplex halimus, A. canescens, A. leucoclada, A. nummularia, A. lentiformis, Salsola vermiculata and Haloxylon aphyllum under semi-arid conditions in Tel Hadya (Syria). Each of these species was evaluated for seedling emergence under two seed treatments (bracteoles removed and non-removed bracteoles) with three seeding rates (10, 30 and 60 seeds per pot), in a completely randomised block design. The results showed a positive effect of seed treatment on seedling emergence for all studied species. The native A. halimus had the highest emergence percentages whereas the introduced A. mummularia, had the lowest. However, there were no significant effects of seeding rates on seedling emergence. These results showed that bracteole removal could improve germination and seedling emergence, and potentially increase the rate of establishment of the species studied. Therefore, when implementing rangeland rehabilitation projects, bracteole removal needs to be considered. The native S. vermiculata should be recommended for direct seeding in the West Asia and North Africa region given its high seedling emergence, known high palatability, nutritive value, and high auto-regeneration performance.
Additional keywords: arid and semi-arid ecosystems, direct seeding, indigenous plants, rangeland restoration, seed scarification treatments.
Introduction
Most rangelands in the West Asia and North Africa (WANA) region are poorly managed, resulting in land degradation, reduced productivity, and loss of plant biodiversity (Hudson et al. 2017). Overgrazing, conversion of rangelands into rainfed cropping systems, land tenure issues, and climate change are major drivers for this degradation (Ates and Louhaichi 2012; Ouled Belgacem and Louhaichi 2013). Several government agencies have becoming increasingly aware of the magnitude of the problem and strive to provide solutions to address rangeland degradation. However, given the low and slow return on investment, governments are not able to finance large-scale projects to effectively restore and develop rangeland resources (Louhaichi et al. 2016a). Therefore, cost-effective techniques for slowing down and eventually reversing the trend of rangeland degradation are needed.
Transplanting shrub species has been extensively used for rangeland rehabilitation in the WANA region due to high transplant survival, effective soil stabilisation, and reduced soil erosion (Louhaichi and Tastad 2010; Louhaichi et al. 2016b). To improve establishment rates, shrub seedlings are usually grown in nurseries and then transplanted in the field (Franco et al. 2006). However, this process is often expensive and time consuming (Douglas et al. 2007) as it requires facilities and labour to grow seedlings for several weeks or months in nurseries. Furthermore, establishing shrubs from nursery-grown seedlings is particularly challenging in arid environments and/or remote rangeland sites due to lack of irrigation and adequate site preparation (e.g. establishment of contour furrows). Besides the irrigation cost, maintenance is needed to replace dead seedlings during the first year of establishment (Palmerlee and Young 2010).
Direct seeding might be a more effective alternative to transplantation of seedlings to improve plant species richness and the productivity of rangelands (Fernández et al. 2012). Direct seeding techniques are common practices for the establishment of most herbaceous pasture species and have been used to successfully revegetate large areas (Malcolm 1994). Although direct seeding is a cost-effective technique to implement large-scale restoration projects, its success in arid regions is limited by harsh environmental factors and poor soil properties, in addition to seed characteristics such as seed dormancy and physical structures (e.g. bracteoles) that interfere with seed germination (Ungar and Khan 2001; García-Fayos et al. 2010; Busso et al. 2012; Raizada and Juyal 2012; Prats et al. 2016).
Many halophytes, in particular Atriplex species, have chemical and morphological characteristics that regulate seed dormancy and germination (Ungar 1978; Khan and Ungar 1985). Atriplex seeds are enclosed within bracteoles that control the timing of water imbibition, a crucial step in the seed germination process (Ungar and Khan 2001). The bracts surrounding the seeds may contain chemical compounds that interfere with germination (Young et al. 1980; Jefferson and Pennacchio 2003). Removal of these bracts has been suggested as a mean of improving seed germination of Atriplex halimus and Salsola vermiculata (Osman and Ghassali 1997).
In most rehabilitation projects, managers tend to increase seeding rate assuming that seeds could have a low germination rate or could be subject to predation by wildlife (Florentine et al. 2013). Direct seeding with a higher seeding rate increases input costs and may lead to inter-plant competition for nutrients and space (Gordon et al. 1989). However, using low seeding rate greatly decreases plant population and results in low productivity (Florentine et al. 2013).
Understanding the factors that affect seedling emergence and plant establishment under rangeland conditions is critical for improving our ability to address the important rehabilitation problems such as low succession and poor spatial distribution of perennial plants (Lesica and Allendorf 1999; Ghassali et al. 2012; Kemp et al. 2013; Porqueddu et al. 2016). Therefore, the objective of this study was to investigate the combined effects of bracteole removal and seeding rate on seedling emergence of seven native and introduced halophytic shrub species that are widely used for rehabilitating degraded rangelands in the WANA region.
Materials and methods
Study site
The study was conducted in Tel Hadya (Syria) at the headquarters of the International Centre for Agricultural Research in the Dry Areas (ICARDA) (36°01N, 36°56E) using outdoor plastic pots of 14 cm diameter and 25 cm depth. Each pot contained 3.5 kg of a 1 : 1 mixture of farm soil and sand topped with a layer of organic sheep manure. The average annual rainfall over two decades in the study site is 340 mm (ICARDA headquarters meteorological station), occurring predominantly from October to May.
Seeds of Atriplex halimus, A. canescens, A. leucoclada, A. nummularia, A. lentiformis, Salsola vermiculata and Haloxylon aphyllum were collected in the autumn of 2011 from the ICARDA pastoretum (Table 1).

|
Experimental procedure
The pots were arranged in a randomised complete block design with five replications. The treatments consisted of removal and non-removal of bracteoles. Bracteoles were manually removed on half of the seeds (termed seeds without bracteoles) of all species by scarification using sand paper 212, P80, and aluminum oxide. The other half of each seed sample retained its bracteoles (termed seeds with bracteoles) and were considered as the control treatments.
The recommended rate for direct seeding (drill-seeding) of most Atriplex species is between 25 and 35 kg ha–1 (seeds with bracteoles) (Watson and O’Leary 1993; Booth 2002). The average weight of 1000 seeds without bracteoles was 0.50 g for A. leucoclada, 0.64 g for A. lentiformis, 0.93 g for A. halimus, 0.72 g for A. nummularia, 1.02 g for S. vermiculata, 1.81 g for H. aphyllum and 2.82 g for A. canescens. Three seeding rates consisting of 10 (low), 30 (medium), and 60 (high) seeds per pot for each species were compared. This resulted in 21 treatment combinations for seeds without bracteoles and seeds with bracteoles. These combinations resulted in 42 three-way treatments (species × bracteole removal × seeding rate).
Seeds without bracteoles and seeds with bracteoles were sown directly in the pots at a depth of 0.5 to 1 cm and patted down firmly on 27 January 2012. Five randomly selected pots were assigned for each treatment combination, for a total of 210 pots. The pots were kept under natural environmental conditions of rainfall, temperature, and light. During the course of this experiment (27 January to 10 March 2012), the site received 121.6 mm of rainfall and the average minimum and maximum temperature varied from 1.9°C to 12.5°C. The long average of rainfall and temperature is presented in Table 2.
|
Emerged seedlings were counted at 10, 15, 20 and 33 days after seeding. Seedling emergence percentages were calculated as the ratio of the number of seedlings that emerged during the monitoring period to the number of seeds sown, and then multiplying this value by 100. The speed of emergence was calculated according to Gairola et al. (2011), using the following formula;

where, n = number of emerged seeds, t = time after seeding.
Effect of machine removal of bracteoles on A. leucoclada
Results from the manual bracteole removal performed on A. leucoclada revealed lower seedling emergence rate compared with seed with bracteoles intact. Therefore, to provide possible explanations, machine removal of bracteoles was performed on A. leucoclada seed using a Perten Laboratory disc Mill 3310 (calibrated from level one to level six). Calibration was set at level four (4), as levels between 1 and 3 resulted in unacceptable mechanical damage to the seed. The experimental design was a randomised complete block, with bracteole removal as the blocking factor, and was conducted in March 2017 at the ICARDA facility in Terbol experimental station, Lebanon.
There were four replicates of 50 seeds of A. leucoclada. Half of the seeds were machine scarified (machine removed bracteoles) whereas the remainder were not (seeds with intact bracteoles). Seeds were exposed to two media (paper and river sand) for evaluation of germination and emergence (International Seed Testing Association 2017). Laboratory conditions were maintained at 20 ± 2°C, with a 16-h light at 75 347 lm m–2 and 8-h darkness regime, for 14 days.
Statistical analyses
Seedling emergence counts were taken on each of four different dates. In order to factor in the effect of time on seedling emergence, linear regressions passing through the initial first seedling emergence day were performed, and the slopes of the regression estimated using the last observation date.
It is possible that the time it takes a seedling to emerge from the soil might surpass the period of observation, for example, 90% absolute seedling emergence percentage may not be reached within a given time period. To account for this, we rather estimated the percentage of the observed maximum emergence which is interpreted as seedling emergence potential. The slope and time to reach 90% emergence are a measure of emergence strength of the species and treatment, which explains the dynamics of emergence. These two estimated parameters were analysed using ANOVA. The pair-wise comparisons of the species under each combination of treatment and time were carried out using Bonferroni test at α = 0.01. Data of the complimentary laboratory experiment were analysed using ANOVA for the two treatments (bracteole removal and growing media). All statistical analyses were carried out using Genstat software (Genstat 2009).
Results and discussion
The effect of bracteole removal on seedling emergence percentage
Three of the species (A. halimus, A. leucoclada, S. vermiculata) are indigenous to the study area (Table 1). There were significant differences in seedling emergence among the species in response to bracteole removal (P < 0.01) and there was an interaction between bracteole removal treatment and species for seedling emergence percentage (P < 0.01). For seeds without bracteoles and seeds with bracteoles, A. halimus and S. vermiculata showed the highest seedling emergence percentages (72.6%, 69.4% and 65.2, 64.5% respectively). Bracteole removal enhanced seedling emergence of A. halimus, A. canescens, A. lentiformis, A. nummularia, and H. aphyllum. There were no significant differences in seedling emergence between seeds without bracteoles and seeds with bracteoles in S. vermiculata (Table 3). These results confirm the common belief that native perennial species (A. halimus and S. vermiculata) are well adapted to the harsh arid environments (recurrent droughts, shallow soils, crusted soil surface, high summer temperature) and perform better than most introduced species (D’Antonio and Meyerson 2002; Niane 2013). These results are similar to the findings of DePuit et al. (1980) where direct seeding of Gardner saltbush (A. gardneri) in disturbed lands experienced limited success due to the presence of seed bracteoles. Similarly, bracteoles of river saltbush (A. amnicola), and wavy-leaved saltbush (A. undulata) reduced final germination percentage and seedling emergence in Western Australia (Stevens et al. 2006).
Overall, the presence of bracteoles reduced seedling emergence except in S. vermiculata, where emergence was similar between seeds with bracteoles (63.7%) and seeds without bracteoles (65.2%). However, seedling emergence of A. leucoclada was significantly higher for non-scarified seeds (34.6%) compared with scarified seeds (22.1%) (Table 3). This could be attributed to mechanical damage caused by the bracteole removal method. It is likely that excessive bracteole removal damage may have occurred due to low precision of manual removal of bracteoles, owed to the fact that the bracteoles of A. leucoclada seeds are much more tightly attached to the endosperm than the rest of the studied species. To test this hypothesis, a precise and adjustable seed threshing machine was used. The difference in seedling emergence for the seed with machine removed bracteoles was significantly higher (P < 0.01) for A. leucoclada, in both paper and river sand media (Fig. 1). Such differences in the response of seeds of different species to different bracteole removal techniques needs to be taken in considerations in rangeland rehabilitation using direct or indirect seeding.

|
In the present study, seeds with bracteoles of S. vermiculata showed similar seedling emergence percentage to seeds without bracteoles (Table 3). This response could be attributed to environmental conditions that contribute to seedling emergence potential, with or without bracteoles (Pearson et al. 2002). Therefore, despite the presence of bracteoles, seeds with bracteoles were still able to demonstrate comparable emergence levels suggesting that bracteoles in this species do not physically hinder germination and they may not harbor chemicals that interfere with germination as occurs with some other shrubs. Ungar and Khan (2001) studied the influence of bracteoles on the germination response of Atriplex prostrata and A. griffithii. They found that attached bracteoles did not inhibit germination of A. prostrata but completely inhibited germination of A. griffthii seeds. Germination of seeds of A. griffthii was also inhibited in the presence of detached bracteoles, suggesting that the reduced germination of A. griffithii may be due to the presence of relatively high concentrations of dissolved salts in the bracteoles.
Although there were some inconsistencies, removing bracteoles from the seed improved seedling emergence for most species, possibly through breaking the seed coat, thus partially exposing the cotyledons to moisture (Table 3). Exposing the cotyledons can permit the process of hydrolysis and hormone activation, leading to an increase in nucleic acid transcription to promote protein synthesis for germination and early seedling growth (Müntz et al. 2001; Okunlola et al. 2011).
Bracteole removal substantially increased seedling emergence. Separating the seeds from the bracteoles could be beneficial for both physical and physiological reasons including (1) the inhibitory effects of the leachate from the bracteoles (many of them contain a high concentration of salt); (2) the physical barrier the bracteoles impose on the seed; (3) the presence of growth regulating substances or allelopathic compounds in the bracteoles that delay germination; (4) a reduction in oxygen diffusion due to bracteole presence; (5) the effect of the bracteoles on the quality of light falling on the seed (Koller 1957; Aizazzi and Argüello 1992; Schütz 2000; Mandak and Pysek 2001; Li et al. 2008). Similarly, Stevens et al. (2006) reported that removing bracteoles increased germination and improved seedling emergence of Atriplex amnicola and Atriplex undulata by 50% and 150%, respectively. Webb et al. (2009) also found that the removal of seed husks and bracts of sawgrass (Cladium jamaicense) enhanced the percentage and success of germination. The inhibition of germination was attributed to the presence of abscisic acid and other compounds stored in the husks and bracts. Osman and Ghassali (1997) also found that seed germination of S. vermiculata and A. halimus was significantly improved by removing fruiting bracts from the fruits (utricles).
In line with the findings of the present study, mechanical bracteole removal also increased seedling emergence of the coated seed of Tylosema esculentum (Travlos et al. 2007). Our findings add to the consensus that removal of bracteoles stimulates germination and increases seedling emergence. The bracteole removal treatments applied in this study and more specifically the mechanical treatment used for A. leucoclada seems to be very effective in protecting the embryo and hence improving seedlings emergence. Therefore, they would be useful in rangeland revegetation by direct seeding.
The effect of bracteole removal on seedling emergence dynamics, speed of emergence, and time taken to reach maximum seedling emergence
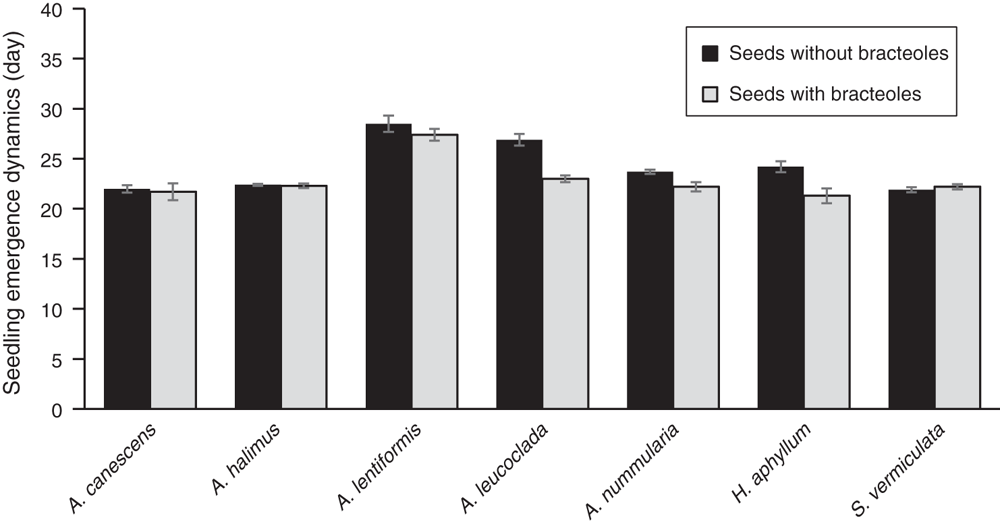
Since no species had reached 90% emergence during the experiment period, the time to 90% observed seedling emergence was estimated from the linear regression. This time and the speed of emergence, showed significant differences in relation to species, bracteole removal, and their interactions (P < 0.01). When compared across all shrub species, bracteole removal resulted in higher seedling emergence percentage per day. The exception was the seeds with bracteole of A. leucoclada, where the opposite was noted. There was no response to bracteole removal in S. vermiculata whereas A. halimus showed the highest seedling emergence percentages per day and together with S. vermiculata recorded the highest mean seedling emergence per day (Fig. 2).

|
Bracteole removal treatments enhanced significantly (P < 0.01) the dynamics of seedling emergence in terms of time to 90% of maximum seedling emergence (seedling emergence potential) compared with seeds with bracteoles, except for Salsola vermiculata. A. lentiformis showed the greatest seedling emergence potential and was the fastest species whereas A. canescens was the slowest (Fig. 3). Overall, all except one species have shown better seedling emergence in response to bracteole removal. Having bracteoles, native halophyte species seem to be more adapted to the arid harsh environmental conditions of the WANA region. Of particular interest is the native S. vermiculata that showed great potential with high seedling emergence even without bracteole removal. This shrub is known for its high palatability and ability to regenerate (Louhaichi et al. 2014) and should be recommended for large-scale restoration in the WANA region. The only problem is that it has a very short seed longevity and therefore should be collected and seeded in the same year (Niane et al. 2013).
|
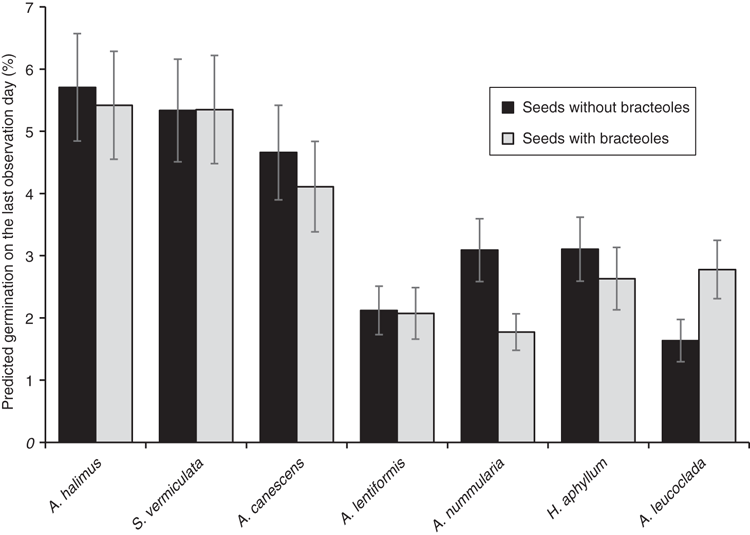
The predicted seedling emergence rate on the last observation date was significantly higher for seeds without bracteoles compared with seeds with bracteoles for all the species except for A. leucoclada and S. vermiculata. The highest and lowest predicted rate of emergence were recorded for A. halimus and A. leucoclada respectively (Fig. 4).
|
Seedling emergence includes two biological steps: seed germination, initiation followed by radicle and shoot elongation. Each step requires different environmental conditions (Roman et al. 2000). The relationship between environmental factors such as climatic conditions, soil, seed, and seedling emergence response, varies with species and the physiological status of the seed (Finch-Savage et al. 2001). However, the interactions among these factors are complex (Vleeshouwers and Kropff 2000). The low percentage and speed of seedling emergence of arid and semi-arid species have been attributed to seed sensitivity to extreme temperatures and moisture availability (Roundy and Biedenbennder 1996; Chesson et al. 2004). Findings from this study suggest that the interaction of species and the presence of bracteoles may significantly contribute to the variability in seedling emergence responses. The initiation of seedling emergence is often more sensitive to water availability (Alvarado and Bradford 2002). This might explain the positive effect of treatment on seedling emergence as the bracteole removal may increase the access to water by the seeds. However, the results did not show any significant benefits of higher seeding rates on seedling emergence, confirming the reports of Linhart (1976) and Florentine et al. (2013). Bracteole removal, in contrast, enhanced both the percentage and speed of seedling emergence of most of the studied species. This is a strong indication of successful establishment of shrub species (Raveneau 2012).
Conclusion
In arid and semi-arid environments, seedling emergence of preferred species may be limited by several factors that regulate seed dormancy, germination and emergence. The success and efficiency of shrub seedling survival and growth under arid conditions depends on removing barriers that inhibit germination and stifle seedling emergence. This study found that bracteole removal from seeds of some shrub species increased seedling emergence percentage and speed. Therefore, seed bracteole removal of shrub species should be considered for large-scale rehabilitation of degraded arid rangelands under the ongoing climate change patterns. This is a crucial step, especially when direct seeding is the only viable option for reversing the trend of rangeland degradation.
Another key finding of this study is the fact that even with the presence of bracteoles, the native halophytic species S. vermiculata and to a lesser degree A. halimus have greater tolerance of extreme temperatures and moisture availability and exhibit high seedling emergence than the introduced species studied. Therefore, these native and well adapted species should be recommended for rehabilitating degraded WANA rangelands.
Nevertheless, to gain a greater understanding of the role of bracteoles in regulating emergence of these rangeland species, further physiological studies are needed to identify factors that minimise seed dormancy. Furthermore, seeding rate should be investigated thoroughly at a larger scale, in the field rather than in pots, and for a longer period to assess factors influencing seedling emergence and establishment.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by the International Centre for Agricultural Research in the Dry Areas (ICARDA), the Arab Fund for Economic and Social Development (AFSED) and the CGIAR Research Program on Livestock (CRP Livestock). The authors thank Mr F. Ghassali and Mr A. K. Salkini for their support implementing the study, while also thanking the Biometrics and Statistics Section, ICARDA, for the statistical support.
References
Aizazzi, M. T., and Argüello, J. A. (1992). Dormancy and germination studies on dispersal units of Atriplex cordobensis (Gandoger et Stucker) (Chenopodiaceae). Seed Science and Technology 20, 401–407.Alvarado, V., and Bradford, K. J. (2002). A hydrothermal time model explains the cardinal temperatures for seed germination. Plant, Cell & Environment 25, 1061–1069.
| A hydrothermal time model explains the cardinal temperatures for seed germination.Crossref | GoogleScholarGoogle Scholar |
Ates, S., and Louhaichi, M. (2012). Reflexions on agropastoralists in the WANA region: challenges and future priorities. Options Méditerranéennes 102, 511–516.
Barrow, J. R., Havstad, K. M., Hubstenberger, J., and McCaslin, B. D. (1997). Seed-borne fungal endophytes on fourwing saltbush, Atriplex canescens. Arid Soil Research and Rehabilitation 11, 307–314.
| Seed-borne fungal endophytes on fourwing saltbush, Atriplex canescens.Crossref | GoogleScholarGoogle Scholar |
Booth, D. T. (2002). Seed longevity and seeding strategies affect sagebrush revegetation. Journal of Range Management 55, 188–193.
| Seed longevity and seeding strategies affect sagebrush revegetation.Crossref | GoogleScholarGoogle Scholar |
Busso, C. A., Bonvissuto, G. L., and Torres, Y. A. (2012). Seedling recruitment and survival of two desert grasses in the Monte of Argentina. Land Degradation & Development 23, 116–129.
| Seedling recruitment and survival of two desert grasses in the Monte of Argentina.Crossref | GoogleScholarGoogle Scholar |
Chesson, P., Gebauer, R. L. E., Schwinning, S., Huntly, N., Wiegand, K., Ernest, M. S. K., Sher, A., Novoplansky, A., and Weltzin, J. F. (2004). Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments. Oecologia 141, 236–253.
| Resource pulses, species interactions, and diversity maintenance in arid and semi-arid environments.Crossref | GoogleScholarGoogle Scholar | 15069635PubMed |
D’Antonio, C., and Meyerson, L. A. (2002). Exotic plant species as problems and solutions in ecological restoration: a synthesis. Restoration Ecology 10, 703–713.
| Exotic plant species as problems and solutions in ecological restoration: a synthesis.Crossref | GoogleScholarGoogle Scholar |
DePuit, E. J., Coenenberg, J. G., and Skilbred, C. L. (1980). Establishment of diverse native plant communities on coal surface-mined lands in Montana as influenced by seeding method, mixture and rate. Montana Agricultural Experiment Station research report 163, Bozeman, MT.
Douglas, G. B., Dodd, M. B., and Power, I. L. (2007). Potential of direct seeding for establishing native plants into pastoral land in New Zealand. New Zealand Journal of Ecology 31, 143–153.
Fernández, C., Vega, J. A., Jiménez, E., Vieira, D. C. S., Merino, A., Ferreiro, A., and Fonturbel, T. (2012). Seeding and mulching + seeding effects on post-fire runoff, soil erosion and species diversity in Galicia (NW Spain). Land Degradation & Development 23, 150–156.
| Seeding and mulching + seeding effects on post-fire runoff, soil erosion and species diversity in Galicia (NW Spain).Crossref | GoogleScholarGoogle Scholar |
Finch-Savage, W. E., Phelps, K., Steckel, J. R. A., Whalley, W. R., and Rowse, H. R. (2001). Seed reserve-dependent and water potential in carrot (Daucus carota L.). Journal of Experimental Botany 52, 2187–2197.
| Seed reserve-dependent and water potential in carrot (Daucus carota L.).Crossref | GoogleScholarGoogle Scholar | 11604458PubMed |
Florentine, S. K., Graz, F. P., Ambrose, G., and O’Brien, L. (2013). The current status of different age, direct-seeded revegetation sites in an agricultural landscape in the Burrumbeet region, Victoria, Australia. Land Degradation & Development 24, 81–89.
| The current status of different age, direct-seeded revegetation sites in an agricultural landscape in the Burrumbeet region, Victoria, Australia.Crossref | GoogleScholarGoogle Scholar |
Franco, J. A., Martínez-Sánchez, J. J., Fernández, J. A., and Bañón, S. (2006). Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. The Journal of Horticultural Science & Biotechnology 81, 3–17.
| Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments.Crossref | GoogleScholarGoogle Scholar |
Gairola, K. C., Nautiyal, A. R., and Dwivedi, A. K. (2011). Effect of temperatures and germination media on seed germination of Jatropha curcas Linn. Advances in Bioresearch 2, 66–71.
García-Fayos, P., Bochet, E., and Cerdà, A. (2010). Seed removal susceptibility through soil erosion shapes vegetation composition. Plant and Soil 334, 289–297.
| Seed removal susceptibility through soil erosion shapes vegetation composition.Crossref | GoogleScholarGoogle Scholar |
Genstat (2009). ‘Genstat for Windows: Introduction.’ 14th edn. (VSN International: Hemel Hempstead, UK.)
Ghassali, F., Salkini, A. K., Petersen, S. L., Niane, A. A., and Louhaichi, M. (2012). Germination dynamics of Acacia species under different seed treatments. Range Management and Agroforestry 33, 37–42.
Gordon, D. R., Menke, J. M., and Rice, K. J. (1989). Competition for soil water between annual plants and blue oak (Quercus douglasii) seedlings. Oecologia 79, 533–541.
| Competition for soil water between annual plants and blue oak (Quercus douglasii) seedlings.Crossref | GoogleScholarGoogle Scholar | 28313489PubMed |
Hudson, L. N., Newbold, T., Contu, S., Hill, S. L. L., Lysenko, I., De Palma, A., and Purvis, A. (2017). The database of the PREDICTS (Projecting Responses of Ecological Diversity In Changing Terrestrial Systems) project. Ecology and Evolution 7, 145–188.
| The database of the PREDICTS (Projecting Responses of Ecological Diversity In Changing Terrestrial Systems) project.Crossref | GoogleScholarGoogle Scholar | 28070282PubMed |
International Seed Testing Association (2017). Rules Proposals for the International Rules for Seed Testing 2017 Edition. Available at: https://www.seedtest.org/upload/cms/user/OGM16–05-Proposed-Changes-to-the-ISTA-Rules-for-20171.pdf (accessed 7 August 2018).
Jefferson, L. V., and Pennacchio, M. (2003). Allelopathic effects of foliage extracts from four Chenopodiaceae species on seed germination. Journal of Arid Environments 55, 275–285.
| Allelopathic effects of foliage extracts from four Chenopodiaceae species on seed germination.Crossref | GoogleScholarGoogle Scholar |
Kemp, D. R., Guodong, H., Xiangyang, H., Michalk, D. L., Fujiang, H., Jianping, W., and Yingjun, Z. (2013). Innovative grassland management systems for environmental and livelihood benefits. Proceedings of the National Academy of Sciences of the United States of America 110, 8369–8374.
| Innovative grassland management systems for environmental and livelihood benefits.Crossref | GoogleScholarGoogle Scholar | 23671092PubMed |
Khan, M. A., and Ungar, I. A. (1985). The role of hormones in regulating the germination of polymorphic seeds and early seedling growth of Atriplex triangularis under saline conditions. Physiologia Plantarum 63, 109–113.
| The role of hormones in regulating the germination of polymorphic seeds and early seedling growth of Atriplex triangularis under saline conditions.Crossref | GoogleScholarGoogle Scholar |
Koller, D. (1957). Germination-regulating mechanisms in some desert seeds, IV. Atriplex dimorphostegia Kar. et Kir. Ecology 38, 1–13.
| Germination-regulating mechanisms in some desert seeds, IV. Atriplex dimorphostegia Kar. et Kir.Crossref | GoogleScholarGoogle Scholar |
Le Houérou, H. N. (2000). Utilization of fodder trees and shrubs in the arid and semiarid zones of West Asia and North Africa. Arid Soil Research and Rehabilitation 14, 101–135.
| Utilization of fodder trees and shrubs in the arid and semiarid zones of West Asia and North Africa.Crossref | GoogleScholarGoogle Scholar |
Lesica, P., and Allendorf, F. W. (1999). Ecological genetics and the restoration of plant communities: mix or match? Restoration Ecology 7, 42–50.
| Ecological genetics and the restoration of plant communities: mix or match?Crossref | GoogleScholarGoogle Scholar |
Li, W., An, P., Liu, X., Khan, M. A., Tsuji, W., and Tanaka, K. (2008). The effect of light, temperature and bracteoles on germination of polymorphic seeds of Atriplex centralasiatica Iljin under saline conditions. Seed Science and Technology 36, 325–338.
| The effect of light, temperature and bracteoles on germination of polymorphic seeds of Atriplex centralasiatica Iljin under saline conditions.Crossref | GoogleScholarGoogle Scholar |
Linhart, Y. B. (1976). Density-dependent seed germination strategies in colonizing versus non-colonizing plant species. Journal of Ecology 64, 375–380.
| Density-dependent seed germination strategies in colonizing versus non-colonizing plant species.Crossref | GoogleScholarGoogle Scholar |
Louhaichi, M., and Tastad, A. (2010). The Syrian Steppe: past trends, current status, and future priorities. Rangelands 32, 2–7.
| The Syrian Steppe: past trends, current status, and future priorities.Crossref | GoogleScholarGoogle Scholar |
Louhaichi, M., Hassan, S., and Clifton, K. (2014). Direct seeding of Salsola vermiculata for rehabilitation of degraded arid and semi-arid rangelands. Range Management and Agroforestry 35, 182–187.
Louhaichi, M., Yigezu, A. Y., Werner, J., Dashtseren, L., El-Shater, T., and Ahmed, M. (2016a). Financial incentives: Possible options for sustainable rangeland management? Journal of Environmental Management 180, 493–503.
| Financial incentives: Possible options for sustainable rangeland management?Crossref | GoogleScholarGoogle Scholar | 27288553PubMed |
Louhaichi, M., Ziadat, F., Ates, S., and Zucca, C. (2016b). Rangeland rehabilitation in the southern part of the Mediterranean basin. Options Méditerranéennes, Series A. Mediterranean 114, 415–418.
Malcolm, C. V. (1994). Use of halophytes forages for rehabilitation of degraded lands. In: ‘Halophytes as Resources for Livestock and for Rehabilitation of Degraded Lands’. (Eds V. R. Squiers and V. T. Ayoub.) pp. 139–163. (Kluwer Academic Publisher: Dordrecht, The Netherlands.)
Mandak, B., and Pysek, P. (2001). The effects of light quality, nitrate concentration and presence of bracteoles on germination of different fruit types in the heterocarpous Atriplex sagittata. Journal of Ecology 89, 149–158.
| The effects of light quality, nitrate concentration and presence of bracteoles on germination of different fruit types in the heterocarpous Atriplex sagittata.Crossref | GoogleScholarGoogle Scholar |
Müntz, K., Belozersky, M. A., Dunaevsky, Y. E., Schlereth, A., and Tiedemann, J. (2001). Stored proteinases and the initiation of storage protein mobilization in seeds during germination and seedling growth. Journal of Experimental Botany 52, 1741–1752.
| Stored proteinases and the initiation of storage protein mobilization in seeds during germination and seedling growth.Crossref | GoogleScholarGoogle Scholar | 11520862PubMed |
Niane, A. A. (2013). Impact of grazing on range plant community components under arid Mediterranean climate in northern Syria. PhD Thesis, Wageningen University, Wageningen, The Netherlands.
Niane, A. A., Struik, C. P., and Bishaw, Z. (2013). Effects of temperature, relative humidity and moisture content on seed longevity of shrubby Russian thistle (Salsola vermiculata L.). Journal of Agricultural Science and Technology B 3, 623–634.
Okunlola, A. I., Adebayo, R. A., and Orimogunje, A. D. (2011). Methods of breaking seed dormancy on germination and early seedling growth of African locust bean (Parkia biglobosa) (JACQ.) Benth. Journal of Horticulture and Forestry 3, 1–6.
Ortiz-Dorda, J., Martínez-Mora, C., Correal, E., Simón, B., and Cenis, J. L. (2005). Genetic structure of Atriplex halimus populations in the Mediterranean Basin. Annals of Botany 95, 827–834.
| Genetic structure of Atriplex halimus populations in the Mediterranean Basin.Crossref | GoogleScholarGoogle Scholar | 15713783PubMed |
Osman, A. E., and Ghassali, F. (1997). Effect of storage conditions and presences of fruiting bracts on the germination of Atriplex halimus and Salsola vermiculata. Experimental Agriculture 33, 149–155.
| Effect of storage conditions and presences of fruiting bracts on the germination of Atriplex halimus and Salsola vermiculata.Crossref | GoogleScholarGoogle Scholar |
Ouled Belgacem, A., and Louhaichi, M. (2013). The vulnerability of native rangeland plant species to global climate change in the West Asia and North African regions. Climatic Change 119, 451–463.
| The vulnerability of native rangeland plant species to global climate change in the West Asia and North African regions.Crossref | GoogleScholarGoogle Scholar |
Palmerlee, A. P., and Young, T. P. (2010). Direct seeding is more cost effective than container stock across ten woody species in California. Nature Plants 11, 89–102.
Pearson, T. R. H., Burslem, D. F. R. P., Mullins, C. E., and Dalling, J. W. (2002). Germination ecology of neotropical pioneers: interacting effects of environmental conditions and seed size. Ecology 83, 2798–2807.
| Germination ecology of neotropical pioneers: interacting effects of environmental conditions and seed size.Crossref | GoogleScholarGoogle Scholar |
Porqueddu, C., Ates, S., Louhaichi, M., Kyriazopoulos, A. P., Moreno, G., del Pozo, A., Ovalle, C., Ewing, M. A., and Nichols, P. G. H. (2016). Grasslands in ‘Old World’ and ‘New World’ Mediterranean climate zones: past trends, current status and future research priorities. Grass and Forage Science 71, 1–35.
| Grasslands in ‘Old World’ and ‘New World’ Mediterranean climate zones: past trends, current status and future research priorities.Crossref | GoogleScholarGoogle Scholar |
Prats, S. A., Cortizo Malvar, M., Simões Vieira, D. C., MacDonald, L., and Keizer, J. J. (2016). Effectiveness of hydromulching to reduce runoff and erosion in a recently burnt pine plantation in central Portugal. Land Degradation & Development 27, 1319–1333.
| Effectiveness of hydromulching to reduce runoff and erosion in a recently burnt pine plantation in central Portugal.Crossref | GoogleScholarGoogle Scholar |
Pyankov, V. I., Black, C. C., Artyusheva, E. G., Voznesenskaya, E. V., Ku, M. S. B., and Edwards, G. E. (1999). Features of photosynthesis in Haloxylon species of Chenopodiaceae that are dominant plants in Central Asia deserts. Plant & Cell Physiology 40, 125–134.
| Features of photosynthesis in Haloxylon species of Chenopodiaceae that are dominant plants in Central Asia deserts.Crossref | GoogleScholarGoogle Scholar |
Raizada, A., and Juyal, G. P. (2012). Tree species diversity, species regeneration and biological productivity of seeded Acacia catechu Willd. in rehabilitated limestone mines in the North West Indian Himalayas. Land Degradation & Development 23, 167–174.
| Tree species diversity, species regeneration and biological productivity of seeded Acacia catechu Willd. in rehabilitated limestone mines in the North West Indian Himalayas.Crossref | GoogleScholarGoogle Scholar |
Raveneau, M. P. (2012). Effect of seed desiccation rate and low temperature on pea germination. PhD Thesis, Ecole supérieure d’agriculture d’Angers, Angers, France.
Roman, E. S., Murphy, S. D., and Swanton, C. J. (2000). Simulation of Chenopodium album seedling emergence. Weed Science 48, 217–224.
| Simulation of Chenopodium album seedling emergence.Crossref | GoogleScholarGoogle Scholar |
Roundy, B. A., and Biedenbennder, S. H. (1996). Germination of warm-season grasses under constant and dynamic temperatures. Journal of Range Management 49, 425–431.
| Germination of warm-season grasses under constant and dynamic temperatures.Crossref | GoogleScholarGoogle Scholar |
Sankary, M. N. (1986). Species distribution and growth in salt affected land of Syria. Reclamation and Revegetation Research 5, 125–143.
Schütz, W. (2000). Ecology of seed dormancy and germination in sedges (Carex). Perspectives in Plant Ecology, Evolution and Systematics 3, 67–89.
| Ecology of seed dormancy and germination in sedges (Carex).Crossref | GoogleScholarGoogle Scholar |
Stevens, J. C., Barrett-Lennard, E. G., and Dixon, K. W. (2006). Enhancing the germination of three fodder shrubs (Atriplex amnicola, A. nummularia, A. undulate; Chenopodiaceae): Implications for optimization of field establishment. Australian Journal of Agricultural Research 57, 1279–1289.
| Enhancing the germination of three fodder shrubs (Atriplex amnicola, A. nummularia, A. undulate; Chenopodiaceae): Implications for optimization of field establishment.Crossref | GoogleScholarGoogle Scholar |
Travlos, I. S., Economou, G., and Karamanos, A. I. (2007). Germination and emergence of the hard seed coated Tylosema esculentum (Burch) A. Schreib in response to different pre-sowing seed treatments. Journal of Arid Environments 68, 501–507.
| Germination and emergence of the hard seed coated Tylosema esculentum (Burch) A. Schreib in response to different pre-sowing seed treatments.Crossref | GoogleScholarGoogle Scholar |
Ungar, I. A. (1978). Halophyte seed germination. Botanical Review 44, 233–264.
| Halophyte seed germination.Crossref | GoogleScholarGoogle Scholar |
Ungar, I. A., and Khan, M. A. (2001). Effect of bracteoles on seed germination and dispersal of two species of Atriplex. Annals of Botany 87, 233–239.
| Effect of bracteoles on seed germination and dispersal of two species of Atriplex.Crossref | GoogleScholarGoogle Scholar |
Vleeshouwers, L. M., and Kropff, M. J. (2000). Modelling field emergence patterns in arable weeds. New Phytologist 148, 445–457.
| Modelling field emergence patterns in arable weeds.Crossref | GoogleScholarGoogle Scholar |
Watson, M. C., and O’Leary, J. W. (1993). Performance of Atriplex species in the San Joaquin valley, California, under irrigation and with mechanical harvests. Agriculture, Ecosystems & Environment 43, 255–266.
| Performance of Atriplex species in the San Joaquin valley, California, under irrigation and with mechanical harvests.Crossref | GoogleScholarGoogle Scholar |
Webb, J., Miao, S., and Zhang, X. (2009). Factors and mechanisms influencing seed germination in a wetland plant sawgrass. Plant Growth Regulation 57, 243–250.
| Factors and mechanisms influencing seed germination in a wetland plant sawgrass.Crossref | GoogleScholarGoogle Scholar |
Young, J. A., Kay, B. L., George, A., and Evans, R. A. (1980). Germination of three species of Atriplex. Agronomy Journal 72, 705–709.
| Germination of three species of Atriplex.Crossref | GoogleScholarGoogle Scholar |



