205 CAPACITATION OF STALLION SPERMATOZOA EVALUATED BY FERTILIZATION OF BOVINE OOCYTES
M. R. Hudson A , G. E. Seidel Jr A , E. L. Squires A , B. E. Spizzirri A , D. J. Walker A and J. K. Graham AARBL, Colorado State University, Fort Collins, CO, USA
Reproduction, Fertility and Development 20(1) 181-182 https://doi.org/10.1071/RDv20n1Ab205
Published: 12 December 2007
Abstract
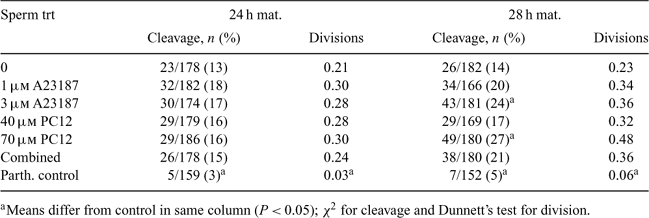
In vitro fertilization in the horse does not work reliably. Several methods of capacitating sperm in other species fail in the horse. The goal of this experiment was to develop a method to capacitate equine spermatozoa using calcium ionophore A23187 or phosphatidylcholine 12 (PC12). We also studied effects of maturing bovine oocytes for 24 or 28 h on fertilizability by capacitated equine sperm, hypothesizing that longer maturation would yield oocytes more easily fertilized by equine spermatozoa. Two sets of bovine oocytes were aspirated from 3 to 8 mm follicles of abattoir ovaries 4 h apart, but fertilized at the same time. On the day of fertilization, semen from 1 of 3 stallions was collected, evaluated, and centrifuged through 33% Percoll to remove seminal plasma. The resultant pellet was extended to 5 × 107 cells mL–1 in M199 containing 0.6% BSA, 2 mm caffeine, and 5 mm CaCl2. Sperm were treated with A23187 (1 or 3 μm) or PC12 (40 or 70 μm) or both A23187 and PC12 (1 μm/40 μm) in 500- μL aliquots. Sperm were incubated at 39°C for 10 min (for A23187 and combination treatments) or 15 min (for PC12 treatments), and then diluted 1:20 for fertilization. Oocytes from each maturation time were fertilized using the same semen preparation for each treatment. Oocytes and sperm were incubated together for 18 h in FCDM in 5% CO2 at 39°C (De La Torre-Sanchez et al. 2006 Reprod. Fertil. Devel. 18, 585–596). Presumptive zygotes were cultured for 30 h in CDM-1, vortexed to remove cumulus cells, and evaluated for cleavage. Oocytes were also co-incubated with killed sperm to determine the level of parthenogenesis. Cleaved embryos were stained with orcein to ensure that each cell had a nucleus. Number of cell divisions were recorded as 0 for a 1-cell, 1 for a 2-cell, 1.5 for a 3-cell, etc. More oocytes cleaved after 28 h (18%) than 24 h (14%) maturation (P < 0.01). Sperm of Stallion 1 resulted in higher overall cleavage (24%) than Stallions 2 or 3 (11 and 12%; P < 0.01). Highest cleavage was seen with 28 h maturation and 70 μm PC12 and 3 μm A23187 (27 and 24%, respectively). The most cell divisions were seen with 28 h maturation and 70 μm PC12 (0.48); 28 of the 49 cleaved in this treatment reached ≥4-cell stage. In conclusion, both A23187 and PC12 were able to capacitate equine sperm in a dose-dependent manner as determined from cleavage of bovine oocytes matured for 28 h; maturation for the conventional 24 h was an inferior model for this purpose.
|


