91 EMBRYO DEVELOPMENT AFTER ICSI OF EQUINE OOCYTES VITRIFIED BEFORE AND AFTER IVM
B. Merlo A , E. Iacono A , S. Colleoni B , E. Dell'Aquila C , C. Galli B and G. Mari AA Veterinary Clinical department, University of Bologna, Bologna, Italy
B Laboratorio di Tecnologie della Riproduzione, C.I.Z. srl, Istituto Sperimentale Italiano Lazzaro Spallanzani, 26100 Cremona, Italy
C Department of animal production, University of Bari, Bari, Italy. Email: gfmari@vet.unibo.it
Reproduction, Fertility and Development 17(2) 195-196 https://doi.org/10.1071/RDv17n2Ab91
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
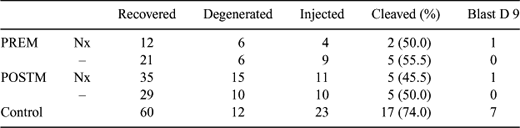
Vitrification has proven to be the method of choice for cryopreservation of mammalian oocytes. In this study, we evaluated in vitro embryonic developmental competence of equine oocytes, vitrified before and after IVM, and fertilized by ICSI. The benefits of the interaction between Naloxone (Nx) and endogenous opioids peptide receptors in different conditions of cellular stress have already been demonstrated (Sheu et al. 1997 Biochem. Biophys. Res. Comm. 231, 12–16). In this study we determined whether addition of Nx to the vitrification solutions can limit the oocyte's damages. COCs collected April to June from abattoir ovaries were: (1) vitrified immediately after recovery (PREM) or (2) matured for 24 h in TCM 199 (Galli et al. 2002 Theriogenology 58, 705–708) before vitrification (POSTM). Half of the oocytes of the two groups were vitrified using solutions supplemented with 10−8 M Nx. Cryoprotectants were loaded in three steps as reported by Maclellan et al. (2002 Theriogenology 58, 911–919). Oocytes were placed on a nylon cryoloop (Hampton Research, Laguna Niguel, CA, USA) and immediately plunged into liquid nitrogen. Oocytes were thawed by immersing the loop sequentially in 0.25 M, 0.188 M, and 0.125 M sucrose in HEPES synthetic oviductal fluid (HSOF) for 30 s per step. PREM oocytes were subjected to 24 h IVM, POSTM were cultured 2–3 h after thawing. Matured oocytes, as assessed by the presence of the first polar body, underwent ICSI. Frozen semen was separated over a discontinuous Percoll gradient and denuded oocytes were injected with a single spermatozoon. Non-vitrified oocytes matured under the same conditions were used as a control. Injected oocytes were cultured in SOFaa until Day 9 (Day 0 day of ICSI). Vitrification was done in five replicates and all oocytes were injected on the same day. Chi-square test was used for statistical analysis (Statistica for Windows; Stat Soft, Inc., Tulsa, OK, USA); significance was assessed at P < 0.05. Results are reported in Table 1. The number of degenerated oocytes and the cleavage rates were not significantly different among treatments (P > 0.05). Within vitrified COCs, only those with Nx in the vitrification solutions reached the blastocyst stage at Day 9; because of the low number of oocytes used in this work, blastocyst rate was not different among treatments. Further studies are needed to evaluate the benefits of adding Nx to oocyte vitrification solutions.
|
This research was funded by MIUR Cofin PRIN 2003.


