36 IMPROVING THE APPLICATION OF NUCLEAR TRANSFER FOR PRODUCING NON-DOMESTIC FELIDS
M.C. Gomez A B , C.E. Pope A , L. Lyons C , A. Cole A , M. Lopez A B , C. Dumas A and B.L. Dresser A DA Audubon Center for Research of Endangered Species, New Orleans, LA, USA
B Department of Animal Sciences, Louisiana State University, Baton Rouge, LA 70803, USA
C School of Veterinary Medicine, University of California-Davis, Davis, CA 95616, USA
D Department of Biological Sciences, University of New Orleans, New Orleans, LA, USA. Email: mgomez@auduboninstitute.org
Reproduction, Fertility and Development 17(2) 168-168 https://doi.org/10.1071/RDv17n2Ab36
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
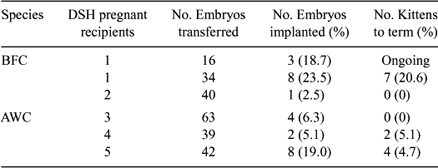
One of the most remarkable aspects of somatic cell nuclear transfer (NT) is the possibility of avoiding extinction when there are few remaining animals of a specific felid population. Previously, we produced live male African Wildcat (AWC; Felis lybica) cloned kittens using inter-species nuclear transfer (Gomez et al. 2004 Cloning and Stem Cells 6, 217–228). The production of females is a primary objective of most breeding programs. Therefore, the purpose of the present study was to determine (1) if we could produce live female AWC cloned kittens at a proportion similar to that previously demonstrated with males, and (2) if our inter-species NT technique used to produce AWC is applicable to in vitro production of another non-domestic felid species. Specifically, we evaluated the in vivo developmental competence of NT embryos derived by fusion of Black footed cat (BFC, Felis nigripes) and AWC fibroblasts with domestic cat (DSH, Felis catus) cytoplasts, after transfer into domestic cat recipients. Fibroblast cell lines were established from skin biopsies of BFC (6-year-old), and AWC (12-year-old) adult females. After at least three passages, cells were serum-starved for 5 days and injected into the perivitelline space of enucleated domestic cat oocytes. Fusion of cell-cytoplast couplets was induced by applying a 3-s AC pre-pulse of 20 V, 1 MHz, followed by two 30-μs DC pulses of 240 V/mm. Fused couplets were activated 2 to 3 h after fusion by exposure to two 60 μsec DC pulses of 120 V/mm, followed by 4 h incubation with 10 μg/mL cycloheximide and 5 μg/mL cytochalasin B. Reconstructed BFC (n = 16) and AWC (n = 536) NT Day 1 embryos were transferred by laparoscopy into the oviducts of 1 and 12 gonadotrophin-treated DSH recipients, respectively, on Day 1 after induced ovulation. Pregnancy was assessed by ultrasonography on Day 22. One cat (100%) receiving BFC NT embryos and 5 (41.6%) cats receiving AWC NT embryos became pregnant. Twenty-three AWC cloned embryos implanted and 11 kittens were born. Three BFC NT embryos implanted and the pregnancy is currently ongoing. AWC cloned kittens were phenotypically and genetically identical to their somatic cell donor. Their clonal identity was assessed by multiplex PCR amplification of 20 microsatellite markers, including seven markers that are known to be on the X chromosome.
In summary, these results indicate that female AWC cloned kittens can be produced and BFC pregnancy can be established in domestic cat recipients. The embryo implantation rate and viability of AWC female cloned embryos was higher than that observed after the transfer of AWC male cloned embryos. The difference may be due to improvements in the NT procedure, rather than to differences in the sex of the cell lines.
|


