275 THE EFFECTS OF MATURATION CULTURE PERIOD ON THE SEX RATIO OF BOVINE IVF EMBRYOS
B. Agung A , P. Wongsrikeao A , M. Murakami A , H. Watari B and T. Otoi AA Laboratory of Animal Reproduction, Department of Veterinary Sciences, Yamaguchi University, Yamaguchi, Japan
B Eiken Chemical Company, Ltd., Tochigi, Japan. Email: agung_bd@mailcity.com
Reproduction, Fertility and Development 17(2) 287-288 https://doi.org/10.1071/RDv17n2Ab275
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
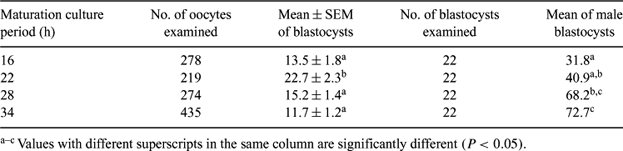
The maturational stage of oocytes at the time of insemination has been suggested as influencing the sex ratio of resulting embryos (Dominko and First, 1997 Theriogenology 47, 1041–1050). However, there are very few reports concerning the relation between the maturation culture period of oocytes and the sex ratio of resulting embryos. The objective of this study was to investigate the effects of maturation culture period of oocytes on the sex ratio of bovine IVF blastocysts, using a novel technique of loop-mediated isothermal amplification (LAMP). Cumulus-oocyte complexes (COCs) were collected from ovaries of slaughtered cows. The COCs were cultured for various times (16, 22, 28 and 34 h) in maturation medium (TCM 199 supplemented with 5% fetal cow serum (FCS), 0.02 mg mL−1 of follicle-stimulating hormone and 50 μg mL−1 of gentamicin). After maturation culture for each period, the oocytes were inseminated with frozen-thawed spermatozoa (4 × 106 spermatozoa mL−1). After incubation with spermatozoa for 5 h, oocytes were transferred into culture medium (TCM199 supplemented with 5% FCS, and 5 μg mL−1 of insulin) and cultured for 7 days at 38.5°C in a humidified atmosphere of 5% CO2 in air. The zona pellucida of blastocysts collected on Day 7 after insemination (Day 0) was removed by brief exposure to 0.2% pronase, and the sex determination of embryos was conducted by the LAMP method, using a bovine embryo sexing kit (Eiken Chemical Co., Ltd., Tochigi, Japan). The rates of blastocyst formation were analyzed by ANOVA, and the sex ratios of embryos were compared by chi-square analysis. As shown in Table 1, the rate of blastocyst formation after insemination was significantly higher (P < 0.05) in the oocytes matured for 22 h than in the oocytes matured for 16, 28, and 34 h. The proportion of male blastocysts derived from oocytes matured for 34 h was significantly higher (P < 0.05) than from oocytes matured for 16 and 22 h. Moreover, the proportions of male blastocysts increased with delaying insemination. These results indicate that skewing of the sex ratio of IVF blastocysts is apparently influenced by the maturation culture period of oocytes.
|


