155 DEVELOPMENT OF BOVINE AGGREGATE EMBRYOS CONSTRUCTED FROM NUCLEAR TRANSFER EMBRYOS AND ELECTROFUSED IVF-DERIVED EMBRYOS
A.M. Landry A , M. Murakami A , R.S. Denniston A , J.L. Williams B , Y. Echelard B and R.A. Godke AA Embryo Biotechnology Laboratory, Department of Animal Sciences, Louisiana State University Agricultural Center, Baton Rouge, LA 70803, USA
B GTC Biotherapeutics, Inc., Framingham, MA, USA. Email: amlandry@agcenter.lsu.edu
Reproduction, Fertility and Development 17(2) 228-228 https://doi.org/10.1071/RDv17n2Ab155
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
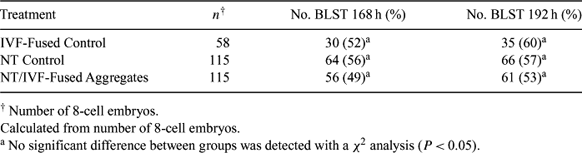
The production of animals by nuclear transfer can be hindered by placental and developmental abnormalities in the fetus. Studies in mice have indicated that tetraploid embryo complementation can be used to rescue embryos with lethal placental deficiencies and produce live offspring. The objectives of this experiment were to produce bovine electrofused embryos by blastomere fusion and to utilize those embryos for aggregation with nuclear transfer (NT) embryos. Oocytes were obtained from a commercial source (BoMed, Madison, WI, USA) and were allocated for use in either NT or in vitro fertilization (IVF). NT embryos were produced using standard procedures. Bovine transgenic fibroblast cells maintained in active culture were used as the nuclear donor. Oocytes were fertilized with frozen semen using standard IVF procedures. Zygotes were observed for cleavage and selected at the 2-cell stage for electrofusion at 28, 30, 32, and 34 h post-insemination in 0.3 M mannitol fusion buffer. Embryos were aligned using a 5 s, 7.5 V AC pulse and were fused using a 1.4 kV cm−1 100 μs DC pulse. Treated embryos were observed after ∼1 h for fusion of cell membranes between the two blastomeres and were returned to culture (IVF-Fused). Good quality 8-cell embryos produced from the NT and IVF-Fused groups were selected for aggregation at 72 h post-insemination. Aggregate embryos were constructed by removing 3–4 blastomeres from an 8-cell NT embryo. The zona pellucida of an 8-cell IVF-Fused embryo was then removed by placing the embryo in a 0.25% pronase solution for approximately 1 min. After the zona pellucida was removed, 3–4 blastomeres were aspirated from the IVF-Fused embryo and were injected into the NT embryo using a glass pipette. Embryos were returned to culture in CR1aa media and were examined at 168 and 192 h post-insemination for blastocyst (BLST) development (Table 1). This study demonstrates that NT/IVF-Fused aggregate embryos can be constructed and develop at the same rate as controls. An attempt was made to determine the nuclear status of the electrofused embryos but technique limitations did not permit differentiation between tetraploid and multinucleate cells. Further research is needed to determine nuclear status of IVF-Fused embryos and the allocation of NT and IVF-Fused cell lineages within the developing embryo.
|


