Accuracy and errors about the human ovary; the good, bad and the ugly
Raymond J. Rodgers A * and Jeffrey B. Kerr B
A * and Jeffrey B. Kerr B
A
B
Abstract
This collection is dedicated to the memory of Professor Ken McNatty and Professor Rex Scaramuzzi, both of whom made outstanding contributions to the understanding of reproductive, and particularly ovarian, biology. In fact, the impetus for this commentary began when the authors questioned why some textbooks continued to print an earlier theory of ovarian development by Haward Sawyer and Ken McNatty (Sawyer et al. 2002), when important additional findings were published in 2013 (Hummitzsch et al. 2013). The authors question why textbooks, websites and YouTube videos continue to present misinformation about the ovary with statements and illustrations that are patently inaccurate or incorrect. We are aware that medical and science textbook publishers may take no responsibility for the accuracy of content by printing a disclaimer to this effect. Webpages and YouTube videos, in the main, exist with no such caveat. Do authors of textbooks accept responsibility to publish up-to-date factual material and avoid demonstrably incorrect information? In some cases, apparently not. Here we will show examples from the ovarian biology that we encounter regularly, that authors often do not check nor update content for the multiple book editions published over decades. If original sources are not consulted by authors, where are they getting their information? Erroneous statements and dogma continue to be represented in scientific literature as established facts. Textbooks, in particular, are supposed to be reliable sources of information. Unfortunately, too many mislead students and scholars and promulgate misinformation. If the contributions of Professor Ken McNatty, Professor Rex Scaramuzzi and others are to be truly valuable, then knowledge amplified by textbooks and the web must at least be accurate.
Keywords: fetus, follicle, human, menstrual cycle, ovary, puberty, textbook, website.
When do primordial follicles begin to grow?
Numerous textbooks and web-based pages and videos assert that after primordial follicles have formed in the human fetal ovary, no further follicular growth or development occurs in fetal life, infancy and childhood. According to these sources, some of these previously quiescent follicles are stimulated to grow only when approaching or reaching puberty (Fig. 1). This storyline is pure fiction. Follicle growth and development occurs in fetal, neonatal, infant and child ovaries and this has been known for more than a century (Keibel and Mall 1912). Depletion of germ cell and primordial follicle numbers is reported in ovaries before birth (Baker 1963) and continues up to and beyond puberty (Wallace and Kelsey 2010). Why, then, would the ovary be described as a dormant organ prior to puberty when it is resorbing and eliminating tens of thousands of atretic follicles? Some of the sources claiming the prepubertal ovary is dormant are medical textbooks (Wheater et al. 1979; Burkitt et al. 1993; Hall and Hall 2020; Ovalle and Nahirney 2020; Standring 2020; Schoenwolf et al. 2021; Carlson 2023; O’Dowd et al. 2023; Pawlina 2023; Mescher 2024) and webpages or YouTube (Table 1). Based upon published research, from mid-gestation onwards, the human fetal ovary may contain a few activated growing follicles. In the third trimester, follicles with a small antral cavity (Fig. 1) can be found and all can undergo atresia. This pattern of follicular growth and degeneration in the human ovary occurs continuously in late fetal life, during infancy and childhood, and has been described many times over the last 50 years (Van Wagenen and Simpson 1965; Lintern-Moore et al. 1974; Peters et al. 1975, 1978). At puberty, with the cyclic stimulation by gonadotrophins, small to medium antral follicles can grow to larger sizes, with usually one reaching a preovulatory size. This follicle can ovulate in response to a surge release of luteinising hormone from the pituitary gland. Additional reports of follicle development prior to puberty are available using histological evidence (Himelstein-Braw et al. 1976; Peters et al. 1976; Himelstein-Braw et al. 1978; Baker and Scrimgeour 1980; Kurilo 1981; Forabosco et al. 1991; Forabosco and Sforza 2007; Overland et al. 2023). All these studies show that the fetal and postnatal ovary are anything but quiescent and are dynamic organs exhibiting constant internal change. The presence of growing antral follicles in human neonatal to prepubertal ovaries is also confirmed using ultrasound (Stanhope et al. 1985; Holm et al. 1995; Kuiri-Hänninen et al. 2011; Chin et al. 2021). A consequence and further evidence of earlier antral follicle growth followed by atresia in fetal, neonatal, infant, and prepubertal human ovaries is the occurrence of ovarian cysts (Strickland 2002; Brandt and Helmrath 2005; Chen et al. 2020). These are usually asymptomatic and resorb without complication.
Follicle development in fetal and postnatal life up to puberty, showing the most advanced follicle types seen with age. Numerous textbooks indicate a long period of ovarian inactivity, whereby the pool of primordial follicles remain quiescent, including at birth, in the infant and in the child. Approaching puberty, some are activated to grow, and small to medium antral follicles appear at this time. However, the research literature shows that when the population of primordial follicles is established in the fetal ovary, follicular growth and development is from that time a basic event occurring continuously and folliculogenesis in infants and children is the norm. In both versions of events, all growing follicles prior to puberty become atretic and disappear. Numbers in brackets are average volumes (in mL) for single ovaries.

| Name | URL | |
|---|---|---|
| Ninja Nerd (2017) YouTube Female Reproductive Cycle: Ovulation. | https://www.youtube.com/watch?v=pzgUbyD6mCM | |
| Lecturio Medical (2018) YouTube Oogenesis and Follicles – Embryology. | https://www.youtube.com/watch?v=7C9JmIA0fbw | |
| Visible Body (2023) Blog The ovarian cycle: a guide. | https://www.visiblebody.com/blog/the-ovarian-cycle-a-guide | |
| National Cancer Institute USA (2024) Ovaries. SEER Training Modules. | https://training.seer.cancer.gov/anatomy/reproductive/female/ovaries.html | |
| Yale Histology (2024) Website. Female Reproductive System Lab. | https://medcell.org/histology/female_reproductive_system_lab.php |
Follicle growth in the reproductive or menstrual cycle
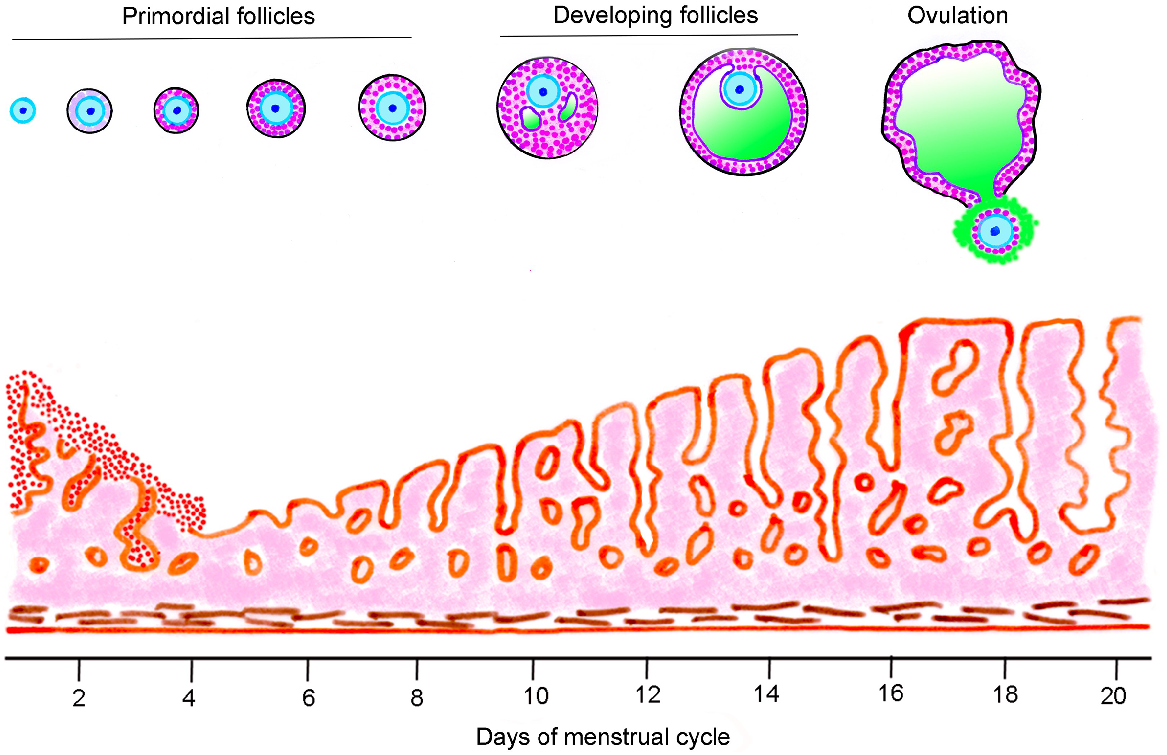
Numerous textbooks of physiology, embryology/developmental biology, histology, medicine and endocrinology, as well as websites, publish figures of the menstrual or reproductive cycle. They show hormonal cycles and stylised changes occurring to follicles and corpora lutea. Most show incorrect information. Their artworks often show a primordial follicle growing into an ovulatory follicle during the course of the follicular phase of a reproductive or menstrual cycle, which is approximately 14 days in humans. An example from the current 42nd edition of Gray’s Anatomy (Standring 2020) is shown in Fig. 2. This graphic is glaringly misleading for two reasons (i) the class of ‘Primordial follicles’ includes growing primary and secondary follicles, which are obviously not primordial follicles and (ii) the time taken for a primordial follicle to mature to ovulation is shown as about 18 days, but this is clearly incorrect. The misinformed illustrations of the whole of folliculogenesis occurring in approximately 2 weeks is not unique to Gray’s Anatomy. It is widely encountered in textbooks (too many to list) and the internet (Table 2). In the human ovary, follicle growth is a very long process (Gougeon 1986; Gougeon 1996). It is estimated that it requires many months for a human primordial follicle to grow into a preovulatory follicle, not merely a couple of weeks (McGee and Hsueh 2000; Gougeon 2010). What really happens in the follicular phase is that a small antral follicle, not a primordial follicle, begins an extended growth phase, eventually culminating in the size of an ovulatory follicle. This is correctly shown in Fig. 3 (Kerr 1999).
Follicle development and uterine mucosa changes up to ovulation in the menstrual cycle. This is part of a larger illustration redrawn from Gray’s Anatomy (Standring 2020). The following features are misleading (i) the heading ‘Primordial follicles’ is erroneous, as it also includes illustrations of primary and secondary follicles (ii) ovulation is shown at day 18, when in fact, it occurs much earlier in the menstrual cycle, and (iii) primordial follicles do not grow and become ovulatory follicles in just over 2 weeks.
| Name | URL | |
|---|---|---|
| Khan Academy (2014) YouTube The ovarian cycle | Reproductive system physiology. | https://www.youtube.com/watch?v=VYSFNwTUkG0 | |
| Ninja Nerd (2016) YouTube Female Reproductive Cycle: Ovulation. | https://www.youtube.com/watch?v=pzgUbyD6mCM | |
| Ninja Nerd (2017) YouTube Female Reproductive Cycle (Menstruation). | https://www.youtube.com/watch?v=EfexbuyIqCY | |
| DrMattDrMike (2021) YouTube Female Reproductive Cycle (Menstrual cycle). | https://www.youtube.com/watch?v=Lzlt1Gk7Riw | |
| DrMattDrMike (2024) YouTube Female Reproductive Cycles Made Easy. | https://www.youtube.com/watch?v=ta4wAINBqio | |
| Histocutup (2024) Website Gynaecology : The Menstrual Cycle | http://www.histocutup.co.uk/Gynaecology/Menstrual.aspx | |
| National Cancer Institute USA (2024) Website. Ovaries. SEER Training Modules. | https://training.seer.cancer.gov/anatomy/reproductive/female/ovaries.html | |
| Wikipedia (2024) Website Menstrual Cycle : Follicular phase. | https://en.wikipedia.org/wiki/Menstrual_cycle#Follicular_phase | |
| Women’s Health Network (2024) Website Your menstrual cycle – the basics. | https://www.womenshealthnetwork.com/pms-and-menstruation/your-menstrual-cycle-the-basics/ |
Simplified scheme of human follicle development shown only during two menstrual cycles. Following an unspecified development time during previous menstrual cycles (dashed line), a limited number of small antral follicles are selected for further growth. In successive cycles, usually just one of these growing follicles will survive to become a dominant follicle available for ovulation. Following oocyte release, the follicle transforms into a corpus luteum that ultimately regresses. Dashed circles represent atresia of some follicles is occurring. M, menses. Redrawn from Kerr (1999).

To illustrate how highly improbable the notion in textbooks is that a primordial follicle grows to the size of an ovulatory follicle in a 2-week follicular phase, consider the following: a primordial follicle is approximately 30 μm in diameter. It grows into an ovulatory follicle approximately 25 mm in diameter, which is a respective 800-fold and 5.74 × 108-fold increase in diameter and volume, and the textbooks imply this takes about 2 weeks. The available evidence for a timeline of human follicular dynamics suggests a newly activated primordial follicle may take up to a year to reach the day of ovulation (Gougeon 1986; Gougeon 1996; McGee and Hsueh 2000; Gougeon 2010) but given the lack of experimental data in humans, there are some caveats. In 1986, Gougeon estimated around 150 days for the primordial to primary transition in humans (Gougeon 1986). By 1996, the model was updated (Gougeon 1996) and this development time was removed and has not been re-instated. Evidence for human follicle development times is available from studies of surgical transplants of frozen–thawed ovarian tissue in cancer patients (Donnez et al. 2004; Li et al. 2010; Donnez et al. 2011), in which preantral or preovulatory follicles appeared approximately 6 months after transplantation. In contrast, when human ovarian tissues are cultured in vitro, primary follicles show accelerated growth to mid- to late- preantral follicles in 8 (McLaughlin et al. 2018) or 21 days, respectively (Xu et al. 2021). Acknowledging these uncertainties, our present understanding of follicle development is shown in Fig. 4. Although the time interval to reach the primary follicle phase is uncertain, it is known that similar, but not identical, groups of small antral follicles (2–5 mm diameter) develop synchronously in two or more waves at regular intervals during the menstrual cycle (Baerwald et al. 2003a; 2003b; Baerwald and Pierson 2020; Bashir et al. 2023). A dominant follicle ≥ 10 mm may continue growth, becoming a preovulatory follicle in the late-follicular phase. Recurrent development and atresia of waves of antral follicles over many menstrual cycles is in marked contrast to the simplistic and flawed concept of Fig. 2.
A possible timeline for folliculogenesis in the human. Time required for transition of primordial into primary follicles is uncertain (by some estimates, approximately 150 days). Growth of preantral follicles to ovulation is estimated at 145 days, or approximately five menstrual cycles. Inset: wave theory of antral follicle development; coloured arcs represent increasing and decreasing size of antral follicles (2–5 mm) as they exhibit growth and atresia; dashed arc, in some women a dominant follicle arises and regresses in the luteal phase; green arc, the growth of the dominant ovulatory follicle; M, menses; dotted line, luteinising hormone concentrations, showing the surge release at ovulation [Inset redrawn from Baerwald et al. (2012)].

Conclusion
The few examples we raised here are not just minor inaccuracies or some esoteric academic points of discussion. They are key pieces of information on how the ovary functions. Unfortunately, now many students and scholars will have studied and learnt incorrect information about the ovary.
How did we get to this situation? The examples of misinformation raised in this article are probably due to authors or artists of textbooks. For many independent textbooks to contain the same misinformation indicates they possibly mirror each other; certainly, the websites and online presentations rely on the textbooks. Maybe the erroneous information in textbooks has come about because the topics covered are so broad that editors cannot reasonably be abreast of a whole area.
How can the situation be rectified? One way already used by some textbooks is for editors or lead authors to coopt authors with specialist knowledge to write or sub-edit individual chapters. From past experience, merely alerting the publishers to misinformation elicits no reply, much less a correction. Perhaps a concerted effort, starting with articles such as this one, might initiate the process to eliminate and correct these inaccuracies.
References
Baerwald A, Pierson R (2020) Ovarian follicular waves during the menstrual cycle: physiologic insights into novel approaches for ovarian stimulation. Fertility and Sterility 114, 443-457.
| Crossref | Google Scholar | PubMed |
Baerwald AR, Adams GP, Pierson RA (2003a) Characterization of ovarian follicular wave dynamics in women. Biology of Reproduction 69, 1023-1031.
| Crossref | Google Scholar |
Baerwald AR, Adams GP, Pierson RA (2003b) A new model for ovarian follicular development during the human menstrual cycle. Fertility and Sterility 80, 116-122.
| Crossref | Google Scholar | PubMed |
Baerwald AR, Adams GP, Pierson RA (2012) Ovarian antral folliculogenesis during the human menstrual cycle: a review. Human Reproduction Update 18, 73-91.
| Crossref | Google Scholar |
Baker TG (1963) A quantitative and cytological study of germ cells in human ovaries. Proceedings of the Royal Society of London. Series B. Biological Sciences 158, 417-433.
| Crossref | Google Scholar | PubMed |
Baker TG, Scrimgeour JB (1980) Development of the gonad in normal and anencephalic human fetuses. Journal of Reproduction and Fertility 60, 193-199.
| Crossref | Google Scholar | PubMed |
Bashir ST, Baerwald AR, Gastal MO, Pierson RA, Gastal EL (2023) Dominant follicle growth patterns and associated endocrine dynamics in anovulatory and ovulatory waves in women. Reproduction and Fertility 4, e220131.
| Crossref | Google Scholar |
Brandt ML, Helmrath MA (2005) Ovarian cysts in infants and children. Seminars in Pediatric Surgery 14, 78-85.
| Crossref | Google Scholar | PubMed |
Chen L, Hu Y, Hu C, Wen H (2020) Prenatal evaluation and postnatal outcomes of fetal ovarian cysts. Prenatal Diagnosis 40, 1258-1264.
| Crossref | Google Scholar | PubMed |
Chin HB, Baird DD, Kaplan SL, Darge K, Adgent MA, Ford EG, Rogan WJ, Stallings VA, Umbach DM (2021) Characterization of ovarian development in girls from birth to 9 months. Paediatric and Perinatal Epidemiology 35, 75-82.
| Crossref | Google Scholar | PubMed |
Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, Martinez-Madrid B, Van Langendonckt A (2004) Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. The Lancet 364, 1405-1410.
| Crossref | Google Scholar |
Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, Pellicer A, Dolmans M-M (2011) Children born after autotransplantation of cryopreserved ovarian tissue. a review of 13 live births. Annals of Medicine 43, 437-450.
| Crossref | Google Scholar | PubMed |
Forabosco A, Sforza C (2007) Establishment of ovarian reserve: a quantitative morphometric study of the developing human ovary. Fertility and Sterility 88, 675-683.
| Crossref | Google Scholar | PubMed |
Forabosco A, Sforza C, De Pol A, Vizzotto L, Marzona L, Ferrario VF (1991) Morphometric study of the human neonatal ovary. The Anatomical Record 231, 201-208.
| Crossref | Google Scholar | PubMed |
Gougeon A (1986) Dynamics of follicular growth in the human: a model from preliminary results. Human Reproduction 1, 81-87.
| Crossref | Google Scholar |
Gougeon A (1996) Regulation of ovarian follicular development in primates: facts and hypotheses. Endocrine Reviews 17, 121-155.
| Crossref | Google Scholar |
Gougeon A (2010) Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Annales d’Endocrinologie 71, 132-143.
| Crossref | Google Scholar | PubMed |
Himelstein-Braw R, Byskov AG, Peters H, Faber M (1976) Follicular atresia in the infant human ovary. Journal of Reproduction and Fertility 46, 55-59.
| Crossref | Google Scholar | PubMed |
Himelstein-Braw R, Peters H, Faber M (1978) Morphological study of the ovaries of leukaemic children. British Journal of Cancer 38, 82-87.
| Crossref | Google Scholar |
Holm K, Mosfeldt Laursen E, Brocks V, Müller J (1995) Pubertal maturation of the internal genitalia: an ultrasound evaluation of 166 healthy girls. Ultrasound in Obstetrics & Gynecology 6, 175-181.
| Crossref | Google Scholar | PubMed |
Hummitzsch K, Irving-Rodgers HF, Hatzirodos N, Bonner W, Sabatier L, Reinhardt DP, Sado Y, Ninomiya Y, Wilhelm D, Rodgers RJ (2013) A new model of development of the mammalian ovary and follicles. PLoS ONE 8, e55578.
| Crossref | Google Scholar |
Kuiri-Hänninen T, Kallio S, Seuri R, Tyrväinen E, Liakka A, Tapanainen J, Sankilampi U, Dunkel L (2011) Postnatal developmental changes in the pituitary-ovarian axis in preterm and term infant girls. The Journal of Clinical Endocrinology & Metabolism 96, 3432-3439.
| Crossref | Google Scholar |
Kurilo LF (1981) Oogenesis in antenatal development in man. Human Genetics 57, 86-92.
| Crossref | Google Scholar | PubMed |
Li J, Kawamura K, Cheng Y, Liu S, Klein C, Liu S, Duan E-K, Hsueh AJW (2010) Activation of dormant ovarian follicles to generate mature eggs. Proceedings of the National Academy of Sciences of the United States of America 107, 10280-10284.
| Crossref | Google Scholar |
Lintern-Moore S, Peters H, Moore GPM, Faber M (1974) Follicular development in the infant human ovary. Journal of Reproduction and Fertility 39, 53-64.
| Crossref | Google Scholar | PubMed |
McGee EA, Hsueh AJW (2000) Initial and cyclic recruitment of ovarian follicles. Endocrine Reviews 21, 200-214.
| Crossref | Google Scholar |
McLaughlin M, Albertini DF, Wallace WHB, Anderson RA, Telfer EE (2018) Metaphase II oocytes from human unilaminar follicles grown in a multi-step culture system. Molecular Human Reproduction 24, 135-142.
| Crossref | Google Scholar |
Overland MR, Li Y, Derpinghaus A, Aksel S, Cao M, Ladwig N, Cunha GR, Himelreich-Perić M, Baskin LS (2023) Development of the human ovary: fetal through pubertal ovarian morphology, folliculogenesis and expression of cellular differentiation markers. Differentiation 129, 37-59.
| Crossref | Google Scholar | PubMed |
Peters H, Byskov AG, Himelstein-Braw R, Faber M (1975) Follicular growth: the basic event in the mouse and human ovary. Journal of Reproduction and Fertility 45, 559-566.
| Crossref | Google Scholar | PubMed |
Peters H, Himelstein-Braw R, Faber M (1976) The normal development of the ovary in childhood. Acta Endocrinologica 82, 617-630.
| Crossref | Google Scholar | PubMed |
Peters H, Byskov AG, Grinsted J (1978) Follicular growth in fetal and prepubertal ovaries of humans and other primates. Clinics in Endocrinology and Metabolism 7, 469-485.
| Crossref | Google Scholar | PubMed |
Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP (2002) Formation of ovarian follicles during fetal development in sheep. Biology of Reproduction 66, 1134-1150.
| Crossref | Google Scholar |
Stanhope R, Adams J, Jacobs HS, Brook CG (1985) Ovarian ultrasound assessment in normal children, idiopathic precocious puberty, and during low dose pulsatile gonadotrophin releasing hormone treatment of hypogonadotrophic hypogonadism. Archives of Disease in Childhood 60, 116-119.
| Crossref | Google Scholar |
Strickland JL (2002) Ovarian cysts in neonates, children and adolescents. Current Opinion in Obstetrics and Gynecology 14, 459-465.
| Crossref | Google Scholar | PubMed |
Wallace WHB, Kelsey TW (2010) Human ovarian reserve from conception to the menopause. PLoS ONE 5, e8772.
| Crossref | Google Scholar |
Xu F, Lawson MS, Bean Y, Ting AY, Pejovic T, De Geest K, Moffitt M, Mitalipov SM, Xu J (2021) Matrix-free 3D culture supports human follicular development from the unilaminar to the antral stage in vitro yielding morphologically normal metaphase II oocytes. Human Reproduction 36, 1326-1338.
| Crossref | Google Scholar |


