Sperm morphology of the Australasian hydromyine rodents and the interactions between the spermatozoon and oocyte at the time of fertilisation
William G. Breed A * , Chris M. Leigh B , Emily Roycroft C and Ingrid Ahmer A
A * , Chris M. Leigh B , Emily Roycroft C and Ingrid Ahmer A
A
B
C
Abstract
This paper explores the morphology of spermatozoa in Australian hydromyine rodents, specifically focusing on the plains mouse (Pseudomys australis), and examines the interactions between sperm and eggs at time of fertilisation.
The aim of this study is to provide an overview of sperm morphology of hydromyine rodents, comparing its morphology across the different species and to investigate the interactions between the gametes at fertilisation in the plains mouse.
We summarise the sperm head morphology of the hydromyine rodents across the six divisions, with emphasis on the structure in the plains mouse and its interactions with the zona pellucida during fertilisation.
Most hydromyine rodents, including the plains mouse, exhibit a highly complex sperm head morphology with two prominent ventral processes in addition to the apical hook. These processes primarily contain filamentous actin with some species of the New Guinea Pogonomys Division having a nuclear extension into the lower process. Nevertheless three species in the Pogonomys Division and a few in the Pseudomys Division have derived sperm heads which lack the ventral processes which in the plains mouse bind the sperm to the zona pellucida around the ovulated oocyte. This may stabilise the sperm head at this time and facilitate zona pellucida penetration and fusion with the oolemma at this time.
The complex sperm head morphology in most of the hydromyine rodents is likely to date back over one million years with, in the plains mouse, interaction between sperm and egg during fertilisation involving sperm head stabilisation and zona pellucida attachment.
These findings suggest in hydromyine rodents valuable insights into the evolutionary development of sperm morphology and sperm-egg interactions during fertilisation, and in particular that the role of the ventral processes may be critical for successful fertilisation in this group. Understanding these processes could give insight into broader studies on reproductive strategies and evolutionary biology in rodents.
Keywords: Australasian rodents, evolution, fertilisation, Hydromyinae, morphology, plains mouse, sperm diversity, sperm-oocyte interactions, ventral processes.
Introduction
The male gamete, the spermatozoon, shows much morphological diversity across species of eutherian mammals. Most rodents in the family Muridae have a characteristic sperm head that contains an apical hook, whose function is somewhat debatable. Some authors suggest that it facilitates attachment of the spermatozoa to the epithelial lining of the upper reaches of the female reproductive tract (Suarez 1987; Timothy Smith and Yanagimachi 1990; Firman and Simmons 2009; Firman et al. 2013), whereas others consider that it may optimise the spermatozoa forming groups to enhance their migration up the female reproductive tract to the site of fertilisation (Moore et al. 2002; Immler et al. 2007; Ramm and Stockley 2010; Šandera et al. 2011; Tourmente et al. 2016; Varea-Sanchez et al. 2016).
The Australasian hydromyine rodents represent an evolutionary radiation of murid rodents that began 8.5 million years ago, giving rise to approximately 160 species of rodents that occur in six divisions in Australasia, New Guinea and nearby islands (Roycroft et al. 2022). Most species contain a spermatozoon that is considerably more morphologically complex than that of other murid rodents as it contains, in addition to the apical hook, two ventral processes that extend from the sperm head to lie just beneath the apical hook. Whereas the apical hook contains an extension of the nucleus and acrosome, together with a perforatorium, the two ventral processes vary in their morphology across the various species of hydromyine rodents but, in most, are largely composed of filamentous actin (Flaherty and Breed 1983, 1987; Flaherty et al. 1983; Breed and Leigh 1991, 2010; Breed 1997; Breed et al. 2000, 2007).
Here we present a brief review of our data on the morphology of the spermatozoa across the six divisions of Australasian hydromyine rodents. We also present an overview of the interactions that take place between the sperm and eggs at the time of fertilisation in the Australian plains mouse, Pseudomys australis, which has a typical hydromyine spermatozoon with an apical hook and two ventral processes.
Materials and methods
To visualise the evolution of sperm head morphology across the Australasian rodents, we plotted scanning electron microscopic images of cauda epididymal sperm onto a phylogeny of representative species from the six divisions of hydromyine rodents (Roycroft et al. 2022), along with one species of Rattus as an outgroup. The images of the sperm heads of the Australasian specimens were obtained from adult males, some of which were wild caught, whereas others were obtained from specimens that were held at either the Western Australian or South Australian Museums (Breed 1983, 1984, 1997; Breed et al. 2007; Breed and Leigh 2010; McLennan et al. 2017; Pahl et al. 2018), whereas images of the New Guinea specimens were obtained largely from specimens held at either the Australian Museum in Sydney or in The Australian National Wildlife collection in Canberra (Breed et al. 2007, 2020).
For determining sperm and egg interactions at the time of fertilisation, male and female plains mice, Pseudomys australis, were obtained from the breeding colony held at The University of Adelaide. To obtain the gametes, individuals were given an overdose of sodium pentobarbitone (Nembutal). Spermatozoa were then obtained from the cauda epididymidis and prepared for light microscopy (LM), scanning electron microscopy (SEM) and/or transmission electron microscopy (TEM). For LM, cauda epididymal sperm were stained with a drop of 4′−6 diamidino-2-phenylindole (DAPI), with fluorescence microscopy being carried out with a UGI filter, U dichroic mirror, and Y455 barrier filter. For demonstration of filamentous actin, the sperm were similarly prepared and then stained with 7-nitrobenz-2-oxa-1,3-diazole-phallacidin (NBD-phallacidin).
For transmission electron microscopy (TEM), small pieces of cauda epididymidis were obtained after administration of an overdose of Nembutal. They were immersed in 3% glutaraldehyde and 2% paraformaldehyde, made up in 0.1 M phosphate buffer, postfixed in 1% osmium tetroxide and, after dehydration, embedded in epoxy resin. One micron thick plastic sections were cut with an ultramicrotome and stained with uranyl acetate and lead citrate. For scanning electron microscopy (SEM), sperm were extruded from the cauda epididymidis into physiological saline, placed on coverslips coated with polylysine, fixed with 3% glutaraldehyde, dehydrated, critical point dried, and coated with gold and palladium. For demonstration of intramembranous proteins in the cell membrane, freeze fracture was performed. For this, cauda sperm were extruded into 0.1 M phosphate buffer, fixed in 2.5% glutaraldehyde, rinsed in fresh buffer, frozen in liquid Freon 22 at −150°C, stored in liquid nitrogen, fractured at −115°C in a Balzers BA360M freeze-etch unit and replicated with platinum and carbon (see Drew et al. 2014).
For investigations of the morphology of the sperm and eggs at the time of fertilisation, adult female plains mice were given an overdose of Nembutal and oocytes were obtained from the oviducts of females after priming with pregnant mare serum gonadotrophin (PMSG) and human chorionic gonadotrophin (hCG) and placing with adult males. Females were anaesthetised with Nembutal and euthanased approximately 8–10 h after mating if it had taken place. The oviducts were then removed and placed in 3% paraformaldehyde and 2% glutaraldehyde, made up in 0.1 M phosphate buffer. After fixation, they were dehydrated and placed in resin. Plastic sections were then cut and prepared for light and transmission electron microscopy. For light microscopy, the sections were placed on microscope slides and stained, whereas for TEM, ultrathin sections were cut, mounted on grids, stained and subsequently examined at 60 or 80 kV with the TEM.
Results
Morphology of spermatozoa from the hydromyine rodents
Murid rodents in the genus Rattus, tribe Rattini, generally contain a sperm head with a single apical hook and a long tail which attaches to the lower concave surface of the sperm head (see Figs 1 and 2 inset). The apical hook contains a nuclear extension, together with part of the acrosome and a perforatorium.
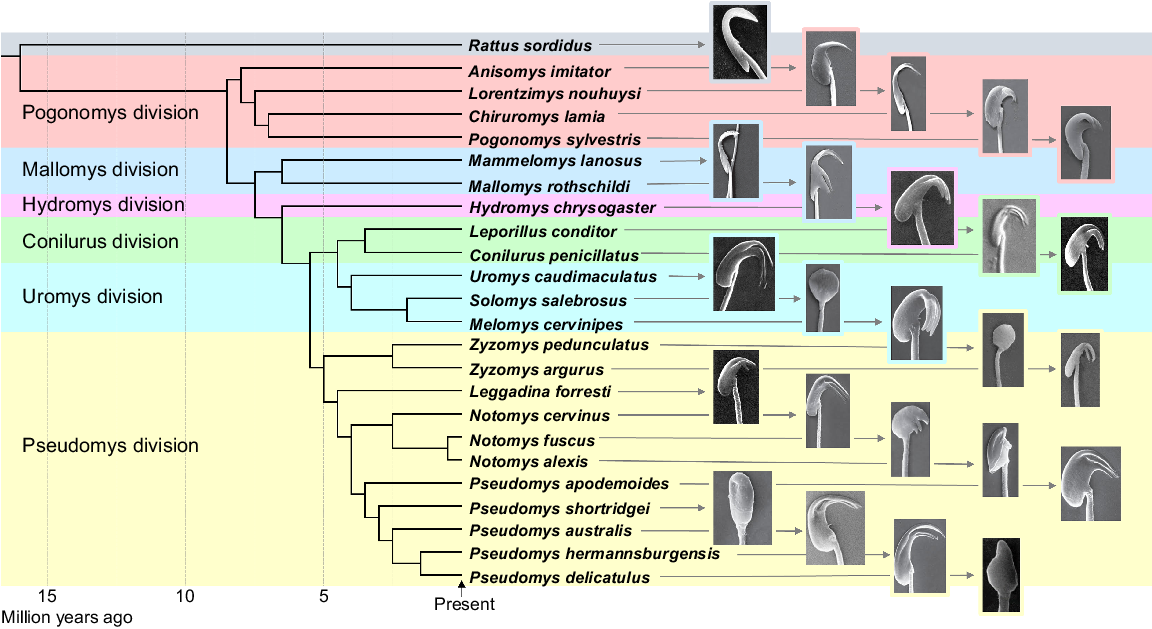
A molecular phylogeny of the Australasian hydromyine rodents, showing the approximate times of divergence of the six divisions (adapted from Roycroft et al. (2022)), together with scanning electron micrograph images of the sperm head morphologies from each of the species indicated.
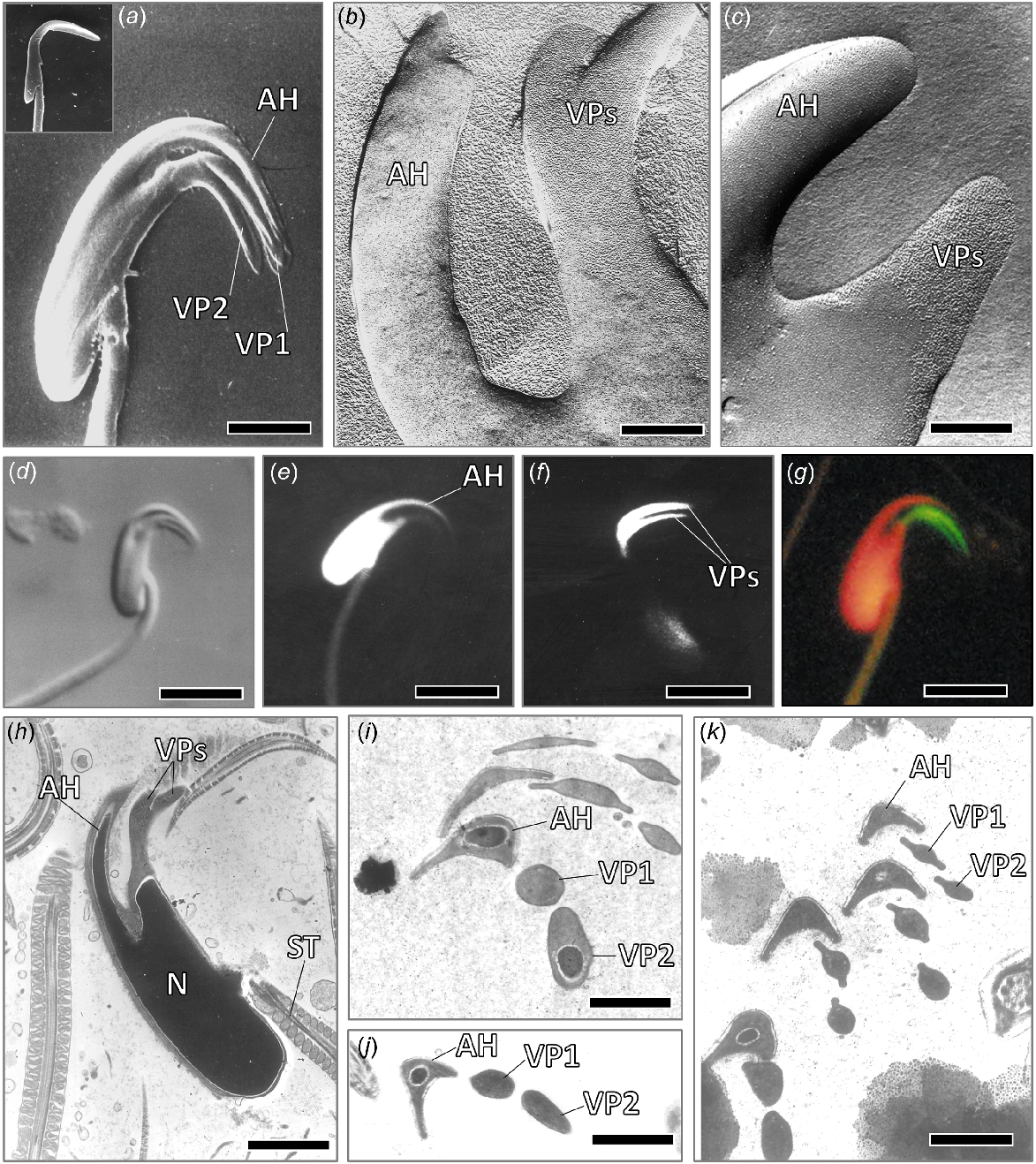
Scanning electron micrographs (a) of sperm heads of cauda epididymal sperm from a bush rat, Rattus fuscipes (inset in a), and a plains mouse, Pseudomys australis (a), showing the presence of two ventral processes (VP1, VP2) in the latter species, but not the former, bar line a = 1.9 μm. Freeze fracture images (b, c) of part of a sperm head from a plains mouse, showing abundant intramembranous particles in the cell membrane of the ventral processes (VPs) but not in the apical hook (AH) (bar line b = 0.4 μm, c = 0.6 μm). Light micrographs (d–g) of sperm heads from cauda epididymidis of a plains mouse, showing (d) Nomarski optics, bar line d = 5 μm; (e) fluorescent image stained with DAPI for DNA, bar line e = 5 μm; (f) fluorescent image stained with Bodipy phallacidin for filamentous actin scale bar 4.2 μm; and (g) fluorescent image stained with DAPI and phallacidin, bar line g = 4.2 μm. In the latter, note strong staining of the two ventral processes, but not the apical hook, with phallacidin (stained green) and DNA in nucleus, including the apical hook (stained red). In (h–k), TEM sections of sperm heads from the plains mouse, with (h) showing longitudinal section through the sperm head, bar line h = 1.5 μm; and (i–k) transverse sections through the apical hook (AH) and the two ventral processes (VP1 and VP2), indicating that the latter become more bilaterally flattened apically (bar line i = 0.7 μm; j, k = 0.8 μm).
The sperm head morphology of the hydromyine rodents varies across the species in the various divisions but in most, it is more complex than that of Rattus. In the two divisions that contain species that largely occur in New Guinea, the Pogonomys and Mallomys divisions, the species Anisomys imitator, Lorentzimys nouhuysi, and Mammelomys lanosus retain a sperm head with a single apical hook but no ventral processes. In Mallomys rothschildi, two ventral processes are present but they differ markedly in their position and form from those of the other species (see Fig. 1), whereas in the several species of both the Chiruromys and Pogonomys genera, ventral processes on the sperm head are clearly present and occur close to the apical hook (for Chiruromys lamia and Pogonomys sylvestris, see Fig. 1).
All species in the Hydromys and Conilurus divisions investigated have typical sperm heads, with an apical hook, together with two ventral processes. The length and orientation of the apical hook and ventral processes are similar to each other for each of the species, albeit they vary somewhat across the species (Fig. 1).
In the Uromys and Pseudomys divisions, most of the species also have a typical sperm head with an apical hook and two ventral processes. However, in the latter division, there are a few species where this is not the case and where a highly divergent sperm head form occurs which, in some, lacks both the apical hook and ventral processes. These include spermatozoa of Solomys salebrosus, Pseudomys delicatulus (and its two close relatives P. pilbarensis and P. mimulus; Roycroft et al. 2024), P. shortridgei, and Zyzomys pedunculatus, all of which have sperm heads that lack both an apical hook and ventral processes (see Fig. 1). In the genus Notomys, N. alexis and N. fuscus both have spermatozoa with a very short apical hook and ventral processes (Fig. 1), with the latter often being absent in the spermatozoa of N. alexis. Conversely, these processes, as well as the apical hook, are well developed in spermatozoa of the closely related species N. cervinus (Fig. 1).
When the sperm of the plains mouse, Pseudomys australis, are stained with NBD-phallacidin for filamentous actin, the two ventral processes stain strongly, unlike the rest of the sperm head (Fig. 2f, g), thus demonstrating the occurrence of an abundance of filamentous actin throughout the length of these processes. Furthermore, freeze fracture shows that the cell membrane of the ventral processes of the spermatozoon has a far greater abundance of intramembranous particles than the cell membrane over the rest of the sperm head (see Fig. 2b, c), suggesting potential receptors for fusing with the zona pellucida.
Morphology of sperm and egg interactions in the plains mouse
Recently ovulated oocytes obtained from the oviduct of the plains mouse (Pseudomys australis) have an adjacent first polar body, with these cells being surrounded by a typical acellular zona pellucida (see Fig. 3a).
Bright field Nomarski light micrographs of (a) a recently ovulated plains mouse oocyte (Oo), showing nucleus (N) and zona pellucida (ZP), bar line = 15 μm; and (b) a spermatozoon bound to the outer surface of the zona pellucida, bar line = 12 μm. (c) LM section though an oocyte, with a sperm head partly within the zona, bar line = 25 μm. Transmission electron micrographs of a spermatozoon in contact with the outer zona pellucida surface, indicating binding by the two ventral processes (d), (e) (bar line = 1 μm); and (f) partly penetrated zona pellucida, showing that the ventral processes of the sperm head increase the size of the hole in the zona during penetration (bar line = 0.6 μm). Transmission electron micrographs (g–i) of cross-sections through the sperm head apical hook (AH) and ventral processes (VP1, VP2) of plains mouse within the cytoplasm of the zygote (Z), showing that the ventral processes (VPs) are still intact together with the apical hook (AH) at this time, shortly after entry into the oocyte cytoplasm. (g) bar line = 4 μm, (h, i) bar line = 0.6, 1.5 μm.
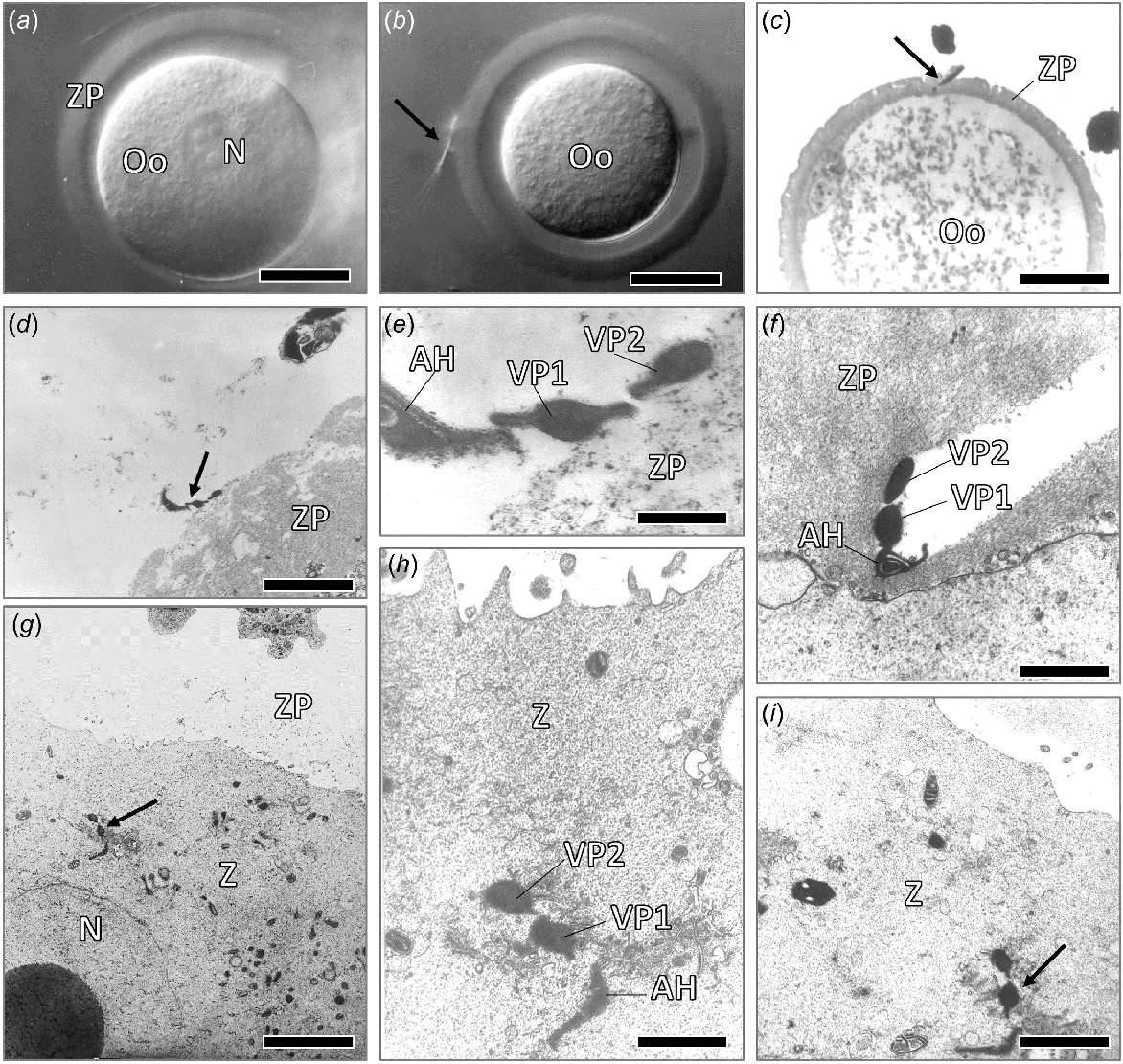
At the time of sperm and egg interactions, the spermatozoon initially binds to the outer surface of the zona pellucida (Fig. 3b), with TEM suggesting that this initial binding occurs by way of receptors on the ventral processes (Fig. 3d, e). Spermatozoa found within the zona matrix result in a hole that appears to be enlarged by the passage of the sperm head ventral processes (see Fig. 3f).
Spermatozoa that had recently entered the oocyte cytoplasm still had the apical hook and two ventral processes largely intact, with transverse sections of both the apical hook and ventral processes of the spermatozoon clearly being evident within the oocyte cytoplasm at this time. The apical hook, which contains the perforatorium and a nuclear extension for much of its length, is generally more bilaterally flattened than that of the ventral processes (Fig. 3g–i).
Discussion
In most species of murid rodents, the sperm head contains an apical hook (Lalli and Clermont 1981; Cummins and Woodall 1985; Oko and Clermont 1988; Pahl et al. 2018), within which there is a perforatorium and an extension of the nucleus, together with part of the acrosome. In the wood mouse (Apodemus spp.), this apical hook around the time of fertilisation appears to aid in spermatozoa forming groups during sperm migration up the female reproductive tract (Moore et al. 2002), whereas in other species it may determine the rate of sperm motility (Immler et al. 2007; Ramm and Stockley 2010; Varea-Sanchez et al. 2016), with species with high levels of sperm competition tending to have spermatozoa with a longer apical hook (Firman and Simmons 2009; Tourmente et al. 2016).
A complex sperm head with an apical hook, together with two ventral processes, is present in most species of the hydromyine rodents in all of the six divisions (see Fig. 1). This suggests that this complex sperm head form evolved in a common ancestor before divergence into the divisions, which occurred in the Miocene and Pliocene eras up to several million years ago. There are, however, a few species which have evolved a very different sperm head morphology. This includes Anisomys imitator and Lorentzimys nouhuysi within the Pogonomys division, and Mammelomys lanosus in the Mallomys division; all three species of which have a sperm head with only a single apical hook but no ventral processes, like that of Rattus, as well as various other south-east Asian species in the subfamily Rattini (Pahl et al. 2018; Breed et al. 2020). However, most of the species within all the divisions in Australia have a sperm head with ventral processes, as well as an apical hook. Species that have spermatozoa with no, or very short, ventral processes include one of the species of Zyzomys, the central rock rat (Z. pedunculatus; Breed et al. 2007) and the spinifex hopping mouse (Notomys alexis), in which variable sperm heads are present that often have no or extremely short ventral processes (Breed 1983; Suttle et al. 1988; Bauer et al. 2005). The heath mouse, (Pseudomys shortridgei), as well as three species of delicate mice (including P. delicatulus) and P. novaehollandiae all have sperm heads with no ventral processes, with only the last species having a sperm head with an apical hook (Breed 1983; McLennan et al. 2017). Sperm–egg interactions in these species have yet to be determined, but the markedly divergent morphology of the sperm heads of these species suggests some differences in sperm–egg interactions at the time of fertilisation, compared to that which occurs in the other species that have a typical hydromyine sperm morphology with two ventral processes.
The occurrence of the complex sperm head with ventral processes that is present in most of the species in the Hydromys, Conilurus, Pseudomys, Uromys and Pogonomys divisions of Australasian rodents suggests that it evolved in an early common ancestor, with these complex sperm heads being retained during the evolution of most of the extant lineages. As these hydromyine rodent divisions diverged up to several million years ago (Rowe et al. 2019; Roycroft et al. 2022; see Fig. 1), it suggests that this highly complex sperm head form with the two ventral processes evolved in a common ancestor in the early Pliocene or late Miocene. In several species in the Pogonomys division, such as Pogonomys sylvestris and Chiruromys lamia, which occur in New Guinea and diverged from the rest of the hydromyine rodents in the late Miocene, the ventral processes are present in the spermatozoa of some of the species. However, the detailed internal structure of the lower ventral process differs from that of the other species in that it contains, in the more caudal process, an extension of the nucleus for most of its length (Breed and Leigh 2010; McLennan et al. 2017), whereas in most hydromyines it terminates near the base of the ventral process. Unlike the function(s) of the apical hook, the data for the two ventral processes in the recently mated plains mouse strongly suggests that these processes facilitate the binding of the spermatozoon to the outer surface of the zona pellucida. These processes may enhance the initial attachment of the spermatozoon to the zona pellucida (McGregor et al. 1989; Breed and Leigh 1991; see Fig. 3b, d, e), with the binding resulting in stabilisation of the sperm head apical hook to the outer surface of the zona pellucida. This may maximise the chances of sperm penetration through the zona matrix and the occurrence of the acrosome reaction. Furthermore, it appears that the size of the penetration hole formed in the zona by the passage of the spermatozoon is somewhat enlarged by the passage of the ventral processes, with these processes subsequently being taken into the ooplasm at the time of sperm–egg fusion.
Conclusion
Since most of the species in all six divisions of the hydromyine rodents have a complex sperm head with two ventral processes, it suggests that this complex sperm form evolved before divergence into the six divisions, with most of the species retaining this complex sperm form. These spermatozoa may be some of the most morphologically complex spermatozoa to have evolved in eutherian mammals.
Data availability
Many of the specimens from which the tissue was obtained are in the various state museums. Most of the plastic and paraffin blocks of tissue used to obtain the data are now present at The School of Animal and Veterinary Sciences, Roseworthy Campus, The University of Adelaide.
References
Bauer M, Leigh C, Peirce E, Breed WG (2005) Comparative study of sperm chromatin condensation in the excurrent ducts of the laboratory mouse Mus musculus and spinifex hopping mouse Notomys alexis. Reproduction, Fertility and Development 17, 611-616.
| Crossref | Google Scholar |
Breed WG (1983) Variation in sperm morphology in the Australian rodent genus, Pseudomys (Muridae). Cell and Tissue Research 229, 611-625.
| Crossref | Google Scholar |
Breed WG (1984) Sperm head structure in the Hydromyinae (Rodentia:Muridae): a further evolutionary development of the subacrosomal space in mammals. Gamete Research 10, 31-44.
| Crossref | Google Scholar |
Breed WG (1997) Evolution of the spermatozoon in Australasian rodents. Australian Journal of Zoology 45, 459-478.
| Crossref | Google Scholar |
Breed WG, Leigh CM (1991) Distribution of filamentous actin in and around spermatids and in spermatozoa of Australian conilurine rodents. Molecular Reproduction and Development 30, 369-384.
| Crossref | Google Scholar |
Breed WG, Leigh CM (2010) The spermatozoon of the old endemic Australo-Papuan and Philippine rodents – its morphological diversity and evolution. Acta Zoologica 91, 279-294.
| Crossref | Google Scholar |
Breed WG, Idriss D, Oko RJ (2000) Protein composition of the ventral processes on the sperm head of Australian hydromyine rodents. Biology of Reproduction 63, 629-634.
| Crossref | Google Scholar |
Breed WG, Leigh CM, Robertson H, Mantellato L, Lambert C, Jequier A, Matson P (2007) Interspecific variation of sperm morphology in the Australian rodent genus Zyzomys. Acta Zoologica 88, 257-263.
| Crossref | Google Scholar |
Breed WG, Leigh CM, Peirce EJ (2020) Reproductive biology of the mice and rats (Family Muridae) in New Guinea – diversity and evolution. Records of the Australian Museum 72, 303-316.
| Crossref | Google Scholar |
Cummins JM, Woodall PF (1985) On mammalian sperm dimensions. Journal of Reproduction and Fertility 75, 153-175.
| Crossref | Google Scholar |
Drew S, Leigh C, Breed WG (2014) Spermatozoa of the old endemic rodents of Australia – the possible functional significance of their ventral processes. Reproduction, Fertility and Development 26, 1183-1187.
| Crossref | Google Scholar |
Firman RC, Simmons LW (2009) Sperm competition and the evolution of the sperm hook in house mice. Journal of Evolutionary Biology 22, 2505-2511.
| Crossref | Google Scholar |
Firman RC, Bentley B, Bowman F, Marchant FG-S, Parthenay J, Sawyer J, Stewart T, O’Shea JE (2013) No evidence of sperm conjugate formation in an Australian mouse bearing sperm with three hooks. Ecology and Evolution 3(7), 1856-1863.
| Crossref | Google Scholar |
Flaherty SP, Breed WG (1983) The sperm head of the plains mouse, Pseudomys australis: ultrastructure and effects of chemical treatments. Gamete Research 8, 231-244.
| Crossref | Google Scholar |
Flaherty SP, Breed WG (1987) Formation of the ventral hooks on the sperm head of the plains mouse, Pseudomys australis. Gamete Research 17, 115-129.
| Crossref | Google Scholar |
Flaherty SP, Breed WG, Sarafis V (1983) Localization of actin in the sperm head of the plains mouse, Pseudomys australis. Journal of Experimental Biology 225, 497-500.
| Crossref | Google Scholar |
Immler S, Moore HDM, Breed WG, Birkhead TR (2007) By hook or by crook? Morphometry, competition, and cooperation in rodent sperm. PLoS ONE 2(1), e170.
| Crossref | Google Scholar |
Lalli M, Clermont Y (1981) Structural changes of the head components of the rat spermatid during late spermiogenesis. American Journal of Anatomy 160, 419-434.
| Crossref | Google Scholar |
McGregor L, Flaherty SP, Breed WG (1989) Structure of the zona pellucida and cumulus oophorus in three species of native Australian rodents. Gamete Research 23, 279-287.
| Crossref | Google Scholar |
McLennan HJ, Lüpold S, Smissen P, Rowe KC, Breed WG (2017) Greater sperm complexity in the Australasian old endemic rodents (Tribe: Hydromyini) is associated with increased levels of inter-male sperm competition. Reproduction, Fertility and Development 29, 921-930.
| Crossref | Google Scholar |
Moore H, Dvoráková K, Jenkins N, Breed W (2002) Exceptional sperm cooperation in the wood mouse. Nature 418, 174-177.
| Crossref | Google Scholar |
Oko R, Clermont Y (1988) Isolation, structure and protein composition of the perforatorium of rat spermatozoa. Biology of Reproduction 39, 673-687.
| Crossref | Google Scholar |
Pahl T, McLennan HJ, Wang Y, Achmadi AS, Rowe KC, Aplin K, Breed WG (2018) Sperm morphology of the Rattini – are the interspecific differences due to variation in intensity of intermale sperm competition? Reproduction, Fertility and Development 30, 1434-1442.
| Crossref | Google Scholar |
Ramm SA, Stockley P (2010) Sperm competition and sperm length influence the rate of mammalian spermatogenesis. Biology Letters 6, 219-221.
| Crossref | Google Scholar |
Rowe KC, Achmadi AS, Fabre P-H, Schenk JJ, Steppan SJ, Esselstyn JA (2019) Oceanic islands of Wallacea as a source for dispersal and diversification of murine rodents. Journal of Biogeography 46, 2752-2768.
| Crossref | Google Scholar |
Roycroft E, Fabre P-H, MacDonald AJ, Moritz C, Moussalli A, Rowe KC (2022) New Guinea uplift opens ecological opportunity across a continent. Current Biology 32, 4215-4224.e3.
| Crossref | Google Scholar |
Roycroft E, Ford F, Ramm T, Schembri R, Breed WG, Burns PA, Rowe KC, Moritz C (2024) Speciation across biomes: rapid diversification with reproductive isolation in the Australian delicate mice. Molecular Ecology 33, e17301.
| Crossref | Google Scholar |
Šandera M, Andrlíková P, Frolíková M, Stopka P (2011) Changes in the curvature of sperm apical hooks in murine rodents. Biologia 66(5), 916-921.
| Crossref | Google Scholar |
Suarez SS (1987) Sperm transport and motility in the mouse oviduct: observations in situ. Biology of Reproduction 36, 203-210.
| Crossref | Google Scholar |
Suttle JM, Moore HDM, Peirce EJ, Breed WG (1988) Quantitative studies on variation in sperm head morphology of the hopping mouse, Notomys alexis. Journal of Experimental Zoology 247, 166-171.
| Crossref | Google Scholar |
Timothy Smith T, Yanagimachi R (1990) The viability of hamster spermatozoa stored in the isthmus of the oviduct: the importance of sperm – epithelium contact for sperm survival. Biology of Reproduction 42, 450-457.
| Crossref | Google Scholar |
Tourmente M, Zarka-Trigo D, Roldan ERS (2016) Is the hook of muroid rodent’s sperm related to sperm train function? Journal of Evolutionary Biology 29, 1168-1177.
| Crossref | Google Scholar |
Varea-Sanchez M, Tourmente M, Bastir M, Roldan ERS (2016) Unraveling the sperm bauplan: relationships between sperm head morphology and sperm function in rodents. Biology of Reproduction 95(1), 25.
| Crossref | Google Scholar |


