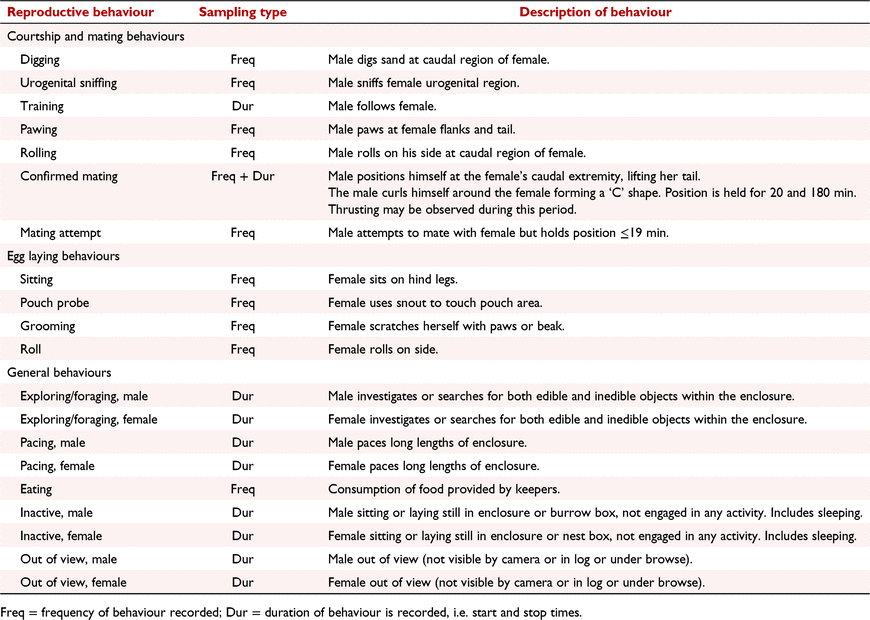
Reproductive behaviour before and after oestrus and oviposition in the captive short-beaked echidna (Tachyglossus aculeatus)
Kate J. Dutton-Regester A * , Alice Roser B , Haley Meer B , Marilyn B. Renfree C , Clive Phillips D E and Stephen D. Johnston
A * , Alice Roser B , Haley Meer B , Marilyn B. Renfree C , Clive Phillips D E and Stephen D. Johnston  A
A
A School of Agriculture and Food Sciences, The University of Queensland, Gatton, Qld 4343, Australia.
B Currumbin Wildlife Sanctuary, Currumbin, Qld 4223, Australia.
C School of BioSciences, The University of Melbourne, Melbourne, Vic. 3010, Australia.
D Estonia University of Life Sciences, Institute of Veterinary Medicine and Animal Science, Tartu, Estonia.
E Curtin University Sustainability Policy Institute, Perth, WA, Australia.
Reproduction, Fertility and Development 34(14) 920-932 https://doi.org/10.1071/RD22092
Published online: 16 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Most of our current knowledge regarding echidna reproductive behaviour is based on qualitative measurements; therefore, it is unclear if specific behavioural cues could be utilised in their captive reproductive management.
Aims: This study aimed to identify quantitative changes in general and reproductive behaviour of echidna breeding pairs and pregnant females that might facilitate the detection of oestrus and impending oviposition and provide a summary of reproductive behaviour observed in a captive colony over a three-year observation period.
Methods: Three echidna breeding pairs and two trios were were monitored daily for seven reproductive and eight general behaviours during the 2020 breeding season. After confirmed copulation, females were monitored for four egg-laying and eight general behaviours until egg incubation. General observations of reproductive behaviours during the 2018–2020 breeding seasons were recorded as part of routine husbandry.
Key results: For breeding pairs, there was a significant rate of change over time before and after copulation for the behaviours ‘urogenital sniffing’, ‘rolling’ and ‘copulation attempt’. For pregnant females, time engaged in ‘pacing’ significantly increased while ‘time eating’ and the ‘qauntity of food eaten’ significanlty decreased on the day of oviposition. We were not able to identify oestrus from specific behaviours, but our observations suggest that the female echidna’s period of receptivity is less than 24 h.
Conclusions: The frequency that males express ‘urogenital sniffing’, ‘rolling’ and ‘copulation attempt’ toward the female can be used to alert zookeepers that copulation has likely occurred. Increased pacing, reduced feeding time and quantity of food eaten can aid zookeepers to identify impending oviposition.
Implications: This study demonstrates that there are quantifiable changes in specific echidna behaviours that can be incorporated into zoo husbandry practices to improve the reproductive management of this species.
Keywords: captive breeding, captive management, egg laying behaviour, oestrus detection, oviposition behaviour, reproductive behaviour, short-beaked echidna, Tachyglossidae.
Introduction
The short-beaked echidna has been housed in zoological institutions for over 100 years (Wallage et al. 2015). Despite their least concern status on the IUCN Redlist (Aplin et al. 2016), echidnas have been notoriously difficult to breed in captivity (Wallage et al. 2015). Nevertheless, the success of captive echidna breeding programs has been steadily increasing over the past decade, with Perth Zoo reporting up to 10 echidna births since 2007 (Ferguson and Turner 2013; Perry et al. 2019), Currumbin Wildlife Sanctuary (CWS) reporting 13 births between 2011 and 2014 (Wallage et al. 2015) and Taronga Zoo having four born between 2016 and 2018 (Perry et al. 2019). This success can most likely be attributed to progress in our understanding of captive echidna reproductive behaviour, resulting in improved husbandry practices such as the provision of increased nutrition prior to the breeding season and the provision of burrow boxes that may facilitate energy conservation (Ferguson and Turner 2013; Wallage et al. 2015).
Echidna behaviours associated with courtship, copulation and oviposition have been described from opportunistic wild encounters and captive based observations. During courtship, echidnas have been shown to form mating ‘trains’ in which one or more males follow a single female (Rismiller and McKelvey 2000; Ferguson and Turner 2013; Wallage et al. 2015). Other courtship behaviours include males probing the female’s urogenital region with their beak, pawing at the female’s flanks and tail and rolling on their side at the female’s caudal region (Wallage et al. 2015). At the time of copulation, the male curls around the female, positioning his urogenital region underneath her, allowing penetration with the penis (Wallage et al. 2015). This position is held, and the male may be observed thrusting for between 20 and 180 min (Rismiller and Seymour 1991; Wallage et al. 2015). During gestation females have been observed to roll on their side or back and probe their pouch and cloaca with their break (Ferguson and Turner 2013). At oviposition, females roll up into a ball-like position, extend the cloaca, and directly deposit the egg into their pouch (Griffiths 1978; Rismiller and McKelvey 2000). During incubation, females typically remain inactive and forego food (Wallage et al. 2015). Despite these observations, many aspects of echidna reproductive behaviour are based on opportunistic observations and remain essentially qualitative in nature.
Oestrus in the female echidna remains elusive, at least to human observers, due to a lack of overt behavioural cues and the difficulty of detecting biologically meaningful changes in plasma oestrogen (Dutton-Regester et al. 2021) and/or faecal oestrogen metabolites (Dutton-Regester et al. submitted to General and Comparative Endocrinology). It is possible that female echidnas undergo a ‘silent’ behavioural oestrus, communicaingt their receptivity by means of chemical signals (i.e. pheromones). This phenomenon has been observed in a wide variety of species (Blissitt et al. 1990; Ziegler et al. 1993; Swaisgood et al. 2002). Indeed, Rismiller (1992) has demonstrated that female echidnas emit a chemical signal during the breeding season that attracts male echidnas.
Oestrus signalling pheromones can induce behavioural changes in the male (Gomez-Diaz and Benton 2013); therefore, it may be possible to observe changes in male echidna behaviour that are reflective of female’s sexual receptivity. If readily discernible specific male behaviours are increased in response to oestrus cues from the female, behavioural observation of the male may have the potential to be used as a non-invasive method for identifying oestrus in captive female echidnas.
Once the female has ovulated and the influence of presumed systemic oestradiol on behaviour has abated, progesterone secreted by the corpus luteum associated with the luteal phase of pregnancy increases (Dutton-Regester et al. 2021), heralding the possibility of further changes in behaviour. In the case of the monotremes, gestation terminates in oviposition (egg laying). An ability to closely monitor or predict egg laying using peri-ovipositional behaviours is likely to be helpful in the reproductive management of this species.
The captive breeding facility at CWS (Dutton-Regester et al. 2021) has provided a valuable opportunity to closely study the reproductive behaviour of echidnas. In an attempt to elucidate behavioural cues that could be used in the reproductive management of captive echidnas, this study aimed to identify quantitative changes in general and reproductive behaviour of breeding pairs and pregnant females that might facilitate the detection of females in oestrus, and predict impending oviposition, respectively. We also provide a summary of the reproductive behaviour observed in the CWS colony over a 3-year observation period.
Methods
Animals and husbandry
The qualitative behavioural study was conducted during June–September 2020 and included seven multiparous female (referred to as F1, F2, etc) and six sexually mature male captive echidnas (Tachyglossus aculeatus aculeatus). These echidnas were a combination of captive and long term wild-caught animals; their precise ages were unknown, but they had been in captivity for a minimum of 15 years. The average weight of both male and female echidnas was 4.5 kg (range: 3.5–6.1 kg, males; 3.5–6.4 kg, females). All echidnas were housed and managed at CWS (28.1356°S, 153.4886°E), Gold Coast, Australia, in a purpose-built breeding centre (see Wallage et al. 2015 for details). Prior to the breeding season, when pregnant or when incubating eggs, female echidnas were individually housed; however, in some cases during the 2019 and 2020 seasons, it was necessary to pair females due to space limitations at CWS. When not housed with females, males were group housed in a separate off-exhibit enclosure (6 m × 3 m). All echidnas were maintained on a beef mince–based diet (Jackson 2003). From November to March, all echidnas were provided with 100 g of feed per animal daily; from April to October, the female portion of the diet was supplemented with fly pupae and olive oil and increased to 150 g per animal daily.
Behavioural observations
Behaviour was recorded with the aid of an infrared motion-detecting surveillance camera (Sony CCD infrared camera, #KTC-79C 4.3-mm lens, Tokyo, Japan) that was installed in the general enclosure and a KOBI CCD infrared dome camera (#K-57HCD 4.3-mm fixed lens, Gold Coast, Australia) mounted within each burrow box (Wallage et al. 2015). Throughout the breeding season cameras recorded continuously to a DVR (KOBI H.264 DVR, Gold Coast, Australia), which allowed direct viewing or downloading to an external hard drive. To differentiate between male and female echidnas when reviewing video footage, a ‘flag’ was created by wrapping reflective safety tape (3M Scotchlite) around a single quill on the dorsal/caudal region of male echidnas (Fig. 1). If two females were present in the same enclosure, a ‘flag’ was placed on both sides of the dorsal/caudal region of one female.
|
|
Study 1a: observations of breeding pairs
An ethogram was developed based on previous observations of wild (Beard and Grigg 2000; Rismiller and McKelvey 2000) and captive echidnas (Ferguson and Turner 2013; Wallage et al. 2015; Table 1). A male was introduced to each female (three singly housed and two pairs) enclosure on June 22. The duration (accuracy ± 1 min) and frequency of seven mutually exclusive reproduction and mating behaviours and eight mutually exclusive general behaviours were analysed retrospectively over a 24-h period (10:00–09:59 h) using continuous focal sampling methods, with behaviours observed being manually recorded by the same observer (KDR). The surveillance system enabled the observer to rewind (e.g. when two echidnas were engaging in independent behaviours) or fast forward (neither echidna active) the video if necessary. Males were removed after they demonstrated loss of interest in the female for four or more consecutive days following a confirmed copulation or when a female started incubation. Incubation was assumed to have started when the female was inactive overnight (Wallage et al. 2015).
|
Study 1b: observations of female echidnas post copulation
After removal of the male from study 1a, female behaviour was intensively monitored until the female started incubation. Behaviour during incubation was monitored and recorded, but not to the same intensity (general observations and notes were made in a dedicated echidna obserbations diary rather than using continuous focal sampling). An ethogram was developed based on previous observations of wild (Beard and Grigg 2000; Rismiller and McKelvey 2000) and captive echidnas (Ferguson and Turner 2013; Table 1). The duration (accuracy ± 1 min) and frequency (number) of four mutually exclusive egg laying behaviours and eight mutually exclusive general behaviours were analysed retrospectively as described for study 1. Pouch young were collected for a parallel study at various stages of development (in progress) and after removal of egg or pouch young, a new male was introduced immediately, and observations continued as per study 1.
Study 2: opportunistic observations of reproductive behaviour
We collated general observations of reproductive behaviours over a 3-year observation period (2018–2020), which involved an additional female echidna (8 in total). Video recordings were viewed by zookeepers and the first author as part of routine daily echidna husbandry during the 2018 and 2019 breeding seasons, but not to the same intensity as that described in Study 1a and b (general observations and notes were made in a dedicated echidna obserbations diary rather than using continuous focal sampling).
Statistical analysis
So that we could quantify behaviour in the 24 h period leading up to copulation/oviposition (i.e. day of copulation/oviposition), and the 6 days before and 6 days after, we defined a day according to the timing of copulation/oviposition, not per calendar day. For example, if a pair concluded copulation at 22:00 h on a Tuesday, day of copulation/oviposition included behaviour data collected between 21:59 h Monday and 22:00 h Tuesday. For each behaviour, observations made over each 24 h period were aggregated to determine the total duration/frequency for each day.
In study 1a, two linear regression models were conducted for each animal for each behaviour. The first model (‘before’) included the 6 days before and the day of confirmed copulation, while the second model (‘after’) included the day of and 6 days after confirmed copulation. The slope (‘m’) values from each linear regression were then used as the response variable in a mixed effects model with animal as a random effect, and before/after presumed oestrus as the fixed effect. Residual plots were used to test data sets for normal distribution. For significant behaviours, a one-way ANOVA followed by a Tukey’s multiple comparisons test was used to test the difference between the duration/frequency on the day of confirmed copulation against the mean of the days before and the mean of the days after confirmed copulation.
In study 1b, a mixed effects model was conducted for each behaviour (excluding ‘quantity of food eaten’) to compare the mean value of the 6 days before oviposition against the value on the day of oviposition. The model was otherwise run and tested as for the previous mixed effects model. For ‘quantity of food eaten’, an ordinal logistic regression model was conducted. Significance levels for all tests were set at P ≤ 0.05. All statistical tests were carried out using the statistical program Minitab (Ver. 21.1.0, 2020).
Ethics statement
This study was approved by the University of Queensland Animal Ethics Committee (SAFS/334/17). Wild short-beaked echidnas were obtained and maintained under the Queensland Government EPA scientific purposes permit (WISP153546614).
Results
Study 1a: observations of breeding pairs
While data collection included seven females, three were later assumed to be acyclic as there was no evidence of cyclicity. Although two pregnancy cycles were recorded for F1, confirmed copulation occurred on the first day of male entry so that no data prior to this was available. Therefore, six cycles from four breeding pairs were available for this analysis.
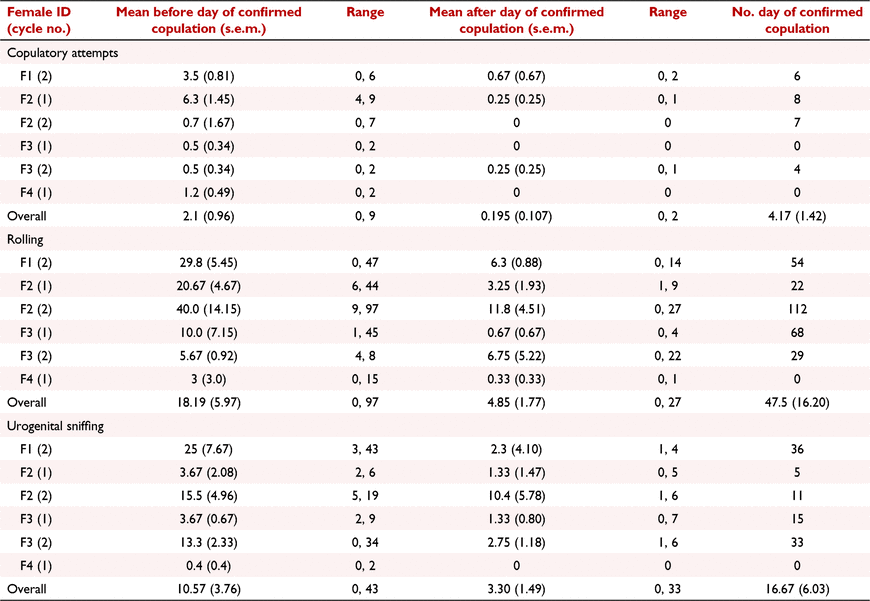
The behaviours ‘urogenital sniffing’, ‘copulatory attempt’, ‘rolling’, ‘digging’, ‘training’, ‘female time active’, ‘male time active’, and ‘female exploring/foraging’ showed a positive rate of change over time before copulation, and negative rate of change over time after copulation. Of these behaviours, regression coefficients for ‘urogenital sniffing’ (mean before: 2.62, mean after: −4.22, SED: ±1.70, P = 0.007, Fig. 2a), ‘copulatory attempt’ (mean before: 0.273, mean after: −1.14, SED: ±0.40, P = 0.022, Fig. 2b), and ‘rolling’ (mean before: 5.95, mean after: −5.40, SED: ±4.60, P = 0.048, Fig. 2b) were significant (Table 2). However, there was considerable inter- and intra- male variability in the frequency that these behaviours were expressed towards the female (Table 3). For example, on the day of confirmed copulation, M4 exhibited ‘rolling’ towards F2 and F3 (cycle 2) a maximum of 112 and 29 times, respectively.
|
|

|
|
For ‘copulatory attempt’ and ‘rolling’, the highest expressions were observed on the day of confirmed copulation in four of six pairings (F1/M1, F2/M3, F3/M4 and F3/M3), but only for a single pairing (F2/M2) for the behaviour ‘urogenital sniffing’ (Table 3). For these behaviours, the mean frequency of the 6 days before confirmed copulation was not significantly different to the frequency on the day of confirmed copulation. However, after confirmed copulation there was a significant decline in the frequency of ‘copulatory attempt’ (mean ± s.e.m.: 0.20 ± 0.02, P = 0.032), and ‘rolling’ (mean ± s.e.m.: 4.85 ± 1.77, P = 0.023) but not ‘urogenital sniffing’ (mean ± s.e.m.: 3.3 ± 1.49, P = 0.094) (Table 3).
The behaviours ‘exploring/foraging male’ and ‘pacing female’ showed a negative rate of change over time before copulation, and a positive rate of change over time after copulation, while ‘pacing male’ showed a positive rate of change both before and after copulation; however, none of these changes in behaviour were significant (Table 3).
Study 1b: observations of female behaviour post copulation
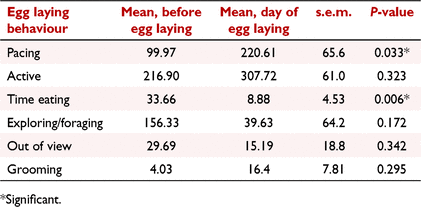
While oviposition was observed on six occasions (from four females), that of F4 could not be included in the data analysis as the male was not removed until the day before oviposition, resulting in inadequate data for meaningful analysis. Consequently, five egg laying events were available from three females. Time engaged in the behaviours ‘pacing’, and ‘time active’, and frequency of the behaviour ‘grooming’, increased on the day of oviposition; however, only ‘pacing’ was significant (regression coefficients, mean before oviposition: 220.61, oviposition day: 99.97, s.e.m.: ± 65.65, P = 0.033; Table 4, Fig. 3a). There was a high level of inter-cycle variation with the time spent ‘pacing’ on the day of oviposition ranging from 7 to 390 min. There was also significant intra- animal cycle variation; for example, the time engaged in ‘pacing’ on the day of oviposition for cycle 1 and 2 of F2 was 214 and 7 min, respectively (Table 5).
|
|
|

|
Time engaged in the behaviours ‘exploring/foraging’, ‘time eating’, the ‘frequency of eating’, and ‘quantity of food eaten’, decreased on the day of oviposition. Of these behaviours, ‘time eating’ (regression coefficients, mean before oviposition: 33.66, oviposition day: 8.88, P = 0.006), and ‘quantity of food eaten’ (P = <0.001) were significant (Fig. 3b, c). Similar to ‘pacing’, there was a high level of inter-cycle variation for both of these behaviours. While time spent eating on the day of egg laying was reduced in four of five cycles, F3 (cycle 1) increased her eating time two-fold. Regarding ‘quantity of food eaten’, no food was consumed in the 24 h prior to oviposition for F1 (cycles 1 and 2) and F2 (cycle 1) and both animals reduced their intake 3 and 1 days before oviposition, respectively. However, F2 (cycle 2) and F3 (cycle 1) consumed all food in the days leading up to oviposition but only ate half of it on the day of oviposition.
Study 2: general descriptive observations of echidna reproductive behaviour (2018–2020)
Sequence of courtship and mating behaviours
Fig. 4 illustrates the general sequence of breeding behaviour displayed by captive male and female echidnas in the CWS breeding colony. Once the male enters the enclosure, short bouts of ‘training’ (following) behaviour begin in addition to frequent urogenital sniffing. At this stage, the female uses avoidance strategies such as termination of the encounter by walking away when approached by the male (see Supplementary material video file ‘training, urogenital sniffing and avoidance’). Subsequently, this progresses to more intense training behaviour which continues over several hours to several days and is intermitted by the male regularly digging at the base of the female’s caudal region (see Supplementary material video file ‘digging’) and rolling on his side in an attempt to copulate with her (see Supplementary material video file ‘rolling’); if the female is not receptive, she continues to demonstrate avoidance behaviours.
|
|
Receptive females were observed to remain stationary (assumed to be standing oestrus), allowing the male to approach (see Supplementary material video file ‘standing oestrus’). Although rarely observed, on two separate occasions, two females were also observed to back their rump into a male’s beak (see Supplementary material video file ‘rump presentation’). One female demonstrated this behaviour several times over a 15 h period (on day 1 of male entry) during which four confirmed copulations, ranging from 50–59 min, and several short mating attempts of less than 10 min were observed. The second female was observed to back her rump into the male’s beak once during a 9 h courtship period; during the first half of this courtship period, 10 successive mating attempts (all <5 min) occurred. The female was observed to lift her tail on the 10th mating attempt and 5 min later she was backing her rump into the male’s beak; this was followed by a brief mating attempt (<2 min). The male subsequently appeared to rest/sleep but was aroused by the female 6 min later, and a further 10 successive mating attempts (all <10 min) were observed. The male trained and attempted to mate with the female for a further 16 days, however, the female demonstrated avoidance behaviour as she had done in the 7 days prior to this described courtship period.
Once the female appeared receptive to the male, the male used his beak to probe the female’s urogenital region and then dig the substrate around this area before rolling on his side adjacent to her caudal region, forming a ‘C’ shaped lateral posture around her rump. The male’s forelimbs appear to place pressure on the female as if to hold her down, while his hindlimbs appeared to guide her tail upward. Once the female’s tail had been raised, the male penetrated his presumably erect penis (not visible) into the female’s urogenital sinus and began thrusting. The male’s thrusting was intermittent, with pauses of 1–18 min (with thrusting bouts ranging from 7–45 min), and the speed and intensity of the thrusting frequently alternated from fast and vigorous to a slower, less vigorous pace. Copulation was terminated when the male withdrew his penis from the female’s urogenital sinus. During copulation, the female remained standing with her tail raised and did not demonstrate any resistance to the male’s advance (see Supplementary material video file ‘copulation’). After successful copulation, the male generally continued intense courtship behaviour for around 2 days and the female subsequently resumed her avoidance strategies. After this, both echidnas engaged in independent activity.
Timing, duration and location of courtship and mating behaviours
When male echidnas were housed with one female, courtship behaviour typically began immediately or within 6 h of male entry (in 94% of 34 echidna pairs) but was also observed up to 12 days later. Courtship continued for mean ± s.e.m.: 10.7 ± 1.9 days (range: 0–26 days) and terminated within mean ± s.e.m.: 2.4 ± 0.3 days (range: 1–3 days) after final observation of copulation (n = 18). In the 34 24 h periods in which confirmed copulations (n = 44) were recorded, typically a single confirmed copulation was observed (n = 29, 85%). However, two (n = 3, 6.8%), three (n = 1, 2.3%) and six (n = 1, 2.3%) confirmed copulations were also observed within a 24 h period, with a mean (±s.e.m.) interval between copulations of 54.8 ± 12.12 min (range: 17–128 min). Confirmed copulations over two consecutive days occurred on four occasions, while others were interspaced by 1, 5, 9 and 31 days; but each only occurred on a single occasion. Thus, 80% of confirmed copulations were confined to a single day. Final observed copulation occurred as early as within 4 h of male entry but was also observed up to 29 days later (mean ± s.e.m.: 12.4 ± 2.1 days, n = 18). The mean duration of copulation was 57.0 ± 6.2 min (range: 20–203 min; n = 44) and typically occurred between 16:00 and 10:30 h. Observed copulations occurred primarily in burrow boxes or logs (n = 40) but occasionally in the open area of the enclosure (n = 3).
Courtship and mating interactions in breeding trios
When housed with two females, the male generally courted both females, alternating between the two, for 2–3 days before focusing his attention on one female. Of the breeding trios observed (n = 4), only one of the two females ever became pregnant. In one example, M4 was housed with two females (F5 and F6) during the 2019 breeding season; all courtship behaviour was directed towards F5, including two assumed copulations (not confirmed due to visibility issues). M4 was replaced by M1 who demonstrated no interest in F5 (later detected to be pregnant by ultrasonography) but courted F6 for two nights (later considered to be in anoestrus), after which, no further interest was shown. Similarly, M5 was housed with F2 and F3 (2020), showing interest in both females for the first 3 days before focusing his attention solely on F2, which resulted in a successful copulation. F3 was later confirmed to be in anoestrus.
Behaviour associated with oviposition
On the day of oviposition, females typically lost interest in food; of the 21 oviposition events recorded, seven were associated with no food having been eaten, eight were associated with < half of the provided food being eaten and four were associated with all food being consumed. 55 to 214 (151.6 ± 20.5) min before oviposition, females (n = 21 oviposition events) generally paced the enclosure (see Supplementary material video file ‘pacing’). which was interrupted by short breaks (periods of inactivity in the open enclosure) and/or would repeatedly enter and exit their burrow box in addition to frequent pouch probing (see Supplementary material video file ‘pouch probe’). At the time of egg laying, females either rolled on their side or sat on their hind legs and extended their head forward towards the cloaca to presumably guide the egg into the pouch (although this was never observed directly); this sequence took 1–2 min and occurred between 17:00 and 09:00 h (n = 21; see Supplementary material video file ‘oviposition’). After oviposition, females resumed pacing and pouch probing for 47.5 ± 9.3 (range: 18–93) min before finding a location to incubate the egg; this was typically in the burrow box or a log (n = 19) or occasionally in the open enclosure (n = 2).
Behaviour during incubation
The duration of incubation when the egg was not prematurely removed was 10.3 ± 0.2 days (n = 15). The egg was removed during incubation of eight females, five of these females resumed activity within 29.8 ± 6.7 h (range: 6–48) of egg removal. The remaining three females continued to demonstrate incubation behaviour until what would have been day 10. On two separate occasions, F3 (2019) continued incubation behaviour for a further 8 and 7 days, respectively, when the egg had been removed on days 8 and 9 of incubation, respectively. Some females (7 of 23 cycles) emerged from incubation to eat but these bouts typically lasted less than 30 min and only occurred once or twice during an individual’s incubation period. Of the seven females that emerged from incubation to eat, two eggs remained viable (29%). For the 16 females that did not eat during incubation, five eggs remained viable (31.3%). One female emerged from incubation on day 7 to eat and resume activity; she was later diagnosed with mastitis and had lost her egg. Over the 3-year study period, six females were) handled two to four times during incubation for blood collection; each female returned to incubation immediately when returned to her enclosure.
Female behaviour while carrying pouch young
For the first 1–3 days after incubation, females (17 of 21 cycles) were generally only active for short periods (20–102 min); however, some females (4 of 21 cycles) were active throughout the night once they were no longer incubating. Regardless, pouch probing/grooming was frequently observed while females were assumed to be carrying pouch young. Most females (18 of 21) ate the full allowance of food provided after incubation, while some (3 of 21) ate 25–75% of the food until days 2–5 post incubation.
Discussion
This study has identified specific behavioural cues that have the potential to be used as non-invasive tools for monitoring reproduction in captive echidna breeding pairs and pregnant females. Leading up to copulation we observed a linear increase in the frequency in which the male would approach the female to sniff her urogenital region, roll at her caudal region and attempt to copulate with her; after copulation, the frequency of these behaviours significantly reduced. On the day of oviposition, female echidnas increased their pacing activity, but reduced feeding time and the quantity of food eaten. These behaviours can aid zookeepers to recognise that a breeding pair have copulated and anticipate the timing of impending oviposition. Additionally, this study identified behaviours indicative of female receptivity that may facilitate the detection and characterisation of oestrus in future studies.
Detection of oestrus in the female echidna by means of behavioural observations remains challenging. While the frequency that the male exhibited ‘rolling’ and ‘copulation attempt’ toward the female generally peaked on the day of confirmed copulation, this was not statistically significant, making it difficult to precisely detect the onset of oestrus. While we focused on male reproductive behaviours directed towards the female in study 1a, our general observations (study 2) revealed that female echidnas may express classic oestrus behaviours commonly observed in livestock and domestic species including standing oestrus and rump presentation (Hurnik et al. 1975; Takahashi 1990) but that direct observation of such behaviours is opportunistic.
Our observation that 80% of confirmed copulations occurred within a single 24-h period suggests that the female echidna’s period of receptivity is less than 24 h. This short window of female receptivity may, in part, explain ‘training’ behaviour (Griffiths 1978), as this close following behaviour ensures that a male is near the female when she becomes receptive. Future behavioural studies that investigate hourly rate of change in male/female interactions (particularly observations of standing oestrus and rump presentation) over the 48 h before and after confirmed copulation may aid in characterising the oestrus period of the echidna.
Our study 1a data show that male echidnas significantly lost interest in females after confirmed copulation. This is supported by our general observation that courtship behaviour terminates approximately two days after confirmed copulation and is also consistent with observations from wild (Rismiller and McKelvey 2000) and other captive echidnas (Ferguson and Turner 2013). Therefore, while we were unable to detect oestrus before copulation using behavioural cues, the significant decline in courtship behaviours directed toward the female after copulation may nevertheless be a useful indicator to zookeepers that copulation has likely occurred, even if coitus is not observed directly. As continuous observation and quantification of the behaviour of a breeding pair is laborious and observation of confirmed copulation is not always possible (Dutton-Regester et al. submitted to General and Comparative Endocrinology), we suggest future studies investigate the reliability of detecting quantifiable changes in ‘rolling’ and ‘copulation attempt’ by observing the first 5 min of each hour between 16:00 and 10:30 h.
Studies of the echidna olfactory region of the brain show that this region is highly evolved and that chemical signals likely play a significant role in mediating complex social behaviours (Harris et al. 2014). Whether chemical signals are involved in signalling female oestrus is unclear as males appear to ‘train’ females (for up to 26 days in this study and 6 weeks in wild populations (Rismiller and McKelvey 2000) until copulation. However, it is possible that chemical signals provide male echidnas with other important information regarding the female’s reproductive status as we observed males to quickly lose interest in pregnant and anoestrous females. Further, after copulation, the 2-day delay in male dispersion (in the wild) or lack of interest (in captive populations) suggests that the male may be waiting for appropriate chemical signals to ensure successful fertilisation has occurred.
On the day of oviposition, female echidnas demonstrated a significant increase in pacing behaviour, but feeding behaviour was suppressed. Our general observations also describe ‘restlessness’ with females repeatedly entering and leaving their burrow. Changes in behaviour in the prepartum period are common in a wide variety of species. For example, studies on domestic cows, goat, sheep and red deer have reported the following behavioural changes as parturition approaches: pacing, pawing, circle walking without an obvious goal, frequent postural changes, reduced eating, and reduced lying duration (Rørvang et al. 2018). These changes in behaviour are likely due to the pain and discomfort associated with parturition (Hydbring et al. 1999; Rørvang et al. 2018) as significant increases in the hormones oxytocin and prostaglandin F and E (PGF and PGE) stimulate intense muscular contractions of the uterus to expel the fetus (O’Brien 1995; Weiss 2000). Similar endocrine changes that promote uterine contractions during oviposition in avian and reptilian species include PGF and PGE in addition to arginine vasotocin, which is biologically similar to oxytocin (Guillette et al. 1991). As the echidna is an egg laying mammal, future studies should investigate PGF and PGE and both oxytocin and arginine vasotocin to determine which of these hormones regulate oviposition in the echidna.
There was variation across cycles in the time engaged in pacing and eating, and the quantity of food eaten; therefore, it is important that zookeeping staff are familiar with the individuality of their breeding females. It is unclear why F2 demonstrated a reduced duration of pacing activity on the day of her second oviposition (7 min) as her overall activity level was consistent with that of previous days. Time eating was clearly reduced in four of five cycles but F3 increased her eating time two-fold. This female’s eating rate was clearly slower than normal as she would typically consume an entire plate of food in approximately 20 min but only managed to consume half of her food over 45 min on the day of oviposition. Regardless, all females noticeably reduced their food intake on the day of oviposition and some up to 3 days before. Therefore, quantity of food eaten demonstrates the most consistent, and clear indication of approaching oviposition for zookeeping staff to incorporate into their daily observations which can be achieved without the need to refer to video. However, our general observations revealed that some females continued to consume all food up until and including the day of oviposition. For these females, duration of pacing and/or eating may be necessary via video observation.
Conclusion
This study demonstrates that there are quantifiable changes in specific echidna behaviours that can be used as tools for the reproductive management of this species. The frequency that males express ‘urogenital sniffing’, ‘rolling’ and ‘copulation attempt’ toward the female can be used to alert zookeepers that copulation has likely occurred. As continuous behaviour monitoring is laborious, we suggest future studies investigate the reliability of detecting quantifiable changes in these behaviours by observing the first 5 min of each hour between 16:00 and 10:30 h. Increased pacing, reduced feeding time and quantity of food eaten can aid zookeepers to identify impending oviposition; however, quantity of food eaten is the most reliable indicator and does not require monitoring via video surveillance. While we were not able to identify female oestrus, our observations suggest that the female echidna’s period of receptivity is less than 24 h and that future studies should investigate hourly rate of change in male/female interactions over the 48 h before and after confirmed copulation to aid in characterising the oestrus period.
Supplementary material
Supplementary material is available online.
Data availability
The data that support this study will be shared upon reasonable request to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was supported by an Australian Council Linkage grant to MBR and SDJ.
Acknowledgements
The authors thank all the keepers at Currumbin Wildlife Sanctuary for their assistance with the handling and husbandry of the echidnas.
References
Aplin K, Dickman C, Salas L, Helgen K (2016) Tachyglossus aculeatus. The IUCN Red List of Threatened Species 2016. Available at https://dx.doi.org/10.2305/IUCN.UK.2016-2.RLTS.T41312A21964662.enBeard, LA, and Grigg, GC (2000). Reproduction in the short-beaked echidna Tachyglossus aculeatus: field observations at an elevated site in South East Queensland. Proceedings of the Linnean Society of New South Wales 122, 89–99.
Blissitt, MJ, Bland, KP, and Cottrell, DF (1990). Discrimination between the odours of fresh oestrous and non-oestrous ewe urine by rams. Applied Animal Behaviour Science 25, 51–59.
| Discrimination between the odours of fresh oestrous and non-oestrous ewe urine by rams.Crossref | GoogleScholarGoogle Scholar |
Dutton-Regester, K, Keeley, T, Fenelon, JC, Roser, A, Meer, H, Hill, A, Pyne, M, Renfree, MB, and Johnston, S (2021). Plasma progesterone secretion during gestation of the captive short-beaked echidna. Reproduction 162, 267–275.
| Plasma progesterone secretion during gestation of the captive short-beaked echidna.Crossref | GoogleScholarGoogle Scholar |
Ferguson, A, and Turner, B (2013). Reproductive parameters and behaviour of captive short-beaked echidna (Tachyglossus aculeatus acanthion) at Perth Zoo. Australian Mammalogy 35, 84–92.
| Reproductive parameters and behaviour of captive short-beaked echidna (Tachyglossus aculeatus acanthion) at Perth Zoo.Crossref | GoogleScholarGoogle Scholar |
Gomez-Diaz, C, and Benton, R (2013). The joy of sex pheromones. EMBO reports 14, 874–883.
| The joy of sex pheromones.Crossref | GoogleScholarGoogle Scholar |
Griffiths M (1978) ‘The biology of monotremes.’ (Academic Press Inc: New York)
Guillette, LJ, Bjorndal, KA, Bolten, AB, Gross, TS, Palmer, BD, Witherington, BE, and Matter, JM (1991). Plasma estradiol-17β, progesterone, prostaglandin F, and prostaglandin E2 concentrations during natural oviposition in the loggerhead turtle (Caretta caretta). General and Comparative Endocrinology 82, 121–130.
| Plasma estradiol-17β, progesterone, prostaglandin F, and prostaglandin E2 concentrations during natural oviposition in the loggerhead turtle (Caretta caretta).Crossref | GoogleScholarGoogle Scholar |
Harris, RL, Holland, BR, Cameron, EZ, Davies, NW, and Nicol, SC (2014). Chemical signals in the echidna: differences between seasons, sexes, individuals and gland types. Journal of Zoology 293, 171–180.
| Chemical signals in the echidna: differences between seasons, sexes, individuals and gland types.Crossref | GoogleScholarGoogle Scholar |
Hurnik, JF, King, GJ, and Robertson, HA (1975). Estrous and related behaviour in postpartum Holstein cows. Applied Animal Ethology 2, 55–68.
| Estrous and related behaviour in postpartum Holstein cows.Crossref | GoogleScholarGoogle Scholar |
Hydbring, E, Madej, A, Macdonald, E, Drugge-boholm, G, Berglund, B, and Olsson, K (1999). Hormonal changes during parturition in heifers and goats are related to the phases and severity of labour. The Journal of Endocrinology 160, 75–85.
| Hormonal changes during parturition in heifers and goats are related to the phases and severity of labour.Crossref | GoogleScholarGoogle Scholar |
Jackson S (2003) ‘Australian mammals: biology and captive management.’ (CSIRO Publishing: Melbourne)
O’Brien, WF (1995). The role of prostaglandins in labor and delivery. Clinics in Perinatology 22, 973–984.
| The role of prostaglandins in labor and delivery.Crossref | GoogleScholarGoogle Scholar |
Perry, T, Toledo-Flores, D, Kang, WX, Ferguson, A, Laming, B, Tsend-Ayush, E, Lim, SL, and Grützner, F (2019). Non-invasive genetic sexing technique for analysis of short-beaked echidna (Tachyglossus aculeatus) populations. Reproduction, Fertility and Development 31, 1289–1295.
| Non-invasive genetic sexing technique for analysis of short-beaked echidna (Tachyglossus aculeatus) populations.Crossref | GoogleScholarGoogle Scholar |
Rismiller PD (1992) Field observations on Kangaroo Island echidnas (Tachyglossus aculeatus multiaculeatus) during the breeding season. In ‘Platypus and echidnas’. (Ed. ML Augee) pp. 101–105. (The Royal Zoological Society of New South Wales: Sydney)
Rismiller, PD, and McKelvey, MW (2000). Frequency of breeding and recruitment in the short-beaked echidna, Tachyglossus aculeatus. Journal of Mammalogy 81, 1–17.
| Frequency of breeding and recruitment in the short-beaked echidna, Tachyglossus aculeatus.Crossref | GoogleScholarGoogle Scholar |
Rismiller, PD, and Seymour, RS (1991). The echidna. Scientific American 264, 96–103.
| The echidna.Crossref | GoogleScholarGoogle Scholar |
Rørvang, MV, Nielsen, BL, Herskin, MS, and Jensen, MB (2018). Prepartum maternal behavior of domesticated cattle: a comparison with managed, feral, and wild ungulates. Frontiers in Veterinary Science 5, 45.
| Prepartum maternal behavior of domesticated cattle: a comparison with managed, feral, and wild ungulates.Crossref | GoogleScholarGoogle Scholar |
Swaisgood, RR, Lindburg, DG, and Zhang, H (2002). Discrimination of oestrous status in giant pandas (Ailuropoda melanoleuca) via chemical cues in urine. Journal of Zoology 257, 381–386.
| Discrimination of oestrous status in giant pandas (Ailuropoda melanoleuca) via chemical cues in urine.Crossref | GoogleScholarGoogle Scholar |
Takahashi, LK (1990). Hormonal regulation of sociosexual behavior in female mammals. Neuroscience & Biobehaviour Reviews 14, 403–413.
| Hormonal regulation of sociosexual behavior in female mammals.Crossref | GoogleScholarGoogle Scholar |
Wallage, A, Clarke, L, Thomas, L, Pyne, M, Beard, L, Ferguson, A, Lisle, A, and Johnston, S (2015). Advances in the captive breeding and reproductive biology of the short-beaked echidna (Tachyglossus aculeatus). Australian Journal of Zoology 63, 181–191.
| Advances in the captive breeding and reproductive biology of the short-beaked echidna (Tachyglossus aculeatus).Crossref | GoogleScholarGoogle Scholar |
Weiss, G (2000). Endocrinology of parturition. The Journal of Clinical Endocrinology & Metabolism 85, 4421–4425.
| Endocrinology of parturition.Crossref | GoogleScholarGoogle Scholar |
Ziegler, TE, Matteri, RL, and Wegner, FH (1993). Detection of urinary gonadotropins in callitrichid monkeys with a sensitive immunoassay based upan a unique monoclonal antibody. American Journal of Primatology 31, 181–188.
| Detection of urinary gonadotropins in callitrichid monkeys with a sensitive immunoassay based upan a unique monoclonal antibody.Crossref | GoogleScholarGoogle Scholar |


