Embryonic diapause in mammals and dormancy in embryonic stem cells with the European roe deer as experimental model
Vera A. van der Weijden A * , Anna B. Rüegg A * , Sandra M. Bernal-Ulloa A and Susanne E. Ulbrich A BA ETH Zurich, Animal Physiology, Institute of Agricultural Sciences, Universitaetstrasse 2, 8092 Zurich, Switzerland.
B Corresponding author. Email: seu@ethz.ch
Reproduction, Fertility and Development 33(2) 76-81 https://doi.org/10.1071/RD20256
Published: 8 January 2021
Journal Compilation © IETS 2021 Open Access CC BY-NC-ND
Abstract
In species displaying embryonic diapause, the developmental pace of the embryo is either temporarily and reversibly halted or largely reduced. Only limited knowledge on its regulation and the inhibition of cell proliferation extending pluripotency is available. In contrast with embryos from other diapausing species that reversibly halt during diapause, embryos of the roe deer Capreolus capreolus slowly proliferate over a period of 4–5 months to reach a diameter of approximately 4 mm before elongation. The diapausing roe deer embryos present an interesting model species for research on preimplantation developmental progression. Based on our and other research, we summarise the available knowledge and indicate that the use of embryonic stem cells (ESCs) would help to increase our understanding of embryonic diapause. We report on known molecular mechanisms regulating embryonic diapause, as well as cellular dormancy of pluripotent cells. Further, we address the promising application of ESCs to study embryonic diapause, and highlight the current knowledge on the cellular microenvironment regulating embryonic diapause and cellular dormancy.
Keywords: dormancy, embryonic diapause, embryonic stem cells, European roe deer Capreolus capreolus.
Embryonic diapause
The time between fertilisation and embryo implantation varies greatly among species. In mice, preimplantation development comprises no more than 5 days (Davidson and Coward 2016). The time until embryo implantation is around 8 days in humans (Niakan et al. 2012), around 18–22 days in cattle (Lonergan et al. 2016) and around 40 days in the mare (Allen and Stewart 2001). This period is prolonged in species that exhibit embryonic diapause, including the European roe deer Capreolus capreolus, in which preimplantation embryo development lasts 4–5 months (Aitken 1974).
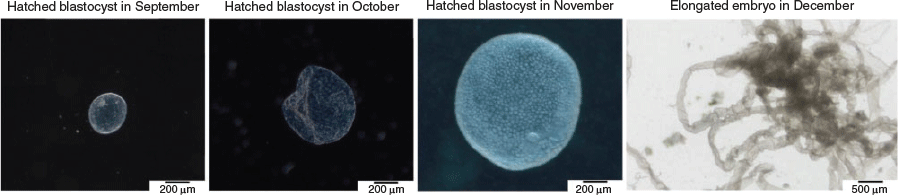
Embryonic diapause is a temporary and reversible arrest of embryo development that was discovered in 1854 in the roe deer (Bischoff 1854). To date, embryonic diapause has been described in more than 100 different mammalian species as an evolutionary conserved mechanism (Fenelon et al. 2014; Renfree and Fenelon 2017). In mammals exhibiting diapause, implantation can be delayed from several days to several months (Renfree and Fenelon 2017). This delay is characterised by a decelerated developmental pace at the hatched blastocyst stage and allows the progeny to be born under the most favourable conditions. The roe deer is the only known ungulate exhibiting embryonic diapause. In contrast with other well-studied diapausing species (e.g. mouse, mink and tammar wallaby), developmental delay in the roe deer has unique features. To date, it remains unknown when and in response to which signal diapause in the roe deer commences. Around Day 14 after fertilisation, expanded blastocysts, hatching and from the zona pellucida completely hatched blastocysts have been found in the uterine horns of roe deer (Drews et al. 2020). From early August until late December, the hatched blastocysts remain free floating in the uterus (Lambert et al. 2001), exhibiting a comparatively long period of diapause (Renfree and Fenelon 2017). Strikingly, development is not completely halted, as shown in many other species. Rather, it features gradual proliferation at a considerably slower pace (Rüegg et al. 2020). The diameter of the blastocyst increases from 0.5 mm in September to approximately 4.0 mm in December (Fig. 1; Drews et al. 2019; Rüegg et al. 2020; van der Weijden and Ulbrich 2020a, 2020b). The end of diapause in the roe deer is marked by a rapid resumption of developmental pace, which morphologically coincides with embryo elongation, a feature shared across ungulates. Because embryo elongation and the following epitheliochorial cotyledonary placentation are comparable, preimplantation development in the roe deer shows morphological similarities to bovine preimplantation development (Short and Hay 1966; Badinga et al. 1994).
Diapause in mice can be induced by ovariectomy (Weitlauf 1974) or reversibly provoked by lactation (McLaren 1968). Moreover, an interspecies embryo transfer experiment, in which sheep embryos were transferred to the mouse uterus, provided evidence that the diapausing mouse uterine environment temporarily induced a proliferation arrest in non-diapausing sheep embryos (Ptak et al. 2012). Thereby, the intrauterine environment received attention as a regulator of decelerated development.
The optimal environment for implantation and establishment of pregnancy depends on the interaction between a developmentally competent embryo and a receptive endometrium. Most embryo losses occur during the peri-implantation period. However, despite the long duration of the peri-implantation period in roe deer, high fertility and little evidence of early embryo losses have been reported (Gaillard et al. 1993; Andersen and Linnell 1996; Focardi et al. 2002; van der Weijden and Ulbrich 2020a). Like the high pregnancy rates in many wildlife species, the estimated implantation rates in roe deer are 92% (van der Weijden and Ulbrich 2020a), and field observations, as well as observations with captive roe deer, have shown a mean of 1.4–2.0 implanted fetuses and live-born fawns per doe, indicating predominantly twin pregnancies (Gaillard et al. 1993; Andersen and Linnell 1996; Focardi et al. 2002). The roe deer is an interesting model species because it can be used to study the regulation of the pace of embryo development and preimplantation embryo development in slow motion.
Embryonic stem cells
After fertilisation, the zygote undergoes several rounds of cleavage to form a blastocyst. Embryonic genome activation (EGA) takes place soon after fertilisation, occurring at the 2-cell stage in murine embryos, the 4-cell stage in porcine embryos, the 4- to 8-cell stage in human embryos and the 8-cell stage in bovine embryos (Flach et al. 1982; Arrell et al. 1991; Niakan et al. 2012; Graf et al. 2014). Following EGA, the embryonic cells show first signs of cellular commitment. The morula and subsequent blastocyst, consisting of the inner cell mass (ICM) and the surrounding trophoblast cells (TB), are formed. During early embryo development, extensive and efficient reprogramming takes place (Fulka et al. 2008). The ICM contains pluripotent stem cells (Evans and Kaufman 1981; Niwa 2007) that retain the ability to unrestrictedly develop into all somatic and germ cells (Wu et al. 2016). Although different to mice, there are similarities in embryo size and the expression of pluripotency markers in bovine and human preimplantation embryos (Simmet et al. 2018a, 2018b). Whether there are similarities between human and roe deer preimplantation embryos remains to be determined.
Embryonic stem cells (ESCs) are derived from the ICM of blastocysts and exhibit a remarkable proliferative capacity, allowing expansion of established cultures in vitro (Evans and Kaufman 1981). Almost 40 years ago, the first ESCs were isolated from diapausing blastocysts derived from ovariectomised mice (Evans and Kaufman 1981; Martin 1981). In 2015, it was shown that proliferating murine ESCs and diapausing mouse blastocysts exhibit highly similar transcriptome profiles (Boroviak et al. 2015). The proliferation of ESCs has subsequently been studied in further detail. The cell cycle machinery of ESCs differs from that of other mammalian cell lines. Cell cycle genes including the S-phase, M-phase and checkpoint genes are essential for the growth of human ESCs (Viner-Breuer et al. 2019). However, ESCs are capable of proliferation in the absence of all G1 cyclins, which are involved in the transition from the G1 to S phase of the cell cycle (Liu et al. 2017). A reduction in the length of both the G1 and the S/G2/M phases was recently related to an increased proliferation rate in mouse ESCs (Waisman et al. 2019). Differences in the regulation of the cell cycle between somatic cells and ESCs may explain why many commonly known cell cycle regulators have not been confirmed to induce diapause in blastocysts. To further study cellular dormancy in a cell culture model, a discrete set of markers serving as dormancy indicators is required.
Pluripotent stem cells in dormancy are thought to exit the cell cycle to survive in a quiescent state (i.e. a reversible state of growth arrest; Vera-Ramirez and Hunter 2017; van Velthoven and Rando 2019). In vitro, cellular dormancy can be induced by starvation or inhibition of key factors involved in proliferation, such as the mammalian target of rapamycin (mTOR) and MYC (Boroviak et al. 2015; Bulut-Karslioglu et al. 2016; Scognamiglio et al. 2016a; Renfree and Fenelon 2017). The use of ESCs as a cellular dormancy model can facilitate the identification of molecular factors regulating embryonic diapause (Bulut-Karslioglu et al. 2016; Scognamiglio et al. 2016b).
Cellular microenvironment driving embryonic diapause and cellular dormancy of pluripotent stem cells
The limited availability and cell numbers of diapausing embryos in vivo and the absence of adequate culture systems mimicking dormancy in vitro have made it challenging to study molecular signalling pathways governing embryonic diapause. Nevertheless, cells within a completely reversibly arrested blastocyst during diapause showed reduced metabolic activity and RNA transcription and no signs of cell division or differentiation (Fu et al. 2014; Renfree and Fenelon 2017). Based on the DNA content, mouse and rat diapausing blastocysts are proposed to be arrested at the G1 phase of the cell cycle (Sherman and Barlow 1972; Surani 1975; Kamemizu and Fujimori 2019). The mVenus-p27K– fusion protein can be used to visualise the G0–G1 transition (Oki et al. 2014), thereby allowing the investigation of the nature of embryonic diapause and cellular dormancy. It is hypothesised that the cellular microenvironment governs embryonic diapause, as well as cellular dormancy. The mTOR signalling pathway, which is highly conserved in mammals (Wolfson and Sabatini 2017), comprises two complexes, namely the mammalian target of rapamycin complex (mTORC) 1 and mTORC2. Via Ras homolog enriched in brain (RHEB), mTORC1 is controlled by nutrients, such as glucose and amino acids (Sancak et al. 2010; Jewell and Guan 2013). Although individual amino acids have been shown to have little effect on mTORC1 activity (Dyachok et al. 2016), mTORC1 signalling is downregulated by the removal of leucine and arginine (Avruch et al. 2009). Further studies defined a set of mTORC1 priming and activating amino acids (Dyachok et al. 2016; Meng et al. 2020), providing evidence that the amino acids in the cellular microenvironment affect cell proliferation. In mouse blastocysts, the inhibition of the expression of the glutamine transporter solute carrier family 38 member 1 (SLC38A1) was found to block the mTOR-dependent diapause state (Hussein et al. 2020). These diapausing embryos had a higher content of free fatty acids and phosphatidylcholine than the non-diapausing control preimplantation blastocysts, but a lower content of triacylglycerol and acylcarnitine (Hussein et al. 2020). Similarly, autophagy, the generation of nutrients required for cell survival during starvation, was activated during diapause (Lee et al. 2011). Autophagy has been shown to be regulated by mTOR (Kim and Guan 2015). Collectively, this indicates that constituents in the cellular microenvironment play a decisive role in regulating embryonic diapause.
Hormone involvement and molecular mechanisms regulating embryonic diapause and cellular dormancy
Circulating hormones are involved in regulating the uterine microenvironment in diapausing mammals (Paria et al. 1998). Neither progesterone nor estrogens altered embryonic metabolism in cultured mouse and rat blastocysts (Weitlauf 1974). However, steroid hormones act on the endometrial cells, which, in turn, secrete a multitude of factors into the uterine lumen (Renfree and Fenelon 2017). Generally, diapause was proposed to be stimulated by the presence or absence of maternal factors in the uterine fluid at the blastocyst stage of development. These factors remain to be identified in many species (Ptak et al. 2012). In mouse, mink and tammar wallaby, implantation-related factors have been found to play a decisive role during blastocyst reactivation. Leukaemia inhibitory factor (LIF) was among the first identified diapause regulating factors. In its absence, blastocysts fail to implant and enter a diapause-like state (Nichols et al. 1996, 2001; Rosario and Stewart 2016). The administration of LIF to ovariectomised mice results in embryo implantation (Chen et al. 2000). The muscle segment homeobox (MSX) genes MSX1 and MSX2 are downregulated by LIF (Cha et al. 2013), and have been suggested to inhibit embryo implantation in mouse, mink, and tammar wallaby. Studies comparing diapausing and reactivated mouse blastocysts reported higher expression of p21 family members in diapausing embryos and higher expression of heparin-binding epidermal growth factor-like growth factor (HB-EGF) in reactivated blastocysts (Hamatani et al. 2004). HB-EGF and erb-b2 receptor tyrosine kinase 4 (ERBB4) are also present during the reactivation of mink and tammar wallaby blastocysts (Fenelon et al. 2017). In addition, FOXO transcription factors and cyclin-dependent kinase inhibitor 1A (CDKN1A) were identified in diapausing mink and tammar wallaby blastocysts, suggesting their role in inhibiting developmental progression (Fenelon et al. 2017). A transcriptome analysis of diapausing mink blastocysts identified an upregulation of genes involved in polyamine synthesis after reactivation (Lefèvre et al. 2011). The importance of polyamines, and especially the rate-limiting enzyme ornithine decarboxylase 1 (ODC1), for diapause entry and reactivation was also confirmed in mice (Fenelon and Murphy 2017). Although an ODC1 inhibitor had no direct effect on cultured blastocysts, the treatment of pregnant mice with an ODC1-inhibitor caused a diapause-like state in blastocysts (Fenelon and Murphy 2017).
Most recently, the inhibition of Myc and its upstream regulator mTOR was shown to induce a diapause-like state in mouse blastocysts cultured ex vivo (Foster et al. 2010; Bulut-Karslioglu et al. 2016; Scognamiglio et al. 2016b). A short, reversible diapause-like state of cellular dormancy lasting 18 h was induced in ESCs and mouse blastocysts using 10058-F4, an inhibitor of the transcription factor c-Myc (Scognamiglio et al. 2016b). Myc is one of four transcription factors capable of causing adult cells to re-differentiate into pluripotent stem cells (Takahashi and Yamanaka 2006), and has been suggested to be involved in oocyte meiotic resumption (Miles et al. 2012). This hints towards a role of Myc in controlling the entry into and exit from cellular dormancy. The inhibition of mTORC1 and mTORC2 with INK128 induced a reversible diapause-like state for up to 30 days and ESC dormancy (Bulut-Karslioglu et al. 2016). However, a recent report stated that this mTOR inhibitor can only induce a diapause-like state in naïve mouse ESC, and that the process was not linked to cellular translation or inhibition of the mTOR pathway (Sousa et al. 2020). In addition, microRNAs (e.g. let-7, which regulates MYC) have been reported as being more abundant during the diapause period (Chang et al. 2009; Cheong et al. 2014; Fenelon et al. 2014). To date, the identification of the molecular factors regulating diapause has been predominantly based on the association of differential gene expression of embryonic diapause versus reactivation. The biological relevance of differentially expressed genes has only been shown for a small subset of candidates. The reversibly inducible dormancy in ESCs offers a promising approach to further our understanding of pluripotent cell dormancy and the molecular signalling pathways involved (Bulut-Karslioglu et al. 2016).
Future directions
The roe deer embryo undergoing diapause has important features to serve as model species in which to investigate the mechanisms driving the pace of embryo development. Maternal factors involved in governing the cell cycle may be elucidated. They may further our understanding of molecular factors in the uterine microenvironment that play a role in stem cell pluripotency (Drews et al. 2019; van der Weijden et al. 2019a, 2019b, 2019c). The findings may be translatable to other species, including bovine and human.
Because proliferation arrest has been shown to be inducible in non-diapause species (Ptak et al. 2012), further findings on the regulation of embryonic diapause may offer novel perspectives. These include insights into the developmental competence of preimplantation embryos, that may enhance our understanding of early embryo mortality in livestock species. As molecular factors inducing dormancy most likely affect the cell cycle by leading to either reduced proliferation or complete cell cycle arrest, the physiological regulation underlying diapause and dormancy may have implications for cell proliferation inhibition to extend pluripotency, induce senescence and to suppress proliferation.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors are active participants of the COST Action CA16119 (CellFit – In vitro 3D total cell guidance and fitness). The writing of this review was funded by the Swiss National Science Foundation SNSF (310030_185026).
References
Aitken, R. J. (1974). Delayed implantation in roe deer (Capreolus capreolus). J. Reprod. Fertil. 39, 225–233.| Delayed implantation in roe deer (Capreolus capreolus).Crossref | GoogleScholarGoogle Scholar | 4851283PubMed |
Allen, W. R., and Stewart, F. (2001). Equine placentation. Reprod. Fertil. Dev. 13, 623–634.
| Equine placentation.Crossref | GoogleScholarGoogle Scholar | 11999314PubMed |
Andersen, R., and Linnell, J. D. (1996). Variation in maternal investment in a small cervid; the effects of cohort, sex, litter size and time of birth in roe deer (Capreolus capreolus) fawns. Oecologia 109, 74–79.
| Variation in maternal investment in a small cervid; the effects of cohort, sex, litter size and time of birth in roe deer (Capreolus capreolus) fawns.Crossref | GoogleScholarGoogle Scholar | 28307615PubMed |
Arrell, V. L., Day, B. N., and Prather, R. S. (1991). The transition from maternal to zygotic control of development occurs during the 4-cell stage in the domestic pig, Sus scrofa: quantitative and qualitative aspects of protein synthesis. Biol. Reprod. 44, 62–68.
| The transition from maternal to zygotic control of development occurs during the 4-cell stage in the domestic pig, Sus scrofa: quantitative and qualitative aspects of protein synthesis.Crossref | GoogleScholarGoogle Scholar |
Avruch, J., Long, X., Ortiz-Vega, S., Rapley, J., Papageorgiou, A., and Dai, N. (2009). Amino acid regulation of TOR complex 1. Am. J. Physiol. Endocrinol. Metab. 296, E592–E602.
| Amino acid regulation of TOR complex 1.Crossref | GoogleScholarGoogle Scholar | 18765678PubMed |
Badinga, L., Michel, F. J., Fields, M. J., Sharp, D. C., and Simmen, R. C. (1994). Pregnancy-associated endometrial expression of antileukoproteinase gene is correlated with epitheliochorial placentation. Mol. Reprod. Dev. 38, 357–363.
| Pregnancy-associated endometrial expression of antileukoproteinase gene is correlated with epitheliochorial placentation.Crossref | GoogleScholarGoogle Scholar | 7980943PubMed |
Bischoff, T. L. W. (1854). ‘Die Entwicklungsgeschichte des Rehes.’ (J. Bicker’sche Buchhandlung: Giessen.)
Boroviak, T., Loos, R., Lombard, P., Okahara, J., Behr, R., Sasaki, E., Nichols, J., Smith, A., and Bertone, P. (2015). Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis. Dev. Cell 35, 366–382.
| Lineage-specific profiling delineates the emergence and progression of naive pluripotency in mammalian embryogenesis.Crossref | GoogleScholarGoogle Scholar | 26555056PubMed |
Bulut-Karslioglu, A., Biechele, S., Jin, H., Macrae, T. A., Hejna, M., Gertsenstein, M., Song, J. S., and Ramalho-Santos, M. (2016). Inhibition of mTOR induces a paused pluripotent state. Nature 540, 119–123.
| Inhibition of mTOR induces a paused pluripotent state.Crossref | GoogleScholarGoogle Scholar | 27880763PubMed |
Cha, J., Sun, X., Bartos, A., Fenelon, J., Lefevre, P., Daikoku, T., Shaw, G., Maxson, R., Murphy, B. D., Renfree, M. B., and Dey, S. K. (2013). A new role for muscle segment homeobox genes in mammalian embryonic diapause. Open Biol. 3, 130035.
| A new role for muscle segment homeobox genes in mammalian embryonic diapause.Crossref | GoogleScholarGoogle Scholar | 23615030PubMed |
Chang, T. C., Zeitels, L. R., Hwang, H. W., Chivukula, R. R., Wentzel, E. A., Dews, M., Jung, J., Gao, P., Dang, C. V., Beer, M. A., Thomas-Tikhonenko, A., and Mendell, J. T. (2009). Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation. Proc. Natl Acad. Sci. USA 106, 3384–3389.
| Lin-28B transactivation is necessary for Myc-mediated let-7 repression and proliferation.Crossref | GoogleScholarGoogle Scholar | 19211792PubMed |
Chen, J. R., Cheng, J. G., Shatzer, T., Sewell, L., Hernandez, L., and Stewart, C. L. (2000). Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology 141, 4365–4372.
| Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis.Crossref | GoogleScholarGoogle Scholar | 11108244PubMed |
Cheong, A. W., Pang, R. T., Liu, W. M., Kottawatta, K. S., Lee, K. F., and Yeung, W. S. (2014). MicroRNA Let-7a and dicer are important in the activation and implantation of delayed implanting mouse embryos. Hum. Reprod. 29, 750–762.
| MicroRNA Let-7a and dicer are important in the activation and implantation of delayed implanting mouse embryos.Crossref | GoogleScholarGoogle Scholar | 24419497PubMed |
Davidson, L. M., and Coward, K. (2016). Molecular mechanisms of membrane interaction at implantation. Birth Defects Res. C Embryo Today 108, 19–32.
| Molecular mechanisms of membrane interaction at implantation.Crossref | GoogleScholarGoogle Scholar | 26969610PubMed |
Drews, B., Rudolf Vegas, A., van der Weijden, V. A., Milojevic, V., Hankele, A. K., Schuler, G., and Ulbrich, S. E. (2019). Do ovarian steroid hormones control the resumption of embryonic growth following the period of diapause in roe deer (Capreolus capreolus)? Reprod. Biol. 19, 149–157.
| Do ovarian steroid hormones control the resumption of embryonic growth following the period of diapause in roe deer (Capreolus capreolus)?Crossref | GoogleScholarGoogle Scholar | 31147267PubMed |
Drews, B., Ulbrich, S. E., Rudolf Vegas, A., Jewgenow, K., Zahmel, J., Roellig, K., Ortmann, S., Hildebrandt, T. B., and Goeritz, F. (2020). Gliding into diapause: early embryo development in roe deer (Capreolus capreolus). Biosci. Proc. 10, ISEDISED12.
| Gliding into diapause: early embryo development in roe deer (Capreolus capreolus).Crossref | GoogleScholarGoogle Scholar |
Dyachok, J., Earnest, S., Iturraran, E. N., Cobb, M. H., and Ross, E. M. (2016). Amino acids regulate mTORC1 by an obligate two-step mechanism. J. Biol. Chem. 291, 22414–22426.
| Amino acids regulate mTORC1 by an obligate two-step mechanism.Crossref | GoogleScholarGoogle Scholar | 27587390PubMed |
Evans, M. J., and Kaufman, M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature 292, 154–156.
| 7242681PubMed |
Fenelon, J. C., and Murphy, B. D. (2017). Inhibition of polyamine synthesis causes entry of the mouse blastocyst into embryonic diapause. Biol. Reprod. 97, 119–132.
| Inhibition of polyamine synthesis causes entry of the mouse blastocyst into embryonic diapause.Crossref | GoogleScholarGoogle Scholar | 28637295PubMed |
Fenelon, J. C., Banerjee, A., and Murphy, B. D. (2014). Embryonic diapause: development on hold. Int. J. Dev. Biol. 58, 163–174.
| Embryonic diapause: development on hold.Crossref | GoogleScholarGoogle Scholar | 25023682PubMed |
Fenelon, J. C., Shaw, G., Frankenberg, S. R., Murphy, B. D., and Renfree, M. B. (2017). Embryo arrest and reactivation: potential candidates controlling embryonic diapause in the tammar wallaby and minkdagger. Biol. Reprod. 96, 877–894.
| Embryo arrest and reactivation: potential candidates controlling embryonic diapause in the tammar wallaby and minkdagger.Crossref | GoogleScholarGoogle Scholar | 28379301PubMed |
Flach, G., Johnson, M. H., Braude, P. R., Taylor, R. A., and Bolton, V. N. (1982). The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1, 681–686.
| The transition from maternal to embryonic control in the 2-cell mouse embryo.Crossref | GoogleScholarGoogle Scholar | 7188357PubMed |
Focardi, S., Pelliccioni, E., Petrucco, R., and Toso, S. (2002). Spatial patterns and density dependence in the dynamics of a roe deer (Capreolus capreolus) population in central Italy. Oecologia 130, 411–419.
| Spatial patterns and density dependence in the dynamics of a roe deer (Capreolus capreolus) population in central Italy.Crossref | GoogleScholarGoogle Scholar | 28547048PubMed |
Foster, D. A., Yellen, P., Xu, L., and Saqcena, M. (2010). Regulation of G1 cell cycle progression: distinguishing the restriction point from a nutrient-sensing cell growth checkpoint(s). Genes Cancer 1, 1124–1131.
| Regulation of G1 cell cycle progression: distinguishing the restriction point from a nutrient-sensing cell growth checkpoint(s).Crossref | GoogleScholarGoogle Scholar | 21779436PubMed |
Fu, Z., Wang, B., Wang, S., Wu, W., Wang, Q., Chen, Y., Kong, S., Lu, J., Tang, Z., Ran, H., Tu, Z., He, B., Zhang, S., Chen, Q., Jin, W., Duan, E., Wang, H., Wang, Y. L., Li, L., Wang, F., Gao, S., and Wang, H. (2014). Integral proteomic analysis of blastocysts reveals key molecular machinery governing embryonic diapause and reactivation for implantation in mice. Biol. Reprod. 90, 52.
| Integral proteomic analysis of blastocysts reveals key molecular machinery governing embryonic diapause and reactivation for implantation in mice.Crossref | GoogleScholarGoogle Scholar | 24451987PubMed |
Fulka, H., St John, J. C., Fulka, J., and Hozak, P. (2008). Chromatin in early mammalian embryos: achieving the pluripotent state. Differentiation 76, 3–14.
| Chromatin in early mammalian embryos: achieving the pluripotent state.Crossref | GoogleScholarGoogle Scholar | 18093226PubMed |
Gaillard, J. M., Delorme, D., and Jullien, J. M. (1993). Effects of cohort, sex, and birth date on body development of roe deer (Capreolus capreolus) fawns. Oecologia 94, 57–61.
| Effects of cohort, sex, and birth date on body development of roe deer (Capreolus capreolus) fawns.Crossref | GoogleScholarGoogle Scholar | 28313858PubMed |
Graf, A., Krebs, S., Zakhartchenko, V., Schwalb, B., Blum, H., and Wolf, E. (2014). Fine mapping of genome activation in bovine embryos by RNA sequencing. Proc. Natl Acad. Sci. USA 111, 4139–4144.
| Fine mapping of genome activation in bovine embryos by RNA sequencing.Crossref | GoogleScholarGoogle Scholar | 24591639PubMed |
Hamatani, T., Daikoku, T., Wang, H., Matsumoto, H., Carter, M. G., Ko, M. S., and Dey, S. K. (2004). Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation. Proc. Natl Acad. Sci. USA 101, 10326–10331.
| Global gene expression analysis identifies molecular pathways distinguishing blastocyst dormancy and activation.Crossref | GoogleScholarGoogle Scholar | 15232000PubMed |
Hussein, A. M., Wang, Y., Mathieu, J., Margaretha, L., Song, C., Jones, D. C., Cavanaugh, C., Miklas, J. W., Mahen, E., Showalter, M. R., Ruzzo, W. L., Fiehn, O., Ware, C. B., Blau, C. A., and Ruohola-Baker, H. (2020). Metabolic control over mTOR-dependent diapause-like state. Dev. Cell 52, 236–250.
| Metabolic control over mTOR-dependent diapause-like state.Crossref | GoogleScholarGoogle Scholar | 31991105PubMed |
Jewell, J. L., and Guan, K. L. (2013). Nutrient signaling to mTOR and cell growth. Trends Biochem. Sci. 38, 233–242.
| Nutrient signaling to mTOR and cell growth.Crossref | GoogleScholarGoogle Scholar | 23465396PubMed |
Kamemizu, C., and Fujimori, T. (2019). Distinct dormancy progression depending on embryonic regions during mouse embryonic diapause. Biol. Reprod. 100, 1204–1214.
| Distinct dormancy progression depending on embryonic regions during mouse embryonic diapause.Crossref | GoogleScholarGoogle Scholar | 30715198PubMed |
Kim, Y. C., and Guan, K. L. (2015). mTOR: a pharmacologic target for autophagy regulation. J. Clin. Invest. 125, 25–32.
| mTOR: a pharmacologic target for autophagy regulation.Crossref | GoogleScholarGoogle Scholar | 25654547PubMed |
Lambert, R. T., Ashworth, C. J., Beattie, L., Gebbie, F. E., Hutchinson, J. S., Kyle, D. J., and Racey, P. A. (2001). Temporal changes in reproductive hormones and conceptus-endometrial interactions during embryonic diapause and reactivation of the blastocyst in European roe deer (Capreolus capreolus). Reproduction 121, 863–871.
| Temporal changes in reproductive hormones and conceptus-endometrial interactions during embryonic diapause and reactivation of the blastocyst in European roe deer (Capreolus capreolus).Crossref | GoogleScholarGoogle Scholar | 11373172PubMed |
Lee, J. E., Oh, H. A., Song, H., Jun, J. H., Roh, C. R., Xie, H., Dey, S. K., and Lim, H. J. (2011). Autophagy regulates embryonic survival during delayed implantation. Endocrinology 152, 2067–2075.
| Autophagy regulates embryonic survival during delayed implantation.Crossref | GoogleScholarGoogle Scholar | 21363932PubMed |
Lefèvre, P. L. C., Palin, M.-F., Beaudry, D., Dobias-Goff, M., Desmarais, J. A., Llerena, E. M., and Murphy, B. D. (2011). Uterine signaling at the emergence of the embryo from obligate diapause. Am. J. Physiol. Endocrinol. Metab. 300, E800–E808.
| Uterine signaling at the emergence of the embryo from obligate diapause.Crossref | GoogleScholarGoogle Scholar |
Liu, L., Michowski, W., Inuzuka, H., Shimizu, K., Nihira, N. T., Chick, J. M., Li, N., Geng, Y., Meng, A. Y., Ordureau, A., Kolodziejczyk, A., Ligon, K. L., Bronson, R. T., Polyak, K., Harper, J. W., Gygi, S. P., Wei, W., and Sicinski, P. (2017). G1 cyclins link proliferation, pluripotency and differentiation of embryonic stem cells. Nat. Cell Biol. 19, 177–188.
| G1 cyclins link proliferation, pluripotency and differentiation of embryonic stem cells.Crossref | GoogleScholarGoogle Scholar | 28192421PubMed |
Lonergan, P., Fair, T., Forde, N., and Rizos, D. (2016). Embryo development in dairy cattle. Theriogenology 86, 270–277.
| Embryo development in dairy cattle.Crossref | GoogleScholarGoogle Scholar | 27158131PubMed |
Martin, G. R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA 78, 7634–7638.
| Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells.Crossref | GoogleScholarGoogle Scholar | 6950406PubMed |
McLaren, A. (1968). A study of balstocysts during delay and subsequent implantation in lactating mice. J. Endocrinol. 42, 453–463.
| A study of balstocysts during delay and subsequent implantation in lactating mice.Crossref | GoogleScholarGoogle Scholar | 4179775PubMed |
Meng, D., Yang, Q., Wang, H., Melick, C. H., Navlani, R., Frank, A. R., and Jewell, J. L. (2020). Glutamine and asparagine activate mTORC1 independently of Rag GTPases. J. Biol. Chem. 295, 2890–2899.
| Glutamine and asparagine activate mTORC1 independently of Rag GTPases.Crossref | GoogleScholarGoogle Scholar | 32019866PubMed |
Miles, J. R., McDaneld, T. G., Wiedmann, R. T., Cushman, R. A., Echternkamp, S. E., Vallet, J. L., and Smith, T. P. (2012). MicroRNA expression profile in bovine cumulus–oocyte complexes: possible role of let-7 and miR-106a in the development of bovine oocytes. Anim. Reprod. Sci. 130, 16–26.
| MicroRNA expression profile in bovine cumulus–oocyte complexes: possible role of let-7 and miR-106a in the development of bovine oocytes.Crossref | GoogleScholarGoogle Scholar | 22269106PubMed |
Niakan, K. K., Han, J., Pedersen, R. A., Simon, C., and Pera, R. A. R. (2012). Human pre-implantation embryo development. Development 139, 829.
| Human pre-implantation embryo development.Crossref | GoogleScholarGoogle Scholar | 22318624PubMed |
Nichols, J., Davidson, D., Taga, T., Yoshida, K., Chambers, I., and Smith, A. (1996). Complementary tissue-specific expression of LIF and LIF-receptor mRNAs in early mouse embryogenesis. Mech. Dev. 57, 123–131.
| Complementary tissue-specific expression of LIF and LIF-receptor mRNAs in early mouse embryogenesis.Crossref | GoogleScholarGoogle Scholar | 8843390PubMed |
Nichols, J., Chambers, I., Taga, T., and Smith, A. (2001). Physiological rationale for responsiveness of mouse embryonic stem cells to gp130 cytokines. Development 128, 2333–2339.
| 11493552PubMed |
Niwa, H. (2007). How is pluripotency determined and maintained? Development 134, 635.
| How is pluripotency determined and maintained?Crossref | GoogleScholarGoogle Scholar | 17215298PubMed |
Oki, T., Nishimura, K., Kitaura, J., Togami, K., Maehara, A., Izawa, K., Sakaue-Sawano, A., Niida, A., Miyano, S., Aburatani, H., Kiyonari, H., Miyawaki, A., and Kitamura, T. (2014). A novel cell-cycle-indicator, mVenus–p27K−, identifies quiescent cells and visualizes G0–G1 transition. Sci. Rep. 4, 4012.
| A novel cell-cycle-indicator, mVenus–p27K−, identifies quiescent cells and visualizes G0–G1 transition.Crossref | GoogleScholarGoogle Scholar | 24500246PubMed |
Paria, B. C., Lim, H., Wang, X. N., Liehr, J., Das, S. K., and Dey, S. K. (1998). Coordination of differential effects of primary estrogen and catecholestrogen on two distinct targets mediates embryo implantation in the mouse. Endocrinology 139, 5235–5246.
| Coordination of differential effects of primary estrogen and catecholestrogen on two distinct targets mediates embryo implantation in the mouse.Crossref | GoogleScholarGoogle Scholar | 9832464PubMed |
Ptak, G. E., Tacconi, E., Czernik, M., Toschi, P., Modlinski, J. A., and Loi, P. (2012). Embryonic diapause is conserved across mammals. PLoS One 7, e33027.
| Embryonic diapause is conserved across mammals.Crossref | GoogleScholarGoogle Scholar | 22427933PubMed |
Renfree, M. B., and Fenelon, J. C. (2017). The enigma of embryonic diapause. Development 144, 3199–3210.
| The enigma of embryonic diapause.Crossref | GoogleScholarGoogle Scholar | 28928280PubMed |
Rosario, G. X., and Stewart, C. L. (2016). The multifaceted actions of leukaemia inhibitory factor in mediating uterine receptivity and embryo implantation. Am. J. Reprod. Immunol. 75, 246–255.
| The multifaceted actions of leukaemia inhibitory factor in mediating uterine receptivity and embryo implantation.Crossref | GoogleScholarGoogle Scholar | 26817565PubMed |
Rüegg, A. B., Bernal-Ulloa, S. M., Moser, F., Rutzen, I., and Ulbrich, S. E. (2020). Trophectoderm and embryoblast proliferate at slow pace in the course of embryonic diapause in the roe deer (Capreolus capreolus). Biosci. Proc. 10, ISEDISED13.
| Trophectoderm and embryoblast proliferate at slow pace in the course of embryonic diapause in the roe deer (Capreolus capreolus).Crossref | GoogleScholarGoogle Scholar |
Sancak, Y., Bar-Peled, L., Zoncu, R., Markhard, A. L., Nada, S., and Sabatini, D. M. (2010). Ragulator–Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141, 290–303.
| Ragulator–Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids.Crossref | GoogleScholarGoogle Scholar | 20381137PubMed |
Scognamiglio, R., Cabezas-Wallscheid, N., Thier, M. C., Altamura, S., Reyes, A., Prendergast, A. M., Baumgartner, D., Carnevalli, L. S., Atzberger, A., Haas, S., von Paleske, L., Boroviak, T., Worsdorfer, P., Essers, M. A., Kloz, U., Eisenman, R. N., Edenhofer, F., Bertone, P., Huber, W., van der Hoeven, F., Smith, A., and Trumpp, A. (2016a). Myc depletion induces a pluripotent dormant state mimicking diapause. Cell 164, 668–680.
| Myc depletion induces a pluripotent dormant state mimicking diapause.Crossref | GoogleScholarGoogle Scholar | 26871632PubMed |
Scognamiglio, R., Cabezas-Wallscheid, N., Thier, M. C., Altamura, S., Reyes, A., Prendergast, Á. M., Baumgärtner, D., Carnevalli, L. S., Atzberger, A., Haas, S., von Paleske, L., Boroviak, T., Wörsdörfer, P., Essers, M. A. G., Kloz, U., Eisenman, R. N., Edenhofer, F., Bertone, P., Huber, W., van der Hoeven, F., Smith, A., and Trumpp, A. (2016b). Myc depletion induces a pluripotent dormant state mimicking diapause. Cell 164, 668–680.
| Myc depletion induces a pluripotent dormant state mimicking diapause.Crossref | GoogleScholarGoogle Scholar | 26871632PubMed |
Sherman, M. I., and Barlow, P. W. (1972). Deoxyribonucleic acid content in delayed mouse blastocysts. J. Reprod. Fertil. 29, 123–126.
| Deoxyribonucleic acid content in delayed mouse blastocysts.Crossref | GoogleScholarGoogle Scholar | 5016999PubMed |
Short, R. V., and Hay, M. F. (1966) Delayed implantation in the roe deer Capreolus capreolus. In ‘Comparative Biology of Reproduction in Mammals’. (Ed. I. W. Rowlands.) pp. 173–194. (Academic Press: New York.)
Simmet, K., Zakhartchenko, V., Philippou-Massier, J., Blum, H., Klymiuk, N., and Wolf, E. (2018a). OCT4/POU5F1 is required for NANOG expression in bovine blastocysts. Proc. Natl Acad. Sci. USA 115, 2770–2775.
| OCT4/POU5F1 is required for NANOG expression in bovine blastocysts.Crossref | GoogleScholarGoogle Scholar | 29483258PubMed |
Simmet, K., Zakhartchenko, V., and Wolf, E. (2018b). Comparative aspects of early lineage specification events in mammalian embryos – insights from reverse genetics studies. Cell Cycle 17, 1688–1695.
| Comparative aspects of early lineage specification events in mammalian embryos – insights from reverse genetics studies.Crossref | GoogleScholarGoogle Scholar | 29995579PubMed |
Sousa, M. I., Correia, B., Rodrigues, A. S., and Ramalho-Santos, J. (2020). Metabolic characterization of a paused-like pluripotent state. Biochim Biophys Acta 1864, 129612.
| Metabolic characterization of a paused-like pluripotent state.Crossref | GoogleScholarGoogle Scholar |
Surani, M. A. (1975). Zona pellucida denudation, blastocyst proliferation and attachment in the rat. J. Embryol. Exp. Morphol. 33, 343–353.
| 1176850PubMed |
Takahashi, K., and Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676.
| Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors.Crossref | GoogleScholarGoogle Scholar | 16904174PubMed |
van der Weijden, V. A., and Ulbrich, S. E. (2020a). Embryonic diapause in roe deer: a model to unravel embryo–maternal communication during pre-implantation development in wildlife and livestock species. Theriogenology 158, 105–111.
| Embryonic diapause in roe deer: a model to unravel embryo–maternal communication during pre-implantation development in wildlife and livestock species.Crossref | GoogleScholarGoogle Scholar | 32947063PubMed |
van der Weijden, V. A., and Ulbrich, S. E. (2020b). Embryonic diapause in the European roe deer (Capreolus capreolus). Biosci. Proc. 10, ISEDISED4.
van der Weijden, V. A., Bick, J., Bauersachs, S., Arnold, G. J., Frohlich, T., Drews, B., and Ulbrich, S. E. (2019a). Uterine fluid proteome changes during diapause and resumption of embryo development in roe deer. Reproduction 158, 13–24.
| Uterine fluid proteome changes during diapause and resumption of embryo development in roe deer.Crossref | GoogleScholarGoogle Scholar | 30933930PubMed |
van der Weijden, V. A., Hankele, A. K., Rüegg, A. B., Schmicke, M., Rehm, K., Bigler, L., and Ulbrich, S. E. (2019b). Progestogen profiling over the course of diapause and resumption of embryo development in the European roe deer. SciMedicine Journal 1, 158–167.
| Progestogen profiling over the course of diapause and resumption of embryo development in the European roe deer.Crossref | GoogleScholarGoogle Scholar |
van der Weijden, V. A., Puntar, B., Vegas, A. R., Milojevic, V., Schanzenbach, C. I., Kowalewski, M. P., Drews, B., and Ulbrich, S. E. (2019c). Endometrial luminal epithelial cells sense embryo elongation in the roe deer independent of interferon-tau. Biol. Reprod. 101, 882–892.
| Endometrial luminal epithelial cells sense embryo elongation in the roe deer independent of interferon-tau.Crossref | GoogleScholarGoogle Scholar | 31317179PubMed |
van Velthoven, C. T. J., and Rando, T. A. (2019). Stem cell quiescence: dynamism, restraint, and cellular idling. Cell Stem Cell 24, 213–225.
| Stem cell quiescence: dynamism, restraint, and cellular idling.Crossref | GoogleScholarGoogle Scholar |
Vera-Ramirez, L., and Hunter, K. W. (2017). Tumor cell dormancy as an adaptive cell stress response mechanism. F1000Res. 6, 2134.
| Tumor cell dormancy as an adaptive cell stress response mechanism.Crossref | GoogleScholarGoogle Scholar | 29263786PubMed |
Viner-Breuer, R., Yilmaz, A., Benvenisty, N., and Goldberg, M. (2019). The essentiality landscape of cell cycle related genes in human pluripotent and cancer cells. Cell Div. 14, 15.
| The essentiality landscape of cell cycle related genes in human pluripotent and cancer cells.Crossref | GoogleScholarGoogle Scholar | 31889988PubMed |
Waisman, A., Sevlever, F., Elías Costa, M., Cosentino, M. S., Miriuka, S. G., Ventura, A. C., and Guberman, A. S. (2019). Cell cycle dynamics of mouse embryonic stem cells in the ground state and during transition to formative pluripotency. Sci. Rep. 9, 8051.
| Cell cycle dynamics of mouse embryonic stem cells in the ground state and during transition to formative pluripotency.Crossref | GoogleScholarGoogle Scholar | 31142785PubMed |
Weitlauf, H. M. (1974). Metabolic changes in the blastocysts of mice and rats during delayed implantation. J. Reprod. Fertil. 39, 213–224.
| Metabolic changes in the blastocysts of mice and rats during delayed implantation.Crossref | GoogleScholarGoogle Scholar | 4604758PubMed |
Wolfson, R. L., and Sabatini, D. M. (2017). The dawn of the age of amino acid sensors for the mTORC1 pathway. Cell Metab. 26, 301–309.
| The dawn of the age of amino acid sensors for the mTORC1 pathway.Crossref | GoogleScholarGoogle Scholar | 28768171PubMed |
Wu, J., Yamauchi, T., and Izpisua Belmonte, J. C. (2016). An overview of mammalian pluripotency. Development 143, 1644.
| An overview of mammalian pluripotency.Crossref | GoogleScholarGoogle Scholar | 27190034PubMed |
* These authors contributed equally to this work.