Extracellular vesicles derived from donor oviduct fluid improved birth rates after embryo transfer in mice
Pengxiang Qu A B * , Yuelei Zhao A * , Rong Wang A , Yali Zhang A , Lu Li A , Jianglin Fan C and Enqi Liu A B DA Laboratory Animal Centre, Xi’an Jiaotong University Health Science Centre, Xi’an, Shaanxi 710061, China.
B Research Institute of Atherosclerotic Disease, Xi’an Jiaotong University Cardiovascular Research Centre, Xi’an, Shaanxi 710061, China.
C Department of Molecular Pathology, Interdisciplinary Graduate School of Medicine and Engineering, University of Yamanashi, Yamanashi 409-3898, Japan.
D Corresponding author. Email: liuenqi@mail.xjtu.edu.cn
Reproduction, Fertility and Development 31(2) 324-332 https://doi.org/10.1071/RD18203
Submitted: 8 January 2018 Accepted: 7 July 2018 Published: 10 September 2018
Journal compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Embryo transfer (ET) is an important procedure for assisted reproduction. However, the relatively lower success rate of ET hampers its application potential. In this study we aimed to elucidate the effects of extracellular vesicles derived from donor oviduct fluid (EDOF) on embryo development after ET. Extracellular vesicles from the oviduct were isolated and purified using ultracentrifugation and identified using transmission electron microscopy, NanoSight, bicinchoninic acid (BCA) protein assay and western blotting. The results revealed that extracellular vesicles were present in donor oviduct fluid in higher concentrations (P < 0.05) and contained more proteins (P < 0.05) than extracellular vesicles derived from recipient oviduct fluid (EROF). EDOF or EROF were supplemented in an ET medium (ETM) and the results showed that EDOF significantly improved birth rate via resisting apoptosis and promoting differentiation. In conclusion, our study indicated that there are differences in EDOF and EROF and that supplementing EDOF to ETM can improve the efficiency of ET; improved ET efficiency promotes the use of gene editing and benefits assisted reproductive technology and animal welfare.
Additional keywords: ammonium, apoptosis, differentiation, embryo transfer.
Introduction
Embryo transfer (ET), which is a crucial procedure for assisted reproduction, has been widely used in producing transgenic animals, breeding and rescuing endangered species. However, a relatively lower success rate of ET has hampered its application potential (Bin Ali et al. 2014; Lin et al. 2015; Gonzalez-Jara et al. 2017). Many factors affect the efficiency of ET, such as the synchronisation of embryo and maternal reproductive system, quality of embryos, site of transplant and ET medium (ETM; Lopez et al. 2014; Lin et al. 2015; Lopez-Cardona et al. 2015). Although the physical and chemical properties of synthetic ETM are similar to those of oviduct and uterine fluids, synthetic ETM lacks extracellular vesicles secreted by the maternal reproductive tract.
Extracellular vesicles are cell-derived and present in various tissue fluids, including blood, urine, oviduct fluid and uterine fluid (Thery et al. 2006). Numerous studies have shown that extracellular vesicles contain proteins, microRNA and other substances that play key roles in intercellular signalling processes (Simons and Raposo 2009). Oviduct-derived extracellular vesicles might play critical roles in embryo development during oviduct–embryo cross-talk (Alminana et al. 2017).
Genetically engineered mice are mainly generated by microinjecting fertilised eggs and then transferring the embryos to the oviduct. During this process, fertilised eggs are obtained from a donor oviduct and then kept in culture medium and ETM, which leads to the loss of extracellular vesicles derived from the donor oviduct fluid (EDOF). In this study, we aimed to elucidate the effects of EDOF on embryo development after ET. For this purpose, we isolated and purified oviduct-derived extracellular vesicles using ultracentrifugation, identified and compared EDOF and extracellular vesicles derived from the recipient oviduct fluid (EROF) using transmission electron microscopy (TEM), NanoSight, bicinchoninic acid (BCA) protein assay and western blotting. We also examined the effect of supplementing EDOF to ETM on embryo development in terms of the birth rate.
Materials and methods
Animals
All mice (C57BL/6) were procured from the Laboratory Animal Centre of Xi’an Jiaotong University and kept under the following conditions: temperature of 24°C ± 1°C, relative humidity of 55% ± 5% and photoperiod of 12 h light : 12 h dark. The animals were given access to water and food ad libidum. The Animal Care and Use Committee of Xi’an Jiaotong University approved all the procedures related to the use of and experimentation on animals.
Collection of embryos and oviduct fluid
Female mice (donors) were injected with 5 IU pregnant mare serum gonadotrophin (PMSG), followed by the injection of 5 IU human chorionic gonadotrophin (hCG) after 2 days to induce superovulation. The donors were then mated overnight with males. Next morning, the donors with vaginal plugs (pregnant) were selected and used for collecting fertilised eggs or embryos. Simultaneously, female mice (recipients) were mated with vasectomised males and those with vaginal plugs (pseudo-pregnant) were selected for ET. The end of a 32-gauge needle was cut and rubbed on the grindstone to make it blunt. Donor females with vaginal plugs were euthanised by cervical dislocation in accordance with the euthanasia of animals and their abdominal cavity was exposed using a pair of sharp scissors. Then, the oviduct and uterus were gently removed and the oviduct was cut and transferred to a 35-mm dish and observed under a stereoscopic microscope. A needle was then inserted at the end of the oviduct and gently pressed on the dish and a syringe containing 0.2 mL of Dulbecco’s phosphate-buffered saline (DPBS) was slowly inserted. The embryos and oviduct fluid were retrieved from the opposite end of the oviduct. Embryos were temporarily cultured in potassium simplex optimisation medium(KSOM) medium supplemented with amino acids (Millipore) in 5% CO2 (v/v) atmosphere at 37.5°C. The embryos were then transferred to the oviducts of the pseudo-pregnant recipients. Oviduct fluid was collected in a centrifuge tube and stored at −80°C in a freezer.
Isolation and identification of extracellular vesicles
Extracellular vesicles were isolated, purified and identified using our published protocol (Qu et al. 2017). Briefly, oviduct fluid was gathered and subjected to differential centrifugation at 4°C (300g, 10 min to separate cells; 2000g, 10 min to separate dead cells; 10 000g, 30 min to separate cell debris, macroparticles and apoptotic bodies). The supernatants thus obtained were ultracentrifuged at 100 000g for 70 min at 4°C. They were then discarded; sediments were retained and suspended in phosphate-buffered saline (PBS) and again centrifuged at 100 000g for 70 min at 4°C. Each pellet was resuspended in 50 µL PBS. The number of mice for isolation of EDOF and EROF for nanoparticle tracking analysis, BCA protein assay and western blotting was the same. To observe the configuration of extracellular vesicles using transmission electron microscope (TEM), the pellet suspension was top-loaded onto 300-mesh grids and then dried. The grids were stained with 2% phosphotungstic acid and visualised under a JEOL JEM-100SX TEM (JEOL). Nanoparticle tracking analysis using NanoSight LM-10 (Malvern) was performed to determine the size, distribution and number of particles in the pellet suspension. The procedure was performed according to the manufacturer’s instructions.
Culture and treatment of embryos
Isolated extracellular vesicles were dissolved in culture medium at different concentrations to determine a suitable concentration. The effects of supplementation of extracellular vesicles to the culture medium on the developmental competency of embryos were assessed based on the cleavage and blastocyst formation rates. The medium used for culturing fertilised embryos was KSOM supplemented with amino acids, covered with mineral oil and cultured in 5% (v/v) CO2 atmosphere at 37.5°C.
Ammonium assay
Ammonium levels in the media were assessed according to the methods previously reported (Gardner and Lane 1993; Lane and Gardner 2003). We used the ultramicrofluorometric technique, which was based on the following reaction: glutamate dehydrogenase α-ketoglutarate + NADH + NH4+ → glutamate + NAD+ + H2O. The reaction reagents had the following composition: 0.24 mM NADH, 0.75 mM NaHCO3, 0.63 mM ADP, 14.15 mM α-ketoglutarate and 3 U mL−1 glutamate dehydrogenase in 157 mM triethanolamine buffer (pH 8.0). A calibration curve of ammonium chloride concentrations of 0–0.5 mM was plotted with each series of assays.
Embryo transfer
Before ET, the embryos were cultured in vitro for 1 h. The recipients were anaesthetised by injecting 100 mg kg−1 bodyweight ketamine and 10 mg kg−1 bodyweight xylazine, and the oviduct was accessed by making a small incision on the recipient’s back (Koutroli et al. 2014). A small cavity was made in the oviduct using a thin needle and 10 embryos at 2-cell stage were inserted using a pipette; this procedure was performed bilaterally and hence, 20 embryos at 2-cell stage were transferred for one mouse. The incision was then closed and the mice were housed in a warm cage under a 50W bulb and mouse eyes were covered until awakening to avoid injury to the eyes.
BCA protein assay and western blotting
Radio immunoprecipitation assay (RIPA) buffer was added to the suspension of extracellular vesicles for membrane lysis. The concentration of proteins derived from extracellular vesicles was determined using Pierce BCA protein assay kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The proteins were separated by sodium dodecyl sulphate (SDS) polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. The membranes were blocked in 5% non-fat milk powder in Tris-buffered saline containing 0.05% Tween 20 (TBST), incubated with primary antibodies to CD9 and HSP70 (extracellular vesicle markers; Abcam), washed three times in TBST and incubated in TBST with 0.2% BSA containing secondary antibodies (Beyotime Biotechnology). Immunoblots were visualised using an enhanced chemiluminescence kit (Millipore).
Real-time polymerase chain reaction (PCR)
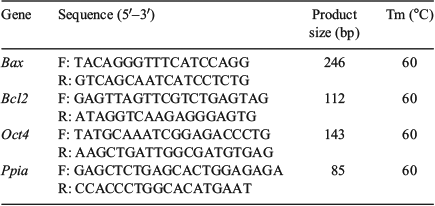
Plugged females were euthanised on 3.5 days post-coitum (dpc) hCG injection, followed by cutting open the abdominal cavity with a pair of sharp scissors. The uterus was then immediately removed and an incision was made near the cervix. Then, a 26-gauge needle was inserted at the junction of the uterus and oviduct and gently pressed on the dish; 0.4 mL of DPBS was then injected using a syringe. Blastocysts that out-flowed from the hole of the uterus were collected. Total RNA was extracted from the blastocysts using Cells-to-Signal Kit (Invitrogen) according to the manufacturer’s protocol. Complementary DNA was synthesised using the PrimeScript RT Reagent Kit (TaKaRa). Real-time PCR was performed using a CFX96 real-time PCR detection system (Bio-Rad) using SYBR Premix Ex TaqII (TaKaRa). Details of the procedure are as follows: thermal cycling conditions of 95°C for 1 min, 40 PCR cycles of 5 s at 95°C for DNA denaturation and 30 s at 60°C for primer annealing and extension, followed by 65°C–95°C for melting (increment: 0.5°C per 5 s). Primers were designed for genes using the Primer3 software (https://github.com/primer3-org/primer3, verified 26 July 2018), optimised and initially screened using similar concentration templates. Details of the primers are described in Table 1. The specificity of the quantitative PCR reaction was confirmed by single peaks in the melting curves. Amplification efficiencies of genes were obtained using the slopes of the standard curves. Twenty embryos per group were processed in each replicate. All the genes examined were quantified more than triplicate and gene expression was calculated relative to that of the housekeeping genes. The relative abundances of gene transcripts were established by testing the data for normality and equal variance using the Levene median test, ANOVA, followed by multiple pair-wise comparisons using Tukey’s test.
|
Immunofluorescence
Immunodetection was based on the detection of POU domain, class 5, transcription factor-1 (Oct-4), a specific marker of inner cell mass (ICM). Embryos were fixed using 4% (v/v) paraformaldehyde (at room temperature (RT) for 2 h), permeabilised using 0.2% (v/v) Triton X-100 (RT for 20 min) and blocked in a blocking liquid (4°C for 12 h). The embryos were then incubated overnight with a primary Oct4 antibody (Sigma-Aldrich) at 4°C, followed by incubation with Alexa Fluor 555 secondary antibody (RT for 2 h; Beyotime). After incubation with primary and secondary antibodies, the embryos were washed thrice in 0.1% PBS-polyvinyl alcohol (PVA; RT for 15 min) and treated with 4,6-diamidino-2-phenylindole hydrochloride (DAPI) for 3 min. After washing and mounting, slides were examined by epifluorescence using a Nikon Eclipse Ti-S microscope and images were captured using a digital camera (Nikon).
Apoptosis detection
Apoptosis detection was performed using the DeadEnd Fluorometric TUNEL System (Promega) according to the manufacturer’s instructions. Briefly, blastocysts were fixed in 4% paraformaldehyde (RT for 2 h), permeabilised in 0.5% Triton X-100 (RT for 5 min) and incubated with fluorescein isothiocyanate (FITC)-conjugated dUTP and terminal deoxynucleotidyl transferase in the dark (37°C for 1 h). The tailing reaction was terminated using 2× sodium chloride/sodium citrate (SSC) in the dark (RT for 15 min). Embryos were then incubated with PBS containing 25 µg mL−1 RNase A in the dark (RT for 30 min). After DAPI staining and washing with PBS in the dark, slides were examined by epifluorescence using the Nikon Eclipse Ti-S microscope and images were captured using a digital camera (Nikon).
Statistical analysis
Cleavage, blastocyst formation and birth rates were analysed using the χ2 test. Apoptotic index was defined as the ratio of the number of apoptotic cells and total cells in blastocysts; ICM : TE was defined as the ratio of ICM and trophectoderm (TE) cells in blastocysts. Apoptotic index, ICM : TE and the relative expression of gene transcripts were analysed using ANOVA. Statistical analyses were conducted using the SPSS software package (SPSS Inc.). Data were expressed as the mean ± s.e.m. and P < 0.05 was considered to be statistically significant.
Results
Presence of extracellular vesicles in oviduct fluid
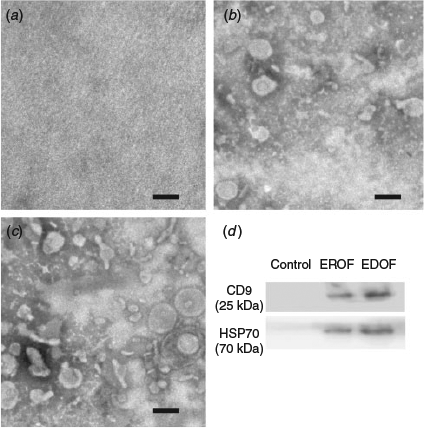
Extracellular vesicles in the oviduct fluid were isolated by ultracentrifugation. PBS without oviduct fluid was used as the control. Electron microscopy results revealed the presence of variable particles in the extracted oviduct fluid (Fig. 1a–c). Nanoparticle tracking analysis showed that diameters of the majority of particles ranged from 30 to 150 nm (Fig. 2a, b). The particles were further characterised by western blotting using specific antibodies against exosomal membrane markers CD9 and HSP70. Clear CD9 and HSP70 signals were detected from the extracts of oviduct fluid obtained from donors and recipients, whereas no signal was detected in the control (Fig. 1d), indicating the presence of EDOF and EROF.
|

|
Characterisation of EDOF and EROF
Nanoparticle tracking analysis was used to measure the quantities of EDOF and EROF. The proportion of EDOF was higher than that of EROF (1.29 × 109 ± 6.32 × 107 particles mL−1 vs 5.90 × 108 ± 5.29 × 106 particles mL−1; P < 0.05; Fig. 2c). Total protein content in EDOF and EROF was measured using the Pierce BCA protein assay. Consistent with the results of the concentration of extracellular vesicles, EDOF showed a higher quantity of proteins than EROF (356.3 ± 28.3 µg mL−1 vs 175.7 ± 44.7 µg mL−1; P < 0.05; Fig. 2d).
Effect of various doses of extracellular vesicles derived from the oviduct fluid on in vitro development of embryos
Extracellular vesicles derived from the oviduct fluid, which were extracted from 1, 10 and 20 recipient or donor mice, were dissolved in a final volume of 10 µL of culture medium. Embryos were cultured from 1-cell stage to the blastocyst stage and the extracellular vesicles were added into the culture medium at the 1-cell stage. The results showed that there was no obvious difference in the blastocyst formation rate when extracellular vesicles extracted from 1, 10 and 20 recipient mice were added into the culture medium (P > 0.05). Furthermore, there was no obvious difference when the extracellular vesicles extracted from 1 and 20 donor mice were added into the culture medium. However, extracellular vesicles extracted from 10 donor mice significantly improved the blastocyst formation rate (Table 2). The results indicated that EDOF from 10 donor mice had significant positive effects on in vitro development of embryos.
EDOF from 10 mice affected the blastocyst formation rate, whereas that from 20 mice did not. The addition of extracellular vesicles might cause changes in the culture medium and ammonium concentration was an important indicator (Lane and Gardner 2003). Thus, we measured the ammonium concentration of the culture medium on Day 3.5. The result showed that ammonium levels in culture medium increased with the increase in the amount of extracellular vesicles added (Table 2). Additionally, ammonium concentration of >300 µM impaired embryo development (Lane and Gardner 2003). Ammonium concentration in the group of EDOF from 20 mice was 333.2 ± 3.2 µM, which was significantly higher than in all other groups. These results indicated that an overdose of extracellular vesicles may be detrimental for embryo development due to the production of excessive ammonium.
Effects of extracellular vesicles derived from oviduct fluid on the development of embryos after ET
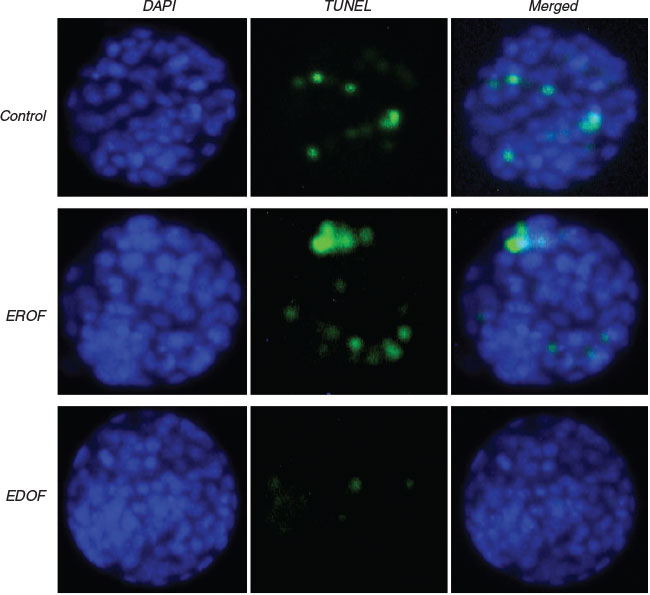
Extracellular vesicles extracted from 10 donors and 10 recipient mice were added into ETM and embryos were then transferred to the oviduct to define the roles of EDOF and EROF in embryo development after ET. The efficiency of embryo collection and development rate in vivo were analysed. Most of the embryos from each group were collected and the blastocyst formation rate in the EDOF group was significantly higher than in other groups (Table 3). The results revealed that in vivo blastocysts in ETM supplemented with EDOF exhibited lower Bcl-2-associated X(Bax) expression, higher B-cell leukemia /lymphoma 2(Bcl2)expression, higher Oct4 expression, lower apoptotic index and higher ICM : TE ratio than those in ETM supplemented with EROF and controls (Figs 3–5 and Table 3). Furthermore, the effects of extracellular vesicles on the development of embryos were evaluated based on the birth rate; EDOF significantly improved the birth rates (Table 3). The results suggest that the supplementation of EDOF to ETM improves the efficiency of ET.
Discussion
Synchronicity of embryos and recipients is an important factor for successful ET. Embryos at 1-cell and 2-cell stages were transferred to the oviduct and blastocysts were then transferred to the uterus to achieve synchronicity for normal development and implantation (Lopez-Cardona et al. 2015). Synchronicity of donors and recipients is also important. Therefore, recipients were mated with vasectomised males to make them more compatible with donors for embryo development (Hasegawa et al. 2017). However, there were still some differences between the donors and recipients. Recipients who mated with vasectomised males lacked spermatozoa; however, donors were mated with males having good reproductive performance. In donors, spermatozoa gathered at the oviductal isthmus interacted with epithelial cells and regulated the expression of genes in the oviduct; they are also reported to upregulate the expression of prostaglandin endoperoxidase synthase 2 and adrenomedullin (Li et al. 2010). Seminal fluid also affected oviduct fluid protein and embryotrophic cytokine levels, which are considered as key regulators of fertilisation and embryo development (Bromfield et al. 2014; Schjenken and Robertson 2015). The interaction of spermatozoa and oviduct can also regulate the capacitation of spermatozoa and limit the opportunity of polyspermy at fertilisation (Miller 2015). After fertilisation, embryos secreting prostaglandin E2 regulate gene expression in the oviduct, which is related to embryo development and immune function (Weber et al. 1991; Lee et al. 2002; Maillo et al. 2016). Our findings suggest the presence of a difference between EDOF and EROF. One of the reasons may be that the former is from hormonally induced superovulation, whereas the latter results from natural cycling. This could account for the differences in the extracellular vesicle concentrations. Furthermore, spermatozoa and fertilised eggs may be responsible for the observed differences between EDOF and EROF.
Extracellular vesicles are important mediators for cell–cell communication and are involved in multiple processes related to reproduction. Extracellular vesicles derived from ovarian follicles participate in the maturation of oocytes via the inclusion of miRNA and proteins (da Silveira et al. 2014, 2015; Santonocito et al. 2014). Extracellular vesicles derived from the oviduct fluid containing plasma membrane Ca2+-ATPase 4a (PMCA4a) play a crucial role in the capacitation and acrosome reaction of spermatozoa (Al-Dossary et al. 2013). Extracellular vesicles derived from uterine fluid can also regulate the reaction between embryos and endometrium (Ng et al. 2013; Machtinger et al. 2016; Familari et al. 2017). Our previous studies demonstrated that embryos can secrete extracellular vesicles, benefiting the development of embryos in vitro (Qu et al. 2017). Additionally, supplementing extracellular vesicles derived from oviduct fluid can improve embryo development in vitro (Alminana et al. 2017). In the present study, EDOF from 10 mice affected blastocyst formation rate, but that from 20 mice did not. The ammonium concentration in the group of EDOF from 20 mice was 333.2 ± 3.2 µM. Ammonium levels in the culture medium increased with the increase in the amount of extracellular vesicles added; extracellular vesicle overdose led to more proteins that may have undergone degradation and produced more ammonium. High levels of ammonium impairs embryo development (Lane et al. 2001; Lane and Gardner 2003), thus implying that an overdose of extracellular vesicles may be detrimental for embryo development due to the production of excessive ammonium.
There is no information regarding the effect of supplementing extracellular vesicles in ETM on the reproductive tract. In this study, we supplemented oviduct-derived extracellular vesicles to ETM and the result demonstrated that supplementing EDOF to ETM improved birth rates, suggesting that EDOF play a positive role in the development of transferred embryos. Moreover, for investigating the effects of extracellular vesicles derived from oviduct fluid, the expression of genes related to apoptosis and development was studied in blastocysts. Bax is an apoptosis activator that is involved in P53-mediated apoptosis, whereas Bcl2 has the ability to block apoptosis. Embryos exhibiting lower Bax and higher Bcl2 levels are considered to be conducive for development (Exley et al. 1999; Guillemin et al. 2009). Oct4 is essential for pluripotency in ICM and embryos with higher Oct4 expression have excellent development potential (Le Bin et al. 2014). Our results revealed lower Bax expression and higher Bcl2 and Oct4 expression in the EDOF-supplemented groups. To further determine the role of extracellular vesicles derived from the oviduct, apoptosis detection and differential staining of ICM : TE of blastocysts was performed. The results showed that embryos in ETM supplemented with EDOF exhibited a lower apoptotic index and higher ICM : TE ratio, suggesting that EDOF improved the competency of embryos to resist apoptosis and promote differentiation. During ET, zygotes or embryos experience fluctuations in temperature, osmotic pressure, oxygen concentration and other external environment factors, which may be prevented by EDOF supplementation.
The rapid development of gene editing relies mainly on microinjection, nuclear transfer and ET (Proudfoot et al. 2015). However, multiple factors are responsible for hampering the application potential of this technology. To obtain better efficiency, various measures have been adopted, such as using high-efficiency gene targeting tools (Zinc-finger nucleases, Transcription activator-like effector nucleases and Clustered, regularly interspaced, short palindromic repeats) and improving the methods of microinjection, nuclear transfer and ET (Bin Ali et al. 2014; Josa et al. 2016; Tan et al. 2016). Undoubtedly, ET is a crucial step in breeding transgenic animals, rescuing endangered species and even performing human-assisted reproduction.
Our findings demonstrated differences between EDOF and EROF. Furthermore, supplementation of EDOF to ETM significantly improved birth rates in mice. In conclusion, the present study provided a new idea for improving the efficiency of ET and contributed to the application of gene editing. It may also have a positive effect on the development of assisted reproduction technology for humans and animals. Moreover, it may be beneficial for animal welfare in terms of reduction in the number of laboratory animals required to obtain sufficient numbers of offspring.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This work was supported in part by the National Natural Science Foundation of China (No. 81270348) and Natural Science Foundation of Shaanxi Province (2014FWPT017).
References
Al-Dossary, A. A., Strehler, E. E., and Martin-Deleon, P. A. (2013). Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm. PLoS One 8, e80181.| Expression and secretion of plasma membrane Ca2+-ATPase 4a (PMCA4a) during murine estrus: association with oviductal exosomes and uptake in sperm.Crossref | GoogleScholarGoogle Scholar |
Alminana, C., Corbin, E., Tsikis, G., Alcantara-Neto, A. S., Labas, V., Reynaud, K., Galio, L., Uzbekov, R., Garanina, A. S., Druart, X., and Mermillod, P. (2017). Oviduct extracellular vesicles protein content and their role during oviduct–embryo cross-talk. Reproduction 154, 153–168.
| Oviduct extracellular vesicles protein content and their role during oviduct–embryo cross-talk.Crossref | GoogleScholarGoogle Scholar |
Bin Ali, R., van der Ahe, F., Braumuller, T. M., Pritchard, C., Krimpenfort, P., Berns, A., and Huijbers, I. J. (2014). Improved pregnancy and birth rates with routine application of nonsurgical embryo transfer. Transgenic Res. 23, 691–695.
| Improved pregnancy and birth rates with routine application of nonsurgical embryo transfer.Crossref | GoogleScholarGoogle Scholar |
Bromfield, J. J., Schjenken, J. E., Chin, P. Y., Care, A. S., Jasper, M. J., and Robertson, S. A. (2014). Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring. Proc. Natl. Acad. Sci. USA 111, 2200–2205.
| Maternal tract factors contribute to paternal seminal fluid impact on metabolic phenotype in offspring.Crossref | GoogleScholarGoogle Scholar |
da Silveira, J. C., Carnevale, E. M., Winger, Q. A., and Bouma, G. J. (2014). Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare. Reprod. Biol. Endocrinol. 12, 44.
| Regulation of ACVR1 and ID2 by cell-secreted exosomes during follicle maturation in the mare.Crossref | GoogleScholarGoogle Scholar |
da Silveira, J. C., Winger, Q. A., Bouma, G. J., and Carnevale, E. M. (2015). Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-beta signalling during follicle development in the mare. Reprod. Fertil. Dev. 27, 897–905.
| Effects of age on follicular fluid exosomal microRNAs and granulosa cell transforming growth factor-beta signalling during follicle development in the mare.Crossref | GoogleScholarGoogle Scholar |
Exley, G. E., Tang, C., McElhinny, A. S., and Warner, C. M. (1999). Expression of caspase and BCL-2 apoptotic family members in mouse preimplantation embryos. Biol. Reprod. 61, 231–239.
| Expression of caspase and BCL-2 apoptotic family members in mouse preimplantation embryos.Crossref | GoogleScholarGoogle Scholar |
Familari, M., Cronqvist, T., Masoumi, Z., and Hansson, S. R. (2017). Placenta-derived extracellular vesicles: their cargo and possible functions. Reprod. Fertil. Dev. 29, 433–447.
| Placenta-derived extracellular vesicles: their cargo and possible functions.Crossref | GoogleScholarGoogle Scholar |
Gardner, D. K., and Lane, M. (1993). Amino acids and ammonium regulate mouse embryo development in culture. Biol. Reprod. 48, 377–385.
| Amino acids and ammonium regulate mouse embryo development in culture.Crossref | GoogleScholarGoogle Scholar |
Gonzalez-Jara, P., Fontela, T., Lopez-Mimbela, E., Cereceda, M., Del Olmo, D., and Moreno, M. (2017). Optimization of the balance between effort and yield in unilateral surgical transfer of mouse embryos. Lab. Anim. 51, 622–628.
| Optimization of the balance between effort and yield in unilateral surgical transfer of mouse embryos.Crossref | GoogleScholarGoogle Scholar |
Guillemin, Y., Lalle, P., Gillet, G., Guerin, J. F., Hamamah, S., and Aouacheria, A. (2009). Oocytes and early embryos selectively express the survival factor BCL2L10. J. Mol. Med. 87, 923–940.
| Oocytes and early embryos selectively express the survival factor BCL2L10.Crossref | GoogleScholarGoogle Scholar |
Hasegawa, A., Mochida, K., Ogonuki, N., Hirose, M., Tomishima, T., Inoue, K., and Ogura, A. (2017). Efficient and scheduled production of pseudopregnant female mice for embryo transfer by estrous cycle synchronization. J. Reprod. Dev. 63, 539–545.
| Efficient and scheduled production of pseudopregnant female mice for embryo transfer by estrous cycle synchronization.Crossref | GoogleScholarGoogle Scholar |
Josa, S., Seruggia, D., Fernandez, A., and Montoliu, L. (2016). Concepts and tools for gene editing. Reprod. Fertil. Dev. 29, 1–7.
| Concepts and tools for gene editing.Crossref | GoogleScholarGoogle Scholar |
Koutroli, E., Alexakos, P., Kakazanis, Z., Symeon, I., Balafas, E., Voyiatzaki, C., and Kostomitsopoulos, N. (2014). Effects of using the analgesic tramadol in mice undergoing embryo transfer surgery. Lab Anim. (NY) 43, 167–172.
| Effects of using the analgesic tramadol in mice undergoing embryo transfer surgery.Crossref | GoogleScholarGoogle Scholar |
Lane, M., and Gardner, D. K. (2003). Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol. Reprod. 69, 1109–1117.
| Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse.Crossref | GoogleScholarGoogle Scholar |
Lane, M., Hooper, K., and Gardner, D. K. (2001). Effect of essential amino acids on mouse embryo viability and ammonium production. J. Assist. Reprod. Genet. 18, 519–525.
| Effect of essential amino acids on mouse embryo viability and ammonium production.Crossref | GoogleScholarGoogle Scholar |
Le Bin, G. C., Munoz-Descalzo, S., Kurowski, A., Leitch, H., Lou, X., Mansfield, W., Etienne-Dumeau, C., Grabole, N., Mulas, C., Niwa, H., Hadjantonakis, A. K., and Nichols, J. (2014). Oct4 is required for lineage priming in the developing inner cell mass of the mouse blastocyst. Development 141, 1001–1010.
| Oct4 is required for lineage priming in the developing inner cell mass of the mouse blastocyst.Crossref | GoogleScholarGoogle Scholar |
Lee, K. F., Yao, Y. Q., Kwok, K. L., Xu, J. S., and Yeung, W. S. (2002). Early developing embryos affect the gene expression patterns in the mouse oviduct. Biochem. Biophys. Res. Commun. 292, 564–570.
| Early developing embryos affect the gene expression patterns in the mouse oviduct.Crossref | GoogleScholarGoogle Scholar |
Li, H. W., Liao, S. B., Chiu, P. C., Tam, W. W., Ho, J. C., Ng, E. H., Ho, P. C., Yeung, W. S., Tang, F., and O., W. S. (2010). Expression of adrenomedullin in human oviduct, its regulation by the hormonal cycle and contact with spermatozoa, and its effect on ciliary beat frequency of the oviductal epithelium. J. Clin. Endocrinol. Metab. 95, E18–E25.
| Expression of adrenomedullin in human oviduct, its regulation by the hormonal cycle and contact with spermatozoa, and its effect on ciliary beat frequency of the oviductal epithelium.Crossref | GoogleScholarGoogle Scholar |
Lin, T., Diao, Y. F., Kang, J. W., Lee, J. E., Kim, D. K., and Jin, D. I. (2015). Tauroursodeoxycholic acid improves the implantation and live-birth rates of mouse embryos. Reprod. Biol. 15, 101–105.
| Tauroursodeoxycholic acid improves the implantation and live-birth rates of mouse embryos.Crossref | GoogleScholarGoogle Scholar |
Lopez, M. J., Garcia, D., Rodriguez, A., Colodron, M., Vassena, R., and Vernaeve, V. (2014). Individualized embryo transfer training: timing and performance. Hum. Reprod. 29, 1432–1437.
| Individualized embryo transfer training: timing and performance.Crossref | GoogleScholarGoogle Scholar |
Lopez-Cardona, A. P., Fernandez-Gonzalez, R., Perez-Crespo, M., Alen, F., de Fonseca, F. R., Orio, L., and Gutierrez-Adan, A. (2015). Effects of synchronous and asynchronous embryo transfer on postnatal development, adult health, and behavior in mice. Biol. Reprod. 93, 85.
| Effects of synchronous and asynchronous embryo transfer on postnatal development, adult health, and behavior in mice.Crossref | GoogleScholarGoogle Scholar |
Machtinger, R., Laurent, L. C., and Baccarelli, A. A. (2016). Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation. Hum. Reprod. Update 22, 182–193.
Maillo, V., Sanchez-Calabuig, M. J., Lopera-Vasquez, R., Hamdi, M., Gutierrez-Adan, A., Lonergan, P., and Rizos, D. (2016). Oviductal response to gametes and early embryos in mammals. Reproduction 152, R127–R141.
| Oviductal response to gametes and early embryos in mammals.Crossref | GoogleScholarGoogle Scholar |
Miller, D. J. (2015). Regulation of sperm function by oviduct fluid and the epithelium: insight into the role of glycans. Reprod Domest Anim. 50, 31–39.
| Regulation of sperm function by oviduct fluid and the epithelium: insight into the role of glycans.Crossref | GoogleScholarGoogle Scholar |
Ng, Y. H., Rome, S., Jalabert, A., Forterre, A., Singh, H., Hincks, C. L., and Salamonsen, L. A. (2013). Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo–endometrial cross talk at implantation. PLoS One 8, e58502.
| Endometrial exosomes/microvesicles in the uterine microenvironment: a new paradigm for embryo–endometrial cross talk at implantation.Crossref | GoogleScholarGoogle Scholar |
Proudfoot, C., Carlson, D. F., Huddart, R., Long, C. R., Pryor, J. H., King, T. J., Lillico, S. G., Mileham, A. J., McLaren, D. G., Whitelaw, C. B., and Fahrenkrug, S. C. (2015). Genome edited sheep and cattle. Transgenic Res. 24, 147–153.
| Genome edited sheep and cattle.Crossref | GoogleScholarGoogle Scholar |
Qu, P., Qing, S., Liu, R., Qin, H., Wang, W., Qiao, F., Ge, H., Liu, J., Zhang, Y., Cui, W., and Wang, Y. (2017). Effects of embryo-derived exosomes on the development of bovine cloned embryos. PLoS One 12, e0174535.
| Effects of embryo-derived exosomes on the development of bovine cloned embryos.Crossref | GoogleScholarGoogle Scholar |
Santonocito, M., Vento, M., Guglielmino, M. R., Battaglia, R., Wahlgren, J., Ragusa, M., Barbagallo, D., Borzi, P., Rizzari, S., Maugeri, M., Scollo, P., Tatone, C., Valadi, H., Purrello, M., and Di Pietro, C. (2014). Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil Steril. 102, 1751–1761.e1.
| Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation.Crossref | GoogleScholarGoogle Scholar |
Schjenken, J. E., and Robertson, S. A. (2015). Seminal fluid signalling in the female reproductive tract: implications for reproductive success and offspring health. Adv. Exp. Med. Biol. 868, 127–158.
| Seminal fluid signalling in the female reproductive tract: implications for reproductive success and offspring health.Crossref | GoogleScholarGoogle Scholar |
Simons, M., and Raposo, G. (2009). Exosomes – vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581.
| Exosomes – vesicular carriers for intercellular communication.Crossref | GoogleScholarGoogle Scholar |
Tan, W., Proudfoot, C., Lillico, S. G., and Whitelaw, C. B. (2016). Gene targeting, genome editing: from Dolly to editors. Transgenic Res. 25, 273–287.
| Gene targeting, genome editing: from Dolly to editors.Crossref | GoogleScholarGoogle Scholar |
Thery, C., Amigorena, S., Raposo, G., and Clayton, A. (2006). Isolation and characterization of exosomes from cell culture supernatants and biological fluids. In ‘Current protocols in cell biology’. pp. 3.22.1–3.22.29. (Wiley: New York.)
Weber, J. A., Freeman, D. A., Vanderwall, D. K., and Woods, G. L. (1991). Prostaglandin E2 secretion by oviductal transport-stage equine embryos. Biol. Reprod. 45, 540–543.
| Prostaglandin E2 secretion by oviductal transport-stage equine embryos.Crossref | GoogleScholarGoogle Scholar |
* Denotes joint first authorship.