Tropical summer induces DNA fragmentation in boar spermatozoa: implications for evaluating seasonal infertility
Santiago T. Peña A B C , Felicity Stone A , Bruce Gummow B D , Anthony J. Parker E and Damien B. B. P. Paris
A B C , Felicity Stone A , Bruce Gummow B D , Anthony J. Parker E and Damien B. B. P. Paris  A F G
A F G
A Discipline of Biomedical Science, College of Public Health, Medical and Veterinary Sciences, James Cook University, Townsville, Qld 4811, Australia.
B Discipline of Veterinary Science, College of Public Health, Medical and Veterinary Sciences, James Cook University, Townsville, Qld 4811, Australia.
C College of Veterinary Medicine, Visayas State University, Baybay City, Leyte, Philippines.
D Faculty of Veterinary Science, University of Pretoria, Onderstepoort, 0110, South Africa.
E College of Food, Agricultural and Environmental Sciences, Ohio State University, Wooster, OH 44691, USA.
F Centre for Tropical Environmental and Sustainability Science, James Cook University, Townsville, Qld 4811, Australia.
G Corresponding author. Email: damien.paris@jcu.edu.au
Reproduction, Fertility and Development 31(3) 590-601 https://doi.org/10.1071/RD18159
Journal Compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Summer infertility continues to undermine pig productivity, costing the pig industry millions in annual losses. The boar’s inefficient capacity to sweat, non-pendulous scrotum and the extensive use of European breeds in tropical conditions, can make the boar particularly vulnerable to the effects of heat stress; however, the link between summer heat stress and boar sperm DNA damage has not yet been demonstrated. Semen from five Large White boars was collected and evaluated during the early dry, late dry and peak wet seasons to determine the effect of seasonal heat stress on the quality and DNA integrity of boar spermatozoa. DNA damage in spermatozoa during the peak wet was 16-fold greater than during the early dry and nearly 9-fold greater than during the late dry season. Sperm concentration was 1.6-fold lower in the peak wet than early dry whereas no difference was found across several motility parameters as determined by computer-assisted sperm analysis. These results demonstrate that tropical summer (peak wet season) induces DNA damage and reduces concentration without depressing motility in boar spermatozoa, suggesting that traditional methods of evaluating sperm motility may not detect inherently compromised spermatozoa. Boar management strategies (such as antioxidant supplementation) need to be developed to specifically mitigate this problem.
Additional keywords: DNA damage, heat stress, oxidative stress, pig, summer infertility, TUNEL.
Introduction
Forty percent of global meat consumption is pork (National Pork Board 2017), with at least four tropical countries (Brazil, Vietnam, The Philippines and Mexico) among the top 10 pork producers in the world (National Pork Board 2014). With rising populations and increasing demand for animal protein, emerging tropical economies in Asia and elsewhere are projected to contribute significantly to the global food crisis (Pingali 2007; Alexandratos and Bruinsma 2012). However, the production efficiency of pigs in tropical and sub-tropical regions is known to be affected by seasonal or summer infertility, a syndrome characterised by an overall reduction in the reproductive performance of the breeding herd. This poor performance can be caused by several factors, including: ambient temperatures greater than the animal’s thermal comfort zone (i.e. 18–20°C; Stone 1982; Prunier et al. 1997), humidity, photoperiod and management practices (Love 1981; Hennessy and Williamson 1984; Auvigne et al. 2010) including genetic background (Sonderman and Luebbe 2008), causing significant reduction to profitability in the pig industry. For example, at least $300 million are lost annually in swine alone and billions across the US livestock industry due to heat stress (St-Pierre et al. 2003).
Summer infertility is mainly characterised by: (1) reduced expression of oestrus in gilts and sows, (2) increased rates of pregnancy failure (Paterson et al. 1978; Hughes and van Wettere 2010) and (3) decreased breeding efficiency in boars (Wettemann et al. 1976; Boma and Bilkei 2006; Auvigne et al. 2010). Even in a temperate climate such as southern France, over a 5-year period, mean fertility, based on ultrasound pregnancy diagnosis 28 days after insemination, was at its lowest in summer (81.2%; end of August) compared with its peak of 86.8% in winter (end of March; Auvigne et al. 2010). In Australia, the adjusted farrowing rate dropped to 77.1% in summer–autumn compared with 91.9% in spring (O’Leary 2010), whereas in the tropical Philippines, farrowing rate, percentage live born, litter size at weaning and pigs weaned per sow per year were significantly lower around the third quarter of the year after exposure to higher ambient temperatures. This was compounded by reduced voluntary feed intake and lower feed quality, with small-to-medium farms being the most severely affected (Vega and Agbisit 2009; Vega et al. 2010).
The boar is particularly vulnerable to the effects of heat stress due to several notable characteristics. Pigs generally are known to be inefficient at sweating (Ingram 1965; Mount 1968). While apocrine sweat glands appear to be abundant in the skin of pigs, they are located deeper in the dermis and in subcutaneous tissue except for eccrine type in the snout and dorsal nasal regions (Sumena et al. 2010). Moreover, the boar scrotum is not pendulous (Knox 2003) and boar spermatozoa tend to be more susceptible to temperature shock (Einarsson et al. 2008). Stone (1982) demonstrated that spermatogenesis in boars is impaired when ambient temperatures rise above 29°C. Thus, heat stress in boars has been shown to result in lower semen volume (Cameron and Blackshaw 1980), reduced sperm concentration (Egbunike and Dede 1980), lower motility and higher rates of abnormal spermatozoa (Egbunike and Dede 1980; Heitman et al. 1984; Barranco et al. 2013), interference in testosterone production (Stone and Seamark 1984), extended ejaculation time (Egbunike and Dede 1980) and reduced libido (Flowers 1997).
Moreover, the relatively high levels of unsaturated fatty acids in the plasma membrane (Cerolini et al. 2001) and low antioxidant activity of seminal plasma (Brzezińska-Ślebodzińska et al. 1995) all contribute to the high sensitivity of boar spermatozoa to peroxidative damage. We have recently proposed that such mechanisms may make boar spermatozoa highly prone to DNA damage during periods of heat stress (Peña et al. 2017). Recent studies in mice have conclusively demonstrated that heat stress induces sperm DNA damage, which causes abnormal and arrested embryo development and ultimately embryo and fetal loss (Paul et al. 2008). This suggests that heat stress-induced DNA damage in boar spermatozoa may contribute significantly to seasonal pregnancy failure and reduced litter size in sows (Peña et al. 2017). In pigs, Didion et al. (2009) have proposed that spermatozoa with greater than 6% DNA fragmentation can cause both decreased farrowing rates and average number of piglets born. However, definitive evidence of the link between heat stress and DNA damage in boar spermatozoa is limited. While boar spermatozoa collected in spring–summer appeared to have a relatively higher percentage of DNA-damaged spermatozoa, a significant increase was only evident in fractionated ejaculates (F1 and F2) from two out of five boars (Zasiadczyk et al. 2015). By contrast, Petrocelli et al. (2015) reported that neither season, photoperiod nor genetic line affected sperm DNA fragmentation. Both studies, however, were conducted in temperate climates where ambient temperatures may not be sufficient to induce significant DNA damage compared with pigs raised in the tropics. Thus, the aim of this study was to determine the effect of seasonal heat stress on the quality and DNA integrity of spermatozoa obtained from boars housed in the dry tropics of Townsville, North Queensland, Australia, a climate more similar to that experienced by pig producers in developing tropical Asian countries.
Materials and methods
Boars and location
Power analysis (PASS 14 Power Analysis and Sample Size Software 2016; NCSS LLC) was undertaken to determine the minimum number of animals needed to show a significant change in sperm DNA damage due to heat stress according to the principles of the three Rs (EU Directive 2010/63/EU; Kilkenny et al. 2010). A sample size of n = 3 boars was sufficient under the following estimated conditions: mean difference in DNA fragmentation index between control boars and heat stressed boars = 24%; standard deviation = ± 10; assuming equal variance (Fernandes et al. 2008; Evenson et al. 2009). As a contingency against failed sample collections and to align with other boar studies (Dubé et al. 2004; Boe-Hansen et al. 2005; Alkmin et al. 2013; Zasiadczyk et al. 2015), n = 5 Large White boars were purchased at 11–12 months of age from a commercial piggery and reared in an open, gable roof-type facility within individual 3 × 3 m pens at the College of Public Health, Medical and Veterinary Sciences, James Cook University, Townsville, Qld, Australia (19°19’46.4′S, 146°45’40.3′E). Boars were exposed to prevailing winds and ambient temperatures throughout the day. Each boar was fed 1.8–2.3 kg day−1 of a commercial pelleted diet (Barastoc; Ridley AgriProducts) to maintain a body score between 3 and 3.5. Water was provided ad libitum via an automatic pig nipple waterer. Experiments were approved by the James Cook University Animal Ethics Committee.
Temperature, relative humidity and temperature-humidity index
Townsville is situated in the dry tropics with a climate that has less rainfall than other comparable regions in the wet tropics (Bureau of Meteorology 2011a). The dry season (late April to October) is typically cooler and dry, while the wet season (November to early April) tends to be hot and wet, with monsoon rains from late December to early April. Mean, minimum and maximum daily temperatures as well as mean, 6:00 a.m. and 3:00 p.m. daily relative humidity (corresponding to the coolest and hottest times of the day respectively; Bureau of Meteorology 2011b) for Townsville were obtained from the Australian Bureau of Meteorology. Mean, minimum and maximum temperature–humidity indices (THI) were generated for each day using mean, minimum and maximum daily temperatures coupled with mean, 6 a.m. and 3 p.m. daily relative humidity values respectively. This was achieved using an online heat index calculator (National Weather Service 2016a), validated and interpreted using a temperature–humidity index chart (Thom 1959; Hahn et al. 2009). Mean values were calculated for all parameters spaning the 42-day period immediately before each seasonal semen collection time point. This period encompasses the ambient environmental conditions to which boars were exposed for one complete cycle of spermatogenesis in the boar (França and Cardoso 1998; França et al. 2005).
Seasonal semen collection and processing
Boars were sexually mature (20–28 months old) at the time of the experiment and met minimum standards of sperm quality (70% motility, 65% morphologically normal spermatozoa and an ejaculate volume of at least 100 mL) in order to qualify for the study. To avoid measuring DNA damage associated with dead or degenerating spermatozoa stored for prolonged periods in the epididymis, semen was routinely collected from boars by the same person 2–3 times every 2 weeks before experimental sampling. One ejaculate was analysed from each of the same n = 5 boars at each sampling time point during the late dry (warm and humid; October 2014), peak wet (hot and wet; February 2015) and early dry (cool and dry; end of May 2015) seasons. Semen was collected using a dummy sow (Minitube, USA) and the gloved hand technique (Hancock and Hovell 1959) into a plastic semen collection bag fitted inside a collection cup and covered with non-woven tissue filters (all Minitube, Vic., Australia) to remove the gel fraction. The collection bag was then placed inside an insulated container containing 38°C water and immediately brought to the laboratory for processing. Raw semen from each boar was diluted 1 : 3 with 38°C pre-warmed Beltsville thawing solution (BTS; pH 7.2; Pursel and Johnson 1975) containing 205 mM D-glucose, 20 mM sodium citrate tribasic dihydrate, 3 mM ethylenediamine tetraacetic acid (EDTA) disodium salt dihydrate, 10 mM potassium chloride, 15 mM sodium bicarbonate and 0.1% (v/v) gentamicin reagent solution (Life Technologies) in nanopure deionised water. All reagents were sourced from Sigma-Aldrich unless otherwise stated. One aliquot was evaluated for sperm concentration by Neubauer haemocytometer, using standard protocols (WHO 2010), a second aliquot was adjusted to 20 × 106 spermatozoa mL−1 in BTS for evaluation of sperm motility characteristics using a computer-assisted sperm analyser (CASA; IVOS Version 10; Hamilton Thorne Research) and a third aliquot was evaluated for DNA damage.
Determination of motility characteristics by CASA
About 3 µL of 20 × 106 spermatozoa mL−1 semen was loaded into each chamber of 38°C pre-warmed Leja Standard Count 4 Chamber Slides (Leja Products) and loaded into the CASA machine. At least 200 spermatozoa across five random fields were examined per sample. Motility characteristics of spermatozoa were analysed as previously described (Peña et al. 2015). The CASA software was calibrated to the following settings: analysis set-up #7: BOAR; frames acquired, 40 s−1; frame rate, 50 Hz; minimum contrast, 60%; minimum cell size, two pixels; minimum static contrast, 30%; straightness threshold, 71.4%; low average-path velocity (VAP) cut-off, 5.0 µm s−1; medium VAP cut-off, 22.0 µm s−1; low straight-line velocity (VSL) cut-off, 11.0 µm s−1; head size (non-motile), two pixels; head intensity (non-motile), 70 pixels; static head size, 0.10–10.0 pixels; static head intensity, 0.10–0.95 pixels; static elongation, 0–60; count slow cells as motile, YES; magnification, 3.20; video source, camera; video frequency, 50; brightfield, NO; illumination intensity, 2381 and temperature, 38°C. The following characteristics were evaluated: total motility, progressive motility of the whole sample, average-path velocity (VAP; µm s−1), straight-line velocity (VSL; µm s−1), curvilinear velocity (VCL; µm s−1), amplitude of lateral head displacement (ALH; µm), beat cross frequency (BCF; Hz), straightness (STR; ratio of VSL/VAP), linearity (LIN; ratio of VSL/VCL) and elongation (ELO; ratio in % of head width to head length) as previously described (Mortimer 2000).
Sperm DNA integrity assay
BTS-diluted semen samples were purified by Percoll gradient centrifugation to remove seminal plasma and possibly dead and damaged spermatozoa (Grant et al. 1994). Two mL of 40% Percoll solution (GE Healthcare) in BTS was layered on top of 2 mL of 80% Percoll solution in BTS in a 15 mL centrifuge tube. Six mL of 1 : 3 diluted semen in BTS solution was layered on top of the Percoll gradients and centrifuged at 700g for 25 min at room temperature. The supernatant was removed and the remaining pellet was washed twice in 5 mL BTS by spinning tubes at 1000g for 5 min each at room temperature. The final sperm pellet was adjusted to 5 × 106 spermatozoa mL−1 in BTS.
Boar spermatozoa were stained using the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay according to the manufacturer’s instructions (In Situ Cell Death Detection Kit, Fluorescein; Roche Diagnostics) with modifications. Briefly, boars were randomly divided and collected in two groups of 2–3 boars to facilitate timely processing. Six control samples (two positive, two negative and two unlabelled) were prepared in parallel using pooled semen from each batch of boars tested and were individually stained for each of the six controls. These were used to accurately gate different populations of spermatozoa in the flow cytometer before experimental samples were analysed (Fig. 1).

|
One mL (5 × 106 spermatozoa) of each sample was used for TUNEL labelling and centrifuged at 720g for 5 min at room temperature. Each sperm pellet was washed twice in 200 µL phosphate-buffered saline (PBS) by centrifugation at 720g for 5 min at room temperature. The final pellet was resuspended in 100 µL PBS to which 100 µL of 4% (w/v) paraformaldehyde in PBS was added to fix spermatozoa for 60 min at room temperature. Thereafter, samples were centrifuged at 720g for 5 min at room temperature and the pellet resuspended in 200 µL PBS and stored at 4°C overnight.
The next day, samples were centrifuged 720g for 5 min at room temperature and pellets resuspended in 100 µL 0.5% Triton X-100 in 0.1% sodium citrate permeabilisation solution then incubated for 30 min at 37°C. Samples were washed twice and resuspended in 200 µL PBS except for positive controls (P1 and P2), which were resuspended in 100 µL 1000 U mL−1 DNase 1 in Roche Buffer 2 (comprising 20 µL 10 U µL−1 Roche DNase 1 stock solution: 500 µL 40 mM Tris-HCl, 2 mM MgCl2 solution, 10 000 U lyophilised DNase 1 and 500 µL glycerol; plus 180 µL Roche Buffer 2: 0.058 g NaCl, 0.099 g MnCl24H2O, 0.0011 g CaCl2 and 0.1864 g KCl in 100 mL 10 mM Tris-HCl solution) and incubated for 30 min at 37°C to induce doubled-stranded DNA breaks. P1 and P2 controls were subsequently washed twice and resuspended in 200 µL PBS before TUNEL labelling.
The TUNEL reaction labels DNA-damaged cells positive for fluorescein isothiocyanate (FITC). All samples were centrifuged 720g for 5 min at room temperature and their sperm pellets subjected to different treatments: unlabelled controls (U1 and U2) were resuspended in 50 µL PBS, negative controls (N1 and N2) were resuspended in 50 µL TUNEL labelling solution without the enzyme whereas positive controls (P1 and P2) and all test samples were resuspended in 50 µL TUNEL reaction mixture containing enzyme. All samples were then incubated for 90 min at 37°C before washing twice in PBS. Thereafter, U2, N2, P2 and all test samples were incubated with 5 µg mL−1 of the nucleic acid stain 4′,6′-diamidino-2-phenylindole (DAPI) in PBS for 20 min at room temperature to ensure that only nucleated TUNEL-positive spermatozoa were accounted for as DNA-damaged cells during analysis by fluorescence activated cell sorting (FACS). The specificity of sperm staining was further validated using fluorescent microscopy, which showed FITC–DAPI-positive DNA-damaged spermatozoa in green alongside DAPI-positive intact nucleated boar spermatozoa in blue (Fig. 1h).
Flow cytometry analysis
All samples were washed twice and resuspended in 2 mM EDTA in PBS and evaluated using a CyanADP flow cytometer (serial number 389; manufactured July 2005; Dako Cytomation). The instrument was not altered and consisted of a fixed-alignment quartz cuvette flow cell and used the following light source and filter combinations: (i) 488 nm Coherent Sapphire solid state; 20 mW for detector FL1 with 95/5 mirror, 530/40 nm filter for FITC and (ii) 405 nm Coherent semiconductor; 50 mW for detector FL6 with 450/50 nm filter for DAPI. Detector voltages were set to: FSC = 220 V, SSC = 330 V, FL1 = 500 V and FL6 = 500 V. Samples were first passed through a 60 µm nylon woven net filter before being loaded onto the machine in 5 mL round-bottom polystyrene tubes. Spermatozoa were identified by their forward and side scatter profiles using a scatter-area versus scatter-height gate previously calibrated specifically for boar spermatozoa. Data were analysed using Summit 4.3 software (Dako Cytomation). The flow cytometer was set to analyse 20 000 cells per sample at ~150 events s−1. Prior to evaluating test samples, control samples were used to accurately define the different cell staining populations delineated into four distinct quadrants by adjusting both vertical and horizontal thresholds: (i) R3, FITC-positive cells only; (ii) R4, both FITC- and DAPI-positive cells; (iii) R5, unstained cells and (iv) R6, DAPI-positive cells only (Fig. 1a–f). Sample N2 (negative control in label solution with DAPI) was used to set a 0.5% threshold cut-off before running all test samples. Cells in R4 were designated as nucleated DNA-damaged spermatozoa, expressed as a percentage of the total number of cells analysed within the gated area (Fig. 1g).
Data presentation and statistical analyses
The Shapiro–Wilk test was used to evaluate normality of the data, while Mauchly’s test of sphericity was used to determine if variances of the difference scores between each within-subject variable were equal. If these assumptions were not met, a log10 transformation of the data (sperm DNA damage, daily mean temperatures, relative humidity) was performed before ANOVA or either the Greenhouse–Geisser or Huynd–Feldt correction was used to interpret significant difference. Given the same boars were used across the three sample time points, data were analysed by single-group or one-way repeated-measures ANOVA to reduce random variance and improve statistical sensitivity, along with pairwise comparisons based on marginal means with Bonferroni adjustments applied (IBM SPSS Version 22; IBM Corporation). Graphs were plotted using Microsoft Excel 2016. P ≤ 0.05 was considered to be statistically significant.
Results
Daily mean, mean minimum and mean maximum temperatures spanning the 42-day period immediately before semen was collected at each time point are shown in Table 1. The peak wet season was significantly hotter for all three temperature measures than early and late dry seasons (P ≤ 0.05; Table 1). Similarly, the daily mean relative humidity spanning the 42-day period immediately before semen collection differed across seasons, with the peak wet season being more humid than early or late dry season (P ≤ 0.05; Table 1). It was typically more humid at 6 a.m. during the coolest part of the day, than at 3 p.m., which was the hottest for all seasons. In this regard, the peak wet season had more humid mornings than the late dry and more humid afternoons than the early dry season (P ≤ 0.05; Table 1). Moreover, the temperature–humidity index was also highest during the peak wet season for all three mean measures than early or late dry seasons (P ≤ 0.05; Table 1).

|
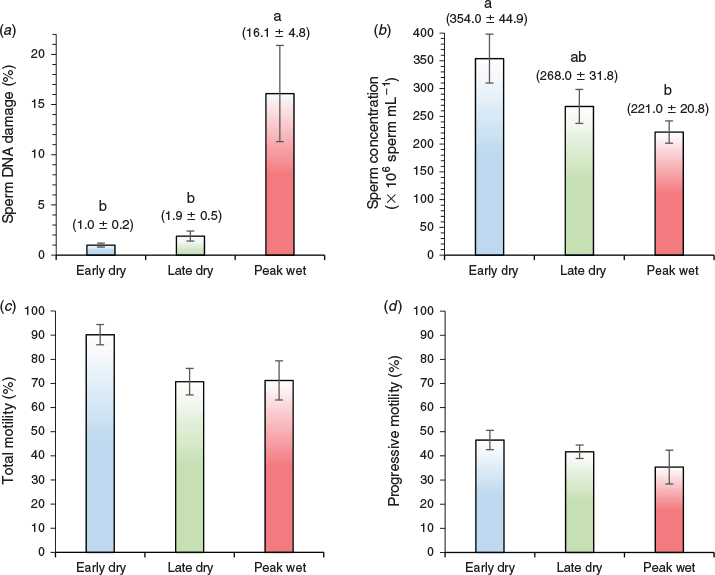
Strikingly, spermatozoa collected during the peak wet season had more than 16-fold higher DNA damage than in the early dry and nearly 9-fold higher DNA damage than in the late dry season (P ≤ 0.05; Fig. 2a). Moreover, semen collected in the peak wet season had significantly lower sperm concentration than early dry but did not differ from the late dry season (P ≤ 0.05; Fig. 2b). Both total and progressive motility of spermatozoa collected in the peak wet season did not differ to that in early or late dry seasons (P > 0.05; Figs 2c and 2d respectively).
Detailed sperm motility and head shape characteristics determined by CASA are shown in Table 2. There was no significant difference observed between seasons for any CASA parameter (P > 0.05). While spermatozoa collected in the early dry appeared to have better curvilinear, straight line and average path velocities (P = 0.08, 0.09 and 0.09 respectively), these were not statistically different from values obtained during the peak wet or late dry seasons (Table 2).

|
Discussion
Heat stress has been widely shown to impede proper growth and reproductive function in domestic animals. Moreover, the negative effect that sperm DNA damage can have on male fertility has been extensively studied in many species, including humans. However, to our knowledge, this is the first study that significantly demonstrates the critical link between ambient environmental heat stress and sperm DNA damage in a domestic production animal (Pacey 2010). Interestingly, our results show that the peak wet tropical summer season found in Townsville, North Queensland, Australia, induces DNA damage and reduces concentration of boar spermatozoa without depressing its motility. This suggests that traditional methods to evaluate sperm motility may not detect inherently compromised spermatozoa, which has important implications for the management and evaluation of seasonal infertility in boars during periods of heat stress.
Predicting overall sperm quality using conventional established laboratory guidelines for semen analyses (i.e. sperm motility, morphology, viability, concentration, etc.) has proven to be controversial or insufficient in determining fertility outcomes in both animals and humans (Love and Kenney 1998; Carrell et al. 2003; García-Macías et al. 2007). Semen known to be normal may in fact carry a subpopulation of DNA-damaged spermatozoa (Dobrinski et al. 1994; Kishikawa et al. 1999). Moreover, DNA-damaged spermatozoa may actually swim and fertilise an oocyte normally (Ahmadi and Ng 1999; Simon and Lewis 2011). However, nuclear damage to spermatozoa can negatively impact breeding efficiency (Evenson 1999) along with early embryonic loss, interrupted embryo development, genetic abnormalities in the offspring and lower pregnancy rates (Sailer et al. 1997; Henkel et al. 2004; Paul et al. 2008; Simon and Lewis 2011). It is likely that the true impact of sperm DNA fragmentation would only manifest as arrested embryo development from the 4-cell stage onward; a period corresponding to genome activation in the pig (Oestrup et al. 2009; Deshmukh et al. 2011). Of concern is the fact that the high rates of sperm DNA fragmentation observed during the peak wet season in our study (without noticeable effect on sperm motility) may currently go undetected by pig industries in tropical and subtropical climates. Moreover, they could significantly contribute to the high rates of embryo loss and pregnancy failure observed in sows during summer infertility (Peña et al. 2017).
Didion et al. (2009) reported that a sperm sample with greater than 6% DNA fragmentation could result in decreased farrowing rates and average number of piglets born. In another study, 0.5 to 0.9 fewer piglets were born per litter when sperm DNA fragmentation was above 2.1% (Boe-Hansen et al. 2008). In humans, 30.3% appears to be the threshold to discriminate between fertile and infertile men (Venkatesh et al. 2011). A similar threshold was reported by Brahem et al. (2011) in men with a history of recurrent pregnancy loss, but with the fertile group showing much lower damage (~10%). From a conservative perspective, the overall threshold appears to be ~8% in boars, 10–20% in bulls and 30% in humans (Rybar et al. 2004; Evenson and Wixon 2006). However, utmost care should be taken when comparing levels of DNA fragmentation determined by the sperm chromatin structure assay (SCSA) and TUNEL assay (Evenson 2016). While pioneering authors are convinced that both assays are correlated in terms of detecting and measuring the same existing DNA strand breaks (Gorczyca et al. 1993), the two techniques differ fundamentally in that TUNEL detects ‘real’ DNA damage and SCSA detects abnormal chromatin structure and ‘potential’ DNA damage that depends on the susceptibility of DNA to denaturation (Henkel et al. 2010). If the level of DNA damage observed in boar spermatozoa in our study represents ‘real’ DNA damage at over 16%, it is highly likely that pregnancy rates and litter sizes in sows fertilised by such spermatozoa will be significantly reduced. Collectively, however, these studies suggest that sperm DNA fragmentation could be a valuable prognostic tool to predict final fertility outcomes in pigs (Simon et al. 2013; Roca et al. 2015).
In one study, Tsakmakidis et al. (2010) found that live morphologically normal spermatozoa and intact sperm DNA in boars accounted for 62.2% and 81.7% respectively of the variability in farrowing rates following artificial insemination. However, such findings appear to present limited value to indicate subfertility in fresh or stored semen from normospermic boars (Waberski et al. 2011). High standards of screening and maintaining boars used in large scale commercial artificial insemination centres may preclude less fertile boars, since up to 95.5% of semen samples collected from Pietrain boars used in such centres have <5% sperm DNA fragmentation (Waberski et al. 2011). Nevertheless, early detection of boars with consistently low sperm DNA damage and good fertilising capacity could prove economically beneficial, especially in overcoming individual variations in boar fertility (Roca et al. 2015). Our boars were pre-screened based on classical semen quality parameters before they qualified for the study, but were not tested for fertility by artificial insemination (AI) or natural mating. Such a scenario is likely to reflect current practices in boar selection in small-to-medium farms in developing countries of the tropics. Moreover, the 16-fold increase in DNA fragmentation observed in our study from 1% in the early dry to over 16% in the peak wet season suggests that even carefully selected commercial AI boars may be prone to considerable sperm DNA damage and reduced fertility if exposed to elevated temperatures. This can be aggravated by the fact that the activity of free radical scavengers such as glutathione peroxidase does not appear to increase in spermatozoa during summer (Argenti et al. 2018).
The same five boars were experimentally subjected to identical environmental, husbandry and sample collection conditions at each time point of our study, in order to demonstrate that seasonal effects were not caused by using different batches of boars. Chronologically these boars first exhibited 2% sperm DNA damage in October 2014 (late dry), then 16% damage in February 2015 (peak wet), followed by 1% damage in May 2015 (early dry season). Thus, DNA damage in our boars is clearly not cumulative, but caused by a cyclical rise in damage during hotter and subsequent recovery during cooler times of the year. Damage is most likely induced during spermatogenesis since it is manifest in all spermatogenic populations during heat stress (Paul et al. 2008). Compared with other species, the DNA of mature boar spermatozoa is highly stable with 10 cysteine groups bound to each protamine molecule (Gosálvez et al. 2011, suggesting that heat stress must interfere during DNA–protamine complexing. Our boars ejaculated regularly before each experimental sample collection and all ejaculates analysed were Percoll purified to avoid measuring possible DNA damage in senescent or degenerating spermatozoa associated with prolonged storage in the epididymis due to sexual rest or pathological anejaculation (Qiu et al. 2012; Serafini et al. 2016). Furthermore, given that the TUNEL assay directly detects ‘real’ DNA damage (Henkel et al. 2010) and we examined at least 20 000 spermatozoa per boar per time point by FACS, the 16-fold increase in sperm DNA damage induced by tropical summer in our study should be regarded as conservative at the very least.
Heat has previously been shown to induce sperm DNA fragmentation in mice. Immersion of the scrotum in 40–42°C water for 30 min causes DNA damage to spermatogonia, spermatocytes, spermatids and spermatozoa, resulting in a disruption to blastocyst formation, implantation failure, pregnancy loss and a distortion in sex-ratio of offspring born (Paul et al. 2008; Pérez-Crespo et al. 2008). In addition to disrupted DNA–protamine complexing mentioned above, the underlying mechanism by which heat causes sperm DNA fragmentation may be attributed to several putative factors. For example, it has been observed that heat stress causes apoptosis and testicular germ cell loss, abnormal expression of several DNA repair genes such as 8-Oxoguanine glycosylase (Ogg1), xeroderma pigmentosum complementation group G (Xpg) and DNA repair and recombination protein (Rad54) as well as reduction in the expression of oxidative stress-induced antioxidants (Rockett et al. 2001; Parrish et al. 2017). Moreover, polyADP ribose polymerase that helps in detection and signalling of DNA strand breaks may also be reduced (Tramontano et al. 2000). Heat stress induced by scrotal immersion in 42°C water for 20 min also causes dissociation in X-Y chromosomes of mice and rats, leading to chromosomally unbalanced gametes, even in heat-adapted animals (van Zelst et al. 1995). We postulate that the above mechanisms may play a significant role in inducing DNA damage in boar spermatozoa during periods of heat stress (Peña et al. 2017).
Mean maximum (33.4 ± 0.2°C) daily temperatures observed during the peak wet in Townsville appear to exceed the 29°C threshold identified by Stone (1982) as the upper critical air temperature at which Large White boars are able to produce normal numbers of spermatozoa. Moreover, even a daily mean temperature of 29.2 ± 0.2°C combined with a daily mean relative humidity of 71.4 ± 1.2 during the peak wet season results in a temperature–humidity index of 92.9 ± 1.1 – at ‘extreme caution’ zone of the heat index chart (National Weather Service 2016b) or between ‘danger’ and ‘emergency’ zones for grower–finisher pigs (Hahn et al. 2009). By comparison, the daily mean THI for the early dry (75.9 ± 0.9) and late dry (73.6 ± 0.4) seasons fall safely outside the ‘alert’, let alone the ‘danger’ zone. Consistent with this, our results show that the concentration of boar spermatozoa decreased significantly in the peak wet season compared with early dry, but was similar to late dry. This is further supported by previous studies (Egbunike and Dede 1980; Sarlós et al. 2011) that showed reduced concentration and total number of boar spermatozoa during the summer–spring period (Zasiadczyk et al. 2015). Collectively, these studies suggest that seasonal heat stress causes disrupted spermatogenesis. Sperm concentration is an important aspect in pig production particularly with artificial insemination operations. Highly concentrated semen of sufficient volume can be economically beneficial as it can be extended into a large number of commercial doses to inseminate many females. Sperm concentration declined by only 1.6-fold in our study. However, compared with the 16-fold increase in sperm DNA damage, it is not clear whether such a reduction in sperm concentration, if left uncompensated, would have a major impact on litter size in sows.
Evaluation of sperm motility by CASA permits the identification of ejaculates that are below optimal standards set by the boar stud, which could otherwise result in lower fertility outcomes in commercial farm production (Holt et al. 1997; Gadea et al. 2004; Vyt et al. 2008). An extensive comparison of insemination records with semen parameters from 45 532 boar ejaculates over a 3-year period revealed that progressive motility, curvilinear velocity and beat cross frequency highly influenced farrowing rate, whereas total motility, average path velocity, straight line velocity and amplitude of lateral head displacement correlated with the total number of piglets born (Broekhuijse et al. 2012a). Other factors that affect overall fertility include boar-related sources of variation (direct boar effect) such as genetic line, technician and AI centre, age of the boar and days between ejaculation (Broekhuijse et al. 2012b). Accordingly, sperm motion characteristics obtained from CASA accounted for 9% of the boar and semen-related variation in farrowing rate and 10% for total number of piglets born (Broekhuijse et al. 2012a). Nevertheless, when viewed on an individual level, the predictive value of motility parameters on conception and farrowing rates was not found to be significant and only became obvious when associated with other parameters (Vyt et al. 2008). Given that sperm DNA integrity was found to account for nearly 82% of the variation in farrowing rates after artificial insemination in one study (Tsakmakidis et al. 2010), it would seem that motility parameters in selected highly fertile boars may have a relatively minimal influence on downstream fertility compared with DNA damage. At the very least, this suggests greater attention be placed on the evaluation of DNA integrity of boar spermatozoa, something that the industry is yet to widely adopt.
Heat stress has been reported to significantly decrease sperm motility (McNitt and First 1970; Wettemann et al. 1979; Heitman et al. 1984; Barranco et al. 2013). However, mean total motility across seasons among our boars was greater than 70%, the cut-off point for sperm motility used in artificial insemination (Holt et al. 1997; Eriksson and Rodriguez-Martinez 2000). Moreover, the motility of spermatozoa collected in the peak wet season did not differ to early or late dry seasons across all CASA parameters we evaluated, despite a 16-fold increase in DNA damage. The difference in results may reflect the use of subjective estimates of sperm motility particularly in early studies (±10%), compared with more precise quantitative measures using CASA in our study. Moreover, the margin reported by Barranco et al. (2013) differed by only 3% total motility between summer and winter collections. Media containing >5 mM bicarbonate have been used to rapidly activate motility in sperm subpopulations, as a more sensitive method to detect differences in fertility between individual boars (Satake et al. 2006). Our study measured motility in BTS medium already containing 15 mM sodium bicarbonate, without detecting noticeable differences between treatment groups. On this basis, we postulate that even objective measures of sperm motility as determined by CASA may not detect DNA-compromised spermatozoa; a view supported by observations that DNA-damaged spermatozoa may actually swim and fertilise oocytes normally (Ahmadi and Ng 1999). As such, evaluation of sperm DNA fragmentation may provide greater insight into potential contributing factors causing poor reproductive performance in the sow during summer infertility (Sutovsky 2015). While evaporative cooling, air conditioning, dripping and fogging systems have been slowly introduced to tropical pig farms in South-East Asia, they are not yet commonplace, especially among older facilities that require extensive investment and redesign (Tantasuparuk and Kunavongkrit 2014). Finding economically affordable strategies to mitigate the effects of heat stress in these countries is a big challenge considering that 70% of pig farms in The Philippines, Vietnam, Laos and Cambodia are small-scale with very limited capital and labour resources (Huynh et al. 2007).
In conclusion, summer heat stress significantly increases sperm DNA damage in boars housed in tropical environments and causes a significant decline in sperm concentration. Sperm motility does not appear to be affected by season and, as such, measurement of this parameter alone may mask inherent deficiencies found in DNA-damaged boar spermatozoa. Evaluation of sperm DNA integrity could provide an important diagnostic tool to further discriminate spermatozoa of low and high quality during summer.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank J. Penny, N. Breen, R. Jack, S. Blyth, V. Simpson, A. Blyth, J. Palpratt and I. Benu for assistance with the boars; R. Jose and L. Woodward for training and access to FACS facilities; Glenley Piggery for animals and Roche for TUNEL assays. The project was funded by a JCU Development Grant to D. P. and by College of Public Health, Medical and Veterinary Sciences PhD Research Funds to S. P., who is also supported by the Australia Awards Scholarship.
References
Ahmadi, A., and Ng, S. C. (1999). Fertilizing ability of DNA-damaged spermatozoa. J. Exp. Zool. 284, 696–704.| Fertilizing ability of DNA-damaged spermatozoa.Crossref | GoogleScholarGoogle Scholar |
Alexandratos, N., and Bruinsma, J. (2012) ‘World Agriculture Towards 2030/2050: The 2012 Revision’. (Food and Agriculture Organization of the United Nations: Rome.)
Alkmin, D. V., Martinez-Alborcia, M. J., Parrilla, I., Vazquez, J. M., Martinez, E. A., and Roca, J. (2013). The nuclear DNA longevity in cryopreserved boar spermatozoa assessed using the sperm-sus-halomax. Theriogenology 79, 1294–1300.
| The nuclear DNA longevity in cryopreserved boar spermatozoa assessed using the sperm-sus-halomax.Crossref | GoogleScholarGoogle Scholar |
Argenti, L. E., Parmeggiani, B. S., Leipnitz, G., Weber, A., Pereira, G. R., and Bustamante-Filho, I. C. (2018). Effects of season on boar semen parameters and antioxidant enzymes in the south subtropical region in Brazil. Andrologia , .
| Effects of season on boar semen parameters and antioxidant enzymes in the south subtropical region in Brazil.Crossref | GoogleScholarGoogle Scholar |
Auvigne, V., Leneveu, P., Jehannin, C., Peltoniemi, O., and Sallé, E. (2010). Seasonal infertility in sows: A five year field study to analyze the relative roles of heat stress and photoperiod. Theriogenology 74, 60–66.
| Seasonal infertility in sows: A five year field study to analyze the relative roles of heat stress and photoperiod.Crossref | GoogleScholarGoogle Scholar |
Barranco, I., Ortega, M. D., Martinez-Alborcia, M. J., Vazquez, J. M., Martinez, E. A., and Roca, J. (2013). Season of ejaculate collection influences the freezability of boar spermatozoa. Cryobiology 67, 299–304.
| Season of ejaculate collection influences the freezability of boar spermatozoa.Crossref | GoogleScholarGoogle Scholar |
Boe-Hansen, G. B., Ersbøll, A. K., Greve, T., and Christensen, P. (2005). Increasing storage time of extended boar semen reduces sperm DNA integrity. Theriogenology 63, 2006–2019.
| Increasing storage time of extended boar semen reduces sperm DNA integrity.Crossref | GoogleScholarGoogle Scholar |
Boe-Hansen, G. B., Christensen, P., Vibjerg, D., Nielsen, M. B., and Hedeboe, A. M. (2008). Sperm chromatin structure integrity in liquid stored boar semen and its relationships with field fertility. Theriogenology 69, 728–736.
| Sperm chromatin structure integrity in liquid stored boar semen and its relationships with field fertility.Crossref | GoogleScholarGoogle Scholar |
Boma, M. H., and Bilkei, G. (2006). Seasonal infertility in Kenyan pig breeding units. Onderstepoort J. Vet. Res. 73, 229–232.
| Seasonal infertility in Kenyan pig breeding units.Crossref | GoogleScholarGoogle Scholar |
Brahem, S., Mehdi, M., Landolsi, H., Mougou, S., Elghezal, H., and Saad, A. (2011). Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss. Urology 78, 792–796.
| Semen parameters and sperm DNA fragmentation as causes of recurrent pregnancy loss.Crossref | GoogleScholarGoogle Scholar |
Broekhuijse, M. L. W. J., Šoštarić, E., Feitsma, H., and Gadella, B. M. (2012a). Application of computer-assisted semen analysis to explain variations in pig fertility. J. Anim. Sci. 90, 779–789.
| Application of computer-assisted semen analysis to explain variations in pig fertility.Crossref | GoogleScholarGoogle Scholar |
Broekhuijse, M. L. W. J., Šoštarić, E., Feitsma, H., and Gadella, B. M. (2012b). The value of microscopic semen motility assessment at collection for a commercial artificial insemination center, a retrospective study on factors explaining variation in pig fertility. Theriogenology 77, 1466–1479.e3.
| The value of microscopic semen motility assessment at collection for a commercial artificial insemination center, a retrospective study on factors explaining variation in pig fertility.Crossref | GoogleScholarGoogle Scholar |
Brzezińska-Ślebodzińska, E., Ślebodziński, A., Pietras, B., and Wieczorek, G. (1995). Antioxidant effect of vitamin E and glutathione on lipid peroxidation in boar semen plasma. Biol. Trace Elem. Res. 47, 69–74.
| Antioxidant effect of vitamin E and glutathione on lipid peroxidation in boar semen plasma.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2011a) Climate of Townsville, http://www.bom.gov.au/qld/townsville/climate_Townsville.shtml [Verified 12 October 2018].
Bureau of Meteorology (2011b) About air temperature data, http://www.bom.gov.au/climate/cdo/about/about-airtemp-data.shtml [Verified 12 October 2018].
Cameron, R. D., and Blackshaw, A. W. (1980). The effect of elevated ambient temperature on spermatogenesis in the boar. J. Reprod. Fertil. 59, 173–179.
| The effect of elevated ambient temperature on spermatogenesis in the boar.Crossref | GoogleScholarGoogle Scholar |
Carrell, D. T., Liu, L., Peterson, C. M., Jones, K. P., Hatasaka, H. H., Erickson, L., and Campbell, B. (2003). Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Arch. Androl. 49, 49–55.
| Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss.Crossref | GoogleScholarGoogle Scholar |
Cerolini, S., Maldjian, A., Pizzi, F., and Gliozzi, T. (2001). Changes in sperm quality and lipid composition during cryopreservation of boar semen. Reproduction 121, 395–401.
| Changes in sperm quality and lipid composition during cryopreservation of boar semen.Crossref | GoogleScholarGoogle Scholar |
Deshmukh, R. S., Østrup, O., Østrup, E., Vejlsted, M., Niemann, H., Lucas-Hahn, A., Petersen, B., Li, J., Callesen, H., and Hyttel, P. (2011). DNA methylation in porcine preimplantation embryos developed in vivo and produced by in vitro fertilization, parthenogenetic activation and somatic cell nuclear transfer. Epigenetics 6, 177–187.
| DNA methylation in porcine preimplantation embryos developed in vivo and produced by in vitro fertilization, parthenogenetic activation and somatic cell nuclear transfer.Crossref | GoogleScholarGoogle Scholar |
Didion, B. A., Kasperson, K. M., Wixon, R. L., and Evenson, D. P. (2009). Boar fertility and sperm chromatin structure status: a retrospective report. J. Androl. 30, 655–660.
| Boar fertility and sperm chromatin structure status: a retrospective report.Crossref | GoogleScholarGoogle Scholar |
Dobrinski, I., Hughes, H. P., and Barth, A. D. (1994). Flow cytometric and microscopic evaluation and effect on fertility of abnormal chromatin condensation in bovine sperm nuclei. J. Reprod. Fertil. 101, 531–538.
| Flow cytometric and microscopic evaluation and effect on fertility of abnormal chromatin condensation in bovine sperm nuclei.Crossref | GoogleScholarGoogle Scholar |
Dubé, C., Beaulieu, M., Reyes-Moreno, C., Guillemette, C., and Bailey, J. L. (2004). Boar sperm storage capacity of BTS and Androhep Plus: viability, motility, capacitation, and tyrosine phosphorylation. Theriogenology 62, 874–886.
| Boar sperm storage capacity of BTS and Androhep Plus: viability, motility, capacitation, and tyrosine phosphorylation.Crossref | GoogleScholarGoogle Scholar |
Egbunike, G. N., and Dede, T. I. (1980). The influence of short-term exposure to tropical sunlight on boar seminal characteristics. Int. J. Biometeorol. 24, 129–135.
| The influence of short-term exposure to tropical sunlight on boar seminal characteristics.Crossref | GoogleScholarGoogle Scholar |
Einarsson, S., Brandt, Y., Lundeheim, N., and Madej, A. (2008). Stress and its influence on reproduction in pigs: a review. Acta Vet. Scand. 50, 48.
| Stress and its influence on reproduction in pigs: a review.Crossref | GoogleScholarGoogle Scholar |
Eriksson, B. M., and Rodriguez-Martinez, H. (2000). Effect of freezing and thawing rates on the post-thaw viability of boar spermatozoa frozen in flatpacks and maxi-straws. Anim. Reprod. Sci. 63, 205–220.
| Effect of freezing and thawing rates on the post-thaw viability of boar spermatozoa frozen in flatpacks and maxi-straws.Crossref | GoogleScholarGoogle Scholar |
Evenson, D. P. (1999). Loss of livestock breeding efficiency due to uncompensable sperm nuclear defects. Reprod. Fertil. Dev. 11, 1–15.
| Loss of livestock breeding efficiency due to uncompensable sperm nuclear defects.Crossref | GoogleScholarGoogle Scholar |
Evenson, D. P. (2016). The sperm chromatin structure assay (scsa®) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility. Anim. Reprod. Sci. 169, 56–75.
| The sperm chromatin structure assay (scsa®) and other sperm DNA fragmentation tests for evaluation of sperm nuclear DNA integrity as related to fertility.Crossref | GoogleScholarGoogle Scholar |
Evenson, D. P., and Wixon, R. (2006). Clinical aspects of sperm DNA fragmentation detection and male infertility. Theriogenology 65, 979–991.
| Clinical aspects of sperm DNA fragmentation detection and male infertility.Crossref | GoogleScholarGoogle Scholar |
Evenson, D. P., Kasperson, K., Wixon, R. L., and Didion, B. A. (2009). Boar fertility and sperm chromatin structure assay defined sperm DNA fragmentation. Reprod. Fertil. Dev. 21, 212.
| Boar fertility and sperm chromatin structure assay defined sperm DNA fragmentation.Crossref | GoogleScholarGoogle Scholar |
Fernandes, C. E., Dode, M. A., Pereira, D., and Silva, A. E. (2008). Effects of scrotal insulation in Nellore bulls (Bos taurus indicus) on seminal quality and its relationship with in vitro fertilizing ability. Theriogenology 70, 1560–1568.
| Effects of scrotal insulation in Nellore bulls (Bos taurus indicus) on seminal quality and its relationship with in vitro fertilizing ability.Crossref | GoogleScholarGoogle Scholar |
Flowers, W. L. (1997). Management of boars for efficient semen production. J. Reprod. Fertil. Suppl. 52, 67–78.
França, L. R., and Cardoso, F. M. (1998). Duration of spermatogenesis and sperm transit time through the epididymis in the Piau boar. Tissue Cell 30, 573–582.
| Duration of spermatogenesis and sperm transit time through the epididymis in the Piau boar.Crossref | GoogleScholarGoogle Scholar |
França, L. R., Avelar, G. F., and Almeida, F. F. L. (2005). Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs. Theriogenology 63, 300–318.
| Spermatogenesis and sperm transit through the epididymis in mammals with emphasis on pigs.Crossref | GoogleScholarGoogle Scholar |
Gadea, J., Selles, E., and Marco, M. A. (2004). The predictive value of porcine seminal parameters on fertility outcome under commercial conditions. Reprod. Domest. Anim. 39, 303–308.
| The predictive value of porcine seminal parameters on fertility outcome under commercial conditions.Crossref | GoogleScholarGoogle Scholar |
García-Macías, V., de Paz, P., Martinez-Pastor, F., Álvarez, M., Gomes-Alves, S., Bernardo, J., Anel, E., and Anel, L. (2007). DNA fragmentation assessment by flow cytometry and sperm-bos-halomax (bright-field microscopy and fluorescence microscopy) in bull sperm. Int. J. Androl. 30, 88–98.
| DNA fragmentation assessment by flow cytometry and sperm-bos-halomax (bright-field microscopy and fluorescence microscopy) in bull sperm.Crossref | GoogleScholarGoogle Scholar |
Gorczyca, W., Traganos, F., Jesionowska, H., and Darzynkiewicz, Z. (1993). Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells: analogy to apoptosis of somatic cells. Exp. Cell Res. 207, 202–205.
| Presence of DNA strand breaks and increased sensitivity of DNA in situ to denaturation in abnormal human sperm cells: analogy to apoptosis of somatic cells.Crossref | GoogleScholarGoogle Scholar |
Gosálvez, J., López‐Fernández, C., Fernández, J. L., Gouraud, A., and Holt, W. V. (2011). Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species. Mol. Reprod. Dev. 78, 951–961.
| Relationships between the dynamics of iatrogenic DNA damage and genomic design in mammalian spermatozoa from eleven species.Crossref | GoogleScholarGoogle Scholar |
Grant, S. A., Long, S. E., and Parkinson, T. J. (1994). Fertilizability and structural properties of boar spermatozoa prepared by Percoll gradient centrifugation. J. Reprod. Fertil. 100, 477–483.
| Fertilizability and structural properties of boar spermatozoa prepared by Percoll gradient centrifugation.Crossref | GoogleScholarGoogle Scholar |
Hahn, G. L., Gaughan, J. B., Mader, T. L., and Eigenberg, R. A. (2009) Thermal indices and their applications for livestock environments. In ‘Livestock Energetics and Thermal Environment Management’. (Ed James A. DeShazer.) pp. 113–130. (American Society of Agricultural and Biological Engineers: St. Joseph, MI.)
Hancock, J., and Hovell, G. (1959). The collection of boar semen. Vet. Rec. 71, e665.
Heitman, H., Cockrell, J. R., and Morrison, S. R. (1984). Cycling ambient temperature effect on boar semen. Anim. Sci. 38, 129–132.
Henkel, R., Hajimohammad, M., Stalf, T., Hoogendijk, C., Mehnert, C., Menkveld, R., Gips, H., Schill, W. B., and Kruger, T. F. (2004). Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertil. Steril. 81, 965–972.
| Influence of deoxyribonucleic acid damage on fertilization and pregnancy.Crossref | GoogleScholarGoogle Scholar |
Henkel, R., Hoogendijk, C. F., Bouic, P. J., and Kruger, T. F. (2010). TUNEL assay and SCSA determine different aspects of sperm DNA damage. Andrologia 42, 305–313.
| TUNEL assay and SCSA determine different aspects of sperm DNA damage.Crossref | GoogleScholarGoogle Scholar |
Hennessy, D. P., and Williamson, P. E. (1984). Stress and summer infertility in pigs. Aust. Vet. J. 61, 212–215.
| Stress and summer infertility in pigs.Crossref | GoogleScholarGoogle Scholar |
Holt, C., Holt, W. V., Moore, H. D., Reed, H. C., and Curnock, R. M. (1997). Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: results of two fertility trials. J. Androl. 18, 312–323.
Hughes, P., and van Wettere, W. (2010) ‘Seasonal Infertility in Pigs’. (Pork Cooperative Research Centre Ltd.: Willaston, SA, Australia.) Available at: http://www.porkcrc.com.au/101217_SI.pdf [Verified 12 October 2018].
Huynh, T., Aarnink, A., Drucker, A., and Verstegen, M. (2007). Pig production in Cambodia, Laos, Philippines, and Vietnam: a review. Asian J. Agric. Dev. 4, 69–90.
Ingram, D. L. (1965). Evaporative cooling in the pig. Nature 207, 415–416.
| Evaporative cooling in the pig.Crossref | GoogleScholarGoogle Scholar |
Kilkenny, C., Browne, W. J., Cuthill, I. C., Emerson, M., and Altman, D. G. (2010). Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 8, e1000412.
| Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research.Crossref | GoogleScholarGoogle Scholar |
Kishikawa, H., Tateno, H., and Yanagimachi, R. (1999). Chromosome analysis of balb/c mouse spermatozoa with normal and abnormal head morphology. Biol. Reprod. 61, 809–812.
| Chromosome analysis of balb/c mouse spermatozoa with normal and abnormal head morphology.Crossref | GoogleScholarGoogle Scholar |
Knox, R. 2003. ‘The Anatomy and Physiology of Sperm Production in Boars’. (Department of Animal Sciences, University of Illinois: Urbana, IL.) Available at http://www.ansci.wisc.edu/jjp1/pig_case/html/library/boara&p.pdf [Verified 12 October 2018].
Love, R. J. (1981). Seasonal infertility in pigs. Vet. Rec. 109, 407–409.
| Seasonal infertility in pigs.Crossref | GoogleScholarGoogle Scholar |
Love, C. C., and Kenney, R. M. (1998). The relationship of increased susceptibility of sperm DNA to denaturation and fertility in the stallion. Theriogenology 50, 955–972.
| The relationship of increased susceptibility of sperm DNA to denaturation and fertility in the stallion.Crossref | GoogleScholarGoogle Scholar |
McNitt, J. I., and First, N. L. (1970). Effects of 72-hour heat stress on semen quality in boars. Int. J. Biometeorol. 14, 373–380.
| Effects of 72-hour heat stress on semen quality in boars.Crossref | GoogleScholarGoogle Scholar |
Mortimer, S. T. (2000). CASA-practical aspects. J. Androl. 21, 515–524.
Mount, L. E. (1968). The climatic physiology of the pig. In ‘Monographs. Physiological Society’. (Ed. E. Arnold.) pp. 1–271. (University of Michigan: Ann Arbor, MI.)
National Pork Board (2014) ‘Pork Quick Facts 2014’. (Pork Check Off, National Pork Board: Des Moines, IA.) Available at http://www.pork.org/pork-quick-facts/ [Verified 12 October 2018].
National Pork Board (2017) ‘World Per Capita Pork Consumption’. (Pork Check Off, National Pork Board: Des Moines, IA.) Available at http://www.pork.org/pork-quick-facts/home/stats/u-s-pork-exports/world-per-capita-pork-consumption-2/ [Verified 12 October 2018].
National Weather Service (NWS) (2016a) ‘Meteorological Conversions and Calculations. Heat Index Calculator’. Available at http://www.wpc.ncep.noaa.gov/html/heatindex.shtml [Verified 12 October 2018].
National Weather Service (NWS) (2016b) ‘Heat Index Chart’. Available at https://www.weather.gov/ffc/hichart [Verified 12 October 2018].
Oestrup, O., Hall, V., Petkov, S. G., Wolf, X. A., Hyldig, S., and Hyttel, P. (2009). From zygote to implantation: morphological and molecular dynamics during embryo development in the pig. Reprod. Domest. Anim. 44, 39–49.
| From zygote to implantation: morphological and molecular dynamics during embryo development in the pig.Crossref | GoogleScholarGoogle Scholar |
O’Leary, S. (2010) ‘Final Report to Pork CRC’. (Pork Cooperative Research Centre Ltd.: Willaston, SA, Australia.)
Pacey, A. A. (2010). Environmental and lifestyle factors associated with sperm DNA damage. Hum. Fertil. 13, 189–193.
| Environmental and lifestyle factors associated with sperm DNA damage.Crossref | GoogleScholarGoogle Scholar |
Parrish, J. J., Willenburg, K. L., Gibbs, K. M., Yagoda, K. B., Krautkramer, M. M., Loether, T. M., and Melo, F. C. S. A. (2017). Scrotal insulation and sperm production in the boar. Mol. Reprod. Dev. 84, 969–978.
| Scrotal insulation and sperm production in the boar.Crossref | GoogleScholarGoogle Scholar |
Paterson, A., Barker, I., and Lindsay, D. (1978). Summer infertility in pigs: its incidence and characteristics in an Australian commercial piggery. Aust. J. Exp. Agric. 18, 698–701.
| Summer infertility in pigs: its incidence and characteristics in an Australian commercial piggery.Crossref | GoogleScholarGoogle Scholar |
Paul, C., Murray, A. A., Spears, N., and Saunders, P. T. (2008). A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction 136, 73–84.
| A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice.Crossref | GoogleScholarGoogle Scholar |
Peña, S., Summers, P., Gummow, B., and Paris, D. B. B. P. (2015). Oviduct binding ability of porcine spermatozoa develops in the epididymis and can be advanced by incubation with caudal fluid. Theriogenology 83, 1502–1513.
| Oviduct binding ability of porcine spermatozoa develops in the epididymis and can be advanced by incubation with caudal fluid.Crossref | GoogleScholarGoogle Scholar |
Peña, S. T., Gummow, B., Parker, A. J., and Paris, D. B. (2017). Revisiting summer infertility in the pig: could heat stress-induced sperm DNA damage negatively affect early embryo development? Anim. Prod. Sci. 57, 1975–1983.
| Revisiting summer infertility in the pig: could heat stress-induced sperm DNA damage negatively affect early embryo development?Crossref | GoogleScholarGoogle Scholar |
Pérez-Crespo, M., Pintado, B., and Gutiérrez-Adán, A. (2008). Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol. Reprod. Dev. 75, 40–47.
| Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice.Crossref | GoogleScholarGoogle Scholar |
Petrocelli, H., Batista, C., and Gosálvez, J. (2015). Seasonal variation in sperm characteristics of boars in southern Uruguay. Rev. Bras. Zootec. 44, 1–7.
| Seasonal variation in sperm characteristics of boars in southern Uruguay.Crossref | GoogleScholarGoogle Scholar |
Pingali, P. (2007). Westernization of Asian diets and the transformation of food systems: implications for research and policy. Food Policy 32, 281–298.
| Westernization of Asian diets and the transformation of food systems: implications for research and policy.Crossref | GoogleScholarGoogle Scholar |
Prunier, A., de Bragança, M. M., and Le Dividich, J. (1997). Influence of high ambient temperature on performance of reproductive sows. Livest. Prod. Sci. 52, 123–133.
| Influence of high ambient temperature on performance of reproductive sows.Crossref | GoogleScholarGoogle Scholar |
Pursel, V. G., and Johnson, L. A. (1975). Freezing of boar spermatozoa: fertilizing capacity with concentrated semen and a new thawing procedure. J. Anim. Sci. 40, 99–102.
| Freezing of boar spermatozoa: fertilizing capacity with concentrated semen and a new thawing procedure.Crossref | GoogleScholarGoogle Scholar |
Qiu, Y., Wang, L.-G., Zhang, L.-H., Li, J., Zhang, A.-D., and Zhang, M.-H. (2012). Sperm chromosomal aneuploidy and DNA integrity of infertile men with anejaculation. J. Assist. Reprod. Genet. 29, 185–194.
| Sperm chromosomal aneuploidy and DNA integrity of infertile men with anejaculation.Crossref | GoogleScholarGoogle Scholar |
Roca, J., Broekhuijse, M., Parrilla, I., Rodriguez‐Martinez, H., Martinez, E., and Bolarin, A. (2015). Boar differences in artificial insemination outcomes: can they be minimized? Reprod. Domest. Anim. 50, 48–55.
| Boar differences in artificial insemination outcomes: can they be minimized?Crossref | GoogleScholarGoogle Scholar |
Rockett, J. C., Mapp, F. L., Garges, J. B., Luft, J. C., Mori, C., and Dix, D. J. (2001). Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol. Reprod. 65, 229–239.
| Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice.Crossref | GoogleScholarGoogle Scholar |
Rybar, R., Faldikova, L., Faldyna, M., Machatkova, M., and Rubes, J. (2004). Bull and boar sperm DNA integrity evaluated by sperm chromatin structure assay in the Czech Republic. Vet. Med. (Praha) 49, 1–8.
Sailer, B. L., Sarkar, L. J., Bjordahl, J. A., Jost, L. K., and Evenson, D. P. (1997). Effects of heat stress on mouse testicular cells and sperm chromatin structure. J. Androl. 18, 294–301.
Sarlós, P., Egerszegi, I., Nagy, S., Fébel, H., and Rátky, J. (2011). Reproductive function of Hungarian Mangalica boars: effect of seasons. Acta Vet. Hung. 59, 257–267.
| Reproductive function of Hungarian Mangalica boars: effect of seasons.Crossref | GoogleScholarGoogle Scholar |
Satake, N., Elliott, R. M., Watson, P. F., and Holt, W. V. (2006). Sperm selection and competition in pigs may be mediated by the differential motility activation and suppression of sperm subpopulations within the oviduct. J. Exp. Biol. 209, 1560–1572.
| Sperm selection and competition in pigs may be mediated by the differential motility activation and suppression of sperm subpopulations within the oviduct.Crossref | GoogleScholarGoogle Scholar |
Serafini, R., McPhail, R. E., Varner, D. D., Blanchard, T. L., Teague, S. R., and Love, C. C. (2016). The relationship between sperm DNA structure and sexual rest in stallions. J. Equine Vet. Sci. 43, S65–S66.
| The relationship between sperm DNA structure and sexual rest in stallions.Crossref | GoogleScholarGoogle Scholar |
Simon, L., and Lewis, S. E. M. (2011). Sperm DNA damage or progressive motility: which one is the better predictor of fertilization in vitro? Syst Biol Reprod Med 57, 133–138.
| Sperm DNA damage or progressive motility: which one is the better predictor of fertilization in vitro?Crossref | GoogleScholarGoogle Scholar |
Simon, L., Proutski, I., Stevenson, M., Jennings, D., McManus, J., Lutton, D., and Lewis, S. E. M. (2013). Sperm DNA damage has a negative association with live-birth rates after IVF. Reprod. Biomed. Online 26, 68–78.
| Sperm DNA damage has a negative association with live-birth rates after IVF.Crossref | GoogleScholarGoogle Scholar |
Sonderman, J. P., and Luebbe, J. J. (2008). Semen production and fertility issues related to differences in genetic lines of boars. Theriogenology 70, 1380–1383.
| Semen production and fertility issues related to differences in genetic lines of boars.Crossref | GoogleScholarGoogle Scholar |
Stone, B. A. (1982). Heat induced infertility of boars: the inter-relationship between depressed sperm output and fertility and an estimation of the critical air temperature above which sperm output is impaired. Anim. Reprod. Sci. 4, 283–299.
| Heat induced infertility of boars: the inter-relationship between depressed sperm output and fertility and an estimation of the critical air temperature above which sperm output is impaired.Crossref | GoogleScholarGoogle Scholar |
Stone, B., and Seamark, R. (1984). Effects of acute and chronic testicular hyperthermia on levels of testosterone and corticosteroids in plasma of boars. Anim. Reprod. Sci. 7, 391–403.
| Effects of acute and chronic testicular hyperthermia on levels of testosterone and corticosteroids in plasma of boars.Crossref | GoogleScholarGoogle Scholar |
St-Pierre, N. R., Cobanov, B., and Schnitkey, G. (2003). Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86, E52–E77.
| Economic losses from heat stress by US livestock industries.Crossref | GoogleScholarGoogle Scholar |
Sumena, K., Lucy, K., Chungath, J., Ashok, N., and Harshan, K. (2010). Regional histology of the subcutaneous tissue and the sweat glands of Large White Yorkshire pigs, Tamilnadu. J. Vet. Anim. Sci. (Lahore) 6, 128–135.
Sutovsky, P. (2015). New approaches to boar semen evaluation, processing and improvement. Reprod. Domest. Anim. 50, 11–19.
| New approaches to boar semen evaluation, processing and improvement.Crossref | GoogleScholarGoogle Scholar |
Tantasuparuk, W., and Kunavongkrit, A. (2014) ‘Pig Production in Thailand, Country Report’. Available at https://www.angrin.tlri.gov.tw/English/2014Swine/p136-144.pdf
Thom, E. C. (1959). The discomfort index. Weatherwise 12, 57–61.
| The discomfort index.Crossref | GoogleScholarGoogle Scholar |
Tramontano, F., Malanga, M., Farina, B., Jones, R., and Quesada, P. (2000). Heat stress reduces poly(adpr)polymerase expression in rat testis. Mol. Hum. Reprod. 6, 575–581.
| Heat stress reduces poly(adpr)polymerase expression in rat testis.Crossref | GoogleScholarGoogle Scholar |
Tsakmakidis, I. A., Lymberopoulos, A. G., and Khalifa, T. A. A. (2010). Relationship between sperm quality traits and field-fertility of porcine semen. J. Vet. Sci. 11, 151–154.
| Relationship between sperm quality traits and field-fertility of porcine semen.Crossref | GoogleScholarGoogle Scholar |
van Zelst, S. J., Zupp, J. L., Hayman, D. L., and Setchell, B. P. (1995). X-Y chromosome dissociation in mice and rats exposed to increased testicular or environmental temperatures. Reprod. Fertil. Dev. 7, 1117–1121.
| X-Y chromosome dissociation in mice and rats exposed to increased testicular or environmental temperatures.Crossref | GoogleScholarGoogle Scholar |
Vega, R., and Agbisit, E., Jr (2009) ‘Analysis and Utilization of Farm Performance Data as a Tool in Enhancing Swine Farm Productivity and Efficiency’. (Philippine Swine Industry Research and Development Foundation: Los Baños.) Available at: http://agris.fao.org/agris-search/search.do?recordID=PH2010000360 [Verified 12 October 2018].
Vega, R. S., Garcia, B. R., Agbisit, E., Calud, A. T., and Villar, E. C. (2010). Performance of commercial piggery farms affected by the third quarter reproduction syndrome. Philipp. J. Vet. Anim. Sci. 36, 63–72.
Venkatesh, S., Singh, A., Shamsi, M. B., Thilagavathi, J., Kumar, R. K., Mitra, D., and Dada, R. (2011). Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod. Sci. 18, 1005–1013.
| Clinical significance of sperm DNA damage threshold value in the assessment of male infertility.Crossref | GoogleScholarGoogle Scholar |
Vyt, P., Maes, D., Quinten, C., Rijsselaere, T., Deley, W., Aarts, M., de Kruif, A., and Soom, A. (2008). Detailed motility evaluation of boar semen and its predictive value for reproductive performance in sows. Vlaams Diergeneeskundig Tijdschrift 77, 291–298.
Waberski, D., Schapmann, E., Henning, H., Riesenbeck, A., and Brandt, H. (2011). Sperm chromatin structural integrity in normospermic boars is not related to semen storage and fertility after routine AI. Theriogenology 75, 337–345.
| Sperm chromatin structural integrity in normospermic boars is not related to semen storage and fertility after routine AI.Crossref | GoogleScholarGoogle Scholar |
Wettemann, R. P., Wells, M. E., Omtvedt, I. T., Pope, C. E., and Turman, E. J. (1976). Influence of elevated ambient temperature on reproductive performance of boars. J. Anim. Sci. 42, 664–669.
| Influence of elevated ambient temperature on reproductive performance of boars.Crossref | GoogleScholarGoogle Scholar |
Wettemann, R. P., Wells, M. E., and Johnson, R. K. (1979). Reproductive characteristics of boars during and after exposure to increased ambient temperature. J. Anim. Sci. 49, 1501–1505.
| Reproductive characteristics of boars during and after exposure to increased ambient temperature.Crossref | GoogleScholarGoogle Scholar |
World Health Organization (WHO) (2010). ‘WHO Laboratory Manual for the Examination and Processing of Human Semen’. 5th edn. (World Health Organization: Geneva.). Available at http://apps.who.int/iris/bitstream/10665/44261/1/9789241547789_eng.pdf [Verified 12 October 2018].
Zasiadczyk, L., Fraser, L., Kordan, W., and Wasilewska, K. (2015). Individual and seasonal variations in the quality of fractionated boar ejaculates. Theriogenology 83, 1287–1303.
| Individual and seasonal variations in the quality of fractionated boar ejaculates.Crossref | GoogleScholarGoogle Scholar |