Mouse double minute homologue 2 (MDM2) downregulation by miR-661 impairs human endometrial epithelial cell adhesive capacity
Amy Winship A C , Amanda Ton A B , Michelle Van Sinderen A B , Ellen Menkhorst A B , Katarzyna Rainczuk A B , Meaghan Griffiths A , Carly Cuman A B and Evdokia Dimitriadis A B C DA Centre for Reproductive Health, Hudson Institute of Medical Research, Clayton, Vic. 3168, Australia.
B Department of Molecular and Translational Sciences, Monash University, Clayton, Vic. 3800, Australia.
C Department of Anatomy and Developmental Biology, Monash University, Clayton, Vic. 3800, Australia.
D Corresponding author. Email: evdokia.dimitriadis@hudson.org.au
Reproduction, Fertility and Development 30(3) 477-486 https://doi.org/10.1071/RD17095
Submitted: 10 March 2017 Accepted: 16 July 2017 Published: 29 August 2017
Journal compilation © CSIRO 2018 Open Access CC BY-NC-ND
Abstract
Human blastocysts that fail to implant following IVF secrete elevated levels of miR-661, which is taken up by primary human endometrial epithelial cells (HEECs) and impairs their adhesive capability. MicroRNA miR-661 downregulates mouse double minute homologue 2 (MDM2) and MDM4 in other epithelial cell types to activate p53; however, this has not been examined in the endometrium. In this study MDM2 protein was detected in the luminal epithelium of the endometrium, the site of blastocyst attachment, during the mid secretory receptive phase of the menstrual cycle. The effects of miR-661 on gene expression in and adhesion of endometrial cells was also examined. MiR-661 overexpression consistently downregulated MDM2 but not MDM4 or p53 gene expression in the Ishikawa endometrial epithelial cell line and primary HEEC. Adhesion assays were performed on the real-time monitoring xCELLigence system and by co-culture using Ishikawa cells and HEECs with HTR8/SVneo trophoblast spheroids. Targeted siRNA-mediated knockdown of MDM2 in endometrial epithelial cells reduced Ishikawa cell adhesion (P < 0.001) and also reduced HTR8/SVneo trophoblast spheroid adhesion to Ishikawa cells (P < 0.05) and HEECs (P < 0.05). MDM2 overexpression using recombinant protein treatment resulted in enhanced HTR8/SVneo trophoblast spheroid adhesion to Ishikawa cells (P < 0.01) and HEECs (P < 0.05). This study highlights a potential new mechanism by which human blastocyst-secreted miR-661 reduces endometrial epithelial cell adhesion; via downregulation of MDM2. These findings suggest that MDM2 contributes to endometrial–blastocyst adhesion, implantation and infertility in women.
Additional keywords: endometrium, gene regulation, implantation, trophoblast.
Introduction
Synchronous embryo and endometrial development are critical for implantation and successful pregnancy. In preparation for implantation, the endometrium undergoes dramatic remodelling during the menstrual cycle. Following menses, the endometrium regenerates throughout the proliferative phase under the influence of oestradiol. Progesterone regulates the secretory phase, whereby the endometrium differentiates and undergoes morphological and functional alterations to become ‘receptive’ during the mid-secretory phase of the menstrual cycle. The blastocyst enters the uterine cavity up to 72 h before its apposition and adhesion to a receptive endometrial luminal epithelium (Norwitz et al. 2001).
Abnormalities in blastocyst adhesion to the endometrial luminal epithelium lead to implantation failure and infertility (Timeva et al. 2014). The identification of novel mechanisms by which endometrial receptivity is regulated could lead to targets for treatment of implantation failure. Current understanding of the critical factors that regulate blastocyst–endometrial interactions in humans is limited. To address this, we previously demonstrated that cultured human IVF blastocysts secrete soluble factors that alter primary human endometrial epithelial cell (HEEC) gene expression and adhesion (Cuman et al. 2013), strongly suggesting that human blastocysts influence endometrial receptivity by altering endometrial adhesive capacity and thus affecting implantation. More recently, we discovered that human blastocyst-secreted small non-coding RNAs, microRNAs (miRs), are taken up by primary HEECs and regulate their adhesion, gene and protein expression (Cuman et al. 2015).
MiRs are short (~23 bases), highly conserved sequences that regulate the expression of 50% of genes in the human genome by binding to specific mRNAs via targets located mostly in their 3′ untranslated regions (UTRs; Bartel 2009; Shukla et al. 2011). The main role of miRs is post-transcriptional regulation by binding to target sequences of specific mRNAs to cause translational arrest or degradation of the targeted mRNA (Bartel 2009). We reported that miR-661 was the most highly expressed miR in spent blastocyst culture medium from blastocysts that failed to implant compared with blastocysts that successfully implanted during IVF cycles (Cuman et al. 2015). Uptake of miR-661 by primary HEECs reduced trophoblast cell line spheroid attachment to HEECs, a finding that was only partly explained by miR-661 regulation of Nectin-1 (Cuman et al. 2015). This implies that other miR-661 target genes likely also contribute to endometrial adhesive capacity, receptivity and implantation.
MiR-661 has ~1000 predicted targets in different cell types, with only two experimentally confirmed in the endometrium: Nectin-1 and metastasis-associated protein 2 (MTA2; Cuman et al. 2015). Confirmed targets of miR-661 in other cell types include the mouse double minute homologues 2 (MDM2) and 4 (MDM4; Hoffman et al. 2014a). MDM2 is a really interesting new gene (RING) finger-containing ubiquitin protein ligase (E3) with a well-established function as a negative regulator of p53 (Haupt et al. 1997). MDM4, a structural homologue of MDM2, also binds to the N-terminus of p53 and is a key inhibitor of p53. It is well established that the p53 pathway is crucial in mediating tumourigenesis (Wade et al. 2013). Interestingly, p53 also plays an important role in blastocyst implantation through regulation of leukaemia inhibitory factor (LIF) in mice (Hu et al. 2007). In humans, a p53 single-nucleotide polymorphism (SNP), referred to as codon P72, has been reported to functionally reduce LIF protein production in cells with this SNP. The codon P72 SNP is enriched in women with recurrent implantation failure (Kang et al. 2009). Furthermore, selected alleles in SNPs in the p53 pathway, including LIF, MDM2 and MDM4, were also enriched in infertile IVF patients compared with fertile women, although their functions are yet to be determined (Kang et al. 2009). Together, this suggests that mediators of the p53 signalling pathway, including MDM2 and MDM4, may play an important role in receptivity, implantation and fertility.
To date, however, MDM2 and MDM4 have not been localised in human endometrium and their potential roles in endometrial epithelial cell function and receptivity have not been investigated. In this study we aimed to localise MDM2 and MDM4 in human endometrium across the menstrual cycle and to determine whether these are targets of miR-661 in endometrial epithelial cells. We subsequently investigated the functional effect of MDM2 knockdown and MDM2 overexpression on endometrial epithelial adhesive capacity during the receptive phase of the menstrual cycle.
Materials and methods
Ethics statement
Written informed consent was obtained from each patient and the study was approved by the Southern Health Research and Ethics Committee (#09317B) at Monash Medical Centre Melbourne, Australia.
Endometrial tissue collection
Endometrial biopsies were collected by curettage from women with regular menstrual cycles throughout the menstrual cycle. Biopsies were obtained from women with known normal fertility during the proliferative (Days 7–17) or early (Days 18–20), mid (Days 21–25) and late secretory phases (Days 26–27) of the menstrual cycle (n = 8 per phase). All women had regular menstrual cycles, were not using intrauterine contraceptives and had not used hormones for at least 3 months before surgery. Biopsies were examined by an experienced gynaecological pathologist to confirm that they showed no evidence of possible endometrial dysfunction. Dating of the menstrual cycle was established from histopathology reports and from histological examination.
Immunohistochemistry
Immunohistochemistry for MDM2 and MDM4 was performed on formalin-fixed endometrial tissue from fertile women across the menstrual cycle (n = 8 per phase). Fixed tissue slides were rehydrated and then antigen retrieval was performed in 0.01 M citrate buffer, followed by 3% hydrogen peroxide in methanol for 10 min to block endogenous peroxidase activity. Tissues were washed with Tris-buffered saline (TBS) and incubated with non-immune block (Dako) for 30 min. Primary antibody was applied and incubated at 4°C overnight, using the following primary antibodies: 5 μg mL−1 anti-MDM2 antibody (#ab38618; Abcam) or 1.4 μg mL−1 anti-MDM4 antibody (#ab154324; Abcam). Negative isotype control rabbit IgG (#E0353; Dako) was applied at the same concentration as the primary antibodies. This was followed by biotinylated horse anti-mouse or goat anti-rabbit IgG (1 : 200; Vector) for 30 min, then streptavidin–biotin–peroxidase complex ABC (Dako) according to the manufacturer’s instructions. Peroxidase activity was visualised by application of diaminobenzidine substrate (Dako) for 2–3 min. Tissues were counterstained with Harris haematoxylin (Sigma-Aldrich), air-dried and mounted. A quality control slide was present in each immunohistochemistry run.
Cell lines and culture
Ishikawa endometrial epithelial cells were provided by Dr M. Nishida (Tsukuba University, Tochigi, Japan) and cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10% fetal calf serum (FCS; Invitrogen). The HTR-8/SVneo trophoblast cell line exhibits features of invasive trophoblast cells, such as human leukocyte antigen-G (extravillous trophoblast marker) and cytokeratin-7 expression (Hannan et al. 2010). These cells were cultivated and maintained in Roswell Park Memorial Institute medium (RPMI) 1640 (Sigma-Aldrich) supplemented with 10% FCS as previously described (Graham et al. 1993). Both cell lines were authenticated by Monash Health Translational Precinct Medical Genomics, June 2016.
Primary human endometrial epithelial cell (HEEC) isolation
Endometrial epithelial cells were prepared as previously published (Cuman et al. 2015). Briefly, endometrial tissue was digested with collagenase and the suspension was filtered through 43- and 11-mm nylon mesh to collect endometrial epithelial glands. The cells and epithelial fragments were collected and resuspended in a 1 : 1 mixture of DMEM/Hams F-12 (Gibco) supplemented with 10% FCS and 1% antibiotic–antimycotic solution (Gibco) and plated. A purity of 95% was necessary for the cells to be used experimentally.
Micro-RNA (miR-661) mimic transfection
HEECs and Ishikawa cells were grown to 70% confluence in 96-well plates and transfected according to manufacturer’s instructions using Lipofectamine RNAimax and 100 nM miR-661 mimic (Life Technologies) for 72 h. A scrambled microRNA sequence (scr; Life Technologies) was used as a control. These treated cells were used to model the endometrial epithelium and perform target gene analysis studies (number of passages: Ishikawa cell line, n = 4 per group; HEECs, n = 6 per group).
In order to more accurately model blastocyst-secreted miR-661 uptake by the endometrium, HTR8/SVneo trophoblast cells were grown to 70% confluence in 96-well plates and transfected with miR-661 mimic or scr control as above. Conditioned medium (CM) was collected from transfected HTR-8/SVneo trophoblast cells after 72 h. The CM was cultured with untreated HEEC in 96-well plates for 8 h to determine the effect on HEEC target gene expression (n = 6 per group). We have previously demonstrated using fluorescein-tagged miR-661 that transfection of HTR-8/SVneo cells using miR-661 mimic results in miR-661 secretion into CM and direct uptake by human endometrial epithelial cells, confirmed by immunofluorescence (Cuman et al. 2015). The transfection efficiency in this study was confirmed by quantitative real-time reverse transcription polymerase chain reaction (RT-PCR).
Small interfering RNA (siRNA) transfection
Ishikawa cells or HEECs (n = 3 per group) were grown to 70% confluence in a 96-well plate (spheroid adhesion) or to 70% confluence in a 48-well plate (real-time adhesion) and transfected with commercially generated and validated ON-TARGET plus SMARTpool siRNA (Dharmacon) that targeted either MDM2 or a non-specific sequence as a scrambled (scr) control. Delivery of the siRNA was performed using Lipofectamine RNAiMAX (Invitrogen) according to manufacturer’s instructions at an end concentration of 20 nM. Cells were transfected for 72 h before RNA collection or before beginning the functional experiments. Confirmation of knockdown of MDM2 expression in Ishikawa cell and HEEC lysates was performed by quantitative real-time RT-PCR.
RNA preparation and quantitative real-time RT-PCR
RNA was extracted from cultured cells using Tri Reagent (Sigma-Aldrich) according to the manufacturer’s instructions. Isolated RNA was reverse transcribed into complimentary DNA with the M-MLV RT system (Life Technologies) by using the TaqMan primer sets for miRs (Applied Biosystems) or oligo primers (Sigma-Aldrich) for non-miRs. Real-time PCR was performed using the TaqMan Fast Universal PCR Master mix (Applied Biosystems) or Power SYBR Green master mix (Applied Biosystems) by using TaqMan probes or specific primer pairs (Table 1). MiR-661 expression levels were normalised against housekeeping control snU6 probes as previously reported (Cuman et al. 2015). Expression of gene targets of miR-661 were normalised against 18s.

|
Real-time cell adhesion assay
Experiments were carried out using the RTCA SP xCELLigence instrument (Roche Diagnostics GmbH), which measures electrical impedance of cells (Winship et al. 2017) and was placed in a humidified incubator maintained at 37°C with 95% air, 5% CO2. For adhesion, MDM2 siRNA or scr control-treated Ishikawa cells (n = 3 per group) were seeded in E-plates at 10 000 cells per well in 5% FCS medium. The plate was monitored once every 15 min for a total of 6 h to examine adhesion. Data was calculated using RTCA software Version 1.2, supplied with the instrument (ACEA) and exported for statistical analysis.
Trophoblast spheroid adhesion assay
To determine the effect of MDM2 on the adhesive properties essential for the attachment of the blastocyst to the endometrium, a co-culture model was established based on a previous publication (Krishnan et al. 2013), with some modification. Spheroids were formed using HTR8/SVneo cells (2000 cells per spheroid) in a Cellstar U-shaped 96-well suspension culture plate (Greiner Bio-One) and incubated at 37°C for 48 h. Ishikawa cells and HEECs were treated with MDM2 siRNA, scr control, 500 ng mL−1 recombinant human MDM2 (based on concentration response data) or phosphate-buffered saline (PBS) vehicle control (n = 3 per group). Spheroids were transferred into a 96-well plate containing treated Ishikawa cells (7 spheroids per well) or HEECs (16 spheroids per well). Spheroid number was determined visually before incubation at 37°C for 4 h (Ishikawa cells) or 2 h (HEECs). Co-culture wells were washed gently with 150 μL serum-free DMEM/F12 medium and the remaining spheroids counted to determine the number of adhered spheroids. Attachment was expressed as a percentage of the original spheroid number.
Statistical analyses
Statistical analysis was carried out using GraphPad Prism Version 6.0 (GraphPad Software). For comparisons of two groups, Student’s t-test was performed, while multiple groups were compared using one-way ANOVA, with Tukey’s post-hoc test. Results of P < 0.05 were considered to be statistically significant.
Results
MDM2 and MDM4 localisation in human endometrium across the menstrual cycle
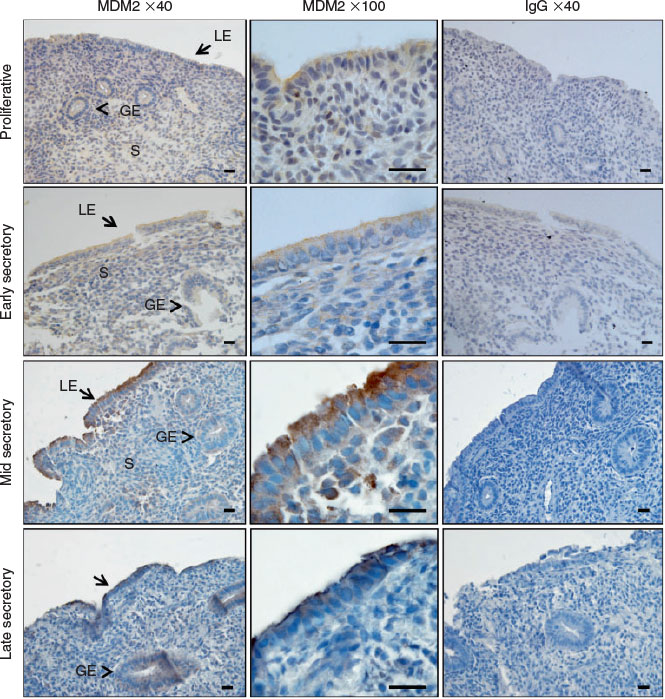
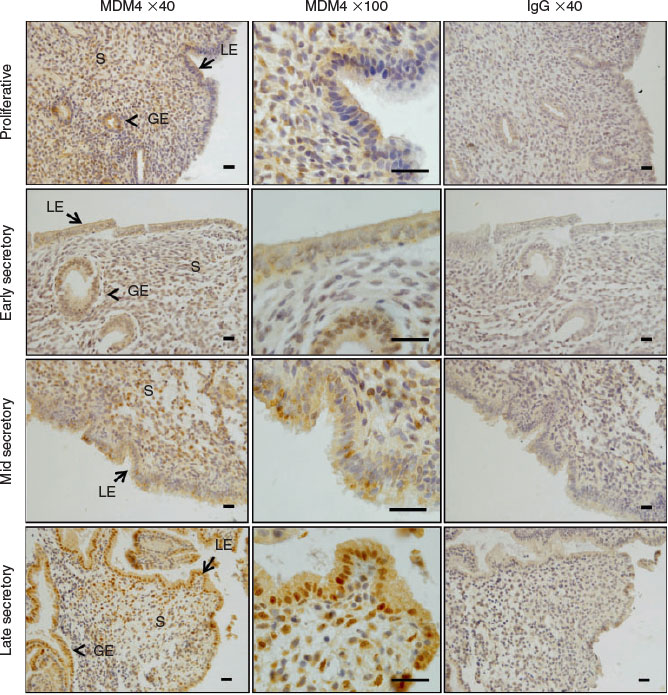
We immunolocalised MDM2 and MDM4 protein in human endometrium across the menstrual cycle. MDM2 localised to the cytoplasm and cell membrane of the luminal epithelium (LE) and staining intensity was high during the mid and late secretory phases in endometrial tissue (Fig. 1a–d). Staining intensity was weak in the glandular epithelium (GE) and stroma throughout all phases of the menstrual cycle and was weak in all cell compartments during the proliferative and early secretory phase. MDM4 localised to the cytoplasm of the GE, LE and the stroma across the menstrual cycle (Fig. 2a–d). Additionally, nuclear MDM4 immunostaining was evident in the LE and GE during the late secretory phase (Fig. 2d).
MiR-661 mimic downregulates MDM2 in endometrial epithelial cells
MiR-661 was overexpressed by transfecting Ishikawa cells or HEECs with miR-661 mimic. MiR-661 was significantly upregulated in both cell types treated with mimic versus scrambled control (Fig. 3a). MiR-661 mimic significantly reduced MDM2 mRNA levels in Ishikawa cells (P < 0.01), but resulted in no significant differences of MDM4 or p53 mRNA compared with the control (Fig. 3b). MDM2 and MDM4 mRNA levels were significantly reduced in primary HEECs transfected with miR-661 mimic compared with control (P < 0.05), whereas p53 mRNA expression was unchanged (Fig. 3c). In order to more accurately model blastocyst-secreted miR-661 being taken up by the endometrium, HTR8/SVneo trophoblast cells were transfected with miR-661 mimic or control. CM from miR-661 or control transfected HTR8/SVneo cells was used to treat HEECs and determine the effect on HEEC gene expression. MDM2 and p53 mRNA levels were significantly reduced following exposure to conditioned medium from HTR8/SVneo trophoblasts transfected with miR-661 mimic versus control (P < 0.05; Fig. 3d). MDM4 mRNA expression was unchanged.

|
MDM2 knockdown decreases endometrial epithelial cell adhesive properties
Quantitative real-time RT-PCR confirmed that transfection of Ishikawa cells and primary HEECs with MDM2 siRNA significantly reduced MDM2 mRNA compared with scr control (P < 0.01; Fig. 4a). Using a real-time adhesion assay, siRNA-mediated MDM2 knockdown significantly reduced Ishikawa cell adhesion by 31% ± 3.8 compared with control at 6 h (P < 0.001; Fig. 4b). To further confirm the effect of MDM2 knockdown on endometrial epithelial adhesive capacity, co-culture adhesion assays were performed using HTR8/SVneo spheroids with MDM2 siRNA or control-treated Ishikawa cells or primary HEECs. HTR8/SVneo trophoblast spheroid adhesion to MDM2 siRNA-treated Ishikawa cells was reduced by 14.66% ± 4.3 compared with scr control (P < 0.05; Fig. 4c). Co-culture of HTR8/SVneo trophoblast spheroids with MDM2 siRNA-treated HEECs resulted in a 38.26% ± 2.0 reduction in spheroid adhesion versus scr control (P < 0.05; Fig. 4d).
MDM2 enhances endometrial epithelial cell adhesive properties
Pretreatment of Ishikawa cells or HEECs with recombinant human MDM2 (500 ng mL−1) for 24 h resulted in increased HTR8/SVneo trophoblast spheroid adhesion to Ishikawa cells by by 24% ± 7.9 compared with control at 4 h (P < 0.01; Fig. 5a) and to HEECs by 22% ± 2.8 compared with control at 4 h (P < 0.05; Fig. 5b).

|
MDM2 regulates p53 and LIF expression in endometrial epithelial cells
MDM2 inhibition by siRNA significantly reduced p53 gene expression in Ishikawa cells (P < 0.05; Fig. 6a), but not HEECs (Fig. 6b). LIF mRNA was significantly reduced following MDM2 knockdown versus control in both Ishikawa cells (Fig. 6a) and HEECs (P < 0.05; Fig. 6b). IL11 and E-cadherin (ECAD) mRNA levels were unchanged in both cell types.

|
Discussion
We have previously demonstrated that human blastocysts that fail to implant secrete elevated levels of miR-661, which is taken up by primary human endometrial epithelial cells and impairs their adhesive capability (Cuman et al. 2015). In the present study we report that miR-661 consistently downregulates MDM2, but not MDM4 or p53 gene expression in human endometrial epithelial cells. MDM2 knockdown reduced endometrial epithelial cell adhesion, while addition of recombinant MDM2 enhanced endometrial epithelial cell adhesive properties. These results highlight a potential new mechanism by which miR-661 reduces endometrial epithelial cell adhesion and receptivity in humans and demonstrates the importance of MDM2 in endometrial receptivity.
MDM2 predominantly localised to the LE in cycling human endometrium, within the cytoplasm and cell membrane, which is consistent with reports in other epithelial cell types (Iwakuma and Lozano 2003). Weak MDM2 staining intensity was observed in the GE and stroma. MDM2 immunostaining intensity in the LE was abundant during the mid and late secretory phase of the menstrual cycle; however, this was not quantified. Cyclic AMP-mediated decidualisation in human endometrial stromal cells was previously reported to downregulate MDM2 mRNA and protein (Pohnke et al. 2004), although MDM2 regulation in the endometrial epithelium has not yet been examined.
MDM4 protein was abundant in the LE, GE and stromal nuclei, but was also present in the cytoplasm in all endometrial compartments. MDM4 contains a nuclear localisation signal, but lacks a nuclear export signal and is unable to bind to and export p53 from the nucleus (Stad et al. 2001). The predominant nuclear presence of MDM4 may be attributed to its role in competitively inhibiting p53 interactions with other proteins. P53 was previously immunolocalised in the cycling human endometrium, with significantly higher protein production during the proliferative versus the secretory phase (Maia et al. 2004). It is possible that MDM4 may contribute to negative regulation of p53 in the endometrium, while MDM2 does not, although this requires further investigation.
MiR-661 consistently downregulated MDM2, but not p53 or MDM4 in Ishikawa cells, primary HEECs and HEECs cultured with miR-661 mimic-treated trophoblast-conditioned medium. Downregulation of MDM2 in endometrial epithelial cells did not coincide with an upregulation in p53, suggesting that MDM2 does not act as a negative regulator of p53 in the endometrium. Interestingly, p53 mRNA was reduced in HEECs treated with trophoblast culture medium containing miR-661. This may be due to other factors present in the trophoblast culture medium, such as other miRs or proteins that alter p53 gene expression. Presently, over 20 miRNAs have been identified as the direct negative regulators of p53 through binding to the 3′-UTR of the p53 mRNA (reviewed Zhang et al. 2015). Of these, numerous miRs confirmed to regulate p53 have also been reported in the endometrium in humans or mice (Panda et al. 2012; Liu et al. 2013; Chen et al. 2016; Liu et al. 2016).
MDM4 expression was reduced in primary HEECs transfected with miR-661 mimic. However, this was not observed in Ishikawa cells or in primary HEECs treated with trophoblast culture medium containing miR-661. It is possible that miR-661 and MDM2 exert differences in target gene regulation between primary human endometrial epithelial cells and the Ishikawa model cell line, highlighting the importance of confirming findings from cell lines using primary cells. It is also likely that factors other than miR-661 were taken up by the HEECs from the culture medium to impair MDM4 regulation by miR-661. Together these findings demonstrate that miR-661 downregulates MDM2 in endometrial epithelial cells more effectively than MDM4, in line with findings in other epithelial cells (Hoffman et al. 2014b).
Although the immunostaining intensity was not quantified across the menstrual cycle, it was clearly evident that MDM2 protein localised to the endometrial LE, the point of contact between an implanting blastocyst and the endometrium during blastocyst adhesion, during the mid-secretory or ‘receptive’ phase in fertile women. However, it is plausible that blastocyst-derived miR-661 could target and downregulate MDM2 in the LE to alter implantation. Indeed MDM2 knockdown impaired Ishikawa cell adhesion, and knockdown in both Ishikawa cells and HEECs significantly reduced trophoblast spheroid adhesion compared with control. Together, this implies that MDM2 is an important regulator of receptivity and that MDM2 has a functional role in endometrial–blastocyst adhesion during implantation.
In support of this hypothesis, the addition of MDM2 protein enhanced trophoblast spheroid adhesion to both Ishikawa cells and HEECs. However, a substantial limitation of this study is that the physiological concentration of MDM2 protein within the uterine cavity remains unknown. The concentration of recombinant MDM2 used in functional adhesion assays in the present study was chosen based on pilot concentration-response data, in which 500 ng mL−1 recombinant MDM2 enhanced spheroid adhesion to endometrial epithelial cells, although did not elicit a complete binding response in all spheroids compared with controls. We have shown that MDM2 localises to the endometrial luminal epithelium cell membrane and cytoplasm. Furthermore, MDM2 is detected in serum (Zhou et al. 2014), together suggesting that MDM2 can be secreted and may be secreted into the endometrial lumen to facilitate adhesion, although this remains to be determined.
Blastocyst implantation is a complex process mediated by many factors. We investigated MDM2 regulation of known receptivity regulators. IL11 is thought to contribute to implantation in humans (Dimitriadis et al. 2007), but has been shown to be fundamental for decidualisation in both humans and mice (Robb et al. 1998; Dimitriadis et al. 2002). MDM2 knockdown did not alter IL11 mRNA expression in endometrial epithelial cells, probably due to the fact that IL11 may play a more crucial role after implantation (Winship et al. 2015). MDM2 and E-cadherin co-localise on the plasma membrane and MDM2 ubiquitinates E-cadherin, decreasing protein levels, in turn impairing cell–cell adhesion, to increase cell motility and invasion in human breast cancer cells (Yang et al. 2006). E-cadherin is essential for the establishment of the epithelial phenotype and the prevention of invasiveness (Behrens 1994). Implantation is an event in which epithelial to mesenchymal transition and trophoblast invasion occur synchronously. However, E-cadherin (ECAD) mRNA was not regulated by MDM2 in the endometrial epithelium. LIF is reported to regulate implantation in humans (Cullinan et al. 1996; Vogiagis et al. 1996; Laird et al. 1997) and is required for implantation in mice (Stewart et al. 1992; White et al. 2007; Menkhorst et al. 2010, 2011). LIF mRNA was significantly reduced in Ishikawa cells and HEECs in response to MDM2 knockdown, which further supports a role for MDM2 in endometrial receptivity and implantation. LIF has been shown to upregulate MDM2 mRNA and protein in order to increase the degradation of p53; hence, LIF and MDM2 both have the ability to negatively regulate p53 in a cancer setting (Yu et al. 2014). However, to our knowledge this is the first report of MDM2 regulation of LIF. This finding highlights a possible mechanism by which MDM2 regulates HEEC adhesion.
Infertility affects ~10–15% of reproductive-aged couples, with an estimated 50% attributed to underlying female infertility (Evers 2002). Pregnancy success rates from assisted reproductive technologies (ART) such as IVF have not significantly altered since their advent; current IVF success rates are ~30% (Kupka et al. 2014). The identification of novel mechanisms by which blastocyst-secreted factors alter the endometrium could lead to targets for treatment of implantation failure, to ultimately improve IVF outcomes. We were the first group to identify a miR, miR-661, that is secreted by blastocysts that fail to implant but is not secreted by blastocysts that successfully implant during IVF (Cuman et al. 2015). It is likely that miR-661 regulates multiple targets within HEECs, with hundreds of different predicted targets, of which only a few have been confirmed in the endometrial epithelium, including MTA2, Nectin-1 (Cuman et al. 2015) and MDM2. Evidently, miR-661 could not only alter embryo implantation, but also reflect embryo implantation potential and be exploited as a biomarker to predict this in an IVF setting. However, it is likely that a panel of miRs, rather than individual molecules could be used to develop such a test. Therefore, it is now crucial to identify and validate additional miRs and their targets.
In summary, this study defines the importance of MDM2 as a regulator of endometrial receptivity. It highlights a new pathway by which human blastocyst-secreted miR-661 regulates MDM2 in the human endometrium, which, in turn, probably contributes to endometrial–blastocyst adhesion, implantation and more broadly, infertility.
Conflict of interest
The authors declare no conflicts of interest.
Acknowledgements
The authors would like to thank Sister Judi Hocking for her assistance and acknowledge all the women that donated endometrial tissue. We acknowledge the Monash IVF embryology team for collecting blastocyst spent media samples. This work was supported by NHMRC Australia Project Grant (1098321) and the Victorian Government’s Operational Infrastructure Support Program. Evdokia Dimitriadis was supported by a NHMRC Australia Senior Research Fellowship (550905). Amy Winship was supported by a Cancer Council Victoria Postdoctoral Fellowship. Amanda Ton was supported by a Monash University Jubilee Honours Scholarship.
References
Bartel, D. P. (2009). MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233.| MicroRNAs: target recognition and regulatory functions.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhs1Kiuro%3D&md5=11639626b613d20937279a61486c48feCAS |
Behrens, J. (1994). Cadherins as determinants of tissue morphology and suppressors of invasion. Acta Anat. (Basel) 149, 165–169.
| Cadherins as determinants of tissue morphology and suppressors of invasion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXmslOiur8%3D&md5=e913b48dcbdc0ba286e09c08367c7686CAS |
Chen, C., Zhao, Y., Yu, Y., Li, R., and Qiao, J. (2016). MiR-125b regulates endometrial receptivity by targeting MMP26 in women undergoing IVF-ET with elevated progesterone on HCG priming day. Sci. Rep. 6, 25302.
| MiR-125b regulates endometrial receptivity by targeting MMP26 in women undergoing IVF-ET with elevated progesterone on HCG priming day.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC28XnsVemsr4%3D&md5=67fa7eefef2690ba2221248b5e2c2a0fCAS |
Cullinan, E. B., Abbondanzo, S. J., Anderson, P. S., Pollard, J. W., Lessey, B. A., and Stewart, C. L. (1996). Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc. Natl. Acad. Sci. USA 93, 3115–3120.
| Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK28XitVCiu7g%3D&md5=e06b4dbf37391bce57bf21eb1bedab5dCAS |
Cuman, C., Menkhorst, E., Rombauts, L., Holden, S., Webster, D., Bilandzic, M., Osianlis, T., and Dimitriadis, E. (2013). Preimplantation human blastocysts release factors that differentially alter human endometrial epithelial cell adhesion and gene expression relative to IVF success. Hum. Reprod. 28, 1161–1171.
| Preimplantation human blastocysts release factors that differentially alter human endometrial epithelial cell adhesion and gene expression relative to IVF success.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXmtlags7o%3D&md5=8542e263b7d4542465393896826bc8faCAS |
Cuman, C., Van Sinderen, M., Gantier, M. P., Rainczuk, K., Sorby, K., Rombauts, L., Osianlis, T., and Dimitriadis, E. (2015). Human blastocyst secreted microRNA regulate endometrial epithelial cell adhesion. EBioMedicine 2, 1528–1535.
| Human blastocyst secreted microRNA regulate endometrial epithelial cell adhesion.Crossref | GoogleScholarGoogle Scholar |
Dimitriadis, E., Robb, L., and Salamonsen, L. (2002). Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells. Mol. Hum. Reprod. 8, 636–643.
| Interleukin 11 advances progesterone-induced decidualization of human endometrial stromal cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XlvFKlsb4%3D&md5=d8839ff3490f14cde9e06b60a017ceadCAS |
Dimitriadis, E., Sharkey, A., Tan, Y., Salamonsen, L. A., and Sherwin, J. (2007). Immunolocalisation of phosphorylated STAT3, interleukin 11 and leukaemia inhibitory factor in endometrium of women with unexplained infertility during the implantation window. Reprod. Biol. Endocrinol. 5, 44–51.
| Immunolocalisation of phosphorylated STAT3, interleukin 11 and leukaemia inhibitory factor in endometrium of women with unexplained infertility during the implantation window.Crossref | GoogleScholarGoogle Scholar |
Evers, J. L. (2002). Female subfertility. Lancet 360, 151–159.
| Female subfertility.Crossref | GoogleScholarGoogle Scholar |
Graham, C. H., Hawley, T. S., Hawley, R. G., MacDougall, J. R., Kerbel, R. S., Khoo, N., and Lala, P. K. (1993). Establishment and characterization of first trimester human trophoblast cells with extended lifespan. Exp. Cell Res. 206, 204–211.
| Establishment and characterization of first trimester human trophoblast cells with extended lifespan.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK3sXks1OisL0%3D&md5=93ec5471eeae2767f9a878da8d1189c8CAS |
Hannan, N. J., Paiva, P., Dimitriadis, E., and Salamonsen, L. A. (2010). Models for study of human embryo implantation: choice of cell lines? Biol. Reprod. 82, 235–245.
| Models for study of human embryo implantation: choice of cell lines?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhsVSns7k%3D&md5=a11fb4e46638aebf4b615c9da83ba67fCAS |
Haupt, Y., Maya, R., Kazaz, A., and Oren, M. (1997). Mdm2 promotes the rapid degradation of p53. Nature 387, 296–299.
| Mdm2 promotes the rapid degradation of p53.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjtlSgtL8%3D&md5=06e44282e45b5d88f2071303598f204cCAS |
Hoffman, Y., Bublik, D. R., Pilpel, Y., and Oren, M. (2014a). miR-661 downregulates both Mdm2 and Mdm4 to activate p53. Cell Death Differ. 21, 302–309.
| miR-661 downregulates both Mdm2 and Mdm4 to activate p53.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhs1OltbrJ&md5=df2759c87950882bdd1087948b105282CAS |
Hoffman, Y., Pilpel, Y., and Oren, M. (2014b). microRNAs and Alu elements in the p53-Mdm2-Mdm4 regulatory network. J. Mol. Cell Biol. 6, 192–197.
| microRNAs and Alu elements in the p53-Mdm2-Mdm4 regulatory network.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXptVCgt7Y%3D&md5=208c7b9aa2d85f86f1cf1e536cea3cc2CAS |
Hu, W., Feng, Z., Teresky, A. K., and Levine, A. J. (2007). p53 regulates maternal reproduction through LIF. Nature 450, 721–724.
| p53 regulates maternal reproduction through LIF.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlKmtbrJ&md5=9292c7fa101b9f2daff977dcf26ac33fCAS |
Iwakuma, T., and Lozano, G. (2003). MDM2, an introduction. Mol. Cancer Res. 1, 993–1000.
| 1:CAS:528:DC%2BD2cXns1Gm&md5=36a8312bfe10553d32e202599c396eeaCAS |
Kang, H. J., Feng, Z., Sun, Y., Atwal, G., Murphy, M. E., Rebbeck, T. R., Rosenwaks, Z., Levine, A. J., and Hu, W. (2009). Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proc. Natl. Acad. Sci. USA 106, 9761–9766.
| Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXotFGjsrY%3D&md5=938d192171f21f290c4dcb9dbd8ccd41CAS |
Krishnan, T., Winship, A., Sonderegger, S., Menkhorst, E., Horne, A. W., Brown, J., Zhang, J. G., Nicola, N. A., Tong, S., and Dimitriadis, E. (2013). The role of leukemia inhibitory factor in tubal ectopic pregnancy. Placenta 34, 1014–1019.
| The role of leukemia inhibitory factor in tubal ectopic pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhsFensbnJ&md5=abab1becbfd9485c21310ca142d80b74CAS |
Kupka, M. S., Ferraretti, A. P., de Mouzon, J., Erb, K., D’Hooghe, T., Castilla, J. A., Calhaz-Jorge, C., De Geyter, C., Goossens, V., European IVF-Monitoring Consortium, for the European Society of Human Reproduction and Embryology (2014). Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE†. Hum. Reprod. 29, 2099–2113.
| Assisted reproductive technology in Europe, 2010: results generated from European registers by ESHRE†.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC2cbms1ektw%3D%3D&md5=375739d78ad2c52d28ee4c2c8be9c1eaCAS |
Laird, S. M., Tuckerman, E. M., Dalton, C. F., Dunphy, B. C., Li, T. C., and Zhang, X. (1997). The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture. Hum. Reprod. 12, 569–574.
| The production of leukaemia inhibitory factor by human endometrium: presence in uterine flushings and production by cells in culture.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXjtVKhsL4%3D&md5=2fdf7f327188e148b1bc990764441273CAS |
Liu, X., Gao, R., Chen, X., Zhang, H., Zheng, A., Yang, D., Ding, Y., Wang, Y., and He, J. (2013). Possible roles of mmu-miR-141 in the endometrium of mice in early pregnancy following embryo implantation. PLoS One 8, e67382.
| Possible roles of mmu-miR-141 in the endometrium of mice in early pregnancy following embryo implantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXhtFWis7vO&md5=914df68b5be1731c9d93e4e57d386914CAS |
Liu, X. J., Bai, X. G., Teng, Y. L., Song, L., Lu, N., and Yang, R. Q. (2016). miRNA-15a-5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. Eur. Rev. Med. Pharmacol. Sci. 20, 3319–3326.
Maia, H., Maltez, A., Studart, E., Athayde, C., and Coutinho, E. M. (2004). Ki-67, Bcl-2 and p53 expression in endometrial polyps and in the normal endometrium during the menstrual cycle. BJOG 111, 1242–1247.
| Ki-67, Bcl-2 and p53 expression in endometrial polyps and in the normal endometrium during the menstrual cycle.Crossref | GoogleScholarGoogle Scholar |
Menkhorst, E., Zhang, J. G., Morgan, P. O., Poulton, I. J., Metcalf, D., Salamonsen, L. A., Sims, N. A., Nicola, N. A., and Dimitriadis, E. (2010). Development of a vaginally applied, non-hormonal contraceptive: the contraceptive efficacy and impact on bone turnover of PEGLA, a long-acting LIF antagonist. J. Reprod. Immunol. 86, 33–34.
| Development of a vaginally applied, non-hormonal contraceptive: the contraceptive efficacy and impact on bone turnover of PEGLA, a long-acting LIF antagonist.Crossref | GoogleScholarGoogle Scholar |
Menkhorst, E., Zhang, J., Sims, N., Morgan, P., Soo, P., Poulton, I., Metcalf, D., Alexandrou, E., Gresle, M., Salamonsen, L., Butzkueven, H., Nicola, N. A., and Dimitriadis, E. (2011). Vaginally administered PEGylated LIF antagonist blocked embryo implantation and eliminated non-target effects on bone in mice. PLoS One 6, e19665.
| Vaginally administered PEGylated LIF antagonist blocked embryo implantation and eliminated non-target effects on bone in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXms1Kgu70%3D&md5=2042f0fff191dbee98f5bc2d410e30ebCAS |
Norwitz, E. R., Schust, D. J., and Fisher, S. J. (2001). Implantation and the survival of early pregnancy. N. Engl. J. Med. 345, 1400–1408.
| Implantation and the survival of early pregnancy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXot1aqu74%3D&md5=d9f8789215a0f65777af8721215d616fCAS |
Panda, H., Chuang, T. D., Luo, X., and Chegini, N. (2012). Endometrial miR-181a and miR-98 expression is altered during transition from normal into cancerous state and target PGR, PGRMC1, CYP19A1, DDX3X, and TIMP3. J. Clin. Endocrinol. Metab. 97, E1316–E1326.
| Endometrial miR-181a and miR-98 expression is altered during transition from normal into cancerous state and target PGR, PGRMC1, CYP19A1, DDX3X, and TIMP3.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhtFGqsr7J&md5=e01711dc8d08f29d174ad373c799c12eCAS |
Pohnke, Y., Schneider-Merck, T., Fahnenstich, J., Kempf, R., Christian, M., Milde-Langosch, K., Brosens, J. J., and Gellersen, B. (2004). Wild-type p53 protein is up-regulated upon cyclic adenosine monophosphate-induced differentiation of human endometrial stromal cells. J. Clin. Endocrinol. Metab. 89, 5233–5244.
| Wild-type p53 protein is up-regulated upon cyclic adenosine monophosphate-induced differentiation of human endometrial stromal cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXovVemtrs%3D&md5=6de1c3695c36d1973a1aebbc54712ae2CAS |
Robb, L., Li, R., Hartley, L., Nandrukar, H., Koentgen, F., and Begley, C. (1998). Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat. Med. 4, 303–308.
| Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK1cXhs1Kjtbo%3D&md5=9ecbb8beb8feb01b7de8e9a263d5bd81CAS |
Shukla, G. C., Singh, J., and Barik, S. (2011). MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol. Cell. Pharmacol. 3, 83–92.
| 1:CAS:528:DC%2BC38XhsVGjtLs%3D&md5=3a814218f713ef41cbde72ea7eeb7d73CAS |
Stad, R., Little, N. A., Xirodimas, D. P., Frenk, R., van der Eb, A. J., Lane, D. P., Saville, M. K., and Jochemsen, A. G. (2001). Mdmx stabilizes p53 and Mdm2 via two distinct mechanisms. EMBO Rep. 2, 1029–1034.
| Mdmx stabilizes p53 and Mdm2 via two distinct mechanisms.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXovFOnsb8%3D&md5=e1d93b4ea7eecce596ffd3b96f497116CAS |
Stewart, C. L., Kaspar, P., Brunet, L. J., Bhatt, H., Gadi, I., Köntgen, F., and Abbondanzo, S. J. (1992). Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature 359, 76–79.
| Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38XlvVGmtLc%3D&md5=803a2f5229eb4cb9a9a3610c7dadbf82CAS |
Timeva, T., Shterev, A., and Kyurkchiev, S. (2014). Recurrent implantation failure: the role of the endometrium. J. Reprod. Infertil. 15, 173–183.
Vogiagis, D., Marsh, M., Fry, R., and Salamonsen, L. (1996). Leukaemia inhibitory factor in human endometrium throughout the menstrual cycle. J. Endocrinol. 148, 95–102.
| Leukaemia inhibitory factor in human endometrium throughout the menstrual cycle.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK287msFSktA%3D%3D&md5=3122f9ed16c6e41c924f1b969316eb2eCAS |
Wade, M., Li, Y. C., and Wahl, G. M. (2013). MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat. Rev. Cancer 13, 83–96.
| MDM2, MDMX and p53 in oncogenesis and cancer therapy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3sXkvFKmtA%3D%3D&md5=a4f917f6c42cfa2a37c8a643d8870192CAS |
White, C. A., Zhang, J.-G., Salamonsen, L. A., Baca, M., Fairlie, W. D., Metcalf, D., Nicola, N. A., Robb, L., and Dimitriadis, E. (2007). Blocking LIF action in the uterus by using a PEGylated antagonist prevents implantation: a nonhormonal contraceptive strategy. Proc. Natl. Acad. Sci. USA 104, 19357–19362.
| Blocking LIF action in the uterus by using a PEGylated antagonist prevents implantation: a nonhormonal contraceptive strategy.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXisVOjuw%3D%3D&md5=90d5da27268ba11af849275dbd6d881cCAS |
Winship, A. L., Koga, K., Menkhorst, E., Van Sinderen, M., Rainczuk, K., Nagai, M., Cuman, C., Yap, J., Zhang, J. G., Simmons, D., Young, M. J., and Dimitriadis, E. (2015). Interleukin-11 alters placentation and causes preeclampsia features in mice. Proc. Natl. Acad. Sci. USA 112, 15928–15933.
| Interleukin-11 alters placentation and causes preeclampsia features in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXhvF2kt7zO&md5=0ecb8cd27d5c2b4d9aef1ac98eb291beCAS |
Winship, A., Van Sinderen, M., Rainczuk, K., and Dimitriadis, E. (2017). Therapeutically blocking interleukin-11 receptor-alpha enhances doxorubicin cytotoxicity in high grade type I endometrioid tumours. Oncotarget 8, 22716–22729.
| Therapeutically blocking interleukin-11 receptor-alpha enhances doxorubicin cytotoxicity in high grade type I endometrioid tumours.Crossref | GoogleScholarGoogle Scholar |
Yang, J. Y., Zong, C. S., Xia, W., Wei, Y., Ali-Seyed, M., Li, Z., Broglio, K., Berry, D. A., and Hung, M. C. (2006). MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation. Mol. Cell. Biol. 26, 7269–7282.
| MDM2 promotes cell motility and invasiveness by regulating E-cadherin degradation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVCltbfJ&md5=c2dfb88cdd3677db14f0034d3269ff87CAS |
Yu, H., Yue, X., Zhao, Y., Li, X., Wu, L., Zhang, C., Liu, Z., Lin, K., Xu-Monette, Z. Y., Young, K. H., Liu, J., Shen, Z., Feng, Z., and Hu, W. (2014). LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers. Nat. Commun. 5, 5218.
| LIF negatively regulates tumour-suppressor p53 through Stat3/ID1/MDM2 in colorectal cancers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2MXksVekt7g%3D&md5=03e01669a884dadab620a1c7b036229bCAS |
Zhang, C., Liu, J., Wang, X., and Feng, Z. (2015). The regulation of the p53/MDM2 feedback loop by microRNAs. RNA Dis. 2, e502.
Zhou, J., Liu, F., Zhang, D., Chen, B., Li, Q., Zhou, L., Lu, L. M., and Tao, L. (2014). Significance of MDM2–309 polymorphisms and induced corresponding plasma MDM2 levels in susceptibility to laryngeal squamous cell carcinoma. DNA Cell Biol. 33, 88–94.
| Significance of MDM2–309 polymorphisms and induced corresponding plasma MDM2 levels in susceptibility to laryngeal squamous cell carcinoma.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC2cXitVygt7s%3D&md5=3b470d8bc642ab7911269c5d40a49a3dCAS |