Predictive value of bovine follicular components as markers of oocyte developmental potential
Satoko Matoba A C , Katrin Bender B , Alan G. Fahey A , Solomon Mamo A , Lorraine Brennan B , Patrick Lonergan A and Trudee Fair A DA School of Agriculture and Food Science, University College Dublin, Belfield, Dublin 4, Ireland.
B Institute of Food and Health, Conway Institute, University College Dublin, Belfield, Dublin 4, Ireland.
C Present address: National Livestock Breeding Center, Odakurahara 1, Nishigo, Fukushima 961-8511, Japan.
D Corresponding author. Email: trudee.fair@ucd.ie
Reproduction, Fertility and Development 26(2) 337-345 https://doi.org/10.1071/RD13007
Submitted: 21 June 2012 Accepted: 18 January 2013 Published: 21 March 2013
Abstract
The follicle is a unique micro-environment within which the oocyte can develop and mature to a fertilisable gamete. The aim of this study was to investigate the ability of a panel of follicular parameters, including intrafollicular steroid and metabolomic profiles and theca, granulosa and cumulus cell candidate gene mRNA abundance, to predict the potential of bovine oocytes to develop to the blastocyst stage in vitro. Individual follicles were dissected from abattoir ovaries, carefully ruptured under a stereomicroscope and the oocyte was recovered and individually processed through in vitro maturation, fertilisation and culture. The mean (± s.e.m.) follicular concentrations of testosterone (62.8 ± 4.8 ng mL–1), progesterone (616.8 ± 31.9 ng mL–1) and oestradiol (14.4 ± 2.4 ng mL–1) were not different (P > 0.05) between oocytes that formed (competent) or failed to form (incompetent) blastocysts. Principal-component analysis of the quantified aqueous metabolites in follicular fluid showed differences between oocytes that formed blastocysts and oocytes that degenerated; l-alanine, glycine and l-glutamate were positively correlated and urea was negatively correlated with blastocyst formation. Follicular fluid associated with competent oocytes was significantly lower in palmitic acid (P = 0.023) and total fatty acids (P = 0.031) and significantly higher in linolenic acid (P = 0.036) than follicular fluid from incompetent oocytes. Significantly higher (P < 0.05) transcript abundance of LHCGR in granulosa cells, ESR1 and VCAN in thecal cells and TNFAIP6 in cumulus cells was associated with competent compared with incompetent oocytes.
Additional keywords: cattle, follicular fluid, IVF, metabolomics, mRNA expression, oocyte quality.
Introduction
As the use of in vitro maturation (IVM) and single embryo transfer (SET) becomes more routine in assisted reproductive technology (ART), the selection of oocytes or embryos with the highest developmental potential is critical to a successful outcome. New technologies, such as transcriptomics, proteomics and metabolomics, have allowed researchers and clinicians to investigate more sophisticated methods for oocyte and embryo selection, with the emphasis on the predictive value of superfluous fluids and tissues, such as follicular fluid, follicle cells and cumulus cells, for non-invasive assessment of oocyte quality (Revelli et al. 2009). Recent studies on bovine oocytes have highlighted the potential of metabolomic technologies in this area (Sinclair et al. 2008; Bender et al. 2010). Both of these studies suggest that metabolomic analysis of follicular fluid may be a useful tool for characterising oocyte quality. Most recent data from human ART research points at the use of a panel of parameters to predict subsequent oocyte developmental potential (Lédée et al. 2007). However, it is often difficult to obtain ‘clean’ data from human ART research as the numbers of tissues analysed in one clinic are often insufficient to generate statistically robust data and therefore datasets may be compiled from more than one clinic using different protocols. Bovine in vitro embryo production provides an excellent resource for the identification of predictive markers of oocyte and embryo potential as large numbers of oocytes are easily retrieved from the ovaries of slaughtered cattle and experimental conditions can be standardised for each trial. From the bovine perspective, markers of oocyte quality have applications in research focussed on mechanisms to improve cow fertility. This study tested the hypothesis that markers in follicular fluid or on cumulus cells are predictive of oocyte development to the blastocyst stage. The specific objective of the present study was to assess the relationship between a panel of non-invasive markers of oocyte competence from follicular fluid and tissues and oocyte development following IVM, in vitro fertilisation (IVF) and in vitro culture (IVC). To this end, the steroid hormone and metabolomic profiles of follicular fluid and candidate gene mRNA expression profiles of granulosa and theca cells were analysed in relation to oocyte development in vitro. In a second experiment, the relationship between the relative abundance of a panel of candidate genes in cumulus-cell biopsies and the developmental competence of the oocyte was assessed.
Materials and methods
All chemicals were obtained from Sigma-Aldrich (Poole, Dorset, UK) unless otherwise indicated.
Experiment 1: relationship between follicular parameters and oocyte developmental competence
Ovary collection, follicle dissection and follicle fluid and tissue collection
Pairs of bovine ovaries were collected at a local abattoir, stored on a per animal basis (n = 49) and transported to the laboratory in phosphate-buffered saline (PBS) at 35°C within 4 h. Individual follicles (n = 213, mean ± s.e.m. diameter 7.92 ± 0.2 mm) were dissected from the ovaries using fine scissors. The diameter of each follicle was measured and the follicles were carefully ruptured using a surgical blade under a stereomicroscope. Once the cumulus–oocyte complex (COC) had been recovered, the follicular fluid was transferred to a 0.6-mL PCR tube and immediately placed on ice until all follicles on a given day were processed (n = 20), after which they were centrifuged for 2 min at 2500g at 4°C to remove any cells and stored at –80°C until steroid and metabolomic analysis. Morphologically-normal COCs were washed in PBS supplemented with 1 mg mL–1 glucose, 36 µg mL–1 pyruvate and 0.5 mg mL–1 bovine serum albumin (BSA) and processed for in vitro embryo production in an individually identifiable manner (Matoba et al. 2010). Concurrent with COC processing, granulosa and theca cells were recovered as previously described (Forde et al. 2008). Following follicle rupture, granulosa cells were scraped from the theca layer by a glass scraper. The theca layer was then peeled away from the stroma with forceps. Given the nature of the cell types, the granulosa cells are presumed to be very pure while some granulosa cell ‘contamination’ of thecal cells cannot be ruled out. Therefore, in line with previous publications, these cells are best referred to as ‘theca-enriched’ cells, although for ease of reading they are referred to as theca cells in the paper. The two cell types were separately transferred to 1.5-mL PCR tubes on ice, centrifuged for 2 min at 21 000g at 4°C and each pellet was immersed in 500 µL RNAlater (1 : 5 w/v) and stored at 4°C for 24 h, after which the RNA later was removed by centrifugation for 10 min at 3000g at 4°C and removal of supernatant and cell pellets were snap frozen in liquid nitrogen and stored at –80°C until RNA extraction. Follicular parameters that were analysed are listed in Table 1.
In vitro embryo production
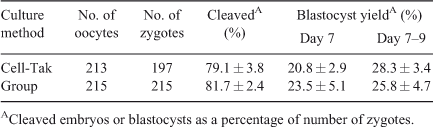
Dishes for in vitro embryo production were prepared with Cell-Tak or a polyester mesh as previously described (Matoba et al. 2010). For IVM, COCs were individually placed in groups of 20 on Cell-Tak in 100-µL droplets of IVM medium (TCM 199 with Earle’s salts, supplemented with 10% (v/v) fetal calf serum (FCS) and 10 ng mL–1 epidermal growth factor). Droplets were overlaid with mineral oil and maturation took place at 39°C for ~24 h in a humidified atmosphere of 5% CO2 in air. Subsequently, IVM medium was replaced with IVF medium (Tyrode’s medium supplemented with 25 mM bicarbonate, 22 mM Na-lactate, 1 mM Na-pyruvate, 6 mg mL–1 fatty acid-free BSA and 10 µg mL–1 heparin–sodium salt (184 U mL–1 heparin; Calbiochem, San Diego, CA, USA)). Each droplet was inseminated with frozen–thawed and Percoll-separated (GE Healthcare Bio-Sciences AB, Uppsala, Sweden) spermatozoa from a single bull (Progressive Genetics, Enfield, Ireland) at 1 × 106 spermatozoa mL–1. Gametes were co-incubated for 18–20 h at 39°C in a humidified atmosphere of 5% CO2 in air. Following IVF, presumptive zygotes were individually denuded by vortexing for 2 min and gentle pipetting with a fine glass pipette and washed in PBS and culture medium. They were then transferred to a new Cell-Tak dish containing 100-µL droplets of synthetic oviduct fluid (SOF) medium (Holm et al. 1999) supplemented with 5% (v/v) FCS and cultured at 39°C for 9 days in an atmosphere of 5% CO2, 5% O2 and 90% N2 in humidified air. Cleavage and blastocyst rates were assessed on Day 2 (i.e. 48 h after insemination; day of IVF = Day 0) and on Days 7 to 9, respectively. Eleven replicates were carried out (~20 follicles or COCs per replicate); each replicate represents a single day of ovary collection.
Follicular fluid steroid concentrations
Concentrations of testosterone (T4), progesterone (P4) and oestradiol (E2) in all follicles were assayed by radio-immuno assay (RIA) using 125I-total testosterone Coat-a-Count kit (Siemens Medical Solution Diagnostics, Los Angeles, CA, USA), 125I Progesterone Coat-a-Count kit (Siemens Medical Solution Diagnostics) and 125I Oestradiol MAIA kit (Adaltis Italia S.p.A., Casalecchio di Reno, Italy). Follicular-fluid samples were diluted 1 : 50 with PBS for T4 and 1 : 100 with PBS for P4 and E2; each assay was carried out in duplicate. The inter-assay coefficients of variation (CV) of T4, P4 and E2 were 18.8, 12.5 and 13.7% for low, 6.9, 3.3 and 6.0% for medium and 7.5, 19.5 and 8.0% for high, respectively. The intra-assay CV of T4, P4 and E2 were 7.6, 11.7 and 11.5% (low), 6.5, 6.6 and 8.4% (medium) and 7.6, 4.9 and 12.0% (high), respectively. The sensitivities of the assays were 0.02 ng tube–1 for T4, 0.02 ng mL–1 for P4 and 0.05 ng mL–1 for E2.
Metabolite extraction and analysis
Metabolomic profiling of the aqueous and fatty-acid components of the follicular fluid was carried out as previously described (Bender et al. 2010) on a subset of samples from oocytes that cleaved and formed blastocysts or cleaved but subsequently degenerated (n = 18, mean ± s.e.m diameter 7.47 ± 0.16 mm). For aqueous metabolite analysis, 40 µL of sample were combined with 160 µL of water and 5 µL of 13C myristic acid (Cambridge Isotopes, Andover, MA, USA) as internal standard before extraction with 800 µL methanol (Jiye et al. 2008). Extracts were dried and samples were derivatised and analysed using an Agilent 7890A GC with an Agilent HP-5 ms 30 m × 250 µm × 0.25 µm column coupled with a 5975C MS (Agilent Technologies Ireland Ltd., Cork, Ireland). Compound identification and calibration was achieved by referencing to in-house standards and an amino-acid mix (1 mM amino acid standard solution; Sigma Aldrich, Buchs, Switzerland) using Agilent Chemstation MSD E.02.00.493. (Agilent Technologies Ireland Ltd.) and by comparison of their mass spectra with those in the National Institute of Standards and Technology (NIST) Library 2.0 (2005).
For the analysis of organic compounds, 50 µL of follicular fluid were combined with 50 µL of water and 10 µL of 2 mg mL–1 pentadecanoic acid (C15 : 0) as internal standard and extracted using a 1 : 2 mixture of chloroform : methanol based on the method of (Bligh and Dyer 1959). Extracts were derivatised by methylation using methanolic BF3. Derivatives were re-suspended in 200 µL hexane and analysed on an Agilent 7890A GC coupled with a 5975C MS with an Agilent HP-5 ms 30 m × 250 µm × 0.25 µm column. Two microlitres were injected in splitless mode, and the initial oven temperature of 70°C was raised to 220°C at 5°C min–1, held for 20 min, and then raised to 320°C at 20°C min–1. Helium was used as carrier gas with a flow of 1.2 mL min–1. Calibration was achieved by comparison of peak areas for fatty acids with reference to a known standard (Supelco 37 compound mix; Supelco, Poole, UK) using Agilent Chemstation MSD E.02.00.493 and by comparison of their mass spectra with those in the National Institute of Standards and Technology (NIST) Library 2.0 (2005). For quality control purposes, two aliquots from a pool of follicular fluid were extracted and analysed in parallel with each batch of samples.
Automatic peak detection was carried out with Agilent Chemstation MSD. Mass spectra deconvolution was performed with AMDIS version 2.65. Peaks with a signal-to-noise ratio (S/N) lower than 30 were rejected. To obtain accurate peak areas for the internal standard and specific peaks or compounds, one quant mass for each peak was specified as target and three masses were selected as qualifiers. Each data file was manually analysed for false positives and negatives in Agilent Chemstation.
The aqueous metabolite data was divided into compounds identified and quantified using external standards and compounds semiquantified relative to the internal standard only. Concentrations given for fatty acids are µg mL–1 + s.e.m.; concentrations for quantified amino acids, glucose and urea are µmol L–1.
RNA isolation and quantitative real-time PCR (Q-PCR)
Excess RNAlater was removed and granulosa and theca cell samples were transferred to RNase/DNase-free tubes for analysis of gene expression by Q-PCR. Total RNA was extracted from granulosa and theca cells originating from individual follicles using the guanidine-based TRI Reagent according to the manufacturer’s instructions. Subsequently, mRNA was isolated from the eluted total granulosa and theca cell RNA using the mRNA Direct TM Micro kit (Invitrogen). Isolated mRNA was eluted in 7 µL of RNase-free water and was DNase treated with DNase I (Invitrogen) resulting in a total volume of 10 µL. Messenger RNA was reverse transcribed using Superscript III (Invitrogen) and random primers (Invitrogen) according to the manufacturer’s instructions (total volume 20 µL). Subsequently, granulosa and theca cell cDNA was diluted (1 : 20) with RNase-free water to a final volume of 380 µL.
Negative controls were generated by omitting RNA from the cDNA synthesis reaction. Reverse transcription PCR employing intron-spanning primers for PPIA was used to confirm successful DNase treatment, generating a 482-bp or 108-bp amplicon in the presence or absence of DNA, respectively. Transcript abundance of candidate genes was analysed in granulosa and theca cell samples from follicles associated with embryos that cleaved and developed to the blastocyst stage (competent, n = 15) and those that cleaved but failed to develop (incompetent, n = 15). The quantification of all gene transcripts was carried out using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA), and performed on the ABI 7300 Real-Time PCR System (Applied Biosystems) using the standard-curve method of analysis. Primers were designed using Primer 3 software (http://primer3.sourceforge.net/; see Tables S1 and S2 available as Supplementary Material to this paper for gene list and primer details). Each reaction was carried out in a total volume of 15 µL, consisting of 4 µL cDNA, 7.5 µL Power SYBR Green PCR Master Mix and the optimum concentration of forward and reverse primers (Eurofins MWG Operon, Ebersberg, Germany) and RNase-free water. Reactions were performed in duplicate in 96-well plates, and four non-template control samples were included for each primer set. The thermal cycling conditions consisted of 50°C for 2 min and 95°C for 10 min, followed by 45 cycles of 95°C for 15 s and annealing for 1 min at 60°C. The specificity of the amplicon was confirmed by melting-curve analysis, consisting of 40 cycles at 95°C for 15 s and 60°C for 1 min, followed by 95°C for 15 s and 60°C for 15 s. The mRNA abundance for each target gene was normalised against the levels of the constitutive housekeeping gene PPIA in all cell types.
Experiment 2: relationship between expression of candidate genes in cumulus cell biopsies and developmental competence of bovine oocytes
Preliminary experiments were carried out to establish the effect of taking a cumulus-cell biopsy from immature and in vitro-matured COCs on development to the blastocyst stage. Based on the results of these experiments, a subsequent experiment was carried out where a biopsy was taken from individual in vitro-matured bovine COCs and snap frozen in liquid nitrogen. The COCs were then fertilised and cultured in vitro in an individually identifiable manner using a polyester mesh system as previously described (Matoba et al. 2010). Following 7 days of culture, cumulus cell biopsies were pooled in groups of five according to whether the oocytes from which they were derived cleaved and developed to the blastocyst stage or cleaved but failed to reach the blastocyst stage (n = 5 replicate pools each).
Statistical analysis
Steroid concentrations (least-square means ± s.e.m.) in follicles that yielded oocytes that developed to the blastocyst stage, that cleaved but failed to develop or that were not cleaved after insemination were compared by analysis of variance (PROC MIXED in SAS, SAS Institute Inc., Cary, NC, USA). Regarding the metabolomic analysis of follicular fluid, principle component analysis (PCA) and partial least-squares discriminant analysis (PLS-DA) was performed using SIMCA-P+11 (Umetrics, Umea, Sweden). The quality of the models formed by PCA and PLS-DA were investigated by interrogation of the R2 and Q2 values. The R2 parameter is a representation of how much of the variation within the dataset is explained by the components of the model. The Q2 parameter gives an indication of how good the model is at class prediction. Correlation analysis, general linear model analysis and Bonferroni posthoc testing was performed using SPSS 14 (IBM, New York, NY, USA). Significance was assumed using P < 0.05 as a cut-off.
Gene expression data were analysed using the standard-curve method. Values were normalised to the average values of the reference gene and means were compared by Student’s t-test. Differences were considered to be significant when P < 0.05.
Results
Relationship between follicular parameters and oocyte developmental competence
Oocyte competence
Developmental data from Experiment 1 indicated that follicle dissection and individual culture on Cell-Tak did not affect cleavage or blastocyst formation rate (Table 2; P > 0.05). In Experiment 2, taking a cumulus biopsy from immature COCs resulted in a significant reduction in subsequent blastocyst development compared with unmanipulated controls, irrespective of whether culture took place in groups (Table S3) or individually in a mesh system (Table S4). In contrast, biopsy of the expanded cumulus cells associated with in vitro-matured oocytes had no detrimental effect on subsequent development (Tables S1 and S2). Thus, subsequent experiments were carried out solely on in vitro-matured oocytes.
|
Follicular T4, P4 and E2 concentration
The mean (± s.e.m.) follicular concentrations of T4, P4 or E2 were not different (P > 0.05) between oocytes that developed to the blastocyst stage (competent oocytes) and oocytes that cleaved after fertilisation and then degenerated (incompetent oocytes; Fig. 1).
Relationship between follicular granulosa and theca cell candidate gene expression and oocyte developmental competence
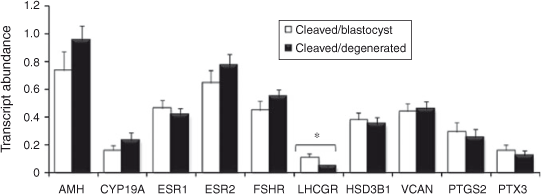
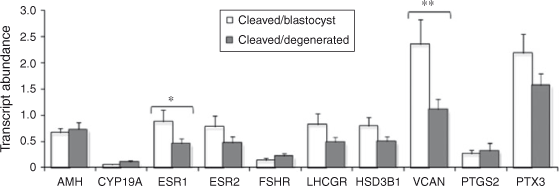
The results are presented in Figs 2 and 3. Briefly, LHCGR mRNA abundance was significantly higher in granulosa cells associated with competent oocytes compared with incompetent oocytes (P < 0.05). The expression of VCAN and ESR1 was higher in thecal cells associated with competent compared with incompetent oocytes (P < 0.05 and P < 0.08, respectively). The abundance of all other transcripts tested was not different between groups.
|
|
Relationship between cumulus cell candidate gene expression and oocyte developmental competence
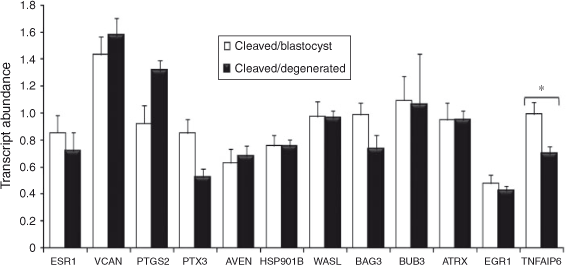
The results are presented in Fig. 4. Briefly, TNFAIP6 mRNA abundance was significantly higher in mature cumulus cells from competent oocytes compared with incompetent oocytes (P < 0.06). The remaining candidate genes were not significantly differentially expressed.
|
Metabolite profile of follicular fluid
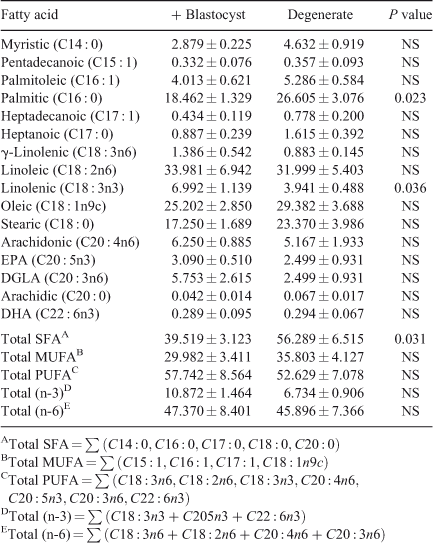
Fatty-acid profile. A total of 16 fatty acids were identified and quantified and the total fatty acid (SFA), total monounsaturated fatty acid (MUFA), total polyunsaturated fatty acid (PUFA), (n-3) PUFA and (n-6) PUFA contents were determined (Table 3). ANOVA analysis of the fatty-acid data showed that follicular fluid from oocytes that cleaved but subsequently degenerated was significantly higher in palmitic acid (P = 0.023) and total SFA (P = 0.031) and significantly lower in linolenic acid (P = 0.036) compared with follicular fluid from competent oocytes (Table 3). Multiple-regression analysis of aqueous compounds with follicle parameters showed that T4 concentrations were positively correlated with arachidonic acid (P < 0.001), pentadecanoic acid (P < 0.001) and heptadecanoic acid (P = 0.045) but was negatively correlated with oleic acid (P = 0.005). P4 concentrations were positively correlated with heptadecanoic acid (P = 0.002) and arachidonic acid (P = 0.045).
|
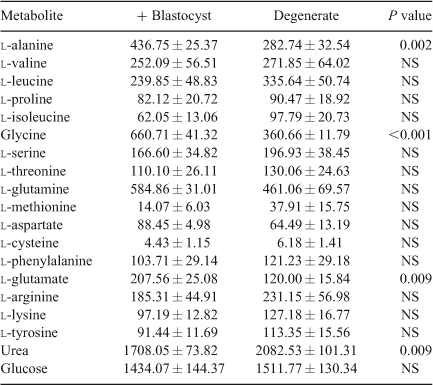
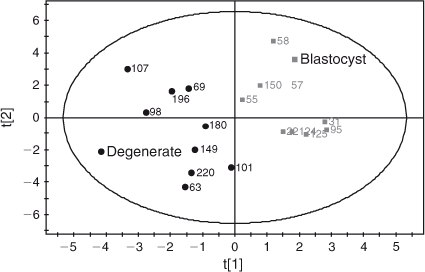
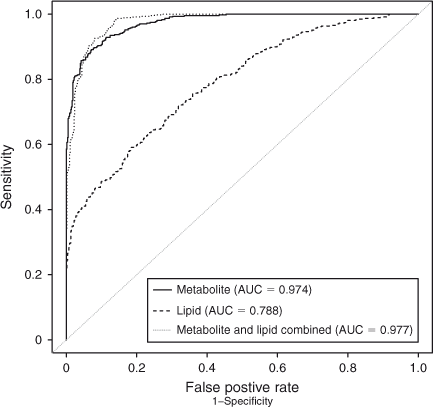
Amino acid profile. A total of 55 aqueous compounds were analysed of which 19 were quantified (Table 4) and 38 semiquantified. Principal-component analysis of the quantified data showed separation between oocytes that formed blastocysts and oocytes that degenerated (R 2 = 0.561). Further analysis using PLS-DA generated a robust model that could predict which oocytes formed blastocysts (R 2 X = 0.552; R 2 Y = 0.874; Q 2 = 0.722; see Fig. 5). Analysis of the corresponding variable importance plots showed that l-alanine, glycine and l-glutamate were positively correlated with blastocyst development. Urea was negatively correlated with blastocyst formation. ANOVA analysis validated these results (Table 4). Multiple-regression analysis of aqueous compounds with follicle parameters showed that l-leucine was positively correlated with T4 (P = 0.03) whereas l-glutamine was negatively correlated with P4 (P = 0.007). Receiver-operating curve analysis was used to identify parameters that were predictive of oocyte development. Follicular steroid concentrations had no predictive value (area under curve (AUC) = 0.35). However, the aqueous metabolites were predictive of potential to develop to the blastocyst stage and a combination of the fatty acids and aqueous data showed a slight improvement in predictive potential (Fig. 6).
|
|
|
Discussion
The objective of the present study was to test the ability of a panel of non-invasive markers from follicular tissue to predict oocyte developmental competence. Following extensive reviews of the literature a panel of candidate genes was assembled for mRNA expression analysis in follicular granulosa and theca cells. The expression of four of the genes was associated with development to the blastocyst stage; LHCGR expression was significantly higher in granulosa cells (GC) associated with competent oocytes compared with incompetent oocytes, VCAN and ESR1 transcript abundance was significantly higher in thecal cells associated with competent compared with incompetent oocytes and TNFAIP6 was significantly higher in post IVM cumulus cells associated with competent compared with incompetent oocytes. Similar findings have been reported in relation to gene expression in human follicular cells as predictors of pregnancy outcome post IVF, where only two genes from a panel of 10 were significantly different despite controlling for embryo quality at the outset (Hamel et al. 2010). The induction of expression of the LHCGR mRNA in GC was recently identified as an early event during the follicle selection process (Luo et al. 2011) and is essential for follicular maturation in the progression from antral to preovulatory stage (Pakarainen et al. 2007). Follicles less than 8 mm in diameter were selected for the present study, excluding the possibility of using selected dominant follicles; therefore, granulosa cell LHCGR expression may also be reflective of follicle viability. Similarly, selective deletion of the Esr1 gene in murine theca cells (thEsr1KO) results in female infertility before the age of 6 months, which is manifested at the level of the ovary by fewer ovulations and therefore fewer corpora lutea, a greater number of early antral follicles and markedly lower or undetectable LH levels in the Esr1KO mice (Lee et al. 2009), again suggesting that higher ESR1 expression is associated with follicle viability. Versican is an abundant extracellular matrix proteoglycan that binds hyaluronic acid and is hormonally regulated in the ovary and is strongly induced at the time of ovulation (Russell et al. 2003b ). Versican is produced by mural granulosa cells in rat and mouse ovaries, but localises selectively to the granulosa–thecal boundary of preovulatory follicles and intensely in expanded COC matrix (Russell et al. 2003b ). Versican is a key substrate for ADAMTS1 proteolysis (Sandy et al. 2001), and versican cleavage occurs rapidly in the expanded COC matrix around the time of ovulation (Russell et al. 2003a , 2003b ). In Adamts1 –/– mice, ovulation rate, fertilisation rate and fertility were reduced by 68–75%, versican cleavage in the expanded COC was 75% lower and structural organisation of the COC matrix was strikingly disrupted. Sites of ADAMTS1 activity localised to the granulosa–thecal interface of ovulating follicles, as well as COC and dysmorphogenesis, were evident in these regions of Adamts1 –/– follicles. Furthermore, the degradation of the COC matrix in oviducts after ovulation was delayed. Our results support the importance of ADAMTS1 in remodelling the extracellular matrix.
Although the present study confirmed the feasibility of individual oocyte tracking during IVC as a model for identifying follicle tissue-derived transcripts as potential biomarkers of oocyte competence, the use of gene-expression analysis of mature cumulus cells as a predictor of oocyte competence was only verified for one previously identified candidate gene (TNFAIP6). TNFAIP6 is a secretory protein of the hyaluronan-binding protein family, which is involved in extracellular matrix formation, playing a role in cumulus cell stabilisation and expansion (Fulop et al. 2003). Our findings cement earlier recommendations of TNFAIP6 as a potential cumulus cell indicator of oocyte competence in cattle (Assidi et al. 2008; Tesfaye et al. 2009). The panel of candidate genes was assembled following a thorough cross-species review of mammalian oocyte and cumulus cell transcriptomic data (O’Shea et al. 2012). The poor correlation between our findings and that of previously published findings is possibly due to the variation in oocyte maturation and sample processing regimes associated with different species. This emphasises the challenge associated with identifying universal somatic cell or follicle-derived predictors of oocyte competence and IVF outcome and highlights the importance of customising biomarker panels according to the oocyte maturation regime within a clinic or laboratory (Grøndahl et al. 2009; Adriaenssens et al. 2010).
Metabolomic analysis of bovine follicular fluid from single-embryo culture
Our recent comparison of the metabolomic profile of preovulatory follicular fluid from nulliparous heifers and postpartum dairy cows has indicated the potential predictive value of metabolomic analysis of follicular fluid for the assessment of oocyte quality (Bender et al. 2010). Consistent with those observations, in the present model both amino acids and fatty acids were found to be predictive of the developmental competence of the oocyte. In contrast to other studies, our results show significant differences in the fatty-acid fraction of competent and degenerate oocytes in terms of their C16 : 0, C18 : 3n3 and saturated fatty-acid concentrations (Sinclair et al. 2008). Follicular fluid from degenerate oocytes was significantly higher in palmitic acid as well as in total saturated fatty acids. The detrimental effects of increased palmitic acid in follicular fluid are well documented in the literature. In vitro studies have shown that increased palmitic acid concentrations resulted in a decreased rate of blastocyst formation (Leroy et al. 2005). Increased concentrations of both palmitic acid and stearic acid impair meiosis, cleavage rate and blastocyst formation (Leroy et al. 2005) as well as exerting adverse effects on both bovine (Vanholder et al. 2005) and human (Mu et al. 2001) granulosa cell growth and function. High concentrations of fatty acids are associated with impaired embryo quality through lipid accumulation (Reis et al. 2003), and developmentally competent human oocytes exhibit low levels of saturated fatty acids (Haggarty et al. 2006). In the present study, the follicular fluid associated with oocytes that formed blastocysts had higher concentrations of the n-3 PUFA linolenic acid. Our previous results demonstrated that follicular-fluid n-3 PUFA levels were higher in a high-fertility bovine model (Bender et al. 2010). Supporting the important role of n-3 PUFA, a recent study demonstrated that a concentration of 50 µM linolenic acid in the culture medium enhanced the number of metaphase II (i.e. mature) stage oocytes resulting from bovine COCs (Marei et al. 2009). Overall, the present results demonstrate the potential role of fatty-acid concentrations in predicting oocyte developmental competence.
The amino acids l-alanine, glycine and glutamate were predictive of the developmental ability of the oocyte. Several studies have described the beneficial effects of alanine (Cetica et al. 2003) and l-alanine and glycine (Lee and Fukui 1996) on embryonic development. Furthermore, l-alanine, glycine, proline, valine and glutamate were proposed to be predictive for oocyte development (Sinclair et al. 2008). Most recently, amino-acid profiling of spent IVM medium revealed higher consumption of glutamine and higher production of alanine during IVM by MII oocytes that failed to cleave by 72 h (Hemmings et al. 2012) Taken together, these findings may be used to optimise in vitro-maturation conditions through supplementation or oocyte profiling.
Linking in with the altered amino-acid levels, it was also found that urea levels were negatively correlated with blastocyst formation. This is consistent with the findings of De Wit et al. (2001) who reported negative effects of urea during IVM on the meiotic progression of bovine oocytes and subsequent cleavage and blastocyst development. An earlier study also reported reduced in vitro blastocyst development rates from oocytes recovered from heifers with increased concentrations of plasma urea (Santos et al. 2009). As urea concentrations have been shown to be similar in plasma and preovulatory follicular fluid in women, the blood plasma data supports our findings (Jozwik et al. 2006).
In conclusion, oocyte developmental potential is reflected in the gene expression signature and metabolomic profile of follicular tissue. Of all parameters measured, receiver-operating characteristic analysis indicated that the best predictor of oocyte developmental competence was the follicular-fluid metabolite profile. Furthermore, when combined, lipid and metabolite profiling improved the predictive capacity.
Acknowledgements
The authors thank Mary Wade, Emma Gallagher, Lydia O’Hara, Yu Fan and Pedro Pires for excellent assistance and staff at Kildare Chilling and Kepak for allowing access to bovine tissues. This work was supported by Science Foundation Ireland (grant number 07/SRC/B1156).
References
Adriaenssens, T., Wathlet, S., Segers, I., Verheyen, G., De Vos, A., Van der Elst, J., Coucke, W., Devroey, P., and Smitz, J. (2010). Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics. Hum. Reprod. 25, 1259–1270.| Cumulus cell gene expression is associated with oocyte developmental quality and influenced by patient and treatment characteristics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXkvFOjsbY%3D&md5=4308a412f14c6dce3b27fc662dea1220CAS | 20228394PubMed |
Assidi, M., Dufort, I., Ali, A., Hamel, M., Algriany, O., Dielemann, S., and Sirard, M. A. (2008). Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate in vitro. Biol. Reprod. 79, 209–222.
| Identification of potential markers of oocyte competence expressed in bovine cumulus cells matured with follicle-stimulating hormone and/or phorbol myristate acetate in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovFWms7o%3D&md5=2d3803a6998081a883758471616f201dCAS | 18417710PubMed |
Bender, K., Walsh, S., Evans, A. C. O., Fair, T., and Brennan, L. (2010). Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows. Reproduction 139, 1047–1055.
| Metabolite concentrations in follicular fluid may explain differences in fertility between heifers and lactating cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXns12qsbs%3D&md5=6169bea462f4d1f1edc632e7125f21c8CAS | 20385782PubMed |
Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911–917.
| A rapid method of total lipid extraction and purification.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaG1MXhtVSgt70%3D&md5=cc01ae73966d033e8c0506071699032aCAS | 13671378PubMed |
Cetica, P., Pintos, L., Dalvit, G., and Beconi, M. (2003). Involvement of enzymes of amino acid metabolism and tricarboxylic acid cycle in bovine oocyte maturation in vitro. Reproduction 126, 753–763.
| Involvement of enzymes of amino acid metabolism and tricarboxylic acid cycle in bovine oocyte maturation in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXmvFWktg%3D%3D&md5=74f471be787999546296e1b51d2dc748CAS | 14748694PubMed |
De Wit, A. A., Cesar, M. L., and Kruip, T. A. (2001). Effect of urea during in vitro maturation on nuclear maturation and embryo development of bovine cumulus–oocyte complexes. J. Dairy Sci. 84, 1800–1804.
| Effect of urea during in vitro maturation on nuclear maturation and embryo development of bovine cumulus–oocyte complexes.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXmtFaqurg%3D&md5=6202a54570e75644b94d4958ffa2c97aCAS | 11518303PubMed |
Forde, N., Rogers, M., Canty, M. J., Lonergan, P., Smith, G. W., Coussens, P. M., Ireland, J. J., and Evans, A. C. O. (2008). Association of the prion protein and its expression with ovarian follicle development in cattle. Mol. Reprod. Dev. 75, 243–249.
| Association of the prion protein and its expression with ovarian follicle development in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovVCrtg%3D%3D&md5=2be58601a75c3f091de8b8b7b472bd56CAS | 17595008PubMed |
Fülöp, C., Szántó, S., Mukhopadhyay, D., Bárdos, T., Kamath, R. V., Rugg, M. S., Day, A. J., Salustri, A., Hascall, V. C., Glant, T. T., and Mikecz, K. (2003). Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development 130, 2253–2261.
| Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice.Crossref | GoogleScholarGoogle Scholar | 12668637PubMed |
Grøndahl, M. L., Borup, R., Lee, Y. B., Myrhøj, V., Meinertz, H., and Sørensen, S. (2009). Differences in gene expression of granulosa cells from women undergoing controlled ovarian hyperstimulation with either recombinant follicle-stimulating hormone or highly purified human menopausal gonadotrophin. Fertil. Steril. 91, 1820–1830.
| Differences in gene expression of granulosa cells from women undergoing controlled ovarian hyperstimulation with either recombinant follicle-stimulating hormone or highly purified human menopausal gonadotrophin.Crossref | GoogleScholarGoogle Scholar | 18439596PubMed |
Haggarty, P., Wood, M., Ferguson, E., Hoad, G., Srikantharajah, A., Milne, E., Hamilton, M., and Bhattacharya, S. (2006). Fatty-acid metabolism in human preimplantation embryos. Hum. Reprod. 21, 766–773.
| Fatty-acid metabolism in human preimplantation embryos.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28Xhs1Sks7w%3D&md5=e4d67533ae3bef7820eb2e45efd0d8b5CAS | 16311299PubMed |
Hamel, M., Dufort, I., Robert, C., Leveille, M. C., Leader, A., and Sirard, M. A. (2010). Identification of follicular marker genes as pregnancy predictors for human IVF: new evidence for the involvement of luteinization process. Mol. Hum. Reprod. 16, 548–556.
| Identification of follicular marker genes as pregnancy predictors for human IVF: new evidence for the involvement of luteinization process.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXptlegt7w%3D&md5=152ce01a88d54e0f3bd0d6138c76bad6CAS | 20610614PubMed |
Hemmings, K. E., Leese, H. J., and Picton, H. M. (2012). Amino acid turnover by bovine oocytes provides an index of oocyte developmental competence in vitro. Biol. Reprod. 86, 1–12.
Holm, P., Booth, P. J., Schmidt, M. H., Greve, T., and Callesen, H. (1999). High bovine blastocyst development in a static in vitro-production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum proteins. Theriogenology 52, 683–700.
| High bovine blastocyst development in a static in vitro-production system using SOFaa medium supplemented with sodium citrate and myo-inositol with or without serum proteins.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c7pvVGnsw%3D%3D&md5=bda1bd1463ad693633a4028a58f68438CAS | 10734366PubMed |
Jiye, A., Huang, Q., Wang, G., Zha, W., Yan, B., Ren, H., Gu, S., Zhang, Y., Zhang, Q., Shao, F., Sheng, L., and Sun, J. (2008). Global analysis of metabolites in rat and human urine based on gas chromatography/time-of-flight mass spectrometry. Anal. Biochem. 379, 20–26.
| Global analysis of metabolites in rat and human urine based on gas chromatography/time-of-flight mass spectrometry.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1czptVWnsg%3D%3D&md5=bc3a0bcc07f9bced89e9b6390addd5cdCAS | 18486586PubMed |
Jozwik, M., Teng, C., and Battaglia, F. C. (2006). Amino acid, ammonia and urea concentrations in human pre-ovulatory ovarian follicular fluid. Hum. Reprod. 21, 2776–2782.
| Amino acid, ammonia and urea concentrations in human pre-ovulatory ovarian follicular fluid.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtVynsr7M&md5=6c961fd957358d10bb798df812dca181CAS | 16950828PubMed |
Lédée, N., Dubanchet, S., Oger, P., Meynant, C., Lombroso, R., Ville, Y., and Chaouat, G. (2007). Uterine receptivity and cytokines: new concepts and new applications. Gynecol. Obstet. Invest. 64, 138–143.
| Uterine receptivity and cytokines: new concepts and new applications.Crossref | GoogleScholarGoogle Scholar | 17934309PubMed |
Lee, E. S., and Fukui, Y. (1996). Synergistic effect of alanine and glycine on bovine embryos cultured in a chemically defined medium and amino acid uptake by in vitro-produced bovine morulae and blastocysts. Biol. Reprod. 55, 1383–1389.
| Synergistic effect of alanine and glycine on bovine embryos cultured in a chemically defined medium and amino acid uptake by in vitro-produced bovine morulae and blastocysts.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXkvFWntQ%3D%3D&md5=21fa10b382a7d4e1bbec953a8eea503cCAS | 8949897PubMed |
Lee, S., Kang, D. W., Hudgins-Spivey, S., Krust, A., Lee, E. Y., Koo, Y., Cheon, Y., Gye, M. C., Chambon, P., and Ko, C. (2009). Theca-specific oestrogen receptor-alpha knockout mice lose fertility prematurely. Endocrinology 150, 3855–3862.
| Theca-specific oestrogen receptor-alpha knockout mice lose fertility prematurely.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXpsV2ru7c%3D&md5=a89dbd56e85fa8273df40278c11528d6CAS | 19423761PubMed |
Leroy, J. L. M. R., Vanholder, T., Mateusen, B., Christophe, A., Opsomer, G., de Kruif, A., Genicot, G., and Van Soom, A. (2005). Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro. Reproduction 130, 485–495.
| Non-esterified fatty acids in follicular fluid of dairy cows and their effect on developmental capacity of bovine oocytes in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXhtFent7rJ&md5=cd82fd88938bb4bfbef1f4cb6ee5e020CAS |
Luo, W., Gumen, A., Haughian, J. M., and Wiltbank, M. C. (2011). The role of luteinizing hormone in regulating gene expression during selection of a dominant follicle in cattle. Biol. Reprod. 84, 369–378.
| The role of luteinizing hormone in regulating gene expression during selection of a dominant follicle in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVelt78%3D&md5=a8dd71fdcdb4cfbc003de19fda82ccbfCAS | 20962252PubMed |
Marei, W. F., Wathes, D. C., and Fouladi-Nashta, A. A. (2009). The effect of linolenic acid on bovine oocyte maturation and development. Biol. Reprod. 81, 1064–1072.
| The effect of linolenic acid on bovine oocyte maturation and development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsV2lt7vL&md5=723304df9e34ac8a15a75e7fa3a466afCAS | 19587335PubMed |
Matoba, S., Fair, T., and Lonergan, P. (2010). Maturation, fertilisation and culture of bovine oocytes and embryos in an individually identifiable manner: a tool for studying oocyte developmental competence. Reprod. Fertil. Dev. 22, 839–851.
| Maturation, fertilisation and culture of bovine oocytes and embryos in an individually identifiable manner: a tool for studying oocyte developmental competence.Crossref | GoogleScholarGoogle Scholar | 20450836PubMed |
Mu, Y. M., Yanase, T., Nishi, Y., Tanaka, A., Saito, M., Jin, C. H., Mukasa, C., Okabe, T., Nomura, M., Goto, K., and Nawata, H. (2001). Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology 142, 3590–3597.
| Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXls1Sqt7o%3D&md5=3b8b1308dfe7898176366412b96ff14dCAS | 11459807PubMed |
O’Shea, L. C., Mehta, J., Lonergan, P., Hensey, C., and Fair, T. (2012). Developmental competence in oocytes and cumulus cells: candidate genes and networks. Syst. Biol. Reprod. Med. 58, 88–101.
| Developmental competence in oocytes and cumulus cells: candidate genes and networks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XktV2nt7k%3D&md5=2693b9071cf618b0084c78bd808b6589CAS | 22313243PubMed |
Pakarainen, T., Ahtiainen, P., Zhang, F. P., Rulli, S., Poutanen, M., and Huhtaniemi, P. (2007). Extragonadal LH/hCG action - not yet time to rewrite textbooks. Mol. Cell. Endocrinol. 269, 9–16.
| Extragonadal LH/hCG action - not yet time to rewrite textbooks.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXjslSqsbk%3D&md5=20ae41927f26f917272e4ba86d511745CAS | 17350753PubMed |
Reis, A., Rooke, J. A., McCallum, G. J., Staines, M. E., Ewen, M., Lomax, M. A., and McEvoy, T. G. (2003). Consequences of exposure to serum, with or without vitamin E supplementation, in terms of the fatty acid content and viability of bovine blastocysts produced in vitro. Reprod. Fertil. Dev. 15, 275–284.
| Consequences of exposure to serum, with or without vitamin E supplementation, in terms of the fatty acid content and viability of bovine blastocysts produced in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXot1Cgur0%3D&md5=e779e7e142fe560e5295d479c49ee5c8CAS | 14588185PubMed |
Revelli, A., Delle Piane, L., Casano, S., Molinari, E., Massobrio, M., and Rinaudo, P. (2009). Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod. Biol. Endocrinol. 7, 40.
| Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics.Crossref | GoogleScholarGoogle Scholar | 19413899PubMed |
Russell, D. L., Doyle, K. M., Ochsner, S. A., Sandy, J. D., and Richards, J. S. (2003a). Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation. J. Biol. Chem. 278, 42 330–42 339.
| Processing and localization of ADAMTS-1 and proteolytic cleavage of versican during cumulus matrix expansion and ovulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXot1amu74%3D&md5=d21e35b9ec48c7f8582150c01b391205CAS |
Russell, D. L., Ochsner, S. A., Hsieh, M., Mulders, S., and Richards, J. S. (2003b). Hormone-regulated expression and localization of versican in the rodent ovary. Endocrinology 144, 1020–1031.
| Hormone-regulated expression and localization of versican in the rodent ovary.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXhslCqtr0%3D&md5=51067f07e7408f21c254c67cc4d5781eCAS | 12586779PubMed |
Sandy, J. D., Westling, J., Kenagy, R. D., Iruela-Arispe, M. L., Verscharen, C., Rodriguez-Mazaneque, J. C., Zimmermann, D. R., Lemire, J. M., Fischer, J. W., Wight, T. N., and Clowes, A. W. (2001). Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4. J. Biol. Chem. 276, 13 372–13 378.
| Versican V1 proteolysis in human aorta in vivo occurs at the Glu441-Ala442 bond, a site that is cleaved by recombinant ADAMTS-1 and ADAMTS-4.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3MXjtFymt70%3D&md5=bd9c3f1befd453c08308d9a05664be4bCAS |
Santos, P., Marques, A., Antunes, G., Chaveiro, A., Andrade, M., Borba, A., and da Silva, F. M. (2009). Effects of plasma urea nitrogen levels on the bovine oocyte ability to develop after in vitro fertilization. Reprod. Domest. Anim. 44, 783–787.
| Effects of plasma urea nitrogen levels on the bovine oocyte ability to develop after in vitro fertilization.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtlSjt7bJ&md5=7b7a1aa203406e7a1e18d5be1b98aeb5CAS | 18992098PubMed |
Sinclair, K. D., Lunn, L. A., Kwong, W. Y., Wonnacott, K., Linforth, R. S., and Craigon, J. (2008). Amino acid and fatty acid composition of follicular fluid as predictors of in vitro embryo development. Reprod. Biomed. Online 16, 859–868.
| Amino acid and fatty acid composition of follicular fluid as predictors of in vitro embryo development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXotFars70%3D&md5=67825ef679c11022cd4c2fc0f8933b62CAS | 18549697PubMed |
Tesfaye, D., Ghanem, N., Carter, F., Fair, T., Sirard, M. A., Hoelker, M., Schellander, K., and Lonergan, P. (2009). Gene expression profile of cumulus cells derived from cumulus-oocyte complexes matured either in vivo or in vitro. Reprod. Fertil. Dev. 21, 451–461.
| Gene expression profile of cumulus cells derived from cumulus-oocyte complexes matured either in vivo or in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXisFemsb8%3D&md5=67727bb4c37b5526a4f3c4fffe627f1cCAS | 19261222PubMed |
Vanholder, T., Leroy, J. L., Soom, A. V., Opsomer, G., Maes, D., Coryn, M., and de Kruif, A. (2005). Effect of non-esterified fatty acids on bovine granulosa cell steroidogenesis and proliferation in vitro. Anim. Reprod. Sci. 87, 33–44.
| Effect of non-esterified fatty acids on bovine granulosa cell steroidogenesis and proliferation in vitro.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXktVOju74%3D&md5=df3d7202ce6adae70e3a89068a8765e3CAS | 15885439PubMed |




