Paradoxical effect of supplementary progesterone between Day 3 and Day 7 on corpus luteum function and conceptus development in cattle
L. O’Hara A , N. Forde A , F. Carter A , D. Rizos B , V. Maillo B , A. D. Ealy C , A. K. Kelly A , P. Rodriguez D , N. Isaka D , A. C. O. Evans A and P. Lonergan A EA School of Agriculture and Food Science, University College Dublin, Belfield, Dublin 4, Ireland.
B Departamento de Reproducción Animal, Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria, Madrid, Spain.
C Department of Animal Sciences, University of Florida, PO Box 110910, Gainesville, FL 32611-0910, USA.
D CEVA Sante Animale, 10 Avenue de la Ballastière 33500 Libourne, France.
E Corresponding author. Email: pat.lonergan@ucd.ie
Reproduction, Fertility and Development 26(2) 328-336 https://doi.org/10.1071/RD12370
Submitted: 13 November 2012 Accepted: 17 January 2013 Published: 26 February 2013
Abstract
The aim of the present study was to investigate the effect of short-term progesterone (P4) supplementation during the early metoestrous period on circulating P4 concentrations and conceptus development in cattle. The oestrous cycles of cross-bred beef heifers were synchronised using a 7-day P4-releasing intravaginal device (PRID® Delta; 1.55 g P4) treatment with administration of a prostaglandin F2α analogue (Enzaprost; CEVA Sante Animale) the day before PRID® Delta removal. Only those heifers recorded in standing oestrus (Day 0) were used. In Experiment 1, heifers were randomly assigned to one of five groups: (1) control: no treatment; (2) placebo: insertion of a blank device (no P4) from Day 3 to Day 7; (3) insertion of a PRID® Delta from Day 3 to Day 7; (4) insertion of a PRID® Delta from Day 3 to Day 5; or (5) insertion of a PRID® Delta from Day 5 to Day 7. In vitro-produced blastocysts were transferred to each heifer in Groups 2–5 on Day 7 (n = 10 blastocysts per heifer) and conceptuses were recovered when heifers were killed on Day 14. Based on the outcome of Experiment 1, in Experiment 2 heifers were artificially inseminated at oestrus and randomly assigned to one of three treatment groups: (1) placebo; (2) PRID from Day 3 to Day 5; or (3) PRID from Day 3 to Day 7. All heifers were killed on Day 16 and recovered conceptuses were incubated in synthetic oviducal fluid medium for 24 h; spent media and uterine flushes were analysed for interferon-τ (IFNT). In both experiments, daily blood samples were taken to determined serum P4 concentrations. Data were analysed using the PROC MIXED procedure of SAS (SAS Institute, Cary, NC, USA). Insertion of a PRID resulted in an increase (P < 0.05) in serum P4 that declined following removal. In Experiment 1, P4 supplementation from Day 3 to Day 5 (17.0 ± 1.4 mm) or Day 3 to Day 7 (11.3 ± 2.3 mm) increased conceptus length compared with placebo (2.1 ± 1.8 mm). Serum P4 was significantly lower from Day 9 to Day 14 (P < 0.05) and the weight of the Day 14 corpus luteum (CL) was lower in the PRID Day 3–7 group than the placebo or control groups. In Experiment 2, supplementation from Day 3 to Day 5 (94.0 ± 18.8 mm) or Day 3 to Day 7 (143.6 ± 20.6 mm) increased conceptus length on Day 16 compared with placebo (50.3 ± 17.4 mm). Serum P4 was significantly lower in the two supplemented groups following PRID removal compared with placebo (P < 0.05) and was associated with a lower CL weight in the Day 3–7 group. Conceptus length was strongly correlated with the IFNT concentration in the uterine flush (r = 0.58; P = 0.011) and spent culture medium (r = 0.68; P < 0.002). The findings of the present study highlight the somewhat paradoxical effects of P4 supplementation when given in the early metoestrous period in terms of its positive effect on conceptus development and its potentially negative effects on CL lifespan.
Additional keywords: conceptus, cow, embryo mortality, fertility, pregnancy.
Introduction
Progesterone (P4) plays a key role in the reproductive events associated with the establishment and maintenance of pregnancy. Elevated concentrations of circulating P4 in the immediate post-conception period stimulate conceptus elongation, increase interferon-τ (IFNT) production and result in higher pregnancy rates in cattle and sheep (Garrett et al. 1988b; Satterfield et al. 2006; Carter et al. 2008). In cattle, most embryonic loss occurs in the period leading up to maternal recognition of pregnancy; a considerable proportion of this loss may be attributable to inadequate circulating P4 concentrations and the subsequent downstream consequences for endometrial gene expression and histotroph secretion into the uterine lumen (Inskeep 2004; Diskin and Morris 2008).
There is a strong correlation between circulating P4 in the days immediately following conception (Days 4–7) and the likelihood of embryo survival beyond Day 30 (Stronge et al. 2005; Diskin et al. 2006; Parr et al. 2012). Interestingly, there is both a linear and quadratic component to the relationship; that is, too much P4 may also lead to a decline in pregnancy rate. Thus, both sub- and supraoptimal concentrations of P4 from Day 4 to Day 7 after AI or a suboptimal rate of increase in the concentration of P4 during this interval are negatively associated with embryo survival.
Data in the literature on the effects of P4 supplementation on pregnancy rate are conflicting. Recent data from our group have demonstrated the following.
-
Significant changes occur in the endometrial transcriptome during both the oestrous cycle and early pregnancy in cattle (Forde et al. 2009, 2011a, 2011b).
-
Elevated P4, achieved by insertion of a P4-releasing intravaginal device on Day 3 after oestrus for a period, results in an advancement in the normal changes that occur over time in the endometrial transcriptome (Forde et al. 2009) and loss of the P4 receptor (Okumu et al. 2010), the consequence of which is advancement in conceptus elongation (Carter et al. 2008).
-
The effect of P4 on conceptus development is mediated exclusively via the endometrium; most convincingly, the embryo does not need to be present in the uterus during the period of P4 elevation in order to benefit from it, suggesting that P4 concentrations before Day 7 are important and that the effect of P4 is via the endometrium and altered histotroph composition (Clemente et al. 2009)
-
Reducing circulating concentrations of P4 during the first 7 days after oestrus results in an alteration in endometrial transcriptome, delayed loss of the P4 receptor and delayed conceptus elongation (Beltman et al. 2009; Forde et al. 2011a).
As well as the beneficial effects of P4 on conceptus elongation, there is also evidence in the literature to suggest that administration of P4 early in the cycle may compromise the function of the corpus luteum (CL), ultimately leading to luteolysis and embryo loss (Ginther 1970; Garrett et al. 1988a; Macmillan and Peterson 1993; Burke et al. 1994; Pope et al. 1995; Van Cleeff et al. 1996). The present study was designed to test the hypothesis that the timing and duration of the rise in P4 concentrations is critical to the success of early embryo development and CL function. Specifically, the aim was to assess the effect of short-term early P4 supplementation (from Day 3 to Day 5; from Day 5 to Day 7; or from Day 3 to Day 7) on circulating P4 concentrations, CL size and subsequent conceptus elongation in beef heifers following embryo transfer (ET) or AI.
Materials and methods
All experimental procedures involving animals were licensed by the Department of Health and Children, Ireland. Protocols were in accord with the Cruelty to Animals Act (Ireland 1876) and the European Community Directive 86/609/EC and were sanctioned by the Institutional Animal Research Ethics Committee. Before inclusion, all heifers were scanned to ensure they were reproductively normal (i.e. cycling and not freemartin) and that they were not pregnant.
Experiment 1
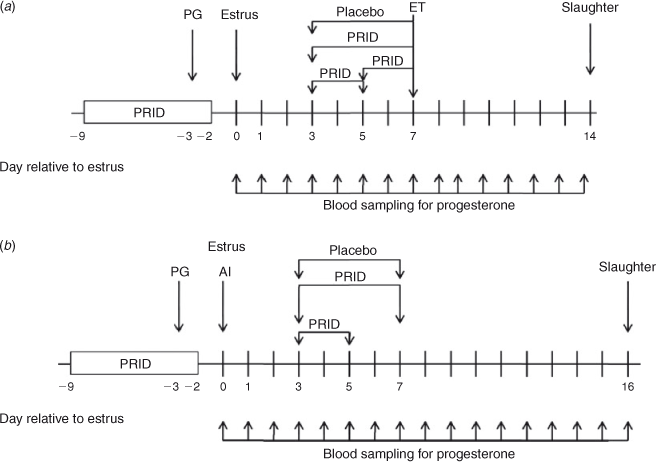
The aim of Experiment 1 was to examine the effect of short-term P4 supplementation between Days 3 and 5, Days 3 and 7 or Days 5 and 7 after oestrus on CL size, circulating P4 concentrations and conceptus development in cattle following transfer of in vitro-produced blastocysts on Day 7.
The experimental design is shown in Fig. 1a. Beef-cross heifers (n = 50), predominantly Charolais and Limousin cross, were used (mean (± s.e.m.) age 26.0 ± 0.4 months; mean weight 575.1 ± 5.6 kg). All animals were housed indoors on slats for the duration of the experiment and were fed a diet consisting of grass and maize silage supplemented with a standard beef ration. The oestrous cycles of all heifers were synchronised using a 7-day P4-releasing intravaginal device (PRID® Delta; Ceva Sante Animale, Libourne, France; containing 1.55 g P4) with administration of a prostaglandin F2α (PGF2α) analogue (Enzaprost; Ceva Sante Animale; 5 mL, equivalent to 25 mg dinoprost) the day before PRID® Delta removal. Heifers were checked for signs of oestrus four times each day commencing 30 h after PRID® Delta withdrawal and only those recorded in standing oestrus (= Day 0) were used.
After synchronisation, heifers were blocked on bodyweight and randomly assigned to one of five treatment groups: (1) control: no treatment; (2) placebo: insertion of a blank device (no P4) from Day 3 to Day 7; (3) insertion of a PRID® Delta from Day 3 to Day 7; (4) insertion of a PRID® Delta from Day 3 to Day 5; or (5) insertion of a PRID® Delta from Day 5 to Day 7. Blood samples were collected daily by jugular venipuncture from Day 0 to Day 14 to determine serum P4 concentrations.
In vitro embryo production
The techniques for producing embryos in vitro have been described in detail elsewhere (Rizos et al. 2002). Immature cumulus–oocyte complexes (COCs) were obtained by aspirating follicles from the ovaries of heifers and cows collected at the time of death. The COCs were matured for 24 h in TCM-199 supplemented with 10% (v/v) fetal calf serum (FCS) and 10 ng mL–1 epidermal growth factor at 39°C in an atmosphere of 5% CO2 in air with maximum humidity. Matured COCs were inseminated with frozen–thawed Percoll-separated bull sperm at a concentration of 1 × 106 spermatozoa mL–1. Gametes were coincubated at 39°C in an atmosphere of 5% CO2 in air with maximum humidity. Semen from the same bull was used throughout. At approximately 20 h post insemination (h.p.i.), presumptive zygotes were denuded of surrounding cumulus cells and accessory spermatozoa and cultured in 500 µL synthetic oviduct fluid (SOF) supplemented with 5% FCS (n = 50 per well) until Day 7.
Embryo transfer and recovery
In vitro-produced Grade 1 blastocysts were transferred to the uterine horn ipsilateral to the CL of synchronised recipients on Day 7 (10 blastocysts per recipient), as described previously (Clemente et al. 2009). All recipients were killed on Day 14 to assess embryo survival. The reproductive tracts of all animals were flushed within 60 min of death with phosphate-buffered saline (PBS) containing 5% FCS. The dimensions (length and width) of each conceptus were measured. In addition, the CL was dissected from the ovary and weighed. Any incidence of uterine infection was recorded.
Experiment 2
Based on the outcome of Experiment 1, the aim of Experiment 2 was to examine the effect of P4 supplementation between Days 3 and 5 or between Days 3 and 7 on CL size, circulating P4 concentrations, conceptus development and IFNT production following AI (i.e. single conceptus).
The experimental design is shown in Fig. 1b. Beef-cross heifers (n = 40), predominantly Charolais and Limousin cross (mean age 22.2 ± 0.4 months; mean weight 595.9 ± 5.1 kg), were housed indoors on slats for the duration of the experiment and managed as described above. The oestrous cycles of all heifers were synchronised as described for Experiment 1 and all heifers seen in standing oestrus (n = 33) were inseminated twice, 12 and 24 h after the onset of oestrus, with semen from a single proven sire.
Heifers were randomly assigned to one of three treatment groups: (1) placebo: insertion of a blank device (no P4) from Days 3 to 7 (n = 12); (2) PRID® Delta from Day 3 to Day 5 (n = 11); or (3) PRID® Delta from Day 3 to Day 7 (n = 10). Blood samples were collected daily to determine P4 concentrations from Day 0 to Day 16. Heifers were killed on Day 16 to assess embryo survival. The CL was dissected from the ovary and weighed. The uterine horn ipsilateral to the CL was flushed with 10 mL PBS. The flush medium was stored at –80°C for analysis of IFNT. Recovered conceptuses (n = 18) were measured and cultured individually for 24 h in 500 μL SOF supplemented with 5% FCS. After 24 h incubation, the spent medium was removed and stored at –80°C for measurement of IFNT.
Progesterone measurement
In both experiments, following collection, blood samples were refrigerated (4°C) for 12–24 h before being centrifuged at 1500g at 4°C for 20 min. Serum was separated and stored at –20°C until assayed to determine P4 concentrations by solid phase radioimmunoassay using the Coat-A-Count Progesterone kit (Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA), as described previously (Forde et al. 2011a). The sensitivity of the assay was 0.03 ng mL–1 P4. The interassay CV for quality control samples was 10.7% (low), 6.2% (medium) and 3.6% (high), respectively. The intraassay CV were 11.7% (low), 6.8% (medium) and 5.4% (high).
Analysis of IFNT
The quantity of biologically active IFNT in conditioned medium was determined using a cytopathic antiviral assay (Michael et al. 2006; Roberts et al. 1989). Samples were examined in duplicate by titrating in a 96-well plate (1 : 3 serial dilution). Madin-Darby bovine kidney (MDBK) cells were added and incubated at 37°C for 24 h in 5% CO2 in humidified air. Cells were challenged with vesicular stomatitis virus for 1 h and then incubated with growth medium (Dulbecco’s modified Eagle’s medium containing 10% FBS) for 18 h. Cell viability was determined after fixation (75% ethanol) using 0.5% (w/v) Gentian violet. The ability of samples to prevent lysis by 50% was compared with a recombinant human interferon-α standard (EMD Biosciences, Gibbstown, NJ, USA; 3.8 × 108 IU mg–1). Data are presented as IU antiviral activity per mL conditioned medium. Unconditioned medium (blanks) did not contain antiviral activity.
Statistical analysis
Heifer was the experimental unit in all models. Data were checked for normality and homogeneity of variance by histograms, qq plots and formal statistical tests as part of the UNIVARIATE procedure of SAS (version 9.1.3; SAS Institute, Cary, NC, USA). Data that were not normally distributed were transformed by raising the variable to the power of lambda. The appropriate lambda value was obtained by conducting a Box–Cox transformation analysis using the TRANSREG procedure of SAS. The natural logarithmic transformation of IFNT was used to normalise data distribution for this variable because preliminary analyses revealed that the distribution of values for this analyte was positively skewed. In addition, embryo width and area required transformation and were raised to the power of 0.25. The transformed data were used to calculate P-values. The corresponding least squares mean ± s.e.m. of the non-transformed data are presented in the Results for clarity. Concentrations of P4 were analysed using repeated-measures with the MIXED procedure of SAS. Fixed effects included experimental treatment, day and their interaction. The interaction term, if not significant (P > 0.10), was subsequently excluded from the final model. Heifer within treatment was included as a random effect. The type of variance–covariance structure used was chosen depending on the magnitude of the Akaike information criterion (AIC) for models run under compound symmetry, unstructured, autoregressive, or Toeplitz variance–covariance structures. The model with the least AIC value was selected. Embryo -elated data were analysed using the PROC MIXED procedure of SAS. The model had experimental treatment as a fixed effect and heifer within treatment was included as a random effect. Differences between treatments were determined by F-tests using Type III sums of squares. The PDIFF command incorporating the Tukey test was applied to evaluate pairwise comparisons between treatment means. Pearson correlation coefficients among traits were determined within day using the CORR procedure of SAS. Correlation coefficients were classified as strong (r > 0.6), moderate (r = 0.4–0.6) or weak (r < 0.4). Changes in P4 concentrations (used in the correlation analyses) during the test period for each animal were computed as the coefficient of the linear regression of measurements upon time (day) using the REG procedure of SAS.
Results
In some tables and figures, the numbers of heifers indicated may be slightly different to the initial number of heifers. This is due to the fact that some heifers were recycled and used again if: (1) they did not show standing oestrus after synchronisation; (2) insufficient embryos were available on the day of transfer (Experiment 1); or (3) they short cycled (both experiments) due to treatment (as evidenced by showing standing heat before time of death and/or a regressed CL or fresh ovulation at the time of death). Thus, in some cases, heifers may contribute to the P4 data, for example, but not to the conceptus or CL data. Numbers of heifers contributing to specific parameters are clearly indicated in the relevant tables and figures.
Experiment 1
Circulating P4 concentrations
Daily P4 concentrations between Day 0 and Day 14 in heifers are shown in Fig. 2. Insertion of a blank (placebo) device had no effect on circulating P4 concentrations compared with control heifers. Insertion of a PRID® Delta on Day 3 or Day 5 resulted in an immediate increase in serum P4 concentrations (P < 0.05) that remained elevated until removal of the device on Day 5 or Day 7. Insertion of a PRID® Delta from Day 3 to Day 7 was followed by a significant reduction (P < 0.05) in serum P4 concentrations from Day 9 to Day 14 compared with the control and placebo groups.

|
Effect of uterine infection and short cycles
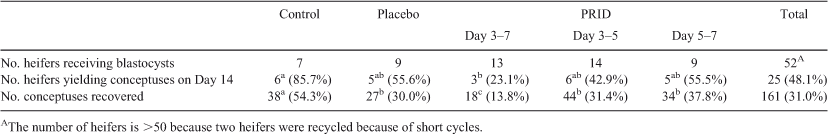
The incidence of uterine infection associated with embryo transfer was higher when the PRID® Delta was removed on the day of ET (Day 5 to Day 7, 21.4%; Day 3 to Day 7, 26.3%) compared with removal on Day 5, 2 days before transfer (5.5%) or the control (0), although the differences did not reach statistical significance (Table 1). One heifer in the placebo group (8.3%) showed evidence of infection.

|
The incidence of short cycles, as evidenced by heifers seen in standing oestrus at the time of death on Day 14 and/or the presence of a fresh CL at the time of death, was highest in groups receiving a PRID® Delta on Day 3 (Day 3–7, 26.3%; Day 3–5, 33.3%) compared with those receiving a PRID® Delta on Day 5 (7.1%) or the placebo (16.7%) or control groups (10.0%), although the differences were not significant (Table 1). The weight of the CL on Day 14 was lowest (P < 0.05) in heifers receiving a PRID® Delta from Day 3 to Day 7 compared with those receiving a placebo or in control heifers (Fig. 3).

|
Conceptus recovery and development
The number of heifers from which conceptuses were recovered on Day 14 is given in Table 2. The overall recovery rate (conceptuses recovered as a proportion of embryos transferred) was 31.0%; significantly fewer embryos (P < 0.05) were recovered in the PRID® Delta Day 3–7 group (13.8%) compared with the other groups. Progesterone supplementation from Day 3 to Day 5 or from Day 3 to Day 7 increased conceptus length and area at Day 14 (P < 0.05; Fig. 4) compared with the placebo group. Supplementation from Day 5 to Day 7 did not affect conceptus dimensions compared with the placebo group.

|
Experiment 2
Circulating P4 concentrations
Daily P4 concentrations between Day 0 and Day 16 in heifers are shown in Fig. 5. Consistent with Experiment 1, insertion of a PRID® Delta on Day 3 resulted in an immediate increase (P < 0.05) in serum P4 concentrations that remained elevated until removal of the device on Day 5 or Day 7. In both cases, following PRID® Delta removal P4 concentrations dropped to significantly lower levels (P < 0.05) than in the placebo and remained lower until Day 16.

|
Conceptus recovery and development
Conceptus recovery rates ranged from 50.0% to 58.3% across the three groups (Table 3). Mean conceptus length was greater (P = 0.004) in those heifers that had received a PRID® Delta from Day 3 to Day 7 (143.6 ± 20.6 mm) compared with those receiving placebo (50.3 ± 18.8 mm). Those receiving a PRID® Delta from Day 3 to Day 5 yielded conceptuses of intermediate length (94.0 ± 15.1 mm; Fig. 6).

|

|
Secretion of IFNT
Concentrations of IFNT were higher in the uterine flush medium from heifers receiving a PRID® Delta from Day 3 to Day 7 compared with those receiving placebo (P = 0.006) and those receiving a PRID® Delta from Day 3 to Day 5 (P = 0.004; Fig. 7). Consistent with these data, following overnight culture in SOF, conceptuses derived from heifers receiving a PRID® Delta from Day 3 to Day 7 secreted significantly more IFNT than did conceptuses from either of the other two groups (P < 0.01).

|
Luteal tissue weight
Luteal tissue weight on Day 16 was lower in heifers receiving a PRID® Delta from Day 3 to Day 7 than in those receiving placebo (4.77 ± 0.59 vs 6.28 ± 0.50 g, respectively; P < 0.05). Those receiving a PRID® Delta from Day 3 to Day 5 had intermediate luteal tissue weight (Fig. 8).

|
Correlation of parameters with conceptus development
Concentrations of P4 on Day 4 (the day after PRID® Delta insertion) were strongly correlated with conceptus length on Day 16 (r = 0.53; P = 0.025), IFNT in the uterine flush (r = 0.68; P = 0.002) and IFNT in the spent culture medium following overnight culture (r = 0.74; P = 0.0004). Similarly, conceptus length on Day 16 was correlated with the concentration of IFNT in the uterine flush (r = 0.58; P = 0.011) and IFNT in the culture medium after overnight culture (r = 0.68; P < 0.002). Concentrations of IFNT in uterine flushings and in spent culture medium from the same conceptus were highly correlated (r = 0.82; P < 0.0001).
Discussion
The main findings of the present study are that short-term P4 supplementation, for as little as 2 days, from Day 3 to Day 7 after oestrus, is sufficient to increase peripheral P4, increase conceptus size and increase IFNT secretion, regardless as to whether ET or AI was used to establish pregnancy. Furthermore, in a proportion of heifers, P4 supplementation was associated with a reduction in CL lifespan.
The data from Experiment 1, in which ET was used to establish pregnancy, on the effects of P4 on conceptus elongation are consistent with previous findings that showed that the embryo does not have to be present in the uterus at the time of elevated P4 in order to benefit from it (Clemente et al. 2009). These data indicate that the well-described association between early increases in P4 after conception and advanced conceptus length is not due to a direct effect of P4 on the embryo, but rather due to P4-induced changes in the environment in which the embryo is developing. This is supported by an advancement or retardation in the normal temporal changes that occur in the endometrial transcriptome in the presence of high (Forde et al. 2009) or low (Forde et al. 2011a) P4, respectively.
Despite the positive effects of elevated P4 on the conceptus, a significant number of treated heifers exhibited short cycles, evidenced by being recorded in standing oestrus before death on Day 14 (Experiment 1) or Day 16 (Experiment 2) and/or the presence of a fresh CL at the time of death. These findings highlight the somewhat paradoxical actions of P4. On the one hand, increased P4 concentrations stimulate conceptus elongation (Garrett et al. 1988a; Satterfield et al. 2006; Carter et al. 2008) via well-characterised changes induced in the endometrial transcriptome (Forde et al. 2009, 2011b) and associated changes in the histotroph (Costello et al. 2010; Hugentobler et al. 2010). Conversely, there is convincing evidence in the literature that administration of P4 early in the cycle may compromise CL function, ultimately leading to luteolysis and embryo loss (Ginther 1970; Garrett et al. 1988a; Burke et al. 1994; Pope et al. 1995; Van Cleeff et al. 1996).
Garrett et al. (1988b) reported that administration of P4 from Day 1 to Day 4 resulted in a transient increase in P4 concentrations that was associated with a marked increase in conceptus length at Day 14. Furthermore, following the last P4 injection, P4 concentrations decreased to levels significantly below the control, in agreement with the present study. Administration of exogenous P4 from Day 1 to Day 4 shortened the interoestrous interval (16.7 days) compared with controls (21.6 days), resulting from an earlier decline in peripheral P4 coincident with an increased pulsatile release of PGFM in both progesterone-treated and control cows (Garrett et al. 1988a). The results indicate that administration of exogenous P4 stimulates an earlier maturation of endometrial development, causing an advanced release of PGF2α, which shortens the interoestrous interval of the cow (Garrett et al. 1988a). Pope et al. (1995) showed that the minimal daily dose of P4 required to stimulate and advance early embryo development in ewes (6 mg) is also the dose that will produce shortened cycles (i.e. it is both luteolytic and embryotrophic).
The timing of the premature elevation of P4 during the metoestrous period is critical to its subsequent effect on luteal lifespan. Ginther (1970) reported the cycle-shortening effect of exogenous P4 when administered from Day 0 to Day 3, from Day 1 to Day 4 or from Day 2 to Day 5. Similarly, Burke et al. (1994) reported that administration of P4 from Day 1 to Day 5, but not from Day 4 to Day 9, reduced CL lifespan. Macmillan and Peterson (1989) reported that when an intravaginal P4 device was inserted from Day 1 to Day 4 of the oestrous cycle, the interoestrous interval was reduced to 7–9 days in 60% of treated cows. Van Cleeff et al. (1996) reported that P4 supplementation within 2 days of insemination for 7 days suppressed fertility in dairy heifers (18.2% vs 46.4%). Interestingly, Ginther (1970) reported that simultaneous administration of P4 and human chorionic gonadotrophin (hCG) prevented the cycle-shortening effect of P4 given on Days 1–4.
Other strategies aimed at increasing peripheral P4 concentrations in the days after oestrus include the administration of hCG (De Rensis et al. 2010; Lonergan 2011; Wiltbank et al. 2012). Most studies have used administration on Day 5 after oestrus with the aim of inducing the ovulation of a dominant follicle and the formation of an accessory CL. In a recent study from our group (Rizos et al. 2012), as well as in the study of Kerbler et al. (1997), P4 concentrations diverged significantly from the control 2–3 days after hCG administration, indicating a trophic effect on the endogenous CL in addition to the formation of an accessory structure.
In Experiment 2, mean conceptus length was greater in heifers that had received a PRID® Delta from Day 3 to Day 7 (143.6 ± 20.6 mm) than those receiving placebo (50.3 ± 18.8 mm). Those receiving a PRID® Delta from Day 3 to Day 5 yielded conceptuses of intermediate length (94.0 ± 15.1 mm). Measurement of antiviral activity indicative of IFNT in the uterine flush and in spent culture medium following overnight culture was highly correlated within embryo. The IFNT concentrations were higher in uterine flush media from heifers receiving a PRID® Delta from Day 3 to Day 7 compared with those receiving placebo and those receiving a PRID® Delta from Day 3 to Day 5. Of a total of 35 heifer uteri flushed, a conceptus was located in 18. With the exception of two heifers, failure to locate a conceptus after flushing the uterus was associated with undetectable IFNT in the flush (n = 17), suggesting that a conceptus was not missed in the flushing procedure.
In conclusion, these data support the positive association between circulating P4 concentrations in the metoestrous period and conceptus elongation. Furthermore, they highlight the paradoxical nature of the P4 effect in that, when administered early, it is associated with short cycles in a proportion of heifers. This highlights the caution that is required when supplementing P4; it may be that strategies aimed at stimulating the development of the endogenous CL (e.g. manipulation of preovulatory follicle development (Wiltbank et al. 2011) or administration of luteotrophic agents such as hCG (De Rensis et al. 2010; Lonergan 2011; Rizos et al. 2012)) rather than supplementation with exogenous P4 will be most effective. Alternatively, a combination of exogenous P4 and luteal support may prove beneficial in achieving a balancing between the apparent negative effects of P4 supplementation on development of the CL and the positive effects on conceptus development.
Acknowledgements
This work was supported by Ceva Sante Animale and Science Foundation Ireland (07/SRC/B1156).
References
Beltman, M. E., Roche, J. F., Lonergan, P., Forde, N., and Crowe, M. A. (2009). Evaluation of models to induce low progesterone during the early luteal phase in cattle. Theriogenology 72, 986–992.| Evaluation of models to induce low progesterone during the early luteal phase in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFGlsrrP&md5=df82d073fb359e6ca7dc763dfec2b657CAS | 19716596PubMed |
Burke, C. R., Mihm, M., Macmillan, K. L., and Roche, J. F. (1994). Some effects of prematurely elevated concentrations of progesterone on luteal and follicular characteristics during the oestrous cycle in heifers. Anim. Reprod. Sci. 35, 27–39.
| Some effects of prematurely elevated concentrations of progesterone on luteal and follicular characteristics during the oestrous cycle in heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2cXktVWhtLo%3D&md5=1d73f71ad6cbf03645a231a00a4542a9CAS |
Carter, F., Forde, N., Duffy, P., Wade, M., Fair, T., Crowe, M. A., Evans, A. C. O., Kenny, D. A., Roche, J. F., and Lonergan, P. (2008). Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers. Reprod. Fertil. Dev. 20, 368–375.
| Effect of increasing progesterone concentration from Day 3 of pregnancy on subsequent embryo survival and development in beef heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXjtVKksLg%3D&md5=64ca51d0d9b619dd0572e72474aeae4aCAS | 18402756PubMed |
Clemente, M., de La Fuente, J., Fair, T., Al Naib, A., Gutierrez-Adan, A., Roche, J. F., Rizos, D., and Lonergan, P. (2009). Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium? Reproduction 138, 507–517.
| Progesterone and conceptus elongation in cattle: a direct effect on the embryo or an indirect effect via the endometrium?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFOgtrvL&md5=6038faeaab282ac53cbf3ad10c7005d7CAS | 19556439PubMed |
Costello, L. M., O’Boyle, P., Godkin, J. D., Diskin, M. G., Hynes, A. C., and Morris, D. G. (2010). Retinol-binding protein (RBP), retinol and β-carotene in the bovine uterus and plasma during the oestrous cycle and the relationship between systemic progesteroneand RBP on Day 7. Reprod. Fertil. Dev. 22, 1198–1205.
| Retinol-binding protein (RBP), retinol and β-carotene in the bovine uterus and plasma during the oestrous cycle and the relationship between systemic progesteroneand RBP on Day 7.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXht1aktLjI&md5=0b7e167b65a0d996f82117c78d30735fCAS | 20883645PubMed |
De Rensis, F., Lopez-Gatius, F., Garcia-Ispierto, I., and Techakumpu, M. (2010). Clinical use of human chorionic gonadotropin in dairy cows: an update. Theriogenology 73, 1001–1008.
| Clinical use of human chorionic gonadotropin in dairy cows: an update.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXktlClu74%3D&md5=46d6c317ee7717eb364c14a20be1e34bCAS | 20116839PubMed |
Diskin, M. G., and Morris, D. G. (2008). Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 43, 260–267.
| Embryonic and early foetal losses in cattle and other ruminants.Crossref | GoogleScholarGoogle Scholar | 18638133PubMed |
Diskin, M. G., Murphy, J. J., and Sreenan, J. M. (2006). Embryo survival in dairy cows managed under pastoral conditions. Anim. Reprod. Sci. 96, 297–311.
| Embryo survival in dairy cows managed under pastoral conditions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28nitFGgsg%3D%3D&md5=e067a53a9bf58dd81f82258a108eac2bCAS | 16963203PubMed |
Forde, N., Carter, F., Fair, T., Crowe, M. A., Evans, A. C. O., Spencer, T. E., Bazer, F. W., McBride, R., Boland, M. P., O’Gaora, P., Lonergan, P., and Roche, J. F. (2009). Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle. Biol. Reprod. 81, 784–794.
| Progesterone-regulated changes in endometrial gene expression contribute to advanced conceptus development in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhtFyhsLbM&md5=b886d18b83f7eafebec7bdb868eac690CAS | 19553605PubMed |
Forde, N., Beltman, M. E., Duffy, G. B., Duffy, P., Mehta, J. P., O’Gaora, P., Roche, J. F., Lonergan, P., and Crowe, M. A. (2011a). Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation. Biol. Reprod. 84, 266–278.
| Changes in the endometrial transcriptome during the bovine estrous cycle: effect of low circulating progesterone and consequences for conceptus elongation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVeltr4%3D&md5=61a2e2dc9f6a05ff10d8194c1307c89aCAS | 20881316PubMed |
Forde, N., Carter, F., Spencer, T. E., Bazer, F. W., Sandra, O., Mansouri-Attia, N., Okumu, L. A., McGettigan, P. A., Mehta, J. P., McBride, R., O’Gaora, P., Roche, J. F., and Lonergan, P. (2011b). Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant? Biol. Reprod. 85, 144–156.
| Conceptus-induced changes in the endometrial transcriptome: how soon does the cow know she is pregnant?Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXotFOlsL4%3D&md5=11f719f39ba17575fcafd885e45e10b9CAS | 21349821PubMed |
Garrett, J. E., Geisert, R. D., Zavy, M. T., Gries, L. K., Wettemann, R. P., and Buchanan, D. S. (1988a). Effect of exogenous progesterone on prostaglandin F2α release and the interestrous interval in the bovine. Prostaglandins 36, 85–96.
| Effect of exogenous progesterone on prostaglandin F2α release and the interestrous interval in the bovine.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1cXlt1aktr4%3D&md5=3cb4e90f75fd1d27fdf2ebce2be96170CAS | 3175025PubMed |
Garrett, J. E., Geisert, R. D., Zavy, M. T., and Morgan, G. L. (1988b). Evidence for maternal regulation of early conceptus growth and development in beef cattle. J. Reprod. Fertil. 84, 437–446.
| Evidence for maternal regulation of early conceptus growth and development in beef cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXhtFKh&md5=51f14d4d7c21acf4ea8c311186fbf6fdCAS | 3199361PubMed |
Ginther, O. J. (1970). Effect of progesterone on length of estrous cycle in cattle. Am. J. Vet. Res. 31, 493–496.
| 1:CAS:528:DyaE3cXht12jtb8%3D&md5=afd2e49be5cc361c71d189e66ab9d154CAS | 5462750PubMed |
Hugentobler, S. A., Sreenan, J. M., Humpherson, P. G., Leese, H. J., Diskin, M. G., and Morris, D. G. (2010). Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood. Reprod. Fertil. Dev. 22, 684–694.
| Effects of changes in the concentration of systemic progesterone on ions, amino acids and energy substrates in cattle oviduct and uterine fluid and blood.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjvF2isLY%3D&md5=3b613adb9726d0a177f46ff72b904e8aCAS | 20353728PubMed |
Inskeep, E. K. (2004). Preovulatory, postovulatory, and postmaternal recognition effects of concentrations of progesterone on embryonic survival in the cow. J. Anim. Sci. 82, E24–E39.
| 15471804PubMed |
Kerbler, T. L., Buhr, M. M., Jordan, L. T., Leslie, K. E., and Walton, J. S. (1997). Relationship between maternal plasma progesterone concentration and interferon-tau synthesis by the conceptus in cattle. Theriogenology 47, 703–714.
| Relationship between maternal plasma progesterone concentration and interferon-tau synthesis by the conceptus in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2sXhvFCltL8%3D&md5=41fe350a21e89f20dad77ab7443f80d1CAS | 16728022PubMed |
Lonergan, P. (2011). Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology 76, 1594–1601.
| Influence of progesterone on oocyte quality and embryo development in cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXhsVanurzI&md5=15dc7ed19b3ee1cf310458ebea657936CAS | 21855985PubMed |
Macmillan, K. L., and Peterson, A. J. (1993). A new intravaginal progesterone releasing device for cattle (CIDR-B) for oestrous synchronisation, increasing pregnancy rates and the treatment of post-partum anoestrus. Anim. Reprod. Sci. 33, 1–25.
| A new intravaginal progesterone releasing device for cattle (CIDR-B) for oestrous synchronisation, increasing pregnancy rates and the treatment of post-partum anoestrus.Crossref | GoogleScholarGoogle Scholar |
Michael, D. D., Alvarez, I. M., Ocon, O. M., Powell, A. M., Talbot, N. C., Johnson, S. E., and Ealy, A. D. (2006). Fibroblast growth factor-2 is expressed by the bovine uterus and stimulates interferon-tau production in bovine trophectoderm. Endocrinology 147, 3571–3579.
| Fibroblast growth factor-2 is expressed by the bovine uterus and stimulates interferon-tau production in bovine trophectoderm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XmtlOrsbk%3D&md5=e801e58c4b7f53fde2988956ddf052f2CAS | 16574787PubMed |
Okumu, L. A., Forde, N., Fahey, A. G., Fitzpatrick, E., Roche, J. F., Crowe, M. A., and Lonergan, P. (2010). The effect of elevated progesterone and pregnancy status on mRNA expression and localisation of progesterone and oestrogen receptors in the bovine uterus. Reproduction 140, 143–153.
| The effect of elevated progesterone and pregnancy status on mRNA expression and localisation of progesterone and oestrogen receptors in the bovine uterus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXhtVWls77E&md5=0ca14c9a1f873a862476b684a5239a45CAS | 20403910PubMed |
Parr, M. H., Mullen, M. P., Crowe, M. A., Roche, J. F., Lonergan, P., Evans, A. C. O., and Diskin, M. G. (2012). Relationship between pregnancy per artificial insemination and early luteal concentrations of progesterone and establishment of repeatability estimates for these traits in Holstein–Friesian heifers. J. Dairy Sci. 95, 2390–2396.
| Relationship between pregnancy per artificial insemination and early luteal concentrations of progesterone and establishment of repeatability estimates for these traits in Holstein–Friesian heifers.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XlvFyksr0%3D&md5=145c1e9d0b7b0f0635d850940dd52063CAS | 22541467PubMed |
Pope, W. F., Cardenas, H., Wiley, T. M., and McClure, K. E. (1995). Dose–response relationships of exogenous progesterone shortly after ovulation on estrous cycle length, blastocyst development and fertility in sheep. Anim. Reprod. Sci. 38, 109–117.
| Dose–response relationships of exogenous progesterone shortly after ovulation on estrous cycle length, blastocyst development and fertility in sheep.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK2MXlt1ertrc%3D&md5=841c8c941de6edfaeaaf5601bb9c5981CAS |
Rizos, D., Ward, F., Duffy, P., Boland, M. P., and Lonergan, P. (2002). Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality. Mol. Reprod. Dev. 61, 234–248.
| Consequences of bovine oocyte maturation, fertilization or early embryo development in vitro versus in vivo: implications for blastocyst yield and blastocyst quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xlt1Giug%3D%3D&md5=f077f10c11fabfb56e4fb3f5fae492a6CAS | 11803560PubMed |
Rizos, D., Scully, S., Kelly, A. K., Ealy, A. D., Moros, R., Duffy, P., Al Naib, A., Forde, N., and Lonergan, P. (2012). Effects of human chorionic gonadotrophin administration on Day 5 after oestrus on corpus luteum characteristics, circulating progesterone and conceptus elongation in cattle. Reprod. Fertil. Dev. 24, 472–481.
| Effects of human chorionic gonadotrophin administration on Day 5 after oestrus on corpus luteum characteristics, circulating progesterone and conceptus elongation in cattle.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38Xjs1Wqsbo%3D&md5=9ed884b2b02064c80dc0e9d3f3e2ca15CAS | 22401279PubMed |
Roberts, R. M., Imakawa, K., Niwano, Y., Kazemi, M., Malathy, P. V., Hansen, T. R., Glass, A. A., and Kronenberg, L. H. (1989). Interferon production by the preimplantation sheep embryo. J. Interferon Res. 9, 175–187.
| Interferon production by the preimplantation sheep embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXitFSlsL8%3D&md5=3be414702fc9e265276f57c0bf4fb33fCAS | 2469745PubMed |
Satterfield, M. C., Bazer, F. W., and Spencer, T. E. (2006). Progesterone regulation of preimplantation conceptus growth and galectin 15 (LGALS15) in the ovine uterus. Biol. Reprod. 75, 289–296.
| Progesterone regulation of preimplantation conceptus growth and galectin 15 (LGALS15) in the ovine uterus.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XnsVWgsbo%3D&md5=f822109fca14186f94595e90d9955f9fCAS | 16707766PubMed |
Stronge, A. J. H., Sreenan, J. M., Diskin, M. G., Mee, J. F., Kenny, D. A., and Morris, D. G. (2005). Post-insemination milk progesterone concentration and embryo survival in dairy cows. Theriogenology 64, 1212–1224.
| Post-insemination milk progesterone concentration and embryo survival in dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpsVCgt7o%3D&md5=050fa34e24ad7530890f3a05957e6561CAS |
Van Cleeff, J., Macmillan, K. L., Drost, M., Lucy, M. C., and Thatcher, W. W. (1996). Effects of administering progesterone at selected intervals after insemination of synchronized heifers on pregnancy rates and resynchronization of returns to service. Theriogenology 46, 1117–1130.
| Effects of administering progesterone at selected intervals after insemination of synchronized heifers on pregnancy rates and resynchronization of returns to service.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28zgtVOgsg%3D%3D&md5=b5f0db99e0fbd839f26249443ce16383CAS | 16727976PubMed |
Wiltbank, M. C., Sartori, R., Herlihy, M. M., Vasconcelos, J. L. M., Nascimento, A. B., Souza, A. H., Ayres, H., Cunha, A. P., Keskin, A., Guenther, J. N., and Gumen, A. (2011). Managing the dominant follicle in lactating dairy cows. Theriogenology 76, 1568–1582.
| Managing the dominant follicle in lactating dairy cows.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3MbnvVCmsA%3D%3D&md5=8da89edcf36f5f4fa78232b2edb96159CAS | 21958644PubMed |
Wiltbank, M. C., Souza, A. H., Carvalho, P. D., Bender, R. W., and Nascimento, A. B. (2012). Improving fertility to timed artificial insemination by manipulation of circulating progesterone concentrations in lactating dairy cattle. Reprod. Fertil. Dev. 24, 238–243.
| Improving fertility to timed artificial insemination by manipulation of circulating progesterone concentrations in lactating dairy cattle.Crossref | GoogleScholarGoogle Scholar |