Bovine spermatozoa react to in vitro heat stress by activating the mitogen-activated protein kinase 14 signalling pathway
Mohammad Bozlur Rahman A E , Leen Vandaele B , Tom Rijsselaere A , Mohamed Shehab El-Deen C , Dominiek Maes A , Mohammed Shamsuddin D and Ann Van Soom AA Department of Reproduction, Obstetrics and Herd Health, Ghent University, Salisburylaan 133, 9820 Merelbeke, Belgium.
B Department of Animal Science, Institute for Agricultural and Fisheries Research, Scheldeweg 68, 9090 Melle, Belgium.
C Department of Animal Production, Suez Canal University, Ismailia 41522, Egypt.
D Department of Surgery and Obstetrics, Bangladesh Agricultural University, Mymensingh 2202, Bangladesh.
E Corresponding author. Email: mohammadbozlur.rahman@ugent.be
Reproduction, Fertility and Development 26(2) 245-257 https://doi.org/10.1071/RD12198
Submitted: 22 June 2012 Accepted: 27 November 2012 Published: 18 January 2013
Abstract
Heat stress has long been recognised as a cause of subfertility in farm animals. The objectives of the present study were to elucidate the effect of heat stress on sperm function and involvement of the mitogen-activated protein kinase (MAPK) 14 signalling pathway. Spermatozoa incubated for 4 h at a physiological temperature (38.5°C) exhibited significantly (P < 0.05) reduced motility, plasma membrane integrity and mitochondrial potential compared with non-incubated spermatozoa; the reductions in these parameters were more severe following incubation at a hyperthermic (41°C) temperature (P < 0.01). Percentages of fertilisation and embryo development were highly affected in spermatozoa incubated at 41°C compared with non-incubated spermatozoa (P < 0.01). Similarly, embryo quality was adversely affected by sperm incubation at 41°C, as indicated by a higher apoptotic cell ratio in Day 7 blastocysts compared with that in the non-incubated control group (14.6% vs 6.7%, respectively; P < 0.01). Using SB203580 (10 µg mL–1), a specific inhibitor of the p38 MAPK pathway, during sperm hyperthermia reduced MAPK14 activation (24.9% vs 35.6%), increased sperm motility (45.8% vs 26.5%) and reduced DNA fragmentation (16.9% vs 23.4%) compared with the untreated control group, but did not improve subsequent fertilisation and embryo development. In conclusion, heat stress significantly affects the potential of spermatozoa to penetrate oocytes, as well as subsequent embryo development and quality. Notably, the data show that the MAPK14 signalling pathway is largely involved in heat-induced sperm damage. However, further research is needed to elucidate other signalling pathways possibly involved in heat-induced sperm damage.
Additional keywords: functional parameters, mitogen-activated protein kinase 14 phosphorylation, terminal deoxyribonucleotidyl transferase-mediated dUTP–digoxigenin nick end-labelling.
Introduction
Mammalian spermatozoa are extremely specialised cells that differ from somatic cells in several features. These unique cells are, in fact, generated via a highly complex process called spermatogenesis. At the end of the spermatogenic cycle, after leaving the testis, spermatozoa become both transcriptionally and translationally inactive cells (Engel et al. 1973; Grunewald et al. 2005). It is believed that the functionality of spermatozoa is largely dependent on the post-translational modifications of proteins in the absence of de novo protein synthesis. This occurs during epididymal maturation and post-ejaculatory capacitation of spermatozoa in the female reproductive tract (Blaquier et al. 1988a, 1988b). The main function of a spermatozoon is to travel through the female genital tract, traverse the zona pellucida of an oocyte and finally deliver the male haploid genome to the oocyte during fertilisation.
The presence of programmed cell death or apoptosis in somatic as well as germ cells, especially during spermatogenesis, is well established (Hikim et al. 2003; Shaha 2007; Jia et al. 2009). However, the presence of apoptotic markers in ejaculated spermatozoa is contentious (Martin et al. 2004; Brum et al. 2008). It was believed that mature spermatozoa do not possess effective mechanisms for protein synthesis or DNA degradation and that the apoptotic cascade does not commence in spermatozoa in the mature state. However, recent data support the notion that apoptosis, or at least apoptotic-like processes, can be induced in ejaculated spermatozoa by a variety of stimuli or stresses (Martin et al. 2007; Ball 2008; Brum et al. 2008). These stresses and stimuli (e.g. heat stress, cryopreservation, oxidative stress and hormone deprivation) have been found to induce signs of apoptosis such as caspase activation, loss of mitochondrial membrane potential (MMP), phosphatidylserine externalisation (PSE) and DNA fragmentation (Aquila et al. 2007; Bejarano et al. 2008; Jia et al. 2009).
Heat stress has long been identified as a possible cause of subfertility in farm animals, especially in hot and humid climates (for a review, see Hansen 2009). However, the molecular basis of heat stress-induced toxicity has not been entirely characterised. In somatic cells, Bellmann et al. (2010) showed that heat stress, just like some degenerative diseases, causes proteins to misfold and aggregate, which, in turn, culminates in cell apoptosis via mitogen-activated protein kinase (MAPK) signalling pathways. The MAPKs are a family of serine threonine kinases that function as critical mediators of a variety of extracellular signals (Johnson and Lapadat 2002; Cowan and Storey 2003; Wada and Penninger 2004). However, it is not clear whether MAPK signalling pathways operate in ejaculated spermatozoa because they are transcriptionally inactive and their translational machinery is thought to be lost with the residual cytoplasm. However, compelling evidence suggests that features typically associated with apoptosis on the plasma membrane in somatic cells, including DNA fragmentation, low MMP, loss of plasma membrane integrity and PSE, are also present in the ejaculated human, boar, horse, bull, rat, hamster and mouse spermatozoa (Aziz et al. 2007; Martin et al. 2007; Brum et al. 2008; Said et al. 2008). In an in vivo study in rats, Jia et al. (2009) showed that MAPK14 is the key signalling pathway involved in heat-induced testicular germ cell apoptosis, inducing Bcl2 phosphorylation, which leads to its inactivation and subsequent activation of the mitochondria-dependent death pathway, mainly as a result of an imbalance in the Bax : Bcl2 ratio. Importantly, the key components of the MAPK signalling pathway, such as extracellular signal-regulated kinase (ERK) and MAPK kinase (MEK), have also been identified within human ejaculated spermatozoa (de Lamirande and Gagnon 2002; O’Flaherty et al. 2005; Almog et al. 2008; Du Plessis et al. 2010).
If we want to understand what happens in terms of the sperm machinery in response to heat stress, gaining an understanding the role of the MAPK14 signalling pathway in this response is highly important. Thus, in the present study we used an in vitro heat stress model that mimics the conditions to which spermatozoa may be exposed to in the reproductive tract of a heat-stressed cow. In the literature, there is no specific information as to the signalling pathway in heat-stressed bovine ejaculated spermatozoa that leads to apoptosis or apoptotic-like cascades. Understanding the signalling pathway involved in heat-induced sperm damage would be an important step towards developing effective strategies by which commercial semen producers could control apoptosis or apoptotic-like cascades, particularly in hot and humid climates. Thus, the first objective of the present study was to elucidate the impact of in vitro heat stress on sperm function, fertilisation and embryo development potential of the ejaculated spermatozoa. Taking into consideration the fact that in ejaculated spermatozoa these effects are due mainly to post-transcriptional modifications, the second objective of the present study was to confirm MAPK14 activation and to investigate the importance of the MAPK14-mediated signalling pathway in decreased sperm motility, increased DNA fragmentation and subsequent impaired fertilisation and embryo development by using the specific p38 MAPK inhibitor SB203580.
Materials and methods
Materials
Tissue culture medium (TCM) 199, gentamicin, basal medium Eagle (BME) amino acids and minimal essential medium (MEM) amino acids were purchased from GIBCO-BRL Life Technologies (Merelbeke, Belgium). Fetal calf serum (FCS) was obtained from Biochrom (Berlin, Germany). The fluorescent probes propidium iodide (PI), Hoechst 33342 and JC-1 were purchased from Molecular Probes (Eugene, OR, USA). Anti-phosphorylated (p-) p38α (Thr180/Tyr182) rabbit polyclonal antibody was obtained from Millipore (Temecula, CA, USA), fluorescein goat anti-rabbit secondary antibody was obtained from Molecular Probes and SB203580 was purchased from Calbiochem (San Diego, CA, USA). Unless stated otherwise, all other materials were obtained from Sigma-Aldrich (St Louis, MO, USA). All media were filtered through a sterile 0.22-µm filter (Millipore, New Bedford, MA, USA) before use.
Semen preparation
In each replicate, four frozen semen straws (0.25 mL) were separated through a discontinuous Percoll gradient (45% and 90% Percoll (v/v); Pharmacia Biotech, Uppsala, Sweden) to obtain viable and motile spermatozoa. The sperm pellet was then resuspended to a concentration of 50 × 106 spermatozoa mL–1 in sperm Tyrode’s albumin-lactate-pyruvate (Sp-TALP) and incubated at 38.5°C and 41°C for 4 h in 5% CO2 in humidified air. In addition, two semen straws were thawed, separated by Percoll gradient centrifugation and diluted to a concentration of 50 × 106 spermatozoa mL–1 in Sp-TALP for use as a non-incubated control. The control semen sample was prepared so that it could be used for fertilisation after the 4-h incubation period for the treated spermatozoa.
Computer-assisted sperm analysis
Computer-assisted sperm analysis (CASA) was performed using a Hamilton Thorne motility analyser (CEROS version 12.3d; Hamilton-Thorne Research, Beverly, MA, USA). The parameter settings were as described in previous studies (Rahman et al. 2011, 2012): frame rate 60 Hz; number of frames 30; minimum contrast 20; minimum cell size 10 pixels; non-motile head size 5 pixels; non-motile head intensity 20; medium path velocity (VAP) cut-off 50 µm s–1; straightness (STR) cut-off 70%; low VAP cut-off 30 µm s–1; and low straight line velocity (VSL) cut-off 15 µm s–1. Using the play-back facility, preliminary trials were performed to evaluate whether the spermatozoa were correctly identified. A 10-μL sample of diluted semen was placed on a prewarmed (37°C) Makler chamber and sperm motility parameters were analysed in five different focus fields using a ×10 negative phase contrast objective.
Sperm functional parameters
Four fluorescent probes (Hoechst 33342, PI, fluorescein isothiocyanate (FITC)-conjugated Pisum sativum agglutinin (PSA) and JC-1) were used to evaluate different sperm functional parameters simultaneously in the same focus field. Hoechst 33342 and PI were used to determine plasma membrane integrity, JC-1 was used to evaluate MMP and FITC-PSA was used to determine acrosomal status. The preparation of the fluorescent probes and sperm staining procedures were as described by Celeghini et al. (2007) with minor modifications. Briefly, semen samples were incubated with the fluorescent probes (5 µg mL–1 Hoechst 33342, 10 µg mL–1 PI, 2 µM JC-1 and 20 µg mL–1 FITC-PSA) for 10 min at 37°C in the dark. After incubation, 5-µL samples of the stained semen were placed on clear glass slides, coverslipped and evaluated immediately by epifluorescence microscopy (Leica DMR; Van Hopplynus, Brussels, Belgium). The spermatozoa were evaluated at ×400 magnification (oil immersion) and at least 200 spermatozoa were examined per slide. The spermatozoa were classified as having either a damaged (red) or intact (violet) plasma membrane, a reacted (yellow) or intact (violet) acrosome or high (orange–red) or low (green) MMP (Fig. 1).
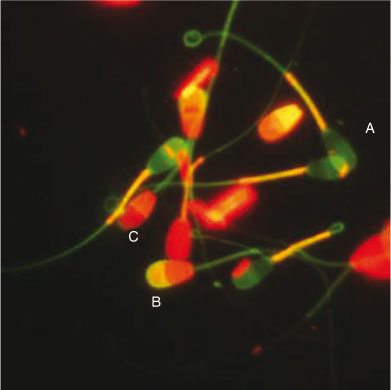
|
Oocyte IVM and IVF
To determine the effects of heat stress on the ability of the spermatozoa to fertilise oocytes, abattoir-derived oocytes were matured and fertilised in vitro. The method for oocyte maturation and fertilisation was as described by Vandaele et al. (2006). Briefly, bovine ovaries were washed three times with warm physiological saline supplemented with kanamycin (25 mg mL–1; GIBCO-BRL Life Technologies, Merelbeke, Belgium). The oocytes were then aspirated from 4–8-mm diameter follicles. Only oocytes with an evenly granulated ooplasm and multilayered (two to six) non-expanded cumulus cells were selected for IVM in modified bicarbonate-buffered TCM 199 supplemented with 20% heat-inactivated FCS (Biochrom). On average, 100–120 oocytes were matured in 500 µL maturation medium in a four-well plate for 20–24 h at 38.5°C in 5% CO2 in humidified air. After 20–24 h maturation, cumulus–oocyte complexes (COCs) were washed with IVF-TALP consisting of bicarbonate-buffered Tyrode solution supplemented with BSA (6 mg mL–1) and heparin (10 µg mL–1) once and transferred to 500 µL IVF medium (including 250 µL sperm suspension in IVF-TALP). The final concentration of spermatozoa was 2 × 106 mL–1 in IVF-TALP. After 20–24 h coincubation, presumptive zygotes were vortexed to remove cumulus cells and excess spermatozoa. Then, the putative zygotes were fixed in fixative solution (2% paraformaldehyde + 2% glutaraldehyde in phosphate-buffered saline (PBS)) and kept at 4°C for 24 h. The fixed presumptive zygotes were subsequently stained with Hoechst 33342 (5 µg mL–1 in PBS) for 10 min in the dark at room temperature. Five to 10 zygotes were placed into a drop of glycerol with 1,4-diazabicyclo (2.2.2) octane (DABCO) on a siliconised slide and coverslipped. The pronuclei (PN) of presumptive zygotes were identified by epifluorescence microscopy (Leica DMR) at a magnification of ×400 (oil immersion). Zygotes with 2PN were considered normally fertilised, whereas those with >2PN were considered to be polyspermic (Thys et al. 2009).
In vitro embryo production
Embryos were cultured using routine in vitro methods (Vandaele et al. 2006). Briefly, oocyte maturation and fertilisation procedures were as described above. For embryo culture, after vortexing presumptive zygotes were washed in Hepes TALP and transferred to 50-µL droplets of synthetic oviducal fluid supplemented with amino acids and FCS (SOFaa + 5% FCS) under mineral oil and cultured in groups of 25 zygotes for up to 168 h post insemination (h.p.i.) at 39°C in 5% CO2, 5% O2, and 90% N2. Embryos were evaluated at 45 h.p.i. for cleavage and at 168 h.p.i. for blastocyst development rates.
Evaluation of apoptotic cell ratio in blastocysts
Paraformaldehyde (4%)-fixed embryos were permeabilised with 0.5% Triton X-100 in PBS for 1 h before being washed with polyvinylpyrrolidone (PVP) solution (1 mg mL–1 in Ca2+- and Mg2+-free PBS). Positive and negative controls were treated with DNase I (50 units mL–1 in PBS) for 1 h at 37°C to ensure detection of strand breaks by terminal deoxyribonucleotidyl transferase-mediated dUTP–digoxigenin nick end-labelling (TUNEL; In Situ Cell Detection kit; Boehringer, Mannheim, Germany). After washing in PVP solution, the positive control and samples were incubated in terminal deoxynucleotidyl transferase for 1 h at 37°C in the dark. Meanwhile, the negative control was incubated in nucleotide mixture only (without transferase). After a second wash in PVP solution, controls and samples were incubated in RNase I (50 mg mL–1 in PBS) for 1 h at room temperature. The nuclei of the embryos were then counterstained with 0.5% (v/v) PI for 1 h at room temperature. Subsequently, embryos were quickly washed in PVP solution and mounted in a drop of DABCO on slides. The embryos were then coverslipped by making vaseline bridges. Embryos were examined by fluorescence microscopy (Leica DMR; magnification ×400, oil immersion). The TUNEL-positive nuclei appeared bright yellowish–green, whereas red (PI) staining was used to determine the total cell number (TCN), enabling identification, localisation and quantification of normal, fragmented and condensed nuclei as defined by Vandaele et al. (2010). Nuclei that appeared TUNEL-positive and condensed or fragmented were recorded as apoptotic.
Assessment of MAPK14 activation in spermatozoa after inhibition of the MAPK14 signalling pathway
An anti-p-MAPK14 antibody was used to identify MAPK14 activation in spermatozoa under non-heat-stressed or heat-stressed conditions. Semen samples were incubated at 38.5°C or 41°C for 4 h with or without SB203580 (10 µg mL–1) and dimethylsulfoxide (DMSO; 1%), alone or in combination. After 4 h incubation, semen samples were centrifuged at 700g for 5 min and the supernatant discarded. The sperm pellet was resuspended in 0.1% Triton X-100 and permeabilised for 10 min at room temperature. After washing in PBS, non-specific sites were blocked by 1% BSA and the spermatozoa were then incubated with anti-p-MAPK14 antibody (1 : 50 dilution) overnight at 4°C. Subsequently, spermatozoa were washed in 1% BSA in PBS before being incubated for 1 h with the secondary antibody (fluorescein goat anti-rabbit antibody; 1 : 200 dilution). After two washes with 1% BSA in PBS, spermatozoa were counterstained with Hoechst 33342 (5 µg mL–1 in PBS). Subsequently, after washing once in PSB at 700g for 3 s at room temperature, the sperm pellet was resuspended in PBS (1 : 30; 5µL sperm pellet in 150 µL PBS). Slides were prepared by putting 5 µL sperm suspension on a drop of glycerol with DABCO and covering them with a coverslip. The slides were then evaluated by epifluorescence microscopy (Leica DMR) at magnification ×400 (oil immersion). At least 200 spermatozoa were analysed from each sample to determine the percentage of MAPK14-activated spermatozoa, which appeared bright yellowish–green (Fig. 2).
Assessment of sperm DNA fragmentation by modified TUNEL assay after inhibition of the MAPK14 signalling pathway
To assess the impact of inhibiting the MAPK14 signalling pathway during heat stress on bovine spermatozoa, we used the specific p38 MAPK inhibitor SB203580 (10 µg mL–1 in DMSO; Calbiochem, San Diego, CA, USA). Stock solutions of 40 µg mL–1 SB203580 were prepared in 1% DMSO and stored at –20°C in the dark until use. The TUNEL assay was used to detect the presence of free 3′-OH terminals in single- and double-stranded sperm DNA. Purified suspensions of spermatozoa were incubated at 38.5°C or 41°C for 4 h with or without 10 µg mL–1 SB203580 and DMSO, alone or in combination. The spermatozoa were then washed with Sp-TALP and incubated in 2 mM dithiothreitol (DTT) in Sp-TALP for 45 min at room temperature (Rahman et al. 2012). The spermatozoa were then centrifuged at 700g for 5 min and washed in Sp-TALP. The sperm pellet was then diluted with PVP solution (1 mg mL–1 in PBS) to a final concentration of 10 × 106 spermatozoa mL–1, from which a 10-µL aliquot was smeared onto a poly-L-lysine-coated microslide. After fixation with 4% paraformaldehyde in PBS (pH 7.4) for 15 min on ice and permeabilisation with 0.5% (v/v) Triton X-100 in PBS and 1% sodium citrate for 30 min at room temperature, the spermatozoa were incubated with TUNEL mixture (terminal deoxynucleotidyl transferase) for 1 h at 37°C in the dark. Both positive (1 mg mL–1 DNAse I) and negative (nucleotide mixture in the absence of transferase) controls were included in each replicate. Hoechst 33342 (5 µg mL–1 in PBS) was used to counterstain sperm DNA. Samples were examined by fluorescence microscopy (Leica DMR; magnification ×400, oil immersion). At least 200 spermatozoa were analysed from each sample to evaluate the percentage of TUNEL-positive spermatozoa (bright green nuclear fluorescence).
Experimental design
Experiment 1: motility and functional parameters of incubated spermatozoa
Sperm samples were subjected to 38.5°C or 41°C at 5% CO2 in air; these temperatures correspond to the characteristic rectal temperatures of cattle under normothermic and hyperthermic conditions, respectively. The non-incubated control consisted of spermatozoa used immediately after Percoll separation. Sperm motility parameters were determined by CASA, whereas sperm functional parameters, such as plasma membrane integrity, MMP and acrosome integrity, were evaluated after staining with a combination of the four fluorochromes. The experiment was repeated four times.
Experiment 2: fertilisation, cleavage and embryo developmental potential of pre-incubated spermatozoa and apoptosis in blastocyst
In all, 966 oocytes were matured in vitro and fertilised with non-incubated spermatozoa (control) or spermatozoa pre-incubated at either 38.5°C or 41°C. At 20–24 h.p.i., presumptive zygotes were denuded and cultured up to Day 7 post insemination. In each replicate, 75 presumptive zygotes were cultured and the remaining zygotes were fixed and subjected to Hoechst 33342 staining to determine normal fertilisation (2PN), polyspermy (>2PN) and sperm penetration (≥2PN) rates. Embryos were evaluated for blastocyst yield on Day 7 post insemination and fixed in 4% paraformaldehyde. Subsequently, the apoptotic cell ratio (ACR) of blastocysts was determined by TUNEL staining. The experiment was repeated three times.
Experiment 3: effects of SB203580 on MAPK14 activation, sperm motility, DNA integrity, fertilisation and embryo development potential of pre-incubated spermatozoa
Before exposing spermatozoa to normal temperatures or heat stress, they were exposed to SB203580 for 4 h. Simultaneously, spermatozoa were incubated without either SB203580 or DMSO (heat-stressed control) or with DMSO alone (vehicle control). All groups were compared with non-incubated control spermatozoa and the heat-stressed control. After 4 h incubation, MAPK14 activation, sperm motility and DNA integrity were evaluated by anti-p-MAPK14 fluorescent staining, CASA and TUNEL staining, respectively. Concomitantly, the incubated semen samples were washed and used for IVF and embryo development. A total of 1616 oocytes was used for IVF and embryo development. The experiment was replicated three times.
Statistical analysis
Univariate analysis of variance (ANOVA) was used for analysis of sperm motility parameters, functional parameters, DNA fragmentation, MAPK14 activation, cleavage and blastocyst development rates and the ACR in blastocysts as dependent variables, with group as the fixed factor and replicate as the random factor (mixed-model ANOVA). Post hoc pairwise comparisons between groups were made using Scheffé’s test. Logistic regression with normal fertilisation (2PN), polyspermy (>2PN) and sperm penetration (≥2PN) as dependent variables and group as the independent variable, including the effect of replicate, was used to analyse normal fertilisation, polyspermy and sperm penetration rates. For all analyses, the assumptions of the models (i.e. normal distribution of data and homogeneity of variances between the groups) were checked using Levene’s test. Two-sided P < 0.05 was considered significant. All statistical analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
Results
Experiment 1
Effects of 4 h incubation at 38.5°C or 41°C on sperm motility parameters
Incubation of spermatozoa at 38.5°C or 41°C for 4 h decreased the percentage of both total and progressive motility, as well as sperm velocities, compared with non-incubated control spermatozoa (Table 1). Notably, total and progressive sperm motility were lower after incubation of spermatozoa at 41°C (23.3% and 15.5%, respectively) compared with motility in non-incubated control spermatozoa (80.3% and 57.3%, respectively; P < 0.001). Furthermore, total and progressive sperm motility were lower for spermatozoa incubated at 41°C compared with those incubated at 38.5°C (P < 0.01).
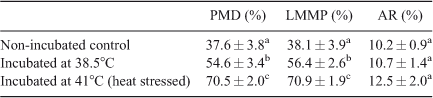
Effects of 4 h incubation at 38.5°C or 41°C on plasma membrane integrity, mitochondrial membrane potential and acrosome status
Marked differences were observed in plasma membrane integrity, and mitochondrial levels between spermatozoa incubated at 38.5°C and 41°C. Incubation of spermatozoa at 38.5°C resulted in significant disruption of plasma membrane integrity and MMP compared with non-incubated control spermatozoa (P < 0.01). Incubation of spermatozoa at 41°C (heat stress) further decreased plasma membrane integrity and MMP compared with non-incubated control spermatozoa (P < 0.01). Spermatozoa with a damaged plasma membrane also exhibited low MMP. However, there were no significant differences in the percentage of acrosome-reacted spermatozoa among groups (Table 2).
|
Experiment 2
Effects of 4 h incubation of spermatozoa at 38.5°C or 41°C on oocyte penetration and fertilisation
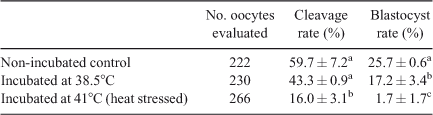
Incubation of spermatozoa for 4 h at either 38.5°C or 41°C significantly reduced percentage oocyte penetration (i.e. embryos with ≥2PN) compared with that of the non-incubated control spermatozoa (55.2% and 21.5% vs 75.3%, respectively; P < 0.05 for both). Although incubation at 38.5°C had no effect on the normal fertilisation rate (determined by the proportion of embryos with 2PN) compared with control (50.8% vs 64.7%; P > 0.05), incubation at 41°C significantly reduced the normal fertilisation rate (20.4%; P < 0.01; Table 3). Conversely, the percentage of oocytes exhibiting polyspermy (i.e. embryos with >2PN) was significantly reduced when oocytes were inseminated with spermatozoa incubated at 41°C compared with non-incubated control spermatozoa (1.1% vs 10.7%, respectively; P < 0.05).
Effects of 4 h incubation of spermatozoa at 38.5°C or 41°C on cleavage and blastocyst rates
When oocytes were fertilised with spermatozoa that had been incubated at 41°C, the proportion of embryos that cleaved to two or more cells was lower than that seen after oocytes were fertilised with spermatozoa incubated at 38.5°C or non-incubated control spermatozoa (16% vs 43% and 59.7%, respectively; P < 0.01; Table 4). There was no significant difference in the proportion of embryos cleaving to two or more cells between oocytes fertilised with spermatozoa incubated at 38.5°C and non-incubated control spermatozoa (P > 0.05). In contrast, blastocyst development was significantly reduced after fertilisation of oocytes with spermatozoa incubated at 38.5°C and 41°C compared with the non-incubated control (17.2% and 1.7% vs 25.7%, respectively; P < 0.05 and P < 0.01, respectively).
|
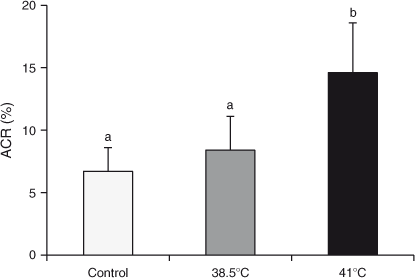
Effects of 4 h incubation of spermatozoa at 38.5°C or 41°C on the ACR in blastocysts
The mean ACR (as determined by the TUNEL assay) was higher in blastocysts developed from oocytes fertilised with spermatozoa incubated at 41°C compared with those from oocytes fertilised with spermatozoa incubated at 38°C or the non-incubated control (14.6% vs 8.4% and 6.7%, respectively; P < 0.01; Fig. 3). There were no differences in the ACR in blastocysts from oocytes fertilised with spermatozoa incubated at 38°C or the non-incubated control (P > 0.05).
|
Experiment 3
Effects of SB203580 on MAPK14 activation in spermatozoa incubated for 4 h at 38.5°C or 41°C
Spermatozoa incubated for 4 h at 38.5°C with or without SB203580 and DMSO alone or in combination did not exhibit any differences in MAPK14 activation. However, MAPK14 activation in these groups was increased compared with that in the non-incubated control group (Fig. 4a). Conversely, incubation of spermatozoa at 41°C significantly increased MAPK14 activation compared with the non-incubated control, which was significantly reduced by SB203580 (35.6% vs 23.9%, respectively; P < 0.01). Alone, DMSO had no effect on MAPK14 activation in spermatozoa incubated at 41°C (42.5%; Fig. 4b).
Effects of SB203580 on the motility of spermatozoa incubated for 4 h at 38.5°C or 41°C
The total and progressive motility of control spermatozoa immediately after Percoll purification were 79.0% and 60.5%, respectively. The addition of SB203580 to semen samples before incubation for 4 h at 41°C effectively protected sperm motility compared with the untreated and DMSO-treated groups (P < 0.01). When semen samples were incubated at 38.5°C, DMSO had a negative effect on sperm motility compared with SB203580 (P < 0.05), indicating that at the concentrations used DMSO has an adverse effect on sperm survival (Table 5).
Effects of SB203580 on DNA fragmentation in spermatozoa incubated for 4 h at 38.5°C or 41°C
Because the MAPK14 signalling pathway is involved in heat responses, we examined whether heat stress could induce DNA fragmentation and, if so, whether SB203580 could prevent such DNA fragmentation. There was no increase in DNA fragmentation in spermatozoa incubated at 38.5°C; however, incubation of spermatozoa at 41°C significantly increased DNA fragmentation (Fig. 5). Notably, SB203580 significantly reduced DNA fragmentation compared with that in the untreated and DMSO-treated spermatozoa incubated at 41°C (P < 0.01).
Effects of SB203580 on the potential for fertilisation and subsequent embryo development
Blockade of the MAPK14 signalling pathway by SB203580 during incubation of spermatozoa for 4 h at 38.5°C or 41°C had no significant effect on percentage fertilisation compared with that in the untreated and DMSO-treated groups incubated at 41°C (P > 0.05; Fig. 6). Similarly, SB203580 had no significant effect on blastocyst development compared with that in the untreated and DMSO-treated groups incubated at 41°C (Fig. 7).
Discussion
In the present study, we investigated the functional modifications of transcriptionally inactive ejaculated bovine spermatozoa induced by heat stress in vitro and, subsequently, the involvement of the MAPK14 signalling pathway. Incubation of bovine spermatozoa under normothermic (38.5°C) or hyperthermic (41°C) conditions significantly reduced sperm motility, plasma membrane integrity, MMP, overall fertilisation and blastocyst development potential. The effects were more severe in spermatozoa incubated at 41°C, a temperature equivalent to the characteristic rectal temperatures recorded in heat-stressed cows, particularly in hot and humid climates. By using an antibody against p-MAPK14, we confirmed heat stress-induced activation of MAPK14. Subsequently, using the specific p38 MAPK inhibitor SB203580, we showed that the MAPK14 pathway played an important role in heat-induced sperm damage and that blocking this pathway protects MAPK14 activation, restores sperm motility and decreases DNA fragmentation. However, blocking of the MAPK14 signalling pathway was not sufficient to augment fertilisation and embryo developmental potential.
The maintenance of testicular temperature 4–5°C below core body temperature in breeding bulls is important for proper spermatogenesis. Increased testicular temperature (~2°C) during the spermatogenic cycle, especially during the spermiogenic and meiotic stages of germ cell development, even for a short period of time (i.e. 48 h) affects sperm motility, morphology, plasma membrane integrity, chromatin protamination and nuclear shape (Rahman et al. 2011). Similarly, after collection of ejaculate, maintenance of the ejaculate temperature close to testicular temperature (~32°C) is important for the maintenance of proper sperm functional properties. After leaving the testicular environment, spermatozoa are, in fact, vulnerable to sudden temperature changes. Even incubation of spermatozoa at normothermic temperatures (39°C) for a short period of time (3 h) compromises their viability and functional competence (Monterroso et al. 1995). In the present study, both total and progressive sperm motility, as well as other parameters of sperm velocity determined by CASA, decreased after spermatozoa were incubated for 4 h at 38.5°C, with even worse effects seen after incubation at 41°C. Similarly, Hendricks et al. (2009) reported that incubation of bovine spermatozoa at 38.5°C for 4 h decreased sperm motility, with slightly more prominent effects when spermatozoa were incubated at 40°C. However, in the present study, incubation of spermatozoa at 41°C had significant adverse effects on sperm motility parameters, which may be due to the fact that the maximum thermotolerance level of the spermatozoa was exceeded. Exposure of spermatogenic cells to heat stress is known to produce reactive oxygen species (ROS; Ishii et al. 2005; Nichi et al. 2006). Because of excessive ROS production, the membrane loses its fluidity and structure, which has been related to poor sperm motility and function (Silva et al. 2007; Du Plessis et al. 2010). Similarly, in the present study we observed that heat stress significantly affects sperm plasma membrane integrity and MMP in a highly correlated manner (r = 0.95). The high correlation between sperm plasma membrane damage and low MMP can be explained by the fact that oxidation of the sperm plasma membrane occurs at the mid-piece (Brouwers and Gadella 2003), the area where the mitochondria are located. Conversely, incubation of spermatozoa at 41°C had no effect on acrosome integrity, as evaluated by FITC-PSA staining. This finding is in accordance with that reported by Silva et al. (2007) and supports the hypothesis that before the acrosome reaction, spermatozoa should undergo capacitation. Moreover, it is also possible that spermatozoa died before starting the acrosome reaction.
The decreased sperm motility and functionality were mirrored by reductions in fertilisation and sperm penetration rates, reflecting the lower ability of incubated spermatozoa to attach to or penetrate the zona pellucida of the oocytes. Studies indicate that the contribution of a spermatozoon to the oocyte is more than delivering the paternal genome, and that the centriole (for a review, see Sutovsky and Schatten 1999), RNA (Ostermeier et al. 2004) and proper epigenetic marks in the form of DNA methylation (for a review, see Miller et al. 2010; Steger et al. 2011) are almost equally important for successful fertilisation and embryo development. Hence, in addition to causing sperm DNA damage (Paul et al. 2008, 2009), it is also possible that heat stress damages the sperm centriole and/or causes aberrant DNA methylation in the male pronucleus that subsequently leads to a delay in fertilisation or fertilisation failure; this would be an interesting area for future research. Incubation of spermatozoa at 39°C for 24 h before fertilisation has been reported to decrease blastocyst developmental competence (Lechniak et al. 2003), which we confirmed in the present study using spermatozoa incubated at 41°C for 4 h. It is conceivable that the quality of blastocysts produced after fertilisation of oocytes with heat-stressed spermatozoa is compromised because of the increased level of sperm DNA damage. Similarly, several studies have reported that mice exposed to scrotal heat stress have an increased percentage of spermatozoa with high levels of DNA damage and, as such, insemination using these spermatozoa ultimately resulted in impaired blastocyst formation (Jannes et al. 1998; Paul et al. 2008; 2009). Further in vitro studies in cattle have confirmed these findings: Walters et al. (2005a, 2005b) observed less blastocyst development with higher ACR after fertilisation of oocytes with semen collected from scrotal-insulated bulls. Thus, the collective data suggest that heat stress to spermatozoa may activate signals for the commencement of death cascades, consequently compromising embryo development and quality. Ishii et al. (2005) speculated that ROS generated from heat stress may function as a type of signal for spermatogenic cell death rather than directly causing oxidative damage to cells.
Therefore, we were interested in investigating the signalling pathway involved in heat-induced sperm damage aiming to shut down the signalling pathway by a specific pharmacological inhibitor whenever necessary. It has been shown that MAPK signalling transduction is activated by a variety of environmental stressors and promotes both apoptosis and the growth inhibition of cells (Cowan and Storey 2003; Wada and Penninger 2004). Specifically, MAPK14 has been identified as a key signalling pathway for heat-induced testicular germ cell apoptosis by resulting in an imbalance in the mitochondrial Bax/Bcl2 ratio (Jia et al. 2009). Therefore, in the present study, we hypothesised that heat stress may activate the MAPK14 signalling pathway in spermatozoa and cause cell death by activating mitochondrial Bax, because Bcl2 is not detected in bovine spermatozoa (Martin et al. 2007). To confirm our hypothesis we used an antibody against p-MAPK14 and observed heat stress-induced MAPK14 activation. Hence, we have illustrated the signalling pathway involved in heat-induced bovine sperm damage and how to inhibit the death cascades (Fig. 8). Subsequently, we inhibited the MAPK14 signalling pathway using SB203580 during heat stress of spermatozoa. Fluorescence investigation proved that SB203580 significantly protected MAPK14 activation during heat stress. In addition, evaluation of sperm motility by CASA revealed that SB203580 effectively protected against the deleterious effects induced by heat stress. We also investigated whether heat stress could induce sperm DNA fragmentation and whether SB203580 could prevent the death cascades. In the sperm DNA fragmentation study, we used a modified TUNEL assay that was originally applied to human, mouse (Mitchell et al. 2011) and bovine (Rahman et al. 2012) spermatozoa because conventional TUNEL assays consistently fail to identify sperm DNA fragmentation (Mitchell et al. 2011). We observed that incubation of spermatozoa at normothermic temperatures for 4 h did not induce sperm DNA fragmentation, because the relative compactness of sperm DNA surrounded by protamine molecules protects against damage (Ward 2010; Mitchell et al. 2011). However, incubation of spermatozoa at normothermic temperatures in the presence of DMSO increased sperm DNA fragmentation. Although we do not know the mechanisms underlying the induction of DNA fragmentation by DMSO, the addition of DMSO as cryoprotectants for bull or stallion spermatozoa has been reported to reduce sperm motility and viability by inhibiting Na+/K+ ion pumps (de la Cueva et al. 1997; Ball and Vo 2001). Notably, incubation of spermatozoa at the hyperthermic temperature significantly augmented DNA fragmentation, which was prevented by SB203580 as a result of blockade of MAKP14 signalling transduction (Fig. 5b).
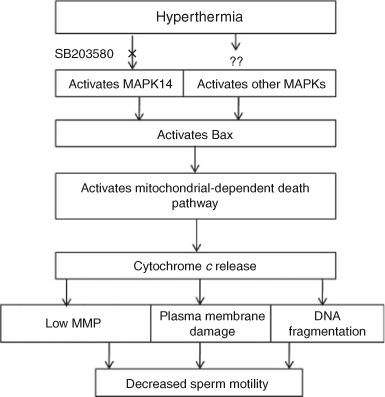
|
Therefore, we speculated that blockade of the MAPK14 signalling pathway by SB203580 in bovine spermatozoa subjected to heat-stress would increase IVF and embryo development rates compared with heat-stressed control or DMSO-treated spermatozoa. Unexpectedly, there were no significant improvements in either the rate of fertilisation or embryo development after blockade of the MAPK14 pathway with SB203580, although there was a trend for an increased fertilisation rate that failed to reach statistical significance (P = 0.08). Higher concentrations of SB203580 (20 and 40 µg mL–1) did not improve the results (data not shown). Thus, it is possible that more than one member of the MAPK family is involved in heat stress-induced sperm damage, and so the following reasons may explain the unexpected outcomes: (1) unlike in the mouse and rat (Jia et al. 2009), heat stress in bovine spermatozoa also activates MAPK1/3 and/or MAPK8 signalling pathways, which may mediate the deleterious effects (this hypothesis requires further investigation before it can be confirmed) and (2) bovine embryo development to the blastocyst stage requires both MAPK and ERK signalling pathways, but these pathway have been found to compensate for one another (Madan et al. 2005). Thus, in the present study, it may be possible that the fertilisation medium contained leftover SB203580 (from semen samples even after washing) that may have blocked MAPK14 signalling in the embryos. However, this is very unlikely because, under these conditions, blockade of the MAPK14 signalling pathway would probably be compensated for by the ERK pathway.
In a practical sense, the effects of heat stress on spermatozoa determined in the present study may reflect the changes occurring in the reproductive tract of heat-stressed cows. In fact, rectal/reproductive tract temperatures in heat-stressed cows have been found to be as high as 41°C (Elvinger et al. 1992; de Castro e Paula et al. 2008). Insemination of cows under such conditions of heat stress has been reported to lead to reductions in field fertility and conception (for a review, see Hansen 2009). Our data support the hypothesis that spermatozoa may be damaged during semen processing or in the reproductive tract of cows, especially under conditions of heat stress. However, in vitro heat stress to spermatozoa does not completely mimic the conditions in vivo in a cow’s reproductive tract, because oviducal fluid and epithelial cells maintain sperm motility and viability (Pollard et al. 1991).
In conclusion, incubation of spermatozoa at temperatures characteristic of normothermia and hyperthermia affected sperm motility and function. Normal fertilisation and cleavage rates were not affected by the incubation of spermatozoa at the normothermic temperature, but embryo development to the blastocyst stage was compromised. Furthermore, incubation of spermatozoa at the hyperthermic temperature severely compromised fertilisation, cleavage and subsequent embryo development potential. We identified MAPK14 as a key signalling pathway by which heat stress activates the intrinsic signal transduction and promotes sperm cell damage. Therefore, we suggest that inhibition of the MAPK14 signalling pathway during heat stress of spermatozoa may be an option for maintaining sperm function under tropical conditions. However, before practical applications can be contemplated, further research is needed to investigate the phosphorylation of mitogen-activated protein kinase-activated protein kinase 2 (MAPKAPK2) and heat shock protein 27 as part of the possible toxicity of the inhibitor on embryos and to elucidate other signalling pathways involved in the damaging effects of heat stress on bovine spermatozoa and subsequent effects on fertilisation and embryo development potential.
Acknowledgements
The authors thank Isabel Lemahieu and Petra Van Damme for their technical assistance. The research was supported financially by Ghent University (grants 01SF1208 and 01SF1409).
References
Almog, T., Lazar, S., Reiss, N., Etkovitz, N., Milch, E., Rahamim, N., Dobkin-Bekman, M., Rotem, R., Kalina, M., Ramon, J., Raziel, A., Brietbart, H., Seger, R., and Naor, Z. (2008). Identification of extracellular signal-regulated kinase 1/2 and p38 MAPK as regulators of human sperm motility and acrosome reaction and as predictors of poor spermatozoan quality. J. Biol. Chem. 283, 14 479–14 489.| Identification of extracellular signal-regulated kinase 1/2 and p38 MAPK as regulators of human sperm motility and acrosome reaction and as predictors of poor spermatozoan quality.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvFyjsL4%3D&md5=395b91768a5682e49197c2d1e48622ccCAS |
Aquila, S., Middea, E., Catalano, S., Marsico, S., Lanzino, M., Casaburi, I., Barone, I., Bruno, R., Zupo, S., and Ando, S. (2007). Human sperm express a functional androgen receptor: effects on PI3K/AKT pathway. Hum. Reprod. 22, 2594–2605.
| Human sperm express a functional androgen receptor: effects on PI3K/AKT pathway.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXhtlektrfL&md5=80f6d1dcf64a32c47f77c51ddda32275CAS | 17656415PubMed |
Aziz, N., Said, T., Paasch, U., and Agarwal, A. (2007). The relationship between human sperm apoptosis, morphology and the sperm deformity index. Hum. Reprod. 22, 1413–1419.
| The relationship between human sperm apoptosis, morphology and the sperm deformity index.Crossref | GoogleScholarGoogle Scholar | 17303629PubMed |
Ball, B. A. (2008). Oxidative stress, osmotic stress and apoptosis: impacts on sperm function and preservation in the horse. Anim. Reprod. Sci. 107, 257–267.
| Oxidative stress, osmotic stress and apoptosis: impacts on sperm function and preservation in the horse.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXoslWkt7c%3D&md5=dfb645a2a5bdabf5a754a24c6c4ec5dfCAS | 18524506PubMed |
Ball, B. A., and Vo, A. (2001). Osmotic tolerance of equine spermatozoa and the effects of soluble cryoprotectants on equine sperm motility, viability, and mitochondrial membrane potential. J. Androl. 22, 1061–1069.
| 1:CAS:528:DC%2BD3MXotlyns7o%3D&md5=cea610611fb5b51f38c4502dd243e644CAS | 11700853PubMed |
Bejarano, I., Lozano, G. M., Ortiz, A., Garcia, J. F., Paredes, S. D., Rodriguez, A. B., and Pariente, J. A. (2008). Caspase 3 activation in human spermatozoa in response to hydrogen peroxide and progesterone. Fertil. Steril. 90, 1340–1347.
| Caspase 3 activation in human spermatozoa in response to hydrogen peroxide and progesterone.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXhtlSrurbK&md5=07faf01885a2aa5fd3ffa4959bf55c6fCAS | 18155705PubMed |
Bellmann, K., Charette, S. J., Nadeau, P. J., Poirier, D. J., Loranger, A., and Landry, J. (2010). The mechanism whereby heat shock induces apoptosis depends on the innate sensitivity of cells to stress. Cell Stress Chaperones 15, 101–113.
| The mechanism whereby heat shock induces apoptosis depends on the innate sensitivity of cells to stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFaqt73E&md5=bb88ee892c7c6a9b6b5924e2788c0712CAS | 19557548PubMed |
Blaquier, J. A., Cameo, M. S., Cuasnicu, P. S., Gonzalez Echeverria, M. F., Pineiro, L., and Tezon, J. G. (1988a). The role of epididymal factors in human sperm fertilizing ability. Ann. N. Y. Acad. Sci. 541, 292–296.
| The role of epididymal factors in human sperm fertilizing ability.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXovVOisQ%3D%3D&md5=76d305460084050574d90191efe53f88CAS | 3195912PubMed |
Blaquier, J. A., Cameo, M. S., Cuasnicu, P. S., Gonzalez Echeverria, M. F., Pineiro, L., Tezon, J. G., and Vazquez, M. H. (1988b). On the role of epididymal factors in sperm fertility. Reprod. Nutr. Dev. 28, 1209–1216.
| On the role of epididymal factors in sperm fertility.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaL1MXkt1WitL8%3D&md5=3ff47dc6f0fc95f125e56e705246cad7CAS | 3075792PubMed |
Brouwers, J. F. H. M., and Gadella, B. M. (2003). In situ detection and localization of lipid peroxidation in individual bovine sperm cells. Free Radic. Biol. Med. 35, 1382–1391.
| In situ detection and localization of lipid peroxidation in individual bovine sperm cells.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXpt1equrg%3D&md5=9fd99b3ee3cfc51cbe921bd5967a8137CAS |
Brum, A. M., Sabeur, K., and Ball, B. A. (2008). Apoptotic-like changes in equine spermatozoa separated by density-gradient centrifugation or after cryopreservation. Theriogenology 69, 1041–1055.
| Apoptotic-like changes in equine spermatozoa separated by density-gradient centrifugation or after cryopreservation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXlvVSltLo%3D&md5=4358b00e67002e4db814002c2c6ece5fCAS | 18378291PubMed |
Celeghini, E. C., de Arruda, R. P., de Andrade, A. F., Nascimento, J., and Raphael, C. F. (2007). Practical techniques for bovine sperm simultaneous fluorimetric assessment of plasma, acrosomal and mitochondrial membranes. Reprod. Domest. Anim. 42, 479–488.
| Practical techniques for bovine sperm simultaneous fluorimetric assessment of plasma, acrosomal and mitochondrial membranes.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2srjtF2qtA%3D%3D&md5=5a3b1a5746e007af1997d3c1b33c37deCAS | 17845603PubMed |
Cowan, K. J., and Storey, K. B. (2003). Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress. J. Exp. Biol. 206, 1107–1115.
| Mitogen-activated protein kinases: new signaling pathways functioning in cellular responses to environmental stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXjt1aqtb0%3D&md5=0f49627d9d7de9fc98b5f578e484e7baCAS | 12604570PubMed |
de Castro e Paula, L. A., Andrzejewski, J., Julian, D., Spicer, L. J., and Hansen, P. J. (2008). Oxygen and steroid concentrations in preovulatory follicles of lactating dairy cows exposed to acute heat stress. Theriogenology 69, 805–813.
| Oxygen and steroid concentrations in preovulatory follicles of lactating dairy cows exposed to acute heat stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXktFClsbs%3D&md5=723b0d4616485933d4f54cf41f938710CAS | 18243293PubMed |
de la Cueva, F. I. C., Pujol, M. R., Rigau, T., Bonet, S., Miro, J., Briz, M., and Rodriguez-Gill, J. E. (1997). Resistance to osmotic stress of horse spermatozoa: The role of ionic pumps and their relationship to cryopreservation success. Theriogenology 48, 947–968.
| Resistance to osmotic stress of horse spermatozoa: The role of ionic pumps and their relationship to cryopreservation success.Crossref | GoogleScholarGoogle Scholar |
de Lamirande, E., and Gagnon, C. (2002). The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion. Mol. Hum. Reprod. 8, 124–135.
| The extracellular signal-regulated kinase (ERK) pathway is involved in human sperm function and modulated by the superoxide anion.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XitFOgsr8%3D&md5=4eab3a859b6146d5775e6042133e49c7CAS | 11818515PubMed |
Du Plessis, S. S., McAllister, D. A., Luu, A., Savia, J., Agarwal, A., and Lampiao, F. (2010). Effects of H2O2 exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels. Andrologia 42, 206–210.
| Effects of H2O2 exposure on human sperm motility parameters, reactive oxygen species levels and nitric oxide levels.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXosF2ls78%3D&md5=d6b350a2ead7c35cba428b8686b29ac6CAS | 20500750PubMed |
Elvinger, F., Natzke, R. P., and Hansen, P. J. (1992). Interactions of heat stress and bovine somatotropin affecting physiology and immunology of lactating cows. J. Dairy Sci. 75, 449–462.
| Interactions of heat stress and bovine somatotropin affecting physiology and immunology of lactating cows.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DyaK38Xht1Wgs7s%3D&md5=62264dfd19f53ce1e05fd9a4e30ddf97CAS | 1560140PubMed |
Engel, J. C., Bernard, E. A., and Wassermann, G. F. (1973). Protein synthesis by isolated spermatozoa from cauda and caput epididymis of rat. Acta Physiol. Lat. Am. 23, 358–362.
| 1:CAS:528:DyaE2cXmvVOhuw%3D%3D&md5=d0596f01fcea1a9337532a07d7d54ee1CAS | 4361620PubMed |
Grunewald, S., Paasch, U., Glander, H. J., and Anderegg, U. (2005). Mature human spermatozoa do not transcribe novel RNA. Andrologia 37, 69–71.
| Mature human spermatozoa do not transcribe novel RNA.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXpslGitLo%3D&md5=26686318d054dad0d1d285fb3bd7b227CAS | 16026427PubMed |
Hansen, P. J. (2009). Effects of heat stress on mammalian reproduction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 3341–3350.
| Effects of heat stress on mammalian reproduction.Crossref | GoogleScholarGoogle Scholar | 19833646PubMed |
Hendricks, K. E. M., Martins, L., and Hansen, P. J. (2009). Consequences for the bovine embryo of being derived from a spermatozoon subjected to post-ejaculatory aging and heat shock: development to the blastocyst stage and sex ratio. J. Reprod. Dev. 55, 69–74.
| Consequences for the bovine embryo of being derived from a spermatozoon subjected to post-ejaculatory aging and heat shock: development to the blastocyst stage and sex ratio.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXktlGgtLw%3D&md5=61928d571702baedc7d3a3aa5162f060CAS |
Hikim, A. P., Lue, Y., Yamamoto, C. M., Vera, Y., Rodriguez, S., Yen, P. H., Soeng, K., Wang, C., and Swerdloff, R. S. (2003). Key apoptotic pathways for heat-induced programmed germ cell death in the testis. Endocrinology 144, 3167–3175.
| Key apoptotic pathways for heat-induced programmed germ cell death in the testis.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD3sXkvF2ktb4%3D&md5=6e21af1d5c986296be213727b2b1c602CAS | 12810573PubMed |
Ishii, T., Matsuki, S., Iuchi, Y., Okada, F., Toyosaki, S., Tomita, Y., Ikeda, Y., and Fujii, J. (2005). Accelerated impairment of spermatogenic cells in sod1-knockout mice under heat stress. Free Radic. Res. 39, 697–705.
| Accelerated impairment of spermatogenic cells in sod1-knockout mice under heat stress.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXltFymurw%3D&md5=3fe9511339d1252534426366781fa270CAS | 16036348PubMed |
Jannes, P., Spiessens, C., Van der Auwera, I., D’Hooghe, T., Verhoeven, G., and Vanderschueren, D. (1998). Male subfertility induced by acute scrotal heating affects embryo quality in normal female mice. Hum. Reprod. 13, 372–375.
| Male subfertility induced by acute scrotal heating affects embryo quality in normal female mice.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK1c3hs12hsg%3D%3D&md5=d5b0e62b4348c677a452ae9629bb7734CAS | 9557841PubMed |
Jia, Y., Castellanos, J., Wang, C., Sinha-Hikim, I., Lue, Y., Swerdloff, R. S., and Sinha-Hikim, A. P. (2009). Mitogen-activated protein kinase signaling in male germ cell apoptosis in the rat. Biol. Reprod. 80, 771–780.
| Mitogen-activated protein kinase signaling in male germ cell apoptosis in the rat.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXjvVSkur4%3D&md5=d4ae4123a4fdce668689b3db3d5ac418CAS | 19109224PubMed |
Johnson, G. L., and Lapadat, R. (2002). Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912.
| Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38Xpt1Wisbc%3D&md5=5d78bf4a79bd3785d151db2879f20b52CAS | 12471242PubMed |
Lechniak, D., Strabel, T., Bousquet, D., and King, A. W. (2003). Sperm pre-incubation prior to insemination affects the sex ratio of bovine embryos produced in vitro. Reprod. Domest. Anim. 38, 224–227.
| Sperm pre-incubation prior to insemination affects the sex ratio of bovine embryos produced in vitro.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s3ntVyhsA%3D%3D&md5=544e6f89f81bf697d16b5facd679b136CAS | 12753558PubMed |
Madan, P., Calder, M. D., and Watson, A. J. (2005). Mitogen-activated protein kinase (MAPK) blockade of bovine preimplantation embryogenesis requires inhibition of both p38 and extracellular signal-regulated kinase (ERK) pathways. Reproduction 130, 41–51.
| Mitogen-activated protein kinase (MAPK) blockade of bovine preimplantation embryogenesis requires inhibition of both p38 and extracellular signal-regulated kinase (ERK) pathways.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXnt1ynsrc%3D&md5=e0d224e28fa9ceafff9b2edbea4d94a0CAS | 15985630PubMed |
Martin, G., Sabido, O., Durand, P., and Levy, R. (2004). Cryopreservation induces an apoptosis-like mechanism in bull sperm. Biol. Reprod. 71, 28–37.
| Cryopreservation induces an apoptosis-like mechanism in bull sperm.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXltFKktLo%3D&md5=0bfa42993e74aa5ac9257bc326f97f11CAS | 14973261PubMed |
Martin, G., Cagnon, N., Sabido, O., Sion, B., Grizard, G., Durand, P., and Levy, R. (2007). Kinetics of occurrence of some features of apoptosis during the cryopreservation process of bovine spermatozoa. Hum. Reprod. 22, 380–388.
| Kinetics of occurrence of some features of apoptosis during the cryopreservation process of bovine spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXotFCqtA%3D%3D&md5=6a8470ad2fc8239c4dab8e26a7286b34CAS | 17092986PubMed |
Miller, D., Brinkworth, M., and Iles, D. (2010). Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 139, 287–301.
| Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXitFKit70%3D&md5=bd833c128e192981c35075fd67475bd8CAS | 19759174PubMed |
Mitchell, L. A., De Iuliis, G. N., and Aitken, R. J. (2011). The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology. Int. J. Androl. 34, 2–13.
| The TUNEL assay consistently underestimates DNA damage in human spermatozoa and is influenced by DNA compaction and cell vitality: development of an improved methodology.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXitlehur4%3D&md5=d9e01a63bfb4c6679aa56b7184a54237CAS | 20158539PubMed |
Monterroso, V. H., Drury, K. C., Ealy, A. D., Edwards, J. L., and Hansen, P. J. (1995). Effect of heat shock on function of frozen/thawed bull spermatozoa. Theriogenology 44, 947–961.
| Effect of heat shock on function of frozen/thawed bull spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD28zgtVGntQ%3D%3D&md5=882d00040228ae08d16585ea769e415aCAS | 16727790PubMed |
Nichi, M., Bols, P. E., Zuge, R. M., Barnabe, V. H., Goovaerts, I. G., Barnabe, R. C., and Cortada, C. N. (2006). Seasonal variation in semen quality in Bos indicus and Bos taurus bulls raised under tropical conditions. Theriogenology 66, 822–828.
| Seasonal variation in semen quality in Bos indicus and Bos taurus bulls raised under tropical conditions.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1cvltVyhsw%3D%3D&md5=040a176e06ffd69963c524b1a5948073CAS | 16529802PubMed |
O’Flaherty, C., de Lamirande, E., and Gagnon, C. (2005). Reactive oxygen species and protein kinases modulate the level of phospho-MEK-like proteins during human sperm capacitation. Biol. Reprod. 73, 94–105.
| Reactive oxygen species and protein kinases modulate the level of phospho-MEK-like proteins during human sperm capacitation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2MXls1Srt7w%3D&md5=e6fe85adf54e7967211cfdac66f52e05CAS | 15772258PubMed |
Ostermeier, G. C., Miller, D., Huntriss, J. D., Diamond, M. P., and Krawetz, S. A. (2004). Reproductive biology: delivering spermatozoan RNA to the oocyte. Nature 429, 154.
| Reproductive biology: delivering spermatozoan RNA to the oocyte.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXjvVKgs7k%3D&md5=d27573b76d852218212a93371df9bdf6CAS | 15141202PubMed |
Paul, C., Murray, A. A., Spears, N., and Saunders, P. T. K. (2008). A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction 136, 73–84.
| A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1cXovVSnsr4%3D&md5=f87e230d3c2be1ce08afb034dbdf6c2bCAS | 18390691PubMed |
Paul, C., Teng, S., and Saunders, P. T. K. (2009). A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death. Biol. Reprod. 80, 913–919.
| A single, mild, transient scrotal heat stress causes hypoxia and oxidative stress in mouse testes, which induces germ cell death.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXlsVWqu7s%3D&md5=d171156e870b8493ccea90740fc1dfccCAS | 19144962PubMed |
Pollard, J. W., Plante, C., King, W. A., Hansen, P. J., Betteridge, K. J., and Suarez, S. S. (1991). Fertilizing-capacity of bovine sperm may be maintained by binding to oviductal epithelial-cells. Biol. Reprod. 44, 102–107.
| Fertilizing-capacity of bovine sperm may be maintained by binding to oviductal epithelial-cells.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3M7pvVemug%3D%3D&md5=3139b1fd7063eeb14b4edbd61acffaf1CAS | 2015341PubMed |
Rahman, M. B., Vandaele, L., Rijsselaere, T., Maes, D., Hoogewijs, M., Frijters, A., Noordman, J., Granados, A., Dernelle, E., Shamsuddin, M., Parrish, J. J., and Van Soom, A. (2011). Scrotal insulation and its relationship to abnormal morphology, chromatin protamination and nuclear shape of spermatozoa in Holstein-Friesian and Belgian blue bulls. Theriogenology 76, 1246–1257.
| Scrotal insulation and its relationship to abnormal morphology, chromatin protamination and nuclear shape of spermatozoa in Holstein-Friesian and Belgian blue bulls.Crossref | GoogleScholarGoogle Scholar | 21777969PubMed |
Rahman, M. B., Vandaele, L., Rijsselaere, T., Zhandi, M., Maes, D., Shamsuddin, M., and Van Soom, A. (2012). Oocyte quality determines bovine embryo development after fertilisation with hydrogen peroxide-stressed spermatozoa. Reprod. Fertil. Dev. 24, 608–618.
| Oocyte quality determines bovine embryo development after fertilisation with hydrogen peroxide-stressed spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XmtVKitbc%3D&md5=8691f515223406ce595005866b711515CAS | 22541549PubMed |
Said, T. M., Agarwal, A., Zborowski, M., Grunewald, S., Glander, H. J., and Paasch, U. (2008). Utility of magnetic cell separation as a molecular sperm preparation technique. J. Androl. 29, 134–142.
| Utility of magnetic cell separation as a molecular sperm preparation technique.Crossref | GoogleScholarGoogle Scholar | 18077822PubMed |
Shaha, C. (2007). Modulators of spermatogenic cell survival. Soc. Reprod. Fertil. Suppl. 63, 173–186.
| 1:CAS:528:DC%2BD1cXpvVykt78%3D&md5=3aa9d8e83bdbce894c3db3bc7c7b0a9bCAS | 17566272PubMed |
Silva, P. F., Gadella, B. M., Colenbrander, B., and Roelen, B. A. (2007). Exposure of bovine sperm to pro-oxidants impairs the developmental competence of the embryo after the first cleavage. Theriogenology 67, 609–619.
| Exposure of bovine sperm to pro-oxidants impairs the developmental competence of the embryo after the first cleavage.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXitlyqug%3D%3D&md5=46c1baec04c5ff3dac99a539e1e18821CAS | 17056104PubMed |
Steger, K., Cavalcanti, M. C. O., and Schuppe, H. C. (2011). Prognostic markers for competent human spermatozoa: fertilizing capacity and contribution to the embryo. Int. J. Androl. 34, 513–527.
| Prognostic markers for competent human spermatozoa: fertilizing capacity and contribution to the embryo.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XivFOgtr4%3D&md5=b44a48d7dda6a55d3720123128d12a08CAS | 21128979PubMed |
Sutovsky, P., and Schatten, G. (1999). Paternal contributions to the mammalian zygote: fertilization after sperm-egg fusion. Int. Rev. Cytol. 195, 1–65.
| Paternal contributions to the mammalian zygote: fertilization after sperm-egg fusion.Crossref | GoogleScholarGoogle Scholar |
Thys, M., Vandaele, L., Morrell, J. M., Mestach, J., Van Soom, A., Hoogewijs, M., and Rodriguez-Martinez, H. (2009). In vitro fertilizing capacity of frozen-thawed bull spermatozoa selected by single-layer (glycidoxypropyltrimethoxysilane) silane-coated silica colloidal centrifugation. Reprod. Domest. Anim. 44, 390–394.
| In vitro fertilizing capacity of frozen-thawed bull spermatozoa selected by single-layer (glycidoxypropyltrimethoxysilane) silane-coated silica colloidal centrifugation.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1MrisFWhtA%3D%3D&md5=bc2f6c81ef11ab658999fb68e8b9fa12CAS | 18992094PubMed |
Vandaele, L., Mateusen, B., Maes, D., de Kruif, A., and Van Soom, A. (2006). Is apoptosis in bovine in vitro produced embryos related to early developmental kinetics and in vivo bull fertility? Theriogenology 65, 1691–1703.
| Is apoptosis in bovine in vitro produced embryos related to early developmental kinetics and in vivo bull fertility?Crossref | GoogleScholarGoogle Scholar | 16280159PubMed |
Vandaele, L., Thys, M., Bijttebier, J., Van Langendonckt, A., Donnay, I., Maes, D., Meyer, E., and Van Soom, A. (2010). Short-term exposure to hydrogen peroxide during oocyte maturation improves bovine embryo development. Reproduction 139, 505–511.
| Short-term exposure to hydrogen peroxide during oocyte maturation improves bovine embryo development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3cXjtV2lsb8%3D&md5=f4c23f42b48145bb0c179246c5896758CAS | 19939885PubMed |
Wada, T., and Penninger, J. M. (2004). Mitogen-activated protein kinases in apoptosis regulation. Oncogene 23, 2838–2849.
| Mitogen-activated protein kinases in apoptosis regulation.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXivFKmsb8%3D&md5=d7e76f07f58dede7f14d0ce93804316bCAS | 15077147PubMed |
Walters, A. H., Eyestone, W. E., Saacke, R. G., Pearson, R. E., and Gwazdauskas, F. C. (2005a). Bovine embryo development after IVF with spermatozoa having abnormal morphology. Theriogenology 63, 1925–1937.
| Bovine embryo development after IVF with spermatozoa having abnormal morphology.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2M7ptFKqtw%3D%3D&md5=37a2be8eb7da634cc1ba464acad481c6CAS | 15823349PubMed |
Walters, A. H., Saacke, R. G., Pearson, R. E., and Gwazdauskas, F. C. (2005b). The incidence of apoptosis after IVF with morphologically abnormal bovine spermatozoa. Theriogenology 64, 1404–1421.
| The incidence of apoptosis after IVF with morphologically abnormal bovine spermatozoa.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2Mvntl2htQ%3D%3D&md5=fd0375bf74cfcc62a80beecb63186246CAS | 15893815PubMed |
Ward, W. S. (2010). Function of sperm chromatin structural elements in fertilization and development. Mol. Hum. Reprod. 16, 30–36.
| Function of sperm chromatin structural elements in fertilization and development.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD1MXhsFOhtrrI&md5=94e9a74eb1960db83f6334a1f838a2a0CAS | 19748904PubMed |










