92 PROCESSING OF POSTMORTEM BOVINE EPIDIDYMAL SPERM AFTER COOLING THE TESTES FOR 24 HOURS
J. R. Saenz A , C. A. Guerrero A , D. Paccamonti B , B. Eilts B , K. R. Bondioli A and R. A. Godke AA Embryo Biotechnology Laboratory, Louisiana State University Agriculture Center, Baton Rouge, LA, USA;
B School of Veterinary Medicine, Louisiana State University, Baton Rouge, LA, USA
Reproduction, Fertility and Development 20(1) 126-127 https://doi.org/10.1071/RDv20n1Ab92
Published: 12 December 2007
Abstract
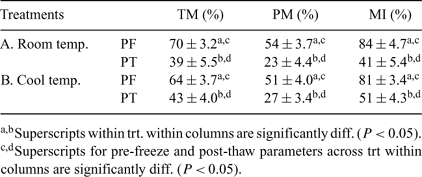
Harvesting and cryopreservation of epididymal sperm from postmortem animals is one way to save gametes from genetically valuable males. Before sperm can be properly harvested from the epididymides, usually the testes need to be cooled and transported to a semen laboratory. Previously, it has been recommended to harvest epididymal sperm from cooled testes in a 4°C cold room. However, a walk-in cold room is not always available. The objective of this study was to hold the testes for 22 h at 4°C, and then remove the epididymal sperm at room temperature (22 to 23°C; Treatment A) or in a cooled environment (4°C; Treatment B). Testicles within the scrotum were collected from sexually mature mixed breed bulls (n = 11) at a local abattoir, placed into plastic bags, and transported (2 h) in a Styrofoam ice chest (pre-cooled with frozen gel packs) to the laboratory. Each pair of testes was then removed from the ice chest and placed into a refrigerator at 4°C for 22 h. After 22 h of cooling, each testicle of the pair was removed from the scrotum and randomly assigned to either Treatment A or Treatment B. The sperm were flushed in a retrograde flow out of a small incision made at the medial section of the cauda epididymides. Total motility (TM) and progressive motility (PM) were determined using an inverted Nikon Diaphot microscope. Membrane integrity (MI) was determined using SYBR 14 and propidium iodide staining under a microscope with epifluorescence detection capability. Sperm in a Triladyl® one-step extender were frozen in 0.5-mL plastic straws in the vapor 2 cm above the LN2. A paired t-test was used for statistical analyses. In summary, there was significant decrease (P ≤ 0.05) from pre-freeze (PF) to post-thaw (PT) for all of the sperm parameters within each treatment (Table 1). However, there were no significant differences between PF and PT sperm parameters between treatments for any of the parameters measured. Post-thaw sperm from all samples had a tendency to swim in a circular pattern after warming, which is a known sign of sperm affected by cold shock. It is likely that during their cold storage, testes were cooled too quickly, inducing cold shock. In summary, cooling bull testes for 24 h and processing them at room temperature produced results similar to those for processing the testes in a cool environment. The PT parameters in this study suggest that these sperm could be used for IVF and/or ICSI procedures.
|


