365 COMPARISON OF IN VITRO FERTILIZING CAPACITY OF FROZEN–THAWED SEX-SORTED AND SEX-SORTED FROZEN–THAWED BULL SPERMATOZOA
V. Malcolm A , M. Marfil A , M. Calvi A , F. Rigali A , M. Pugliese A , J. Gutierrez A , M. Panarace A and M. Medina AAGoyaike S.A.A.C.I. y F., Ea San Joaquin CC 37, Carmen de areco CP 6725, Buenos Aires, Argentina
Reproduction, Fertility and Development 19(1) 298-298 https://doi.org/10.1071/RDv19n1Ab365
Submitted: 12 October 2006 Accepted: 12 October 2006 Published: 12 December 2006
Abstract
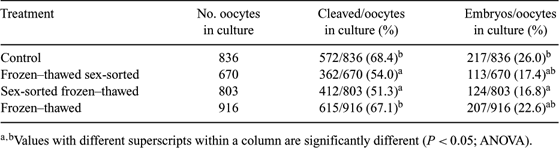
Sperm sexing has become a world-wide technology, now available in many countries. The method has been incorporated into many reproductive technologies such as embryo production (Zhang et al. 2003 Theriogenology 60, 1657–1663), but sex-sorting is limited when bulls are located far from sorters or when only frozen semen is available. Previous studies on sexing frozen–thawed spermatozoa have been done in rams, which resulted in retention of the spermatozoan functional capacities (Hollinshead et al. 2004 Reproduction 127, 557–568). In vitro characteristics were assessed in bulls after sexing of thawed sperm (Hollinshead et al. 2004 Theriogenology 62, 958–968); however, the fertilizing capacity of frozen–thawed sex-sorted (FTSS) spermatozoa was not tested. The aim of the present study was to compare cleavage and embryo development rate among frozen–thawed (FT), sex-sorted frozen–thawed (SSFT), and FTSS bull spermatozoa. For FT, sperm were diluted to a final concentration of 60 × 106 sperm/mL, packaged in 0.5-mL straws, and frozen. In SSFT, spermatozoa were sex-sorted by flow cytometry following Schenk protocols (1999 Theriogenology 52, 1375–1391). Three × 106 spermatozoa were packaged into 0.25-mL straws and frozen. The final treatment (FTSS) consisted of thawing 6 to 10 frozen straws of 4 different bulls containing an average of 25 × 106 spermatozoa and centrifuging at 600g for 15 min at 21°C to extract cryodiluent. Spermatozoa were diluted and stained with Hoechst 33342 (stain concentration of 112.5 µM, the same used for SSFT treatment) following Schenk sexed-semen protocols (1999), sex-sorted by a flow cytometer, and collected in Tris-base extender containing 20% egg yolk. For each ejaculate frozen–thawed, SSFT and FTSS spermatozoa were prepared for oocyte in vitro fertilization. Also, semen from a bull routinely used as a control in the laboratory was added for a better comparison of results. Oocytes from a slaughterhouse were processed following standard in vitro fertilization procedures (Ferré 2002 Theriogenology 57, 664) 4 times for each bull, and comparison was made between treatments. Results were analyzed by ANOVA. No significant differences were observed among bulls (data not shown) (P > 0.05). Although embryo development rate was statistically different between sexed and non-sexed groups (P < 0.05), results showed that frozen–thawed bull spermatozoa can be sex-sorted and used for in vitro fertilization with comparable developmental rates comparable to those when frozen sexed semen is used (Table 1). This opens a new commercial window for cases where pre-selected sexed embryos from bulls that are not in AI centers are desired, also giving an opportunity for dead bulls. Nevertheless, since a large number of straws are necessary, further studies must be carried out to make this procedure more efficient and economically profitable.
|


