370 HISTONE PHOSPHORYLATION PATTERNS AND CHROMOSOMAL STABILITY OF CULTURED BOVINE FIBROBLASTS
A. Giraldo A , J. Lynn B , R. Godke A , J. Jenkins C and K. Bondioli AA Department of Animal Sciences, Louisiana State Agricultural Center, Baton Rouge, LA 70803, USA;
B Department of Biological Sciences, Louisiana State University, Baton Rouge, LA 70803, USA;
C USGS, National Wetlands Research Center, Lafayette, LA 70506, USA
Reproduction, Fertility and Development 18(2) 290-290 https://doi.org/10.1071/RDv18n2Ab370
Published: 14 December 2005
Abstract
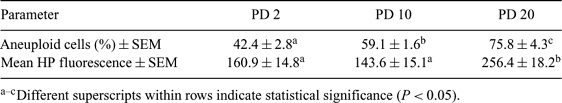
The low percentage of cloned offspring produced by nuclear transfer has been attributed to a variety of factors, including aneuploidy of the donor cells. Previous reports indicate that cultured bovine fibroblasts have a significantly higher level of aneuploidy in late passages than in early passages (Giraldo et al. 2005 J. Reprod. Fert. Dev. 17, 167). Phosphorylation of histone H3 at Serine 10 (Ser10) has been shown to be involved in chromosome compaction during cell division.Abnormal phosphorylation of this histone residue during metaphase could lead to abnormal chromosome segregation and extensive chromosome loss during mitosis. Suboptimal culture conditions may lead to abnormal histone H3 phosphorylation (HP) patterns, ultimately inducing missegregation and loss of chromosomes. The objective of the present study was to determine if the high percentage of aneuploid bovine fibroblast cells observed after long-term culture is associated with an abnormal HP pattern. Four bovine fibroblast cell lines were established from 40- to 60-day fetuses. Cells were cultured in DMEM supplemented with 10% fetal bovine serum and 1% penicillin and streptomycin in 5% CO2 at 37°C and passaged at confluence. Relative levels of HP were determined in three different replicates at population doublings (PD) 2, 10, and 20. Cells were fixed and incubated with an anti-phosphorylated histone H3 (Ser10) antibody, labeled with a secondary antibody, counterstained with propidium iodide, and analyzed for HP fluorescence by flow cytometry. The number of chromosomes was also determined in counts of 800 metaphases. Differences in aneuploidy and HP fluorescence of cells in metaphase among PD were analyzed by χ2 two-way ANOVA, respectively (P < 0.05). The percentages of aneuploid cells in each of the cell lines increased progressively with duration of culture and were elevated from the start. Multinucleated cells were frequently observed after prolonged time in culture in all of the cell lines. The mean phosphorylated histone levels (relative fluorescence intensity) in cells during metaphase were 180.0, 131.5, 174.7, and 157.6 in PD 2; 170.4, 105.72, 145.8, and 152.7 in PD 10; and 274.0, 251.6, 191.4, and 308.3 in PD 20 for the four cell lines, respectively. No difference in HP levels was observed between PD 2 and PD 10. The average of HP during metaphase for the cell lines increased significantly from 160.9 at early passage to 256.3 at late passage (see Table 1). The increase in levels of HP occurred concurrently with the high percentage of aneuploid cells after extended time in culture. These data are consistent with the hypothesis that aneuploid cells observed after long in vitro culture are associated with abnormal HP patterns.
|
This study was supported by Grants in Aid of Research Program (GIAR) from Sigma Xi to A.G.


