6 VITRIFICATION OF BOVINE EMBRYOS WITHOUT ANIMAL-DERIVED PRODUCTS
D. Walker A and G. Seidel AAAnimal Reproduction and Biotechnology Laboratory, Colorado State University, Fort Collins, CO 80521, USA. Email: gseidel@colostate.edu
Reproduction, Fertility and Development 17(2) 153-153 https://doi.org/10.1071/RDv17n2Ab6
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
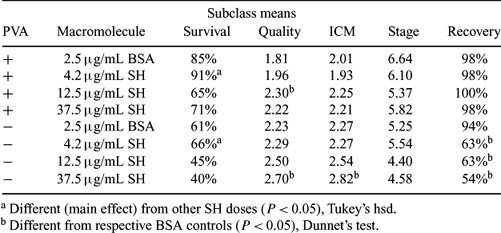
Media free of animal products would facilitate import and export requirements for embryos. Our goal was to test replacement of bovine serum albumin (BSA) (2.5 μg/mL) with sodium hyaluronate (SH) (4.2 μg/mL, 12.5 μg/mL, or 37.5 μg/mL) as the macromolecule in a very simple system in which embryos were vitrified in 0.25-mL straws suitable for direct embryo transfer. Day 7 blastocysts (n = 384) were produced in vitro with semen of 3 bulls, 2 replicates each. For vitrification, embryos were placed into chemically defined, HEPES-buffered medium (HCDM-2) and then transferred to V1 (5 M ethylene glycol, 0.5 M galactose in HCDM-2 plus BSA or SH) for 3 min. Next, embryos were placed in a 6-μL drop of V2 (7 M ethylene glycol, 0.5 M galactose, and 18% w/v Ficoll 70 in HCDM-2 plus BSA or SH) for 45 s. During equilibration, dilution medium (0.5 M galactose in HCDM-2 plus BSA or SH) was aspirated into 0.25 mL straws, followed by the 6-μL drop of V2 plus embryos, and a final short column of dilution medium. When 45 s had elapsed, the heat-sealed end of straw was dipped into liquid nitrogen to cover the embryo and then plunged slowly. Straws were thawed in air for 10 s and then in 37°C water for 20 s. Straws were then shaken like a clinical thermometer 4 times to mix columns, and held in 37°C water for 10 min before expelling embryos to be rinsed and cultured in CDM-2 + 5% FCS. Survival (as determined by expansion of blastocysts), quality score (1 = excellent, 2 = fair, 3 = poor), inner cell mass quality (ICM) (1 = large and compact, 2 = clearly visible, 3 = not discernable), and blastocyst stage (5 = early, 6 = full, 7 = expanded, 8 = hatching, 9 = hatched) were evaluated 24 h post-thaw and analyzed by ANOVA. Due to poor embryo recovery from straws in replicates 1–3, 0.1% polyvinyl alcohol (PVA) was added to vitrification solutions in replicates 4–6. Survival was calculated as a percentage of noncryopreserved controls in the same replicate (control: survival 93%, quality 1.52, ICM 1.43, stage 7.47). The lowest dose of SH replaced BSA efficaciously with this vitrification procedure (Table 1). Addition of PVA greatly improved all responses (main effects): recovery 99% vs. 69%; survival 78% vs. 53%; quality 2.07 vs. 2.43; ICM 2.10 vs. 2.47; stage 5.98 vs. 4.94; all P < 0.001. Successful vitrification of embryos in solutions containing 4.2 μg/mL SH plus PVA in place of BSA in 0.25 mL straws, from which embryos can be transferred directly, further increases the appeal of vitrification as an alternative to conventional cryopreservation.
|


