278 MEIOTIC RESUMPTION IN VITRO OF CANINE OOCYTES: COMPARATIVE METHODS
L. Bogliolo A , F. Ariu A , M.T. Zedda A , S. Pau A and S. Ledda AAObstetrics Section of the Institute of General Pathology, Pathological Anatomy and Veterinary Obstetrics-Surgery Clinic, University of Sassari, Sassari, Italy. Email: nuvola@uniss.it
Reproduction, Fertility and Development 17(2) 289-289 https://doi.org/10.1071/RDv17n2Ab278
Submitted: 1 August 2004 Accepted: 1 October 2004 Published: 1 January 2005
Abstract
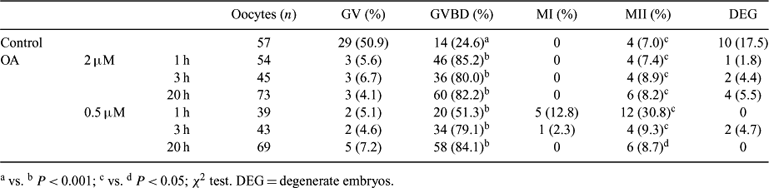
In the bitch, mechanisms responsible for the meiotic resumption and progression of the oocytes are not known. In order to better understand cellular signals involved in canine oocyte meiosis, the present study was performed to investigate the ability of bitch oocytes to resume meiosis in vitro (1) after culture with okadaic acid (OA), and (2) after cell fusion with bovine MII oocytes. For this purpose oocytes were collected from ovaries of bitches undergoing ovariectomy. Ovaries were sliced repeatedly to release oocytes; only cumulus-oocytes complexes with two or more dense layer of cumulus cells, darkly granulated cytoplasm, and >110 μm in diameter were selected for experiments. In the first experiment, oocytes were pre-incubated for different times (1, 3, 20 h) in TCM 199 + 20% FCS + 0.5 μM or 2 μM OA and thereafter cultured for 48 h in the same medium without OA. A group of oocytes was matured in TCM 199 + 20% FCS for 72 h as control. At the end of culture, oocytes were stained with glycerol-Hoechst 33342 to evaluate meiotic stage. Results indicated that incubation with 2 μM OA for 1, 3, and 20 h determined a significantly higher (P < 0.001) meiotic resumption (GVBD) of canine oocytes compared to that in the control group, but the percentage of oocytes reaching MI and MII did not increase. Similar results were obtained after culture with 0.5 μM OA for 3 and 20 h. However, meiotic progression to MI and MII was significantly improved (P < 0.05) after incubation with 0.5 μM OA for 1 h. In the second experiment canine oocytes at GV stage were fused with MII bovine oocytes matured in vitro. This experiment was designed to test whether the high activity of MPF of MII bovine oocytes was able to determine modification of GV of canine oocytes. The zonae pellucidae from both GV and MII oocytes were removed using 0.1% pronase. Pairs of oocytes (n = 37) were agglutinated in medium containing 250 mg/mL phytohemoagglutinin placed between two electrodes in 0.5% glucose fusion medium, fused with a single pulse of direct current (1.25 KV/cm for 80 ms) and cultured for 2–3 h in TCM 199 + 10% FCS. After culture fused partners (n = 36) were stained with glycerol-Hoechst 33342 and evaluated. Results indicated that the fusion of MII bovine oocytes to GV reporter canine oocytes failed to induce nuclear membrane disassembly, chromatin condensation, or modification of canine nuclei in all of the fused combinations. These data suggest that OA induces meiotic resumption of canine oocytes. However, the cell fusion results seem to indicate that increased levels of MPF are not able to determine cell cycle progression. Furthermore, incubation times and concentration of okadaic acid could be refined to optimize a system for meiotic maturation of bitch oocytes.
|
This work was supported by MIUR (ex 40%).


