Postnatal cadmium administration affects the presence and distribution of carbohydrates in the sperm membrane during maturation in the epididymis in adult Wistar rats
Joel Hernández-Rodríguez A , Edith Arenas-Ríos B , Irma Jiménez-Morales C , Edith Cortés-Barberena C , Sergio Montes D , Rosa María Vigueras-Villaseñor E and Marcela Arteaga-Silva
A , Edith Arenas-Ríos B , Irma Jiménez-Morales C , Edith Cortés-Barberena C , Sergio Montes D , Rosa María Vigueras-Villaseñor E and Marcela Arteaga-Silva  B F
B F
A Doctorado en Ciencias Biológicas y de la Salud, Universidad Autónoma Metropolitana, Av. San Rafael Atlixco 186, C.P. 09340, Ciudad de México, México.
B Departamento de Biología de la Reproducción, Universidad Autónoma Metropolitana-Iztapalapa, Av. San Rafael Atlixco 186, C.P. 09340, Ciudad de México, México.
C Departamento de Ciencias de la Salud, Universidad Autónoma Metropolitana-Iztapalapa, Av. San Rafael Atlixco 186, C.P. 09340, Ciudad de México, México.
D Departamento de Neuroquímica, Instituto Nacional de Neurología y Neurocirugía, Manuel Velasco Suárez, Insurgentes Sur 3877, Col. La Fama, C.P. 14269, Ciudad de México, México.
E Instituto Nacional de Pediatría, Calzada México Xochimilco No. 101, Col. San Lorenzo Huipulco, Tlalpan, C.P. 14370, Ciudad de México, México.
F Corresponding author. Email: asm@xanum.uam.mx
Reproduction, Fertility and Development 33(5) 349-362 https://doi.org/10.1071/RD20167
Submitted: 25 June 2020 Accepted: 14 January 2021 Published: 19 February 2021
Abstract
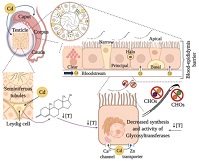
Cadmium (Cd) is a heavy metal related to a decrease in sperm parameters. The transit of spermatozoa through the epididymis is necessary to generate changes in the sperm membrane, such as the assembly of various carbohydrates that are added to the spermatazoan’s surface to prepare it for successful fertilisation of the oocyte. No studies have yet analysed whether Cd alters the presence and distribution of these carbohydrates. We aimed to evaluate the changes induced by Cd in the distribution pattern of N-acetylglucosamine, sialic acid, mannose and fucose on the sperm membrane in the epididymis (e.g. caput, corpus, cauda) and if it alters the epididymal epithelium. Male Wistar pups were treated with Cd doses (0.125, 0.25 and 0.5 mg/kg) on postnatal days 1–49. At postnatal day 90, they were humanely killed, sperm samples were obtained from the epididymis and tissue samples were taken for histological analysis. Cd concentrations in the blood and epididymis increased in proportion to the dose administered and decreased the serum testosterone levels and sperm quality. Histological analysis revealed alterations in the epithelium in all Cd-treated groups. Cd altered the distribution patterns of carbohydrates and fluorescence indices. All these alterations affected the structure and functioning of sperm.
Keywords: cadmium, infertility, sperm epididymal maturation, membrane carbohydrates, testosterone.
References
Adamkovicova, M., Toman, R., Cabaj, M., Massanyi, P., Martiniakova, M., Omelka, R., Krajcovicova, V., and Duranova, H. (2014). Effects of Subchronic Exposure to Cadmium and Diazinon on Testis and Epididymis in Rats. Sci. World J. 2014, 632581.| Effects of Subchronic Exposure to Cadmium and Diazinon on Testis and Epididymis in Rats.Crossref | GoogleScholarGoogle Scholar |
Agency for Toxic Substances and Disease Registry (ATSDR) (2012). Toxicological profile for cadmium. Division of Toxicology and Human Health Sciences, Environmental Toxicology Branch, Atlanta, GA. pp. 487. Available at: https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf.
Alaee, S., Talaiekhozani, A., Rezaei, S., Alaei, K., and Yousefian, E. (2014). Cadmium and male infertility. J. Infertil. Reprod. Biol. 2, 62–69.
Aliabadi, E., Karimi, F., and Talaei-Khozani, T. (2013). Effects of L-Carnitine and Pentoxifylline on Carbohydrate Distribution of Mouse Testicular Sperm Membrane. Iran. J. Med. Sci. 38, 107–115.
| 23825890PubMed |
Alkhedaide, A., Alshehri, Z., Sabry, A., Abdel-Ghaffar, T., Soliman, M., and Attia, H. (2016). Protective effect of grape seed extract against cadmium-induced testicular dysfunction. Mol. Med. Rep. 13, 3101–3109.
| Protective effect of grape seed extract against cadmium-induced testicular dysfunction.Crossref | GoogleScholarGoogle Scholar | 26935153PubMed |
Arain, M. B., Kazi, T., Baig, J., Afridi, H., Sarajuddin, , Brehman, K., Panhwar, H., and Arain, S. (2014). Co-exposure of arsenic and cadmium through drinking water and tobacco smoking: Risk assessment on kidney dysfunction. Environ. Sci. Pollut. Res. Int. 22, 350–357.
| Co-exposure of arsenic and cadmium through drinking water and tobacco smoking: Risk assessment on kidney dysfunction.Crossref | GoogleScholarGoogle Scholar | 25074830PubMed |
Arenas-Ríos, E., León-Galván, M., Mercado, P., and Rosado, A. (2005). Superoxide dismutase, catalase, and glutathione peroxidase during epididymal maturation and prolonged storage of spermatozoa in the Mexican big-eared bat (Corynorhinus mexicanus). Can. J. Zool. 83, 1556–1565.
| Superoxide dismutase, catalase, and glutathione peroxidase during epididymal maturation and prolonged storage of spermatozoa in the Mexican big-eared bat (Corynorhinus mexicanus).Crossref | GoogleScholarGoogle Scholar |
Arrotia, K., Garcia, P., Barbieri, M., Justino, M., and Violin, L. (2012). The Epididymis: Embryology, Structure, Function And Its Role In Fertilization And Infertility. Embryology: Updates and Highlights on Classic Topics. (Ed. L.A. Violin-Pereira.) pp. 41–66. (InTech)
Arteaga-Silva, M., Arenas-Rios, E., Bonilla-Jaime, H., Damian-Matzumura, P., Limon-Morales, O., Hernandez-Rodriguez, J., and Marquez-Aguiluz, D. (2021). Neuroendocrine effects of cadmium exposure on male reproductive functions. Front Biosci (Landmark Ed) 26, 286–326.
| Neuroendocrine effects of cadmium exposure on male reproductive functions.Crossref | GoogleScholarGoogle Scholar | 33049671PubMed |
Asadi, M. H., Zafari, F., Sarveazad, A., Abbasi, M., Safa, M., Koruji, M., Yari, A., and Alizadeh Miran, R. (2013). Saffron Improves Epididymal Sperm Parameters in Rats Exposed to Cadmium. Nephrourol. Mon. 6, e12125.
| Saffron Improves Epididymal Sperm Parameters in Rats Exposed to Cadmium.Crossref | GoogleScholarGoogle Scholar | 24719804PubMed |
Badr, F., and El-Habit, O. (2018). Heavy Metal Toxicity Affecting Fertility and Reproduction of Males. In ‘Bioenvironmental Issues Affecting Men’s Reproductive and Sexual Health’, pp. 293–304. (Academic Press)
Bashir, N., Manoharav, V., and Prabu, S. M. (2014). Cadmium Toxicity: Oxidative Stress and Organ Dysfunction. RRJoT. 4, 1–19.
Benoff, S., Hauser, R., Marmar, J., Hurley, I., Napolitano, B., and Centola, G. (2009). Cadmium Concentrations in Blood and Seminal Plasma: Correlations with Sperm Number and Motility in Three Male Populations (Infertility Patients, Artificial Insemination Donors, and Unselected Volunteers). Mol. Med. 15, 248–262.
| Cadmium Concentrations in Blood and Seminal Plasma: Correlations with Sperm Number and Motility in Three Male Populations (Infertility Patients, Artificial Insemination Donors, and Unselected Volunteers).Crossref | GoogleScholarGoogle Scholar | 19593409PubMed |
Carré, J., Gatimel, N., Moreau, J., Parinaud, J., and Léandri, R. (2017). Does air pollution play a role in infertility?: a systematic review. Environ. Health 16, 82.
| Does air pollution play a role in infertility?: a systematic review.Crossref | GoogleScholarGoogle Scholar | 28754128PubMed |
Carvelli, L., Bannoud, N., Aguilera, A., Sartor, T., Malossi, E., and Sosa, M. (2013). Testosterone influences the expression and distribution of the cation-dependent mannose-6-phosphate receptor in rat epididymis. Implications in the distribution of enzymes. Andrologia 46, 224–230.
| Testosterone influences the expression and distribution of the cation-dependent mannose-6-phosphate receptor in rat epididymis. Implications in the distribution of enzymes.Crossref | GoogleScholarGoogle Scholar | 23290006PubMed |
Cervantes, M. I., Arenas-Ríos, E., Miguel Ángel, L., Ricardo, L., Demetrio, A., and Adolfo, R. (2008). Spermatozoa Epididymal Maturation in the Mexican Big-Eared Bat (Corynorhinus Mexicanus). Syst Biol Reprod Med 54, 196–204.
| Spermatozoa Epididymal Maturation in the Mexican Big-Eared Bat (Corynorhinus Mexicanus).Crossref | GoogleScholarGoogle Scholar | 18942027PubMed |
Cheon, Y. P., and Kim, C. (2015). Impact of glycosylation on the unimpaired functions of the sperm. Clin. Exp. Reprod. Med. 42, 77–85.
| Impact of glycosylation on the unimpaired functions of the sperm.Crossref | GoogleScholarGoogle Scholar | 26473106PubMed |
Cichy, B., Jaroszek, H., and Paszek, A. (2014). Cadmium in phosphate fertilizers; ecological and economical aspects. Chemik Science-Technique-Market 68, 837–842.
Cornwall, G. A. (2014). Role of posttranslational protein modifications in epididymal sperm maturation and extracellular quality control. In ‘Posttranslational Protein Modifications in the Reproductive System. Advances in Experimental Medicine and Biology, vol. 759’. (Ed. P. Sutovsky) pp. 159–180. (Springer: New York, NY.)
Cortés, P. P., Orihuela, P., Zúñiga, L., Velásquez, L., and Croxatto, H. (2004). Sperm Binding to Oviductal Epithelial Cells in the Rat: Role of Sialic Acid Residues on the Epithelial Surface and Sialic Acid-Binding Sites on the Sperm Surface1. Biol. Reprod. 71, 1262–1269.
| Sperm Binding to Oviductal Epithelial Cells in the Rat: Role of Sialic Acid Residues on the Epithelial Surface and Sialic Acid-Binding Sites on the Sperm Surface1.Crossref | GoogleScholarGoogle Scholar | 15201197PubMed |
Cui, X., Jing, X., Wu, X., and Yan, M. (2016). Protective effect of resveratrol on spermatozoa function in male infertility induced by excess weight and obesity. Mol. Med. Rep. 14, 4659–4665.
| Protective effect of resveratrol on spermatozoa function in male infertility induced by excess weight and obesity.Crossref | GoogleScholarGoogle Scholar | 27748829PubMed |
Darbre, P. D. (2003). Underarm cosmetics and breast cancer. J. Appl. Toxicol. 23, 89–95.
| Underarm cosmetics and breast cancer.Crossref | GoogleScholarGoogle Scholar | 12666152PubMed |
Díaz, M. C., González, N., Zanuzzi, C., Najle, R., and Barbeito, C. (2017). Lectin histochemistry for detecting cadmium-induced changes in the glycosylation pattern of rat placenta. Biotech. Histochem. 92, 36–45.
| Lectin histochemistry for detecting cadmium-induced changes in the glycosylation pattern of rat placenta.Crossref | GoogleScholarGoogle Scholar | 28166424PubMed |
Dohle, G.R., Arver, S., Bettocchi, C., Jones, T.H. and Kliesch, S. (2015). Guidelines on Male Hypogonadism. (European Association of Urology.) Available at http://uroweb.org/guideline/male-hypogonadism/.
Domino, S. E., Zhang, L., Gillespie, P., Saunders, T., and Lowe, J. (2001). Deficiency of Reproductive Tract α(1,2)Fucosylated Glycans and Normal Fertility in Mice with Targeted Deletions of the FUT1 or FUT2 α(1,2)Fucosyltransferase Locus. Mol. Cell. Biol. 21, 8336–8345.
| Deficiency of Reproductive Tract α(1,2)Fucosylated Glycans and Normal Fertility in Mice with Targeted Deletions of the FUT1 or FUT2 α(1,2)Fucosyltransferase Locus.Crossref | GoogleScholarGoogle Scholar | 11713270PubMed |
Dubé, E., and Cyr, D. (2013). The blood-epididymis barrier and human male fertility. In ‘Biology and Regulation of Blood-Tissue Barriers. Advances in Experimental Medicine and Biology, vol. 763’. (Ed. C. Y. Cheng.) pp. 218–236. (Springer: New York, NY.)
Durairajanayagam, D. (2018). Lifestyle causes of male infertility. Arab J. Urol. 16, 10–20.
| Lifestyle causes of male infertility.Crossref | GoogleScholarGoogle Scholar | 29713532PubMed |
Farquhar, C., and Marjoribanks, J. (2018). Assisted reproductive technology: an overview of Cochrane Reviews. Cochrane Database Syst. Rev. 8, CD010537.
| Assisted reproductive technology: an overview of Cochrane Reviews.Crossref | GoogleScholarGoogle Scholar | 30117155PubMed |
Fernandez-Fuertes, B., Blanco-Fernandez, A., Reid, C., Meade, K., Fair, S., and Lonergan, P. (2018). Removal of sialic acid from bull sperm decreases motility and mucus penetration ability but increases zona pellucida binding and polyspermic penetration in vitro. Reproduction 155, 481–492.
| Removal of sialic acid from bull sperm decreases motility and mucus penetration ability but increases zona pellucida binding and polyspermic penetration in vitro.Crossref | GoogleScholarGoogle Scholar | 29618635PubMed |
Fierro, R., Foliguet, B., Grignon, G., Daniel, M., Bene, M., Faure, G., and Barbarino-monnier, P. (1996). Lectin-Binding Sites on Human Sperm During Acrosome Reaction: Modifications Judged by Electron Microscopy/Flow Cytometry. Arch. Androl. 36, 187–196.
| Lectin-Binding Sites on Human Sperm During Acrosome Reaction: Modifications Judged by Electron Microscopy/Flow Cytometry.Crossref | GoogleScholarGoogle Scholar | 8743350PubMed |
Galicia-García, V., Rojas-Lopez, M., Rojas, R., Olaiz, G., and Rios, C. (1997). Cadmium levels in maternal, cord and newborn blood in Mexico city. Toxicol. Lett. 91, 57–61.
| Cadmium levels in maternal, cord and newborn blood in Mexico city.Crossref | GoogleScholarGoogle Scholar | 9096287PubMed |
Gamzu, R., Yogev, L., Amnon, B., Kleiman, S., Hauser, R., Lessing, J., Paz, G., and Yavetz, H. (2002). The Expression of Mannose-Ligand Receptor is Correlated with Sperm Morphology. Arch. Androl. 48, 475–480.
| The Expression of Mannose-Ligand Receptor is Correlated with Sperm Morphology.Crossref | GoogleScholarGoogle Scholar | 12425765PubMed |
Gervasi, M. G., and Visconti, P. (2017). Molecular changes and signaling events occurring in spermatozoa during epididymal maturation. Andrology 5, 204–218.
| Molecular changes and signaling events occurring in spermatozoa during epididymal maturation.Crossref | GoogleScholarGoogle Scholar | 28297559PubMed |
González-Santos, J., Ávalos-Rodríguez, A., Martínez-García, J., Rosales-Torres, A., and Herrera-Barragán, J. (2019). Sperm Morphophysiology in Different Sections of the Rooster Reproductive Tract. Int. J. Morphol. 37, 861–866.
| Sperm Morphophysiology in Different Sections of the Rooster Reproductive Tract.Crossref | GoogleScholarGoogle Scholar |
Government of Mexico (2001). NOM-062-ZOO-1999: Norma Oficial Mexicana, Especificaciones técnicas para la producción, cuidado y uso de animales de laboratorio. (Government of Mexico).
Hall, J. C., and Killian, G. (1987). Changes in Rat Sperm Membrane Glycosidase Activities and Carbohydrate and Protein Contents Associated with Epididymal Transit. Biol. Reprod. 36, 709–718.
| Changes in Rat Sperm Membrane Glycosidase Activities and Carbohydrate and Protein Contents Associated with Epididymal Transit.Crossref | GoogleScholarGoogle Scholar | 3593843PubMed |
Hamada, T., Tanimoto, A., and Sasaguri, Y. (1997). Apoptosis induced by cadmium. Apoptosis 2, 359–367.
| Apoptosis induced by cadmium.Crossref | GoogleScholarGoogle Scholar | 14646532PubMed |
Henson, M. C., and Chedrese, P. (2004). Endocrine Disruption by Cadmium, a Common Environmental Toxicant with Paradoxical Effects on Reproduction. Exp. Biol. Med. (Maywood) 229, 383–392.
| Endocrine Disruption by Cadmium, a Common Environmental Toxicant with Paradoxical Effects on Reproduction.Crossref | GoogleScholarGoogle Scholar | 15096650PubMed |
Hermo, L., Oliveira, R., Smith, C., Au, C., and Bergeron, J. (2019). Dark side of the epididymis: tails of sperm maturation. Andrology 7, 566–580.
| Dark side of the epididymis: tails of sperm maturation.Crossref | GoogleScholarGoogle Scholar | 31102346PubMed |
Inskeep, P. B., and Hammerstedt, R. (1982). Changes in Metabolism of Ram Sperm Associated With Epididymal Transit or Induced by Exogenous Carnitine. Biol. Reprod. 27, 735–743.
| Changes in Metabolism of Ram Sperm Associated With Epididymal Transit or Induced by Exogenous Carnitine.Crossref | GoogleScholarGoogle Scholar | 7139017PubMed |
Iusem, N. D., De Larminat, M., Tezon, J., Blaquier, J., and Belocopitow, E. (1984). Androgen Dependence of Protein N-Glycosylation in Rat Epididymis. Endocrinology 114, 1448–1453.
| Androgen Dependence of Protein N-Glycosylation in Rat Epididymis.Crossref | GoogleScholarGoogle Scholar | 6231179PubMed |
Ji, Y. L., Wang, H., Liu, P., Wang, Q., Zhao, X., Meng, X., Yu, T., Zhang, H., Zhang, C., Zhang, Y., and Xu, D. (2010). Pubertal cadmium exposure impairs testicular development and spermatogenesis via disrupting testicular testosterone synthesis in adult mice. Reprod. Toxicol. 29, 176–183.
| Pubertal cadmium exposure impairs testicular development and spermatogenesis via disrupting testicular testosterone synthesis in adult mice.Crossref | GoogleScholarGoogle Scholar | 19897027PubMed |
Jiménez, I., González-Márquez, H., Ortiz, R., Herrera, J., García, A., Betancourt, M., and Fierro, R. (2003). Changes in the distribution of lectin receptors during capacitation and acrosome reaction in boar spermatozoa. Theriogenology 59, 1171–1180.
| Changes in the distribution of lectin receptors during capacitation and acrosome reaction in boar spermatozoa.Crossref | GoogleScholarGoogle Scholar | 12527065PubMed |
Jiménez, I., Fierro, R., González-Márquez, H., Mendoza-Hernández, G., Romo, S., and Betancourt, M. (2006). Carbohydrate affinity chromatography indicates that arylsulfatase-a from capacitated boar sperm has mannose and N-acetylglucosamine/sialic acid residues. Arch. Androl. 52, 455–462.
| Carbohydrate affinity chromatography indicates that arylsulfatase-a from capacitated boar sperm has mannose and N-acetylglucosamine/sialic acid residues.Crossref | GoogleScholarGoogle Scholar | 17050327PubMed |
Kerkhofs, S., Dubois, V., De Gendt, K., Helsen, C., Clinckemalie, L., Spans, L., Schuit, F., Boonen, S., Vanderschueren, D., Saunders, P., Verhoeven, G., and Claessens, F. (2012). A role for selective androgen response elements in the development of the epididymis and the androgen control of the 5 α reductase II gene. FASEB J. 26, 4360–4372.
| A role for selective androgen response elements in the development of the epididymis and the androgen control of the 5 α reductase II gene.Crossref | GoogleScholarGoogle Scholar | 22798427PubMed |
Khan, M. A., Khan, S., Khan, A., and Alam, M. (2017). Soil contamination with cadmium, consequences and remediation using organic amendments. Sci. Total Environ. 601–602, 1591–1605.
| Soil contamination with cadmium, consequences and remediation using organic amendments.Crossref | GoogleScholarGoogle Scholar | 28609847PubMed |
Knazicka, Z., Forgacs, Z., Lukacova, J., Roychoudhury, S., Massanyi, P., and Lukac, N. (2015). Endocrine disruptive effects of cadmium on steroidogenesis: Human adrenocortical carcinoma cell line NCI-H295R as a cellular model for reproductive toxicity testing. J Environ. Sci. Health. A Tox. Hazard. Subst. Environ. Eng. 50, 348–356.
| Endocrine disruptive effects of cadmium on steroidogenesis: Human adrenocortical carcinoma cell line NCI-H295R as a cellular model for reproductive toxicity testing.Crossref | GoogleScholarGoogle Scholar | 25723060PubMed |
Kumar, N., and Singh, A. (2015). Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 8, 191–196.
| Trends of male factor infertility, an important cause of infertility: A review of literature.Crossref | GoogleScholarGoogle Scholar | 26752853PubMed |
Lacorte, L. M., Seiva, F., Rinaldi, J., Delella, F., Moroz, A., Sarobo, C., Godinho, A., Fávaro, W., Fernandes, A., and Felisbino, S. (2013). Caffeine reduces cadmium accumulation in the organism and enhances the levels of antioxidant protein expression in the epididymis. Reprod. Toxicol. 35, 137–143.
| Caffeine reduces cadmium accumulation in the organism and enhances the levels of antioxidant protein expression in the epididymis.Crossref | GoogleScholarGoogle Scholar | 23099337PubMed |
Lafuente, A. (2013). The hypothalamic–pituitary–gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches. Food Chem. Toxicol. 59, 395–404.
| The hypothalamic–pituitary–gonadal axis is target of cadmium toxicity. An update of recent studies and potential therapeutic approaches.Crossref | GoogleScholarGoogle Scholar | 23811532PubMed |
Lamas, C. A., Cuquetto-Leite, L., do Nascimento da Silva, E., Thomazini, B. F., Cordeiro, G., Predes, F. S., Gollücke, A., and Dolder, H. (2017). Grape juice concentrate alleviates epididymis and sperm damage in cadmium-intoxicated rats. Int. J. Exp. Pathol. 98, 86–99.
| Grape juice concentrate alleviates epididymis and sperm damage in cadmium-intoxicated rats.Crossref | GoogleScholarGoogle Scholar | 28581201PubMed |
Lee, J. (2018). Morphological changes of cauda epididymis, sperm infiltration into cauda epididymis, sperm storage and sperm disappearance of cauda epididymis in Rhinolophus ferrumequinum korai (Chiroptera: Rhinolophidae). Eur. Zool. J. 85, 118–127.
| Morphological changes of cauda epididymis, sperm infiltration into cauda epididymis, sperm storage and sperm disappearance of cauda epididymis in Rhinolophus ferrumequinum korai (Chiroptera: Rhinolophidae).Crossref | GoogleScholarGoogle Scholar |
Li, Y., Wu, J., Zhou, W., and Gao, E. (2015). Association between environmental exposure to cadmium and human semen quality. Int. J. Environ. Health Res. 26, 175–186.
| Association between environmental exposure to cadmium and human semen quality.Crossref | GoogleScholarGoogle Scholar | 26249156PubMed |
Li, X., Guo, J., Jiang, X., Sun, J., Tian, L., Jiao, R., Tang, Y., and Bai, W. (2019). Cyanidin-3-O-glucoside protects against cadmium-induced dysfunction of sex hormone secretion via the regulation of hypothalamus-pituitary-gonadal axis in male pubertal mice. Food Chem. Toxicol. 129, 13–21.
| Cyanidin-3-O-glucoside protects against cadmium-induced dysfunction of sex hormone secretion via the regulation of hypothalamus-pituitary-gonadal axis in male pubertal mice.Crossref | GoogleScholarGoogle Scholar | 31014900PubMed |
Li, Y., Huang, Y., He, B., Liu, R., Qu, G., Yin, Y., Shi, J., Hu, L., and Jiang, G. (2020). Cadmium-binding proteins in human blood plasma. Ecotoxicol. Environ. Saf. 188, 109896.
| Cadmium-binding proteins in human blood plasma.Crossref | GoogleScholarGoogle Scholar | 31732271PubMed |
Long, J. A. (2014). Applied andrology in chickens and turkeys. In ‘Animal andrology: theories and applications’. (Eds P. Chenoweth and S. Lorton.) pp. 197–225. (CABI)
Lucio, R. A., Tlachi, J. L., López, A. A., Zempoalteca, R., and Velázquez-Moctezuma, J. (2009). Analysis of the parameters of the ejaculate in the laboratory Wistar rat: technical description. Vet Mex. 40, 405–415.
Ma, X., Pan, Q., Feng, Y., Choudhury, B., Ma, Q., Gagneux, P., and Ma, F. (2016). Sialylation Facilitates the Maturation of Mammalian Sperm and Affects Its Survival in Female Uterus. Biol. Reprod. 94, 123.
| Sialylation Facilitates the Maturation of Mammalian Sperm and Affects Its Survival in Female Uterus.Crossref | GoogleScholarGoogle Scholar | 27075617PubMed |
Marettová, E., Maretta, M., and Legáth, J. (2015). Toxic effects of cadmium on testis of birds and mammals: A review. Anim. Reprod. Sci. 155, 1–10.
| Toxic effects of cadmium on testis of birds and mammals: A review.Crossref | GoogleScholarGoogle Scholar | 25726439PubMed |
Naiho, A., Ekene, E., Ebeye, M., Olowe, G., and Odigie, M. (2018). Cadmium Chloride Reduces Testicular and Epididymal Weights with Degenerative Histoarchitectural Changes in Testis and Gland of Wistar Rats. Journal of Applied Life Sciences International 18, 1–7.
| Cadmium Chloride Reduces Testicular and Epididymal Weights with Degenerative Histoarchitectural Changes in Testis and Gland of Wistar Rats.Crossref | GoogleScholarGoogle Scholar |
Nateghian, Z., and Aliabadi, E. (2019). Aspects of Environmental Pollutants on Male Fertility and Sperm Parameters. J. Environ. Treat. Tech. 8, 299–309.
National Institutes of Health (NIH) (2011). ‘Guide for the Care and Use of Laboratory Animals, National Research Council (US), Committee for the Update of the Guide for the Care and Use of Laboratory Animals.’ (Washington (DC): National Academies Press.)
Nnadi, , and Ngozi, N. (2018). Effects of Lead and Cadmium Concentration on Blood and Semen of Male Patients Consulting Fertility Clinic, Abakiliki, South-East Nigeria. Int. J. Prog. Sci. Technol.s 7, 127–134.
O’Hara, L., Welsh, M., Saunders, P., and Smith, L. (2010). Androgen Receptor Expression in the Caput Epididymal Epithelium Is Essential for Development of the Initial Segment and Epididymal Spermatozoa Transit. Endocrinology 152, 718–729.
| Androgen Receptor Expression in the Caput Epididymal Epithelium Is Essential for Development of the Initial Segment and Epididymal Spermatozoa Transit.Crossref | GoogleScholarGoogle Scholar | 21177831PubMed |
Pirard, C., Compere, S., Firquet, K., and Charlier, C. (2018). The current environmental levels of endocrine disruptors (mercury, cadmium, organochlorine pesticides and PCBs) in a Belgian adult population and their predictors of exposure. Int. J. Hyg. Environ. Health 221, 211–222.
| The current environmental levels of endocrine disruptors (mercury, cadmium, organochlorine pesticides and PCBs) in a Belgian adult population and their predictors of exposure.Crossref | GoogleScholarGoogle Scholar | 29146212PubMed |
Povey, A. C., and Stocks, S. (2010). Epidemiology and trends in male subfertility. Hum Fertil (Camb) 13, 182–188.
| Epidemiology and trends in male subfertility.Crossref | GoogleScholarGoogle Scholar | 21117926PubMed |
Ram, P. A., Cardullo, R., and Millette, C. (1989). Expression and topographical localization of cell surface fucosyltransferase activity during epididymal sperm maturation in the mouse. Gamete Res. 22, 321–332.
| Expression and topographical localization of cell surface fucosyltransferase activity during epididymal sperm maturation in the mouse.Crossref | GoogleScholarGoogle Scholar | 2707732PubMed |
Robaire, B., and Hamzeh, M. (2011). Androgen Action in the Epididymis. J. Androl. 32, 592–599.
| Androgen Action in the Epididymis.Crossref | GoogleScholarGoogle Scholar | 21764895PubMed |
Robaire, B., and Hinton, B. T. (2015). The Epididymis. In ‘Knobil and Neill’s Physiology of Reproduction, 4th edn.’ (Eds T.M. Plant, A.J. Zeleznik.) . pp. 619–677. (Elsevier).
Sengupta, P. (2012). Environmental and occupational exposure of metals and their role in male reproductive functions. Drug Chem. Toxicol. 36, 353–368.
| Environmental and occupational exposure of metals and their role in male reproductive functions.Crossref | GoogleScholarGoogle Scholar | 22947100PubMed |
Sengupta, P. (2014). Metals and male reproduction: The possible mechanisms. Adv. Biomed. Res. 3, 129.
| Metals and male reproduction: The possible mechanisms.Crossref | GoogleScholarGoogle Scholar | 24949300PubMed |
Sharma, A. (2017). Male Infertility; Evidences, Risk Factors, Causes, Diagnosis and Management in Human. Ann. Clin. Lab. Res. 05, 1–10.
| Male Infertility; Evidences, Risk Factors, Causes, Diagnosis and Management in Human.Crossref | GoogleScholarGoogle Scholar |
Sharma, V., Ichikawa, M., and Freeze, H. (2014). Mannose metabolism: More than meets the eye. Biochem. Biophys. Res. Commun. 453, 220–228.
| Mannose metabolism: More than meets the eye.Crossref | GoogleScholarGoogle Scholar | 24931670PubMed |
Sharma, H., Rawal, N., and Mathew, B. B. (2015). The characteristics, toxicity and effects of cadmium. Int. J. Nanotechnol. Nanosci. 3, 1–9.
Skolarczyk, J., Pekar, J., Skolarczyk, J., Skórzyńska-Dziduszko, K., Małecka-Massalska, T., and Budzyński, M. (2017). The impact of cadmium on male infertility. J. Elem. 2018, 35–44.
| The impact of cadmium on male infertility.Crossref | GoogleScholarGoogle Scholar |
Sullivan, R., and Mieusset, R. (2016). The human epididymis: its function in sperm maturation. Hum. Reprod. Update 22, 574–587.
| The human epididymis: its function in sperm maturation.Crossref | GoogleScholarGoogle Scholar | 27307387PubMed |
Tajiri, S., Fukui, T., Sawaguchi, A., and And Yoshinaga, K. (2012). Cell- and region-specific expression of sugar chains in the mouse epididymal epithelium using lectin histochemistry combined with immunohistochemistry. Okajimas Folia Anat. Jpn. 88, 141–146.
| Cell- and region-specific expression of sugar chains in the mouse epididymal epithelium using lectin histochemistry combined with immunohistochemistry.Crossref | GoogleScholarGoogle Scholar | 22645905PubMed |
Tariba Lovaković, B. (2020). Cadmium, arsenic, and lead: elements affecting male reproductive health. Curr. Opin. Toxicol. 19, 7–14.
| Cadmium, arsenic, and lead: elements affecting male reproductive health.Crossref | GoogleScholarGoogle Scholar |
Tecle, E., and Gagneux, P. (2015). Sugar‐coated sperm: Unraveling the functions of the mammalian sperm glycocalyx. Mol. Reprod. Dev. 82, 635–650.
| Sugar‐coated sperm: Unraveling the functions of the mammalian sperm glycocalyx.Crossref | GoogleScholarGoogle Scholar | 26061344PubMed |
Tourzani, D. A., Paudel, B., Miranda, P., Visconti, P., and Gervasi, M. (2018). Changes in Protein O-GlcNAcylation During Mouse Epididymal Sperm Maturation. Front. Cell Dev. Biol. 6, 60.
| Changes in Protein O-GlcNAcylation During Mouse Epididymal Sperm Maturation.Crossref | GoogleScholarGoogle Scholar | 29942801PubMed |
Tulsiani, D. R. (2003). Glycan modifying enzymes in luminal fluid of rat epididymis: Are they involved in altering sperm surface glycoproteins during maturation? Microsc. Res. Tech. 61, 18–27.
| Glycan modifying enzymes in luminal fluid of rat epididymis: Are they involved in altering sperm surface glycoproteins during maturation?Crossref | GoogleScholarGoogle Scholar | 12672119PubMed |
Tulsiani, D. R. (2006). Glycan-modifying enzymes in luminal fluid of the mammalian epididymis: An overview of their potential role in sperm maturation. Mol. Cell. Endocrinol. 250, 58–65.
| Glycan-modifying enzymes in luminal fluid of the mammalian epididymis: An overview of their potential role in sperm maturation.Crossref | GoogleScholarGoogle Scholar | 16413674PubMed |
Tulsiani, D. R., and Abou-Haila, A. (2012). Biological Processes that Prepare Mammalian Spermatozoa to Interact with an Egg and Fertilize It. Scientifica (Cairo) 2012, 607427.
| Biological Processes that Prepare Mammalian Spermatozoa to Interact with an Egg and Fertilize It.Crossref | GoogleScholarGoogle Scholar | 24278720PubMed |
Tulsiani, D. R., Skudlarek, M., Nagdas, S., and Orgebin-Crist, M. (1993). Purification and characterization of rat epididymal-fluid α-d-mannosidase: similarities to sperm plasma-membrane α-d-mannosidase. Biochem. J. 290, 427–436.
| Purification and characterization of rat epididymal-fluid α-d-mannosidase: similarities to sperm plasma-membrane α-d-mannosidase.Crossref | GoogleScholarGoogle Scholar | 8452531PubMed |
Wang, S., Ren, X., Hu, X., Zhou, L., Zhang, C., and Zhang, M. (2019). Cadmium-induced apoptosis through reactive oxygen species-mediated mitochondrial oxidative stress and the JNK signaling pathway in TM3 cells, a model of mouse Leydig cells. Toxicol. Appl. Pharmacol. 368, 37–48.
| Cadmium-induced apoptosis through reactive oxygen species-mediated mitochondrial oxidative stress and the JNK signaling pathway in TM3 cells, a model of mouse Leydig cells.Crossref | GoogleScholarGoogle Scholar | 30796935PubMed |
World Health Organization (WHO) (2020a). Infertility is a global public health issue. Available at: https://www.who.int/reproductivehealth/topics/infertility/perspective/en/.
World Health Organization (WHO) (2020b). ‘WHO Laboratory Manual For The Examination And Processing Of Human Semen.’ Available at: https://www.who.int/reproductivehealth/publications/infertility/9789241547789/en.
Wu, L., and Sampson, N. (2013). Fucose, Mannose, and β-N-Acetylglucosamine Glycopolymers Initiate the Mouse Sperm Acrosome Reaction through Convergent Signaling Pathways. ACS Chem. Biol. 9, 468–475.
| Fucose, Mannose, and β-N-Acetylglucosamine Glycopolymers Initiate the Mouse Sperm Acrosome Reaction through Convergent Signaling Pathways.Crossref | GoogleScholarGoogle Scholar | 24252131PubMed |
Wu, X., Guo, X., Wang, H., Zhou, S., Li, L., Chen, X., Wang, G., Liu, J., Ge, H., and Ge, R. (2017). A brief exposure to cadmium impairs Leydig cell regeneration in the adult rat testis. Sci. Rep. 7, .
| A brief exposure to cadmium impairs Leydig cell regeneration in the adult rat testis.Crossref | GoogleScholarGoogle Scholar | 29273765PubMed |
Xin, A., Cheng, L., Diao, H., Wu, Y., Zhou, S., Shi, C., Sun, Y., Wang, P., Duan, S., Zheng, J., Wu, B., Yuan, Y., Gu, Y., Chen, G., Sun, X., Shi, H., Tao, S., and Zhang, Y. (2016). Lectin binding of human sperm associates with DEFB126 mutation and serves as a potential biomarker for subfertility. Sci. Rep. 6, 20249.
| Lectin binding of human sperm associates with DEFB126 mutation and serves as a potential biomarker for subfertility.Crossref | GoogleScholarGoogle Scholar | 26832966PubMed |
Yari, A., Asadi, M., Bahadoran, H., Dashtnavard, H., Imani, H., and Naghii, M. (2009). Cadmium Toxicity in Spermatogenesis and Protective Effects of l-Carnitine in Adult Male Rats. Biol. Trace Elem. Res. 137, 216–225.
| Cadmium Toxicity in Spermatogenesis and Protective Effects of l-Carnitine in Adult Male Rats.Crossref | GoogleScholarGoogle Scholar | 20012383PubMed |
Yari, A., Sarveazad, A., Asadi, E., Raouf Sarshoori, J., Babahajian, A., Amini, N., Amidi, F., Bahadoran, H., Joghataei, M., Asadi, M., and Shams, A. (2016). Efficacy of Crocus sativusL. on reduction of cadmium-induced toxicity on spermatogenesis in adult rats. Andrologia 48, 1244–1252.
| Efficacy of Crocus sativusL. on reduction of cadmium-induced toxicity on spermatogenesis in adult rats.Crossref | GoogleScholarGoogle Scholar | 27135275PubMed |
Zhao, L. L., Ru, Y., Liu, M., Tang, J., Zheng, J., Wu, B., Gu, Y., and Shi, H. (2017). Reproductive effects of cadmium on sperm function and early embryonic development in vitro. PLoS One 12, e0186727.
| Reproductive effects of cadmium on sperm function and early embryonic development in vitro.Crossref | GoogleScholarGoogle Scholar | 29095856PubMed |


