Effects of bone morphogenetic protein 15 (BMP15) knockdown on porcine testis morphology and spermatogenesis
Tao Tang A * , Qiyuan Lin
A * , Qiyuan Lin  A * , Yufeng Qin A , Xinyu Liang A , Yang Guo A , Peiqing Cong A , Xiaohong Liu A , Yaosheng Chen A B and Zuyong He
A * , Yufeng Qin A , Xinyu Liang A , Yang Guo A , Peiqing Cong A , Xiaohong Liu A , Yaosheng Chen A B and Zuyong He  A B
A B
A State Key Laboratory of Biocontrol, School of Life Sciences, Sun Yat-sen University, Guangzhou 510006, PR China.
B Corresponding authors. Email: chyaosh@mail.sysu.edu.cn; zuyonghe@foxmail.com
Reproduction, Fertility and Development 32(11) 999-1011 https://doi.org/10.1071/RD20056
Submitted: 2 March 2020 Accepted: 5 June 2020 Published: 30 June 2020
Abstract
Bone morphogenetic protein 15 (BMP15) is a member of the transforming growth factor-β (TGFB) superfamily that plays an essential role in mammalian ovary development, oocyte maturation and litter size. However, little is known regarding the expression pattern and biological function of BMP15 in male gonads. In this study we established, for the first time, a transgenic pig model with BMP15 constitutively knocked down by short hairpin (sh) RNA. The transgenic boars were fertile, but sperm viability was decreased. Further analysis of the TGFB/SMAD pathway and markers of reproductive capacity, namely androgen receptor and protamine 2, failed to identify any differentially expressed genes. These results indicate that, in the pig, the biological function of BMP15 in the development of male gonads is not as crucial as in ovary development. However, the role of BMP15 in sperm viability requires further investigation. This study provides new insights into the role of BMP15 in male pig reproduction.
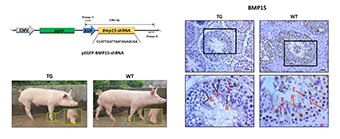
Additional keyword: transgenic pig.
References
Aaltonen, J., Laitinen, M. P., Vuojolainen, K., Jaatinen, R., Horelli-Kuitunen, N., Seppa, L., Louhio, H., Tuuri, T., Sjoberg, J., Butzow, R., Hovata, O., and Dale, L. (1999). Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis. J. Clin. Endocrinol. Metab. 84, 2744–2750.| Human growth differentiation factor 9 (GDF-9) and its novel homolog GDF-9B are expressed in oocytes during early folliculogenesis.Crossref | GoogleScholarGoogle Scholar | 10443672PubMed |
Almeida, F. F. L., Leal, M. C., and França, L. R. (2006). Testis morphometry, duration of spermatogenesis, and spermatogenic efficiency in the wild boar (Sus scrofa scrofa). Biol. Reprod. 75, 792–799.
| Testis morphometry, duration of spermatogenesis, and spermatogenic efficiency in the wild boar (Sus scrofa scrofa).Crossref | GoogleScholarGoogle Scholar |
Boukari, K., Meduri, G., Brailly-Tabard, S., Guibourdenche, J., Ciampi, M. L., Massin, N., Martinerie, L., Picard, J.-Y., Rey, R., Lombès, M., and Young, J. (2009). Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testis development. J. Clin. Endocrinol. Metab. 94, 1818–1825.
| Lack of androgen receptor expression in Sertoli cells accounts for the absence of anti-Mullerian hormone repression during early human testis development.Crossref | GoogleScholarGoogle Scholar | 19276236PubMed |
Carrell, D. T., Emery, B. R., and Hammoud, S. (2007). Altered protamine expression and diminished spermatogenesis: what is the link? Hum. Reprod. Update 13, 313–327.
| Altered protamine expression and diminished spermatogenesis: what is the link?Crossref | GoogleScholarGoogle Scholar | 17208950PubMed |
Chang, C., Chen, Y. T., Yeh, S. D., Xu, Q., Wang, R. S., Guillou, F., Lardy, H., and Yeh, S. (2004). Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells. Proc. Natl Acad. Sci. USA 101, 6876–6881.
| Infertility with defective spermatogenesis and hypotestosteronemia in male mice lacking the androgen receptor in Sertoli cells.Crossref | GoogleScholarGoogle Scholar | 15107499PubMed |
Christoforou, E. R., and Pitman, J. L. (2019). Intra-follicular growth differentiation factor 9 : bone morphogenetic 15 ratio determines litter size in mammals. Biol. Reprod. 100, 1333–1343.
| Intra-follicular growth differentiation factor 9 : bone morphogenetic 15 ratio determines litter size in mammals.Crossref | GoogleScholarGoogle Scholar | 30698706PubMed |
Ciller, I. M., Palanisamy, S. K., Ciller, U. A., and McFarlane, J. R. (2016). Postnatal expression of bone morphogenetic proteins and their receptors in the mouse testis. Physiol. Res. 65, 673–682.
| Postnatal expression of bone morphogenetic proteins and their receptors in the mouse testis.Crossref | GoogleScholarGoogle Scholar | 26988160PubMed |
De Gendt, K., Swinnen, J. V., Saunders, P. T., Schoonjans, L., Dewerchin, M., Devos, A., Tan, K., Atanassova, N., Claessens, F., Lecureuil, C., Heyns, W., and Carmeliet, P. (2004). A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis. Proc. Natl Acad. Sci. USA 101, 1327–1332.
| A Sertoli cell-selective knockout of the androgen receptor causes spermatogenic arrest in meiosis.Crossref | GoogleScholarGoogle Scholar | 14745012PubMed |
Dube, J. L., Wang, P., Elvin, J., Lyons, K. M., Celeste, A. J., and Matzuk, M. M. (1998). The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol. Endocrinol. 12, 1809–1817.
| The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes.Crossref | GoogleScholarGoogle Scholar | 9849956PubMed |
Ducy, P., and Karsenty, G. (2000). The family of bone morphogenetic proteins. Kidney Int. 57, 2207–2214.
| The family of bone morphogenetic proteins.Crossref | GoogleScholarGoogle Scholar | 10844590PubMed |
Epifano, O., and Dean, J. (2002). Genetic control of early folliculogenesis in mice. Trends Endocrinol. Metab. 13, 169–173.
| Genetic control of early folliculogenesis in mice.Crossref | GoogleScholarGoogle Scholar | 11943561PubMed |
Galloway, S. M., McNatty, K. P., Cambridge, L. M., Laitinen, M. P., Juengel, J. L., Jokiranta, T. S., McLaren, R. J., Luiro, K., Dodds, K. G., Montgomery, G. W., Beattie, A. E., and Davis, G. H. (2000). Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner. Nat. Genet. 25, 279–283.
| Mutations in an oocyte-derived growth factor gene (BMP15) cause increased ovulation rate and infertility in a dosage-sensitive manner.Crossref | GoogleScholarGoogle Scholar | 10888873PubMed |
Gilchrist, R. B., Lane, M., and Thompson, J. G. (2008). Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update 14, 159–177.
| Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality.Crossref | GoogleScholarGoogle Scholar | 18175787PubMed |
Guo, Q. Y., Gao, Z. Z., Zhao, L., He, J. P., and Dong, C. S. (2013). Expression of growth differentiation factor 9 (GDF9), ALK5, and claudin-11 in adult alpaca testis. Acta Histochem. 115, 16–21.
| Expression of growth differentiation factor 9 (GDF9), ALK5, and claudin-11 in adult alpaca testis.Crossref | GoogleScholarGoogle Scholar | 22459938PubMed |
Holdcraft, R. W., and Braun, R. E. (2004). Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids. Development 131, 459–467.
| Androgen receptor function is required in Sertoli cells for the terminal differentiation of haploid spermatids.Crossref | GoogleScholarGoogle Scholar | 14701682PubMed |
Hu, J., Chen, Y. X., Wang, D., Qi, X., Li, T. G., Hao, J., Mishina, Y., Garbers, D. L., and Zhao, G. Q. (2004). Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity. Dev. Biol. 276, 158–171.
| Developmental expression and function of Bmp4 in spermatogenesis and in maintaining epididymal integrity.Crossref | GoogleScholarGoogle Scholar | 15531371PubMed |
Knapczyk-Stwora, K., Grzesiak, M., and Slomczynska, M. (2014). Altered expression of 3beta-HSD, CYP17 and 17beta-HSD in the foetal porcine gonads in response to anti-androgen flutamide exposure. Reprod. Domest. Anim. 49, 725–733.
| Altered expression of 3beta-HSD, CYP17 and 17beta-HSD in the foetal porcine gonads in response to anti-androgen flutamide exposure.Crossref | GoogleScholarGoogle Scholar | 25130044PubMed |
Knight, P. G., and Glister, C. (2006). TGF-beta superfamily members and ovarian follicle development. Reproduction 132, 191–206.
| TGF-beta superfamily members and ovarian follicle development.Crossref | GoogleScholarGoogle Scholar | 16885529PubMed |
Liu, X., Wang, M., Qin, Y., Shi, X., Cong, P., Chen, Y., and He, Z. (2018). Targeted integration in human cells through single crossover mediated by ZFN or CRISPR/Cas9. BMC Biotechnol. 18, 66.
| Targeted integration in human cells through single crossover mediated by ZFN or CRISPR/Cas9.Crossref | GoogleScholarGoogle Scholar | 30340581PubMed |
Mayer, A., Fouquet, B., Pugeat, M., and Misrahi, M. (2017). BMP15 ‘knockout-like’ effect in familial premature ovarian insufficiency with persistent ovarian reserve. Clin. Genet. 92, 208–212.
| BMP15 ‘knockout-like’ effect in familial premature ovarian insufficiency with persistent ovarian reserve.Crossref | GoogleScholarGoogle Scholar | 28094433PubMed |
McIntosh, C. J., Lun, S., Lawrence, S., Western, A. H., McNatty, K. P., and Juengel, J. L. (2008). The proregion of mouse BMP15 regulates the cooperative interactions of BMP15 and GDF9. Biol. Reprod. 79, 889–896.
| The proregion of mouse BMP15 regulates the cooperative interactions of BMP15 and GDF9.Crossref | GoogleScholarGoogle Scholar | 18633140PubMed |
McNatty, K. P., Moore, L. G., Hudson, N. L., Quirke, L. D., Lawrence, S. B., Reader, K., Hanrahan, J. P., Smith, P., Groome, N. P., Laitinen, M., Ritvos, O., and Juengel, J. L. (2004). The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology. Reproduction 128, 379–386.
| The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology.Crossref | GoogleScholarGoogle Scholar | 15454632PubMed |
Monestier, O., Servin, B., Auclair, S., Bourquard, T., Poupon, A., Pascal, G., and Fabre, S. (2014). Evolutionary origin of bone morphogenetic protein 15 and growth and differentiation factor 9 and differential selective pressure between mono- and polyovulating species. Biol. Reprod. 91, 83.
| Evolutionary origin of bone morphogenetic protein 15 and growth and differentiation factor 9 and differential selective pressure between mono- and polyovulating species.Crossref | GoogleScholarGoogle Scholar | 25100713PubMed |
Montgomery, G. W., Galloway, S. M., Davis, G. H., and McNatty, K. P. (2001). Genes controlling ovulation rate in sheep. Reproduction 121, 843–852.
| Genes controlling ovulation rate in sheep.Crossref | GoogleScholarGoogle Scholar | 11373170PubMed |
Nagyova, E., Nemcova, L., Bujnakova, M., Lynarcikova, A., Blaha, M., Prochazka, R., and Scsukova, S. (2017). Effect of bone morphogenetic protein-15 on gonadotropin-stimulated synthesis of hyaluronan and progesterone in porcine ovarian follicle. J. Physiol. Pharmacol. 68, 683–691.
| 29375042PubMed |
Nicholls, P. K., Harrison, C. A., Gilchrist, R. B., Farnworth, P. G., and Stanton, P. G. (2009). Growth differentiation factor 9 is a germ cell regulator of Sertoli cell function. Endocrinology 150, 2481–2490.
| Growth differentiation factor 9 is a germ cell regulator of Sertoli cell function.Crossref | GoogleScholarGoogle Scholar | 19106224PubMed |
Ogawa, S., Konta, A., Kimata, M., Ishii, K., Uemoto, Y., and Satoh, M. (2019). Estimation of genetic parameters for farrowing traits in purebred Landrace and Large White pigs. Anim. Sci. J. 90, 23–28.
| Estimation of genetic parameters for farrowing traits in purebred Landrace and Large White pigs.Crossref | GoogleScholarGoogle Scholar | 30370591PubMed |
Otsuka, F., Yao, Z., Lee, T., Yamamoto, S., Erickson, G. F., and Shimasaki, S. (2000). Bone morphogenetic protein-15. Identification of target cells and biological functions. J. Biol. Chem. 275, 39523–39528.
| Bone morphogenetic protein-15. Identification of target cells and biological functions.Crossref | GoogleScholarGoogle Scholar | 10998422PubMed |
Paulini, F., and Melo, E. O. (2011). The role of oocyte-secreted factors GDF9 and BMP15 in follicular development and oogenesis. Reprod. Domest. Anim. 46, 354–361.
| The role of oocyte-secreted factors GDF9 and BMP15 in follicular development and oogenesis.Crossref | GoogleScholarGoogle Scholar | 21198974PubMed |
Peng, J., Li, Q., Wigglesworth, K., Rangarajan, A., Kattamuri, C., Peterson, R. T., Eppig, J. J., Thompson, T. B., and Matzuk, M. M. (2013). Growth differentiation factor 9 : bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc. Natl Acad. Sci. USA 110, E776–E785.
| Growth differentiation factor 9 : bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions.Crossref | GoogleScholarGoogle Scholar | 23382188PubMed |
Persani, L., Rossetti, R., Di Pasquale, E., Cacciatore, C., and Fabre, S. (2014). The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum. Reprod. Update 20, 869–883.
| The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders.Crossref | GoogleScholarGoogle Scholar | 24980253PubMed |
Prapa, E., Vasilaki, A., Dafopoulos, K., Katsiani, E., Georgoulias, P., Messini, C. I., Anifandis, G., and Messinis, I. E. (2015). Effect of anti-Mullerian hormone (AMH) and bone morphogenetic protein 15 (BMP-15) on steroidogenesis in primary-cultured human luteinizing granulosa cells through Smad5 signalling. J. Assist. Reprod. Genet. 32, 1079–1088.
| Effect of anti-Mullerian hormone (AMH) and bone morphogenetic protein 15 (BMP-15) on steroidogenesis in primary-cultured human luteinizing granulosa cells through Smad5 signalling.Crossref | GoogleScholarGoogle Scholar | 26003656PubMed |
Puglisi, R., Montanari, M., Chiarella, P., Stefanini, M., and Boitani, C. (2004). Regulatory role of BMP2 and BMP7 in spermatogonia and Sertoli cell proliferation in the immature mouse. Eur. J. Endocrinol. 151, 511–520.
| Regulatory role of BMP2 and BMP7 in spermatogonia and Sertoli cell proliferation in the immature mouse.Crossref | GoogleScholarGoogle Scholar | 15476453PubMed |
Qin, Y., Tang, T., Li, W., Liu, Z., Yang, X., Shi, X., Sun, G., Liu, X., Wang, M., Liang, X., Cong, P., Mo, D., Liu, X., Chen, Y., and He, Z. (2019). Bone morphogenetic protein 15 knockdown inhibits porcine ovarian follicular development and ovulation. Front. Cell Dev. Biol. 7, 286.
| Bone morphogenetic protein 15 knockdown inhibits porcine ovarian follicular development and ovulation.Crossref | GoogleScholarGoogle Scholar | 31803742PubMed |
Schneider, S., Balbach, M., Jan, F. J., Fietz, D., Nettersheim, D., Jostes, S., Schmidt, R., Kressin, M., Bergmann, M., Wachten, D., Steger, K., and Schorle, H. (2016). Re-visiting the protamine-2 locus: deletion, but not haploinsufficiency, renders male mice infertile. Sci. Rep. 6, 36764.
| Re-visiting the protamine-2 locus: deletion, but not haploinsufficiency, renders male mice infertile.Crossref | GoogleScholarGoogle Scholar | 27833122PubMed |
Schulze, M., Henning, H., Rüdiger, K., Wallner, U., and Waberski, D. (2013). Temperature management during semen processing: Impact on boar sperm quality under laboratory and field conditions. Theriogenology 80, 990–998.
| Temperature management during semen processing: Impact on boar sperm quality under laboratory and field conditions.Crossref | GoogleScholarGoogle Scholar | 23987989PubMed |
Shimasaki, S., Moore, R. K., Otsuka, F., and Erickson, G. F. (2004). The bone morphogenetic protein system in mammalian reproduction. Endocr. Rev. 25, 72–101.
| The bone morphogenetic protein system in mammalian reproduction.Crossref | GoogleScholarGoogle Scholar | 14769828PubMed |
Srisuwatanasagul, K., Prapaiwan, N., Srisuwatanasagul, S., Kunavongkrit, A., and Roongsitthichai, A. (2018). Immunohistochemical study of Ki-67 protein, androgen receptor, and estrogen receptor beta in testicular tissues of male pigs immunocastrated with different times of GnRH vaccination. Anat. Histol. Embryol. 47, 475–480.
| Immunohistochemical study of Ki-67 protein, androgen receptor, and estrogen receptor beta in testicular tissues of male pigs immunocastrated with different times of GnRH vaccination.Crossref | GoogleScholarGoogle Scholar | 30014509PubMed |
Sun, L. P., Song, Y. P., Du, Q. Z., Song, L. W., Tian, Y. Z., Zhang, S. L., Hua, G. H., and Yang, L. G. (2014). Polymorphisms in the bone morphogenetic protein 15 gene and their effect on sperm quality traits in Chinese Holstein bulls. Genet. Mol. Res. 13, 1805–1812.
| Polymorphisms in the bone morphogenetic protein 15 gene and their effect on sperm quality traits in Chinese Holstein bulls.Crossref | GoogleScholarGoogle Scholar | 24668668PubMed |
van Caam, A., Madej, W., Garcia de Vinuesa, A., Goumans, M. J., Ten Dijke, P., Blaney Davidson, E., and van der Kraan, P. (2017). TGFbeta1-induced SMAD2/3 and SMAD1/5 phosphorylation are both ALK5-kinase-dependent in primary chondrocytes and mediated by TAK1 kinase activity. Arthritis Res. Ther. 19, 112.
| TGFbeta1-induced SMAD2/3 and SMAD1/5 phosphorylation are both ALK5-kinase-dependent in primary chondrocytes and mediated by TAK1 kinase activity.Crossref | GoogleScholarGoogle Scholar | 28569204PubMed |
Van den Broeke, A., Aluwé, M., Janssens, S., Wauters, J., Vanhaecke, L., Buys, N., Millet, S., and Tuyttens, F. A. M. (2015). The effect of the MC4R gene on boar taint compounds, sexual maturity and behaviour in growing-finishing boars and gilts. Animal 9, 1688–1697.
| The effect of the MC4R gene on boar taint compounds, sexual maturity and behaviour in growing-finishing boars and gilts.Crossref | GoogleScholarGoogle Scholar | 26155873PubMed |
Vílchez, M. C., Santangeli, S., Maradonna, F., Gioacchini, G., Verdenelli, C., Gallego, V., Peñaranda, D. S., Tveiten, H., Pérez, L., Carnevali, O., and Asturiano, J. F. (2015). Effect of the probiotic Lactobacillus rhamnosus on the expression of genes involved in European eel spermatogenesis. Theriogenology 84, 1321–1331.
| Effect of the probiotic Lactobacillus rhamnosus on the expression of genes involved in European eel spermatogenesis.Crossref | GoogleScholarGoogle Scholar | 26271165PubMed |
Wang, H., Yuan, Q., Sun, M., Niu, M., Wen, L., Fu, H., Zhou, F., Chen, Z., Yao, C., Hou, J., Shen, R., and Lin, Q. (2017). BMP6 regulates proliferation and apoptosis of human Sertoli cells via Smad2/3 and cyclin D1 pathway and DACH1 and TFAP2A activation. Sci. Rep. 7, 45298.
| BMP6 regulates proliferation and apoptosis of human Sertoli cells via Smad2/3 and cyclin D1 pathway and DACH1 and TFAP2A activation.Crossref | GoogleScholarGoogle Scholar | 28387750PubMed |
Wasilewska, K., Zasiadczyk, Ł., Fraser, L., Mogielnicka-Brzozowska, M., and Kordan, W. (2016). The benefits of cooling boar semen in long-term extenders prior to cryopreservaton on sperm quality characteristcs. Reprod. Domest. Anim. 51, 781–788.
| The benefits of cooling boar semen in long-term extenders prior to cryopreservaton on sperm quality characteristcs.Crossref | GoogleScholarGoogle Scholar | 27554400PubMed |
Wu, Z., Xu, Z., Zou, X., Zeng, F., Shi, J., Liu, D., Urschitz, J., Moisyadi, S., and Li, Z. (2013). Pig transgenesis by piggyBac transposition in combination with somatic cell nuclear transfer. Transgenic Res. 22, 1107–1118.
| Pig transgenesis by piggyBac transposition in combination with somatic cell nuclear transfer.Crossref | GoogleScholarGoogle Scholar | 23857557PubMed |
Yadav, H., and Lal, B. (2017). BMP15 in catfish testis: cellular distribution, seasonal variation, and its role in steroidogenesis. Steroids 125, 114–123.
| BMP15 in catfish testis: cellular distribution, seasonal variation, and its role in steroidogenesis.Crossref | GoogleScholarGoogle Scholar | 28711705PubMed |
Yan, C., Wang, P., DeMayo, J., DeMayo, F. J., Elvin, J. A., Carino, C., Prasad, S. V., Skinner, S. S., Dunbar, B. S., Dube, J. L., Celeste, A. J., and Matzuk, M. M. (2001). Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol. Endocrinol. 15, 854–866.
| Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function.Crossref | GoogleScholarGoogle Scholar | 11376106PubMed |
Yan, Q., Yang, H., Yang, D., Zhao, B., Ouyang, Z., Liu, Z., Fan, N., Ouyang, H., Gu, W., and Lai, L. (2014). Production of transgenic pigs over-expressing the antiviral gene Mx1. Cell Regen. (Lond.) 3, 11.
| Production of transgenic pigs over-expressing the antiviral gene Mx1.Crossref | GoogleScholarGoogle Scholar | 25408889PubMed |
Zalata, A. A., Mokhtar, N., Atwa, A., Khaled, M., and Shaker, O. G. (2016). The role of protamine 2 gene expression and caspase 9 activity in male infertility. J. Urol. 195, 796–800.
| The role of protamine 2 gene expression and caspase 9 activity in male infertility.Crossref | GoogleScholarGoogle Scholar | 26392304PubMed |
Zhao, G. Q., Deng, K., Labosky, P. A., Liaw, L., and Hogan, B. L. (1996). The gene encoding bone morphogenetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse. Genes Dev. 10, 1657–1669.
| The gene encoding bone morphogenetic protein 8B is required for the initiation and maintenance of spermatogenesis in the mouse.Crossref | GoogleScholarGoogle Scholar | 8682296PubMed |
Zhao, G. Q., Liaw, L., and Hogan, B. L. (1998). Bone morphogenetic protein 8A plays a role in the maintenance of spermatogenesis and the integrity of the epididymis. Development 125, 1103–1112.
| 9463357PubMed |
Zhao, G. Q., Chen, Y. X., Liu, X. M., Xu, Z., and Qi, X. (2001). Mutation in Bmp7 exacerbates the phenotype of Bmp8a mutants in spermatogenesis and epididymis. Dev. Biol. 240, 212–222.
| Mutation in Bmp7 exacerbates the phenotype of Bmp8a mutants in spermatogenesis and epididymis.Crossref | GoogleScholarGoogle Scholar | 11784057PubMed |


