Oral COVID-19 antiviral prescribing in Australian general practice – a retrospective observational study
Judith Thomas A * , Abbish Kamalakkannan A , Mirela Prgomet A , Karina Gardner B , Precious McGuire C , Geoffrey Campbell D Andrew Georgiou AA
B
C
D
Abstract
This study aimed to gain a comprehensive understanding of oral COVID-19 antiviral prescribing in Australian general practice.
The study was a retrospective observational cohort study. Routinely collected, de-identified, Australian general practice consultation and prescription data was used with permission from participating primary health networks, from 938 general practices between March 2022 and September 2023. Study cohorts were patients aged ≥15 encompassing (i) an ‘antiviral cohort’ comprising patient records with ≥1 oral COVID-19 antiviral prescription/s and (ii) a ‘comparison cohort’ of remaining records. Primary outcome measures were the frequency and type of oral COVID-19 antivirals prescribed, and sociodemographic and health characteristics of patients prescribed antivirals. Secondary measures were the frequency of antiviral repeat prescribing and consultation modality.
Within the study population of 3,813,051 patients, oral COVID-19 antivirals were prescribed to 3.39% (129,267) of patients including 14.79% (82,215/555,757) of patients aged ≥70 years. Molnupiravir prescribing exceeded nirmatrelvir–ritonavir across all study months. Proportionally, antiviral prescribing was higher in the female population (74,709/2,059,676: 3.63%), Victoria (81,184/2,222,837: 3.65%), residing in high socioeconomic advantage areas (87,530/2,224,501: 3.93%), and ages 80–84 years (16,419/100,911: 16.27%). Of patients prescribed COVID-19 antivirals, 7.27% (9402/129,267) had repeat prescribing. Cardiovascular and musculoskeletal conditions were the most prevalent chronic diagnoses, and telehealth (58,660/107,727: 54.45%) exceeded face-to-face consultations.
Oral COVID-19 antiviral prescribing volumes in general practice may serve as an indicator of periods of increased transmission of COVID-19, through increases in prescribing activity. Telehealth exceeding face-to-face for oral COVID-19 antiviral prescribing supports continued access to telehealth to reduce exposure to COVID-19 and provide time-critical access to treatment.
Keywords: antiviral, Australia, COVID-19, general practice, molnupiravir, nirmatrelvir–ritonavir, prescribing, SARS-CoV-2, telehealth.
Introduction
Over the course of the COVID-19 pandemic a range of strategies were adopted to reduce the spread and health impacts of the SARS-CoV-2 virus, including antiviral and therapeutic treatment options (Andrews et al. 2024). A broad range of COVID-19 treatments are currently available with varying pharmacodynamics (Andrews et al. 2024) and indications based on severity of infection and/or a patient’s risk profile for hospitalisation (World Health Organization 2023; Andrews et al. 2024).
In Australia, the Therapeutic Goods Administration provided provisional approval for two oral COVID-19 antiviral medications, molnupiravir and nirmatrelvir–ritonavir, on 18 January 2022 (Australian Government Department of Health and Aged Care 2022a, 2022b). These antivirals were subsequently listed on the Pharmaceutical Benefits Scheme (PBS) in March and May 2022 respectively, improving access and reducing costs for eligible patients (Allard et al. 2023). Australian eligibility criteria for doctors and authorised nurse practitioners to prescribe PBS-subsidised oral COVID-19 antivirals include non-hospitalised adults with specified risk factors for severe outcomes (e.g. age, residing in residential aged care, severity of infection, healthcare access, time from symptom onset, history of COVID-19 hospitalisation, and comorbidities) (Australian Government Department of Health and Aged Care 2022a, 2022b, 2023). The initial criteria were expanded during 2022–2023 (previously summarised (Allard et al. 2023)), and in March 2024 criteria amendments specified that prescribing molnupiravir required documentation in a patient’s record of reasons why nirmatrelvir–ritonavir could not be prescribed (Australian Government Department of Health and Aged Care 2022a).
As COVID-19 continues to circulate within Australia, general practitioners will have a continuing role in prescribing oral COVID-19 antivirals. Despite the potential to reduce severe outcomes and hospitalisation in high-risk patients (Paraskevis et al. 2023; Van Heer et al. 2023; Andrews et al. 2024), there is limited information regarding oral COVID-19 antiviral prescribing in Australian general practice. The Government Department of Health and Aged Care provides data on total monthly PBS prescriptions dispensed for oral COVID-19 antivirals, by state and territory and age groups (Australian Government Department of Health and Aged Care 2024). PBS prescription and expenditure reports and item reports also provide high-level data. There are notable gaps in information regarding oral COVID-19 antiviral prescribing in general practice, including the sociodemographic characteristics of patients receiving prescriptions, repeat prescribing, or the extent of telehealth uptake for oral COVID-19 antiviral prescribing (given patients seeking treatment would be COVID-19 positive at the time of consultation). Greater understanding of the uptake of oral COVID-19 antivirals can help inform future directions for eligibility criteria, provide an understanding of the characteristics of patients receiving treatment, and identify demands on general practice.
The aim of the current study is to provide a comprehensive understanding of oral COVID-19 antiviral prescribing in Australian general practice. The study explores prescriptions issued in general practice which may or may not have been dispensed, thus reflecting oral antiviral prescribing only, and not dispensing or treatment. The primary objectives of the study are: to determine the frequency and type of oral COVID-19 antivirals prescribed, and describe the sociodemographic and health characteristics of patients prescribed oral COVID-19 antivirals. The secondary objectives are: to determine the frequency of repeat prescribing for oral COVID-19 antivirals, and examine the utilisation of telehealth for prescribing oral COVID-19 antivirals.
Study context
On 19 July 2022, two Medicare Benefits Schedule (MBS) item numbers were introduced for general practice telehealth-phone consultations (in addition to existing telehealth item numbers) specifically for assessing a patient’s eligibility for oral COVID-19 antivirals, where the patient was Medicare eligible and the consultation duration was at least 20 min (Australian Government Department of Health and Aged Care – MBS Online 2022). In October 2022, requirements for mandatory isolation and reporting of COVID-19 rapid antigen test results ceased in NSW (NSW Health 2022) and Victoria’s pandemic declaration ended (The Hon Daniel Andrews MP Premier 2022). The study period fell within the Omicron waves of COVID-19 in Australia (COVID-19 Epidemiology and Surveillance Team 2023).
Methods
Study design and setting
This retrospective observational study was conducted using routinely collected, de-identified, general practice data provisioned by the data custodians, Outcome Health, using their Population Level Analysis and Reporting Program (POLAR) (Pearce et al. 2019) and Aurora research platforms. Data were provided with permission from participating Primary Health Networks (PHNs). The study dataset included patient consultation and prescription data between 1 March 2022 and 30 September 2023 inclusive, from 938 participating Australian general practices across four PHNs (organisations established by the government to independently manage primary health care services within their region), in the states of NSW (n = 2) and Victoria (n = 2). A unique privacy-protected dataset ID was assigned to each patient by the data custodian to track activity and engagement of an individual patient across multiple general practice locations. Data were provisioned with approval from PHNs to authorised users on a secure server hosted within Outcome Health’s (ISO27001 compliant) infrastructure. To meet ethics and governance requirements, any analysis resulting in patient numbers less than 25 (or calculatable from results) could not be included or reported, hence eligibility criteria for the study included patients aged ≥15 years with a binary sex value. Data were analysed using Stata 18 (StataCorp 2023). Strengthening the reporting of observational studies in epidemiology (STROBE) guidelines were used for reporting this study. The study included prescriptions issued in general practice (which may or may not have been dispensed) to reflect oral antiviral prescribing only (not dispensing or treatment).
Study cohorts
Of 4,611,550 records assessed for eligibility, 798,499 did not meet study criteria (797,482 did not meet age criteria; 1017 did not meet sex criteria or had missing values), giving a total study population of 3,813,051 unique individuals. In the dataset, age was provided in 5-year intervals. To capture all adults, we included patients aged ≥15 years, resulting in 2,222,837 patients from Victoria and 1,553,109 from NSW representing approximately 41.7% and 23.5% of persons aged ≥15 years in each state respectively (using 2021 census data) (Australian Bureau of Statistics 2021a). The data also included 37,105 patients from other Australian states and territories that had consultations in the included general practices. Two cohorts were derived from the eligible study population: (i) an ‘antiviral cohort’ comprising all records with one or more prescriptions for an oral COVID-19 antiviral and (ii) a ‘comparison cohort’ comprising all remaining records with no recorded oral COVID-19 antiviral prescriptions.
Data analysis
Frequency and type of oral COVID-19 antivirals were calculated as counts of (i) the number of prescriptions issued in total and by month and (ii) the number of individual patients prescribed an antiviral as a total and according to medication group.
Patient sociodemographic variables are reported for age group, sex, state of residence, and socioeconomic grouping. Socioeconomic groupings were established using Australian local government area (LGA) of residence to determine the Index of Relative Socioeconomic Advantage and Disadvantage deciles (Australian Bureau of Statistics 2021b), with deciles then combined into five socioeconomic groups: ‘Low’ (deciles 1 and 2), ‘Low–Medium’ (deciles 3 and 4), ‘Medium’ (deciles 5 and 6), ‘Medium–High’ (deciles 7 and 8), and ‘High’ (deciles 9 and 10). Health characteristics report the presence or absence of chronic conditions documented in diagnosis data, which were grouped into pre-defined categories in a data field mapped by the data custodian according to Systematized Nomenclature of Medicine (SNOMED) codes.
Patients in the antiviral cohort were further categorised into one of three groups defined as receiving one or more prescriptions during the study period for: (i) molnupiravir only; (ii) ritonavir–nirmatrelvir only; or (iii) both medications. The term ‘first-time prescription’ is henceforth used to specify that the data refers to individual patients who received at least one prescription in the allocated medication grouping (see Supplementary Table S1).
As 5 days is the duration of a course of each antiviral and additional courses of medication require a new prescription (Australian Government Department of Health and Aged Care 2022a, 2022b), repeat prescriptions were defined as any prescription for an oral COVID-19 antiviral medication issued 6 or more days after a previous oral COVID-19 antiviral prescription. This criterion was used to address repeats issued due to lost prescriptions or pharmacy supply issues, giving a more accurate representation of repeated prescribing for subsequent infections.
Consultation modality, determined from structured MBS billing item numbers, was assigned to one of three categories: (i) face-to-face, (ii) telehealth-phone, or (iii) telehealth-video. Analyses included counts of consultations where an oral COVID-19 antiviral was prescribed by modality, month, and proportions by age group.
Bias
To address representative bias associated with unequal distribution of sex, state, and socioeconomic groups within the total study population, proportions were calculated within subgroups rather than across subgroups.
Ethics approval
Outcome Health has prior ethical approval from the Royal Australian College of General Practitioners NREEC (Protocol ID: 17-008 POLAR GP data warehouse) and covers the collection, transfer and storage of data in the POLAR GP data warehouse. The Macquarie University Human Research Ethics Committee provided project-specific approval (project ID: 6756).
Results
Study population
Descriptive statistics for the comparison and antiviral cohorts between 1 March 2022 and 30 September 2023 are presented in Table 1. Descriptive statistics for the antiviral cohort are presented in Table 2.
| Comparison cohort n (% of cohort) | Antiviral cohort n (% of cohort) | Total population n (% of total) | ||
|---|---|---|---|---|
| No. of patients | 3,683,784 | 129,267 | 3,813,051 | |
| Sex | ||||
| Female | 1,984,967 (53.88) | 74,709 (57.79) | 2,059,676 (54.02) | |
| Male | 1,698,817 (46.12) | 54,558 (42.21) | 1,753,375 (45.98) | |
| State of residence | ||||
| Victoria | 2,141,653 (58.14) | 81,184 (62.80) | 2,222,837 (58.30) | |
| NSW | 1,505,629 (40.87) | 47,480 (36.73) | 1,553,109 (40.73) | |
| Other A | 36,502 (0.99) | 603 (0.47) | 37,105 (0.97) | |
| Age group (years) | ||||
| 15–19 | 241,405 (6.55) | 139 (0.11) | 241,544 (6.33) | |
| 20–24 | 295,564 (8.02) | 542 (0.42) | 296,106 (7.77) | |
| 25–29 | 349,450 (9.49) | 862 (0.67) | 350,312 (9.19) | |
| 30–34 | 372,959 (10.12) | 1194 (0.92) | 374,153 (9.81) | |
| 35–39 | 360,556 (9.79) | 1699 (1.31) | 362,255 (9.50) | |
| 40–44 | 321,187 (8.72) | 2296 (1.78) | 323,483 (8.48) | |
| 45–49 | 278,801 (7.57) | 2805 (2.17) | 281,606 (7.39) | |
| 50–54 | 281,914 (7.65) | 5742 (4.44) | 287,656 (7.54) | |
| 55–59 | 253,087 (6.87) | 7069 (5.47) | 260,156 (6.82) | |
| 60–64 | 244,775 (6.64) | 10,423 (8.06) | 255,198 (6.69) | |
| 65–69 | 210,544 (5.72) | 14,281 (11.05) | 224,825 (5.90) | |
| 70–74 | 163,297 (4.43) | 24,844 (19.22) | 188,141 (4.93) | |
| 75–79 | 129,868 (3.53) | 24,526 (18.97) | 154,394 (4.05) | |
| 80–84 | 84,492 (2.29) | 16,419 (12.70) | 100,911 (2.65) | |
| 85+ | 95,885 (2.60) | 16,426 (12.71) | 112,311 (2.95) | |
| Socioeconomic group (based on LGA of residence) | ||||
| Low (deciles 1 and 2) | 217,944 (5.92) | 5357 (4.14) | 223,301 (5.86) | |
| Low–Medium (deciles 3 and 4) | 30,126 (0.82) | 823 (0.64) | 30,949 (0.81) | |
| Medium (deciles 5 and 6) | 557,648 (15.14) | 14,651 (11.33) | 572,299 (15.01) | |
| Medium–High (deciles 7 and 8) | 660,797 (17.94) | 19,093 (14.77) | 679,890 (17.83) | |
| High (deciles 9 and 10) | 2,136,971 (58.01) | 87,530 (67.71) | 2,224,501 (58.34) | |
| Not specified | 80,298 (2.18) | 1813 (1.40) | 82,111 (2.15) | |
| Molnupiravir n (% of category) | Nirmatrelvir– ritonavir n (% of category) | Both medications n (% of category) | Percentage of total study population in age group prescribed any antiviral A | ||
|---|---|---|---|---|---|
| Total cohort | 88,316 | 38,270 | 2681 | – | |
| Age group (years) | |||||
| 15–29 B | 898 (1.02) | 596 (1.56) | 49 (1.83) | 0.17% | |
| 30–34 | 695 (0.79) | 469 (1.23) | 30 (1.12) | 0.32% | |
| 35–39 | 1019 (1.15) | 635 (1.66) | 45 (1.68) | 0.47% | |
| 40–44 | 1416 (1.60) | 817 (2.13) | 63 (2.35) | 0.71% | |
| 45–49 | 1770 (2.00) | 954 (2.49) | 81 (3.02) | 1.00% | |
| 50–54 | 3562 (4.03) | 2066 (5.40) | 114 (4.25) | 2.00% | |
| 55–59 | 4557 (5.16) | 2354 (6.15) | 158 (5.89) | 2.72% | |
| 60–64 | 6797 (7.70) | 3430 (8.96) | 196 (7.31) | 4.08% | |
| 65–69 | 9555 (10.82) | 4423 (11.56) | 303 (11.30) | 6.35% | |
| 70–74 | 16,078 (18.21) | 8222 (21.48) | 544 (20.29) | 13.20% | |
| 75–79 | 16,768 (18.99) | 7240 (18.92) | 518 (19.32) | 15.89% | |
| 80–84 | 12,104 (13.71) | 4011 (10.48) | 304 (11.34) | 16.27% | |
| 85+ | 13,097 (14.83) | 3053 (7.98) | 276 (10.29) | 14.63% | |
| Female n (% female) | Male n (% male) | Total n (%) | ||
|---|---|---|---|---|
| Medication | ||||
| Molnupiravir | 50,188 (67.18) | 38,128 (69.89) | 88,316 (68.32) | |
| Nirmatrelvir–ritonavir | 22,888 (30.64) | 15,382 (28.19) | 38,270 (29.61) | |
| Both medications | 1633 (2.19) | 1048 (1.92) | 2681 (2.07) | |
A ‘first-time prescription’ refers to individual patients who received a prescription (one or more) per medication group. ‘Both’ includes individual patients who received a prescription for one oral COVID-19 antiviral medication (molnupiravir or nirmatrelvir–ritonavir) and then, at a separate time during the study period, received a prescription for the other oral COVID-19 antiviral (molnupiravir or nirmatrelvir–ritonavir) i.e. had received prescriptions for ‘both’ oral COVID-19 antivirals.
Objective 1 – frequency and type of antiviral prescribing
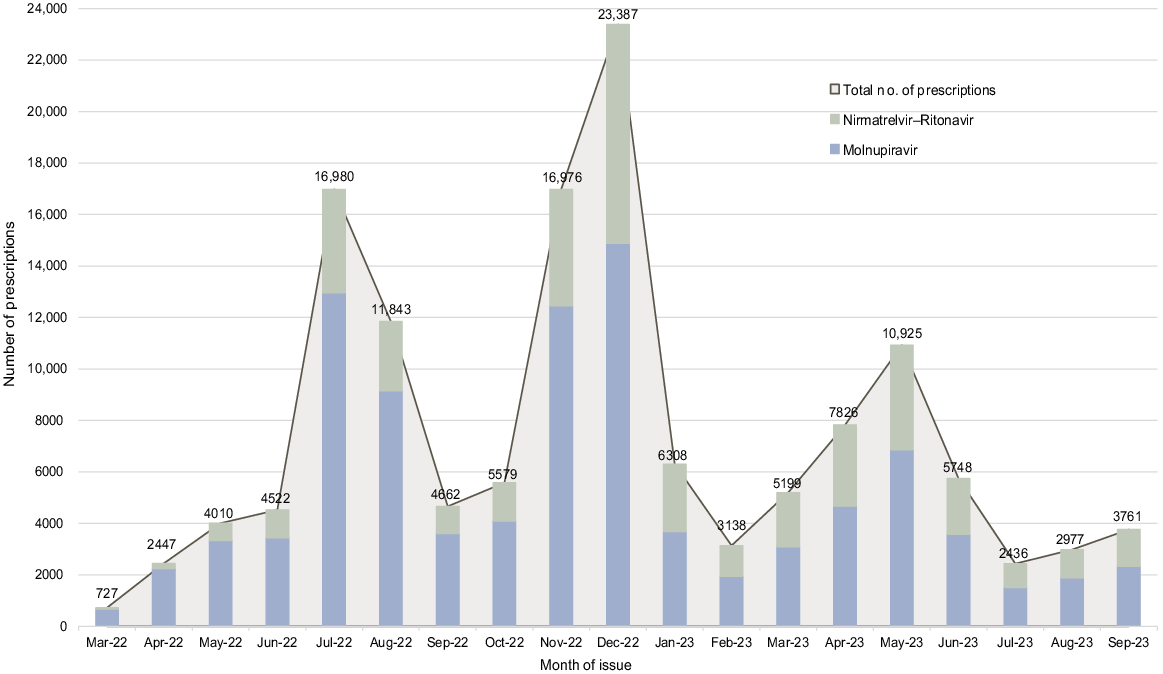
Within the total study population of 3,813,051 patients, 139,451 prescriptions for one or more oral COVID-19 antiviral medications were issued to 129,267 (3.39%) individual patients. The number of prescriptions issued per month is presented in Fig. 1. Of patients prescribed one or more oral COVID-19 antiviral medications, ‘first-time prescriptions’ were issued to 68.32% (88,316/129,267) of patients for molnupiravir only, 29.61% of patients (38,270/129,267) for nirmatrelvir–ritonavir only, and 2.07% (2681/129,267) received prescriptions for both medications. The number of prescriptions for molnupiravir exceeded nirmatrelvir–ritonavir across all months with prescribing peaking in December and July 2022. Over time, the relative proportion of prescribing of molnupiravir decreased with a concurrent increase in the proportion of nirmatrelvir–ritonavir prescribing.
Objective 2 – sociodemographic and health characteristics
The percentage of each age group who received one or more oral COVID-19 antiviral prescriptions by medication group is presented in Fig. 2. The highest percentage occurred in the 80–84 (16,419/100,911: 16.27%) and 75–79 (24,526/154,394 15.89%) year age groups with percentage prescribing increasing with age until a slight decrease for ages 85 years and above. There is a notable increase between ages 65–69 (14,281/224,825: 6.35%) to ages 70–74 years (24,844/188,141: 13.20%). Across all age groups the percentage of prescriptions for molnupiravir was higher than nirmatrelvir–ritonavir.
Percentage of the total study population in each age group prescribed a first-time oral COVID-19 antiviral by medication group – 1 March 2022–30 September 2023. Age groups 15–19, 20–24, and 25–29 years have been combined to meet data custodian ethics and governance requirements. The graph presents the percentage of the total study population (n = 3,813,051) in each age group who were prescribed a first-time oral COVID-19 antiviral medication by medication group (molnupiravir only, nirmatrelvir–ritonavir only or both medications). The percentage value on the top of each bar represents the total percentage within the age-group for the total population (i.e. the graph can be read as – ‘within the total study population, 16.27% of all persons aged 80–84 years received one or more prescriptions for an oral COVID-19 antiviral’).

Within the total study population, oral COVID-19 prescribing was proportionally higher in females (74,709/2,059,676: 3.63%) than males (54,558/1,753,375: 3.11%). Within the antiviral cohort of 129,267 patients, prescriptions were issued to 74,709 females (57.79%) and 54,558 males (42.21%). Results for prescribing by medication type and sex are presented in Table 2.
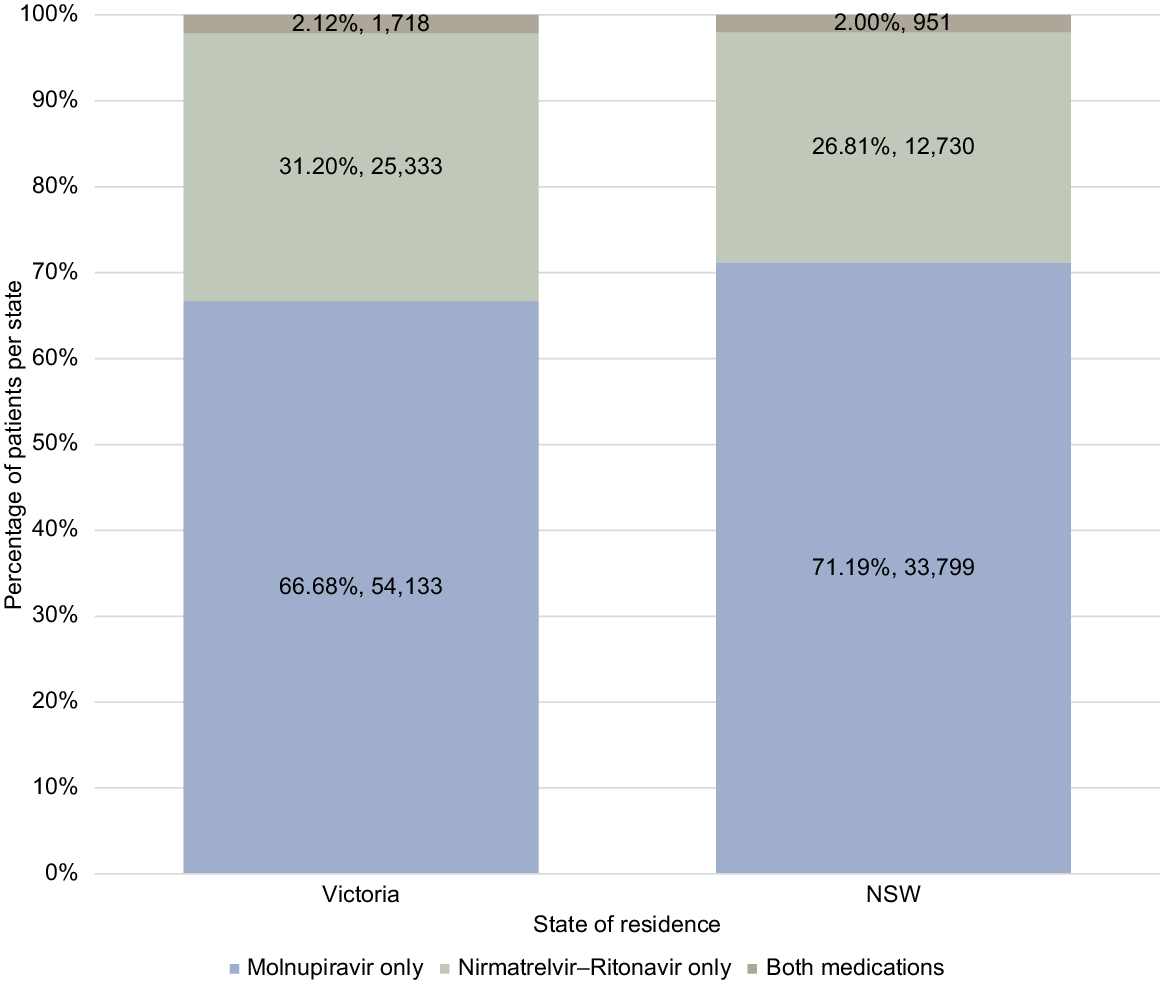
Within the total study population, oral COVID-19 prescribing was proportionally higher in the Victorian (81,184/2,222,837: 3.65%) than NSW population (47,480/1,553,109: 3.06%). Within the total antiviral cohort of 129,267 patients, 81,184 (62.80%) resided in Victoria, 47,480 (36.73%) in NSW, and 603 (0.47%) in other states or territories. Fig. 3 presents the results for NSW and Victoria by medication group demonstrating a slightly higher percentage of prescribing for molnupiravir in NSW and a higher percentage of nirmatrelvir–ritonavir prescribing in Victoria.
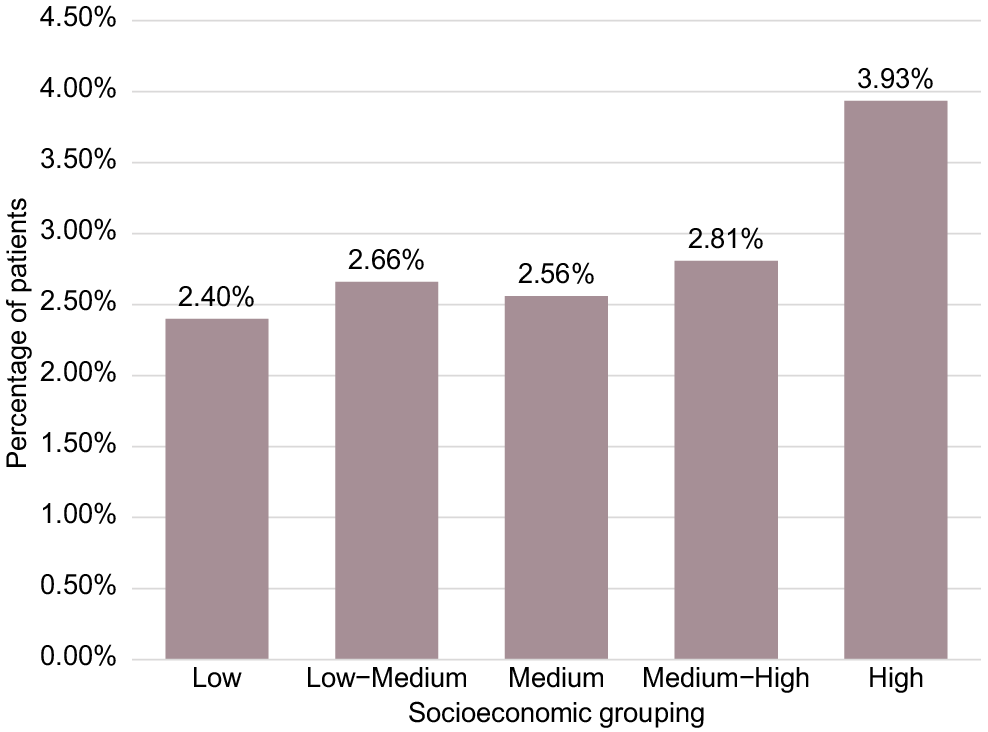
The socioeconomic profile of the total study population (Table 1) shows the population was predominantly from High (58.34%), Medium–High (17.83%), and Medium (15.01%) socioeconomic groups, with Low–Medium (0.81%) and Low (5.86%) under-represented in the study population. As such, all analyses are presented as proportions within each individual grouping rather than across the antiviral cohort. The percentage of oral COVID-19 antiviral prescribing within each socioeconomic grouping in the study population is presented in Fig. 4 (based on the results presented in Table 1), demonstrating the highest percentage prescribing in the High socioeconomic grouping (3.93%).
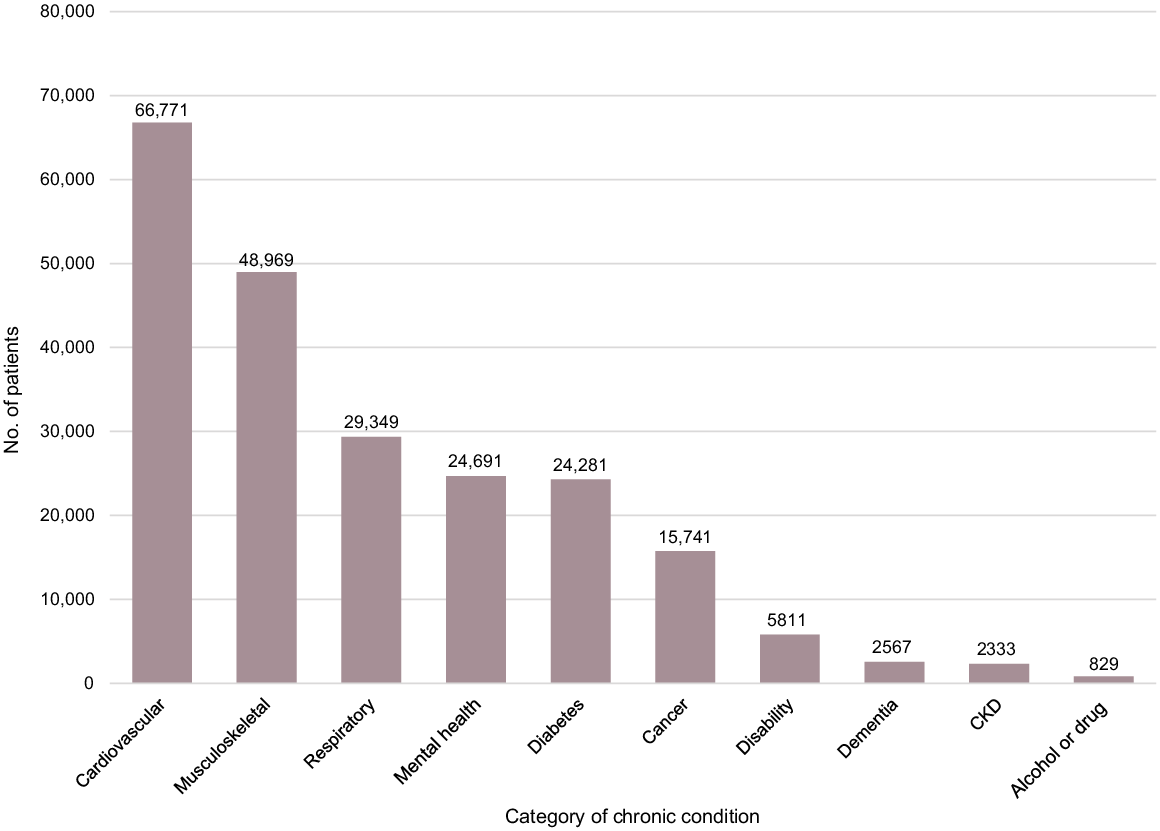
Within the antiviral cohort, 79.39% (102,631/129,267) of patients had one or more chronic conditions documented in their records. The top 10 chronic conditions for these patients are presented in Fig. 5 with cardiovascular, musculoskeletal, and respiratory conditions the three most common categories of chronic conditions.
Top 10 chronic conditions in patients prescribed oral COVID-19 antiviral medications – 1 March 2022–30 September 2023. CKD, Chronic Kidney Disease. Note the graph totals exceed the number of patients in the antiviral cohort as it is possible for a single patient to have multiple chronic conditions.
Objective 3 – repeated prescribing
Within the antiviral cohort, 92.73% (119,865/129,267) of patients were prescribed an antiviral once only with 7.27% (9402/129,267) having repeat prescribing. The number and percentage of patients receiving repeat oral COVID-19 prescriptions are presented in Fig. 6. The median time between repeat prescriptions was 197 days (IQR: 119–289) and the mean time was 204.89 days (s.d.: 117.12, range 6–547).
Number of oral COVID-19 antiviral prescriptions per patient in the antiviral cohort, during the study period – 1 March 2022–30 September 2023. A repeated prescription for oral COVID-19 antivirals is defined as a prescription issued 6 or more days after a previous prescription (see Methods for detailed explanation). Numerical values included on the graph bars are the number (n) and percentage (%) for each category.

Objective 4 – telehealth
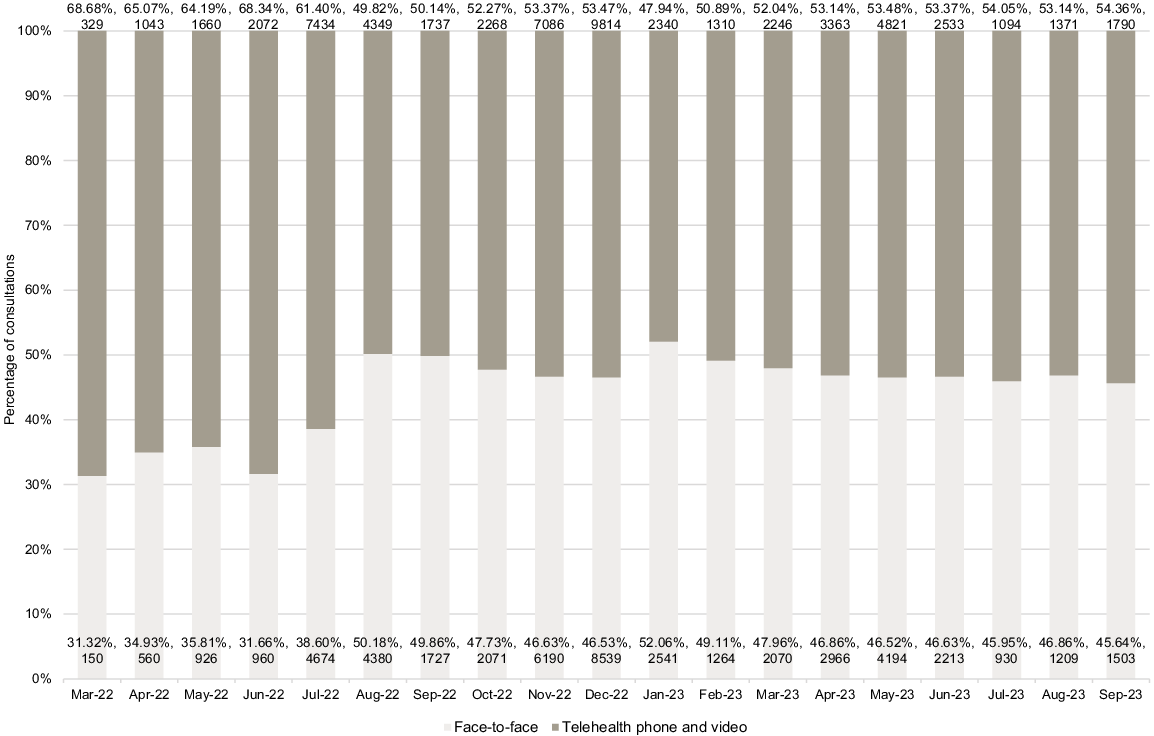
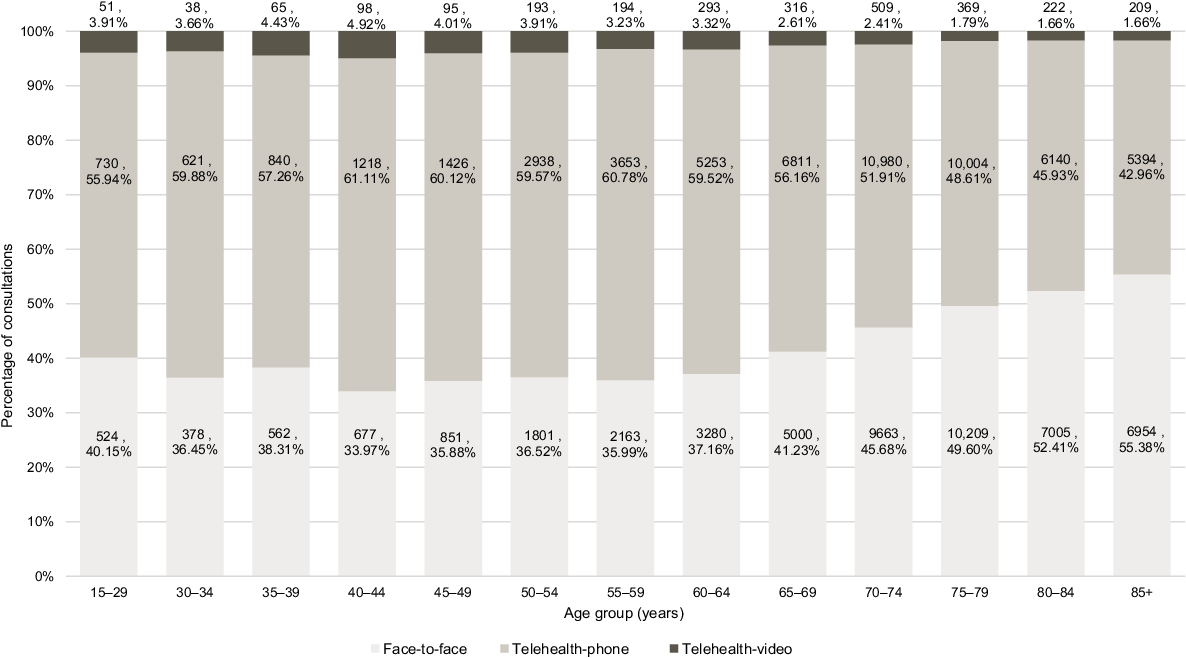
Consultation modality data was available for 107,727 consultations during which an oral COVID-19 antiviral was prescribed including 49,067 (45.55%) face-to-face and 58,660 (54.45%) telehealth (comprising 56,008 telehealth-phone and 2652 telehealth-video) consultations. Consultation modality by age group is presented in Fig. 7 demonstrating telehealth modalities (phone–video combined) exceeded face-to-face consultations for all age groups under 80 years. Telehealth utilisation by month is presented in Fig. 8 demonstrating higher use of telehealth in the earlier months of March–July 2022 and then levelling out with telehealth modalities continuing to exceed face-to-face all months from February 2023 onwards.
Percentage of consultations with an oral COVID-19 antiviral prescribed by modality and age group – 1 March 2022–30 September 2023. Numerical values included on the graph bars are the number (n) and percentage (%) for each group. The graph can be read as e.g. ‘of all persons aged 70–74 years who received at least one prescription for an oral COVID-19 antiviral, 51.91% of consultations were via telephone’. Age groups 15–19, 20–24, and 25–29 years have been combined to meet data custodian ethics and governance requirements.
Discussion
This large general practice observational study of 3,813,051 patients determined 3.39% of all patients and 14.79% of patients aged ≥70 years received at least one prescription for an oral COVID-19 antiviral between March 2022 and September 2023. Molnupiravir prescribing exceeded nirmatrelvir–ritonavir across all months, with prescribing volumes peaking in July and December 2022 and May 2023. Proportionally, oral COVID-19 antiviral prescribing was higher in the female population, Victoria (vs NSW), higher socioeconomic deciles, and ages 80–84 years. Within the antiviral cohort, 7.27% had repeat prescribing and 79.39% had chronic conditions, with cardiovascular and musculoskeletal conditions the most prevalent chronic diagnoses. Telehealth (phone or video) was widely utilised for prescribing oral COVID-19 antivirals and exceeded face-to-face consultations overall.
Temporal patterns of oral COVID-19 antiviral prescribing in our study align with an Australian study and government PBS dispensing graphs which demonstrate pharmacy dispensing peaks in July and December 2022 (Australian Government Department of Health and Aged Care 2024; Lopez et al. 2024) and May 2023 in both states (Australian Government Department of Health and Aged Care 2024), with the July peak occurring during the third Omicron wave in Australia (COVID-19 Epidemiology and Surveillance Team 2023). Epidemiological reporting of confirmed and probable COVID-19 cases indicate the fourth and fifth Omicron waves peaked during December 2022 and May 2023 in Australia (COVID-19 Epidemiology and Surveillance Team 2023), also corresponding with the prescribing peaks in our study. A US study of the association between oral COVID-19 antiviral dispensing and subsequent COVID-19 case numbers found a strong correlation noting, however, that this may underestimate case numbers in non-high-risk populations (Kaul et al. 2023). These findings suggest that, in the absence of mandatory reporting, oral COVID-19 antiviral prescribing volumes in general practice could serve as an indicator of periods of increased transmission of COVID-19.
Our temporal data also provide unique insight into nirmatrelvir–ritonavir prescribing in the months prior to the medication being listed on the PBS (270 prescriptions), reflecting patients who received non-subsidised ‘private prescriptions’ (Australian Government Department of Health and Aged Care 2023) requiring payment of the full price of the medication (~A$1000 (Sheppeard 2022)) if dispensed. In our study, molnupiravir prescribing exceeded nirmatrelvir–ritonavir across all months and age groups. Higher proportions of molnupiravir compared to nirmatrelvir–ritonavir treatment have also been reported in the literature (Lopez et al. 2024), in Victorian studies (Van Heer et al. 2023; Clarke et al. 2024), and is reflected in PBS monthly data for NSW–Victoria combined with molnupiravir items processed exceeding nirmatrelvir–ritonavir between May 2022 and September 2023 (Australian Government – Services Australia 2024). Our findings contrast with a South Australian hospital study in August 2022 that reported 19/49 patients receiving nirmatrelvir–ritonavir and 7/49 receiving molnupiravir (Al Naji et al. 2024). The context of the healthcare setting is likely an important factor for prescribing, with our results specifically reflecting patients contacting or presenting to general practice with non-severe COVID-19. Our findings may also reflect changes to prescribing criteria over time as Fig. 1 demonstrates the proportion of molnupiravir prescriptions decreasing over time with a concurrent increase in nirmatrelvir–ritonavir. The temporal changes in prescribing patterns likely reflect changes in prescribing guidance and eligibility criteria which took place in July and November 2022 and January, April, and July 2023 (Allard et al. 2023; Australian Government Department of Health and Aged Care 2023).
Consistent with eligibility criteria (Australian Government Department of Health and Aged Care 2022a, 2022b, 2023), our study found an increased proportion of prescribing in patients in older age groups and a high proportion of patients receiving oral COVID-19 antivirals having chronic conditions. Determining the most prevalent categories of chronic conditions provides insight into the health characteristics of patients seeking antiviral treatment. Higher proportions of prescribing for females and high socioeconomic advantage in our study echo findings from Victorian research, including a study of oral COVID-19 antiviral effectiveness in ages ≥70 years which reported adults who received treatment were more likely female and from higher socioeconomic areas (Van Heer et al. 2023), and a report of a 15% lower likelihood of oral antiviral treatment when comparing lowest to highest indices of relative socio-economic disadvantage (Allard et al. 2023). The consistency of these findings affirm the need for better understanding of the barriers and facilitators (Clarke et al. 2024) to oral COVID-19 antiviral uptake in adults from lower socioeconomic areas.
Within our antiviral cohort 7.27% of patients had repeated oral COVID-19 antiviral prescribing. The duration between repeat prescribing (range: 6–547 days) suggests that prescribing may have occurred not only for re-infections but also for ongoing infections. As repeat prescribing represents eligible patients who actively sought treatment, it can’t be used as a proxy measure of re-infection rates, however, it does provide evidence of the need for repeat prescribing and the time span between repeat or continuing infections.
Telehealth utilisation within the antiviral cohort exceeded face-to-face overall, across most months and in age groups <80 years, with telehealth-phone far exceeding telehealth-video. This low uptake of telehealth-video is consistent with published literature on telehealth use across many aspects of Australian general practice (Imai et al. 2022; Wabe et al. 2022; Snoswell et al. 2024). Telehealth utilisation in our study exceeds the overall percentage of telehealth consultations in Australian general practice reported at 20% in 2023 (Snoswell et al. 2024). In comparison, studies of prescribing via telehealth in NSW and Victorian general practice have found higher rates of medication prescribing in face-to-face than telehealth consultations during 2020 (Wabe et al. 2022), and initially higher antibiotic prescribing for respiratory conditions via face-to-face with telehealth prescribing rates increasing between April 2020 and November 2021 (Imai et al. 2022). Our results therefore suggest that prescribing for SARS-CoV-2 infections may be particularly well suited to telehealth, especially given the time-critical criteria for commencing treatment (Australian Government Department of Health and Aged Care 2022a, 2022b). Our telehealth findings strongly support the need for continued access to telehealth for COVID-19 positive patients as an integral model of care for the delivery of primary care services, by allowing primary health care teams to provide time critical health care while reducing their exposure to the SARS-CoV-2 virus.
Limitations
This study may not be generalisable beyond the Australian general practice setting, or be reflective of other authorised community (non-hospital) prescribers of COVID-19 antivirals. Our data reflect prescriptions issued in the included general practices which may or may not have been dispensed, thus reflecting increased incidence of COVID-19 infection leading to increased prescribing of oral COVID-19 antiviral medicines. Although our comparison cohort did not receive oral COVID-19 antiviral prescriptions from included general practices, it is possible that patients may have been prescribed COVID-19 treatment in other hospital or healthcare settings, in which case our results would underestimate the need for treatment. Our study included health characteristics of patients at the time of receiving oral COVID-19 antiviral prescriptions, however, vaccination status, severity of infection, and patient outcomes were beyond the scope of the study. Our study is reflective of prescribing that occurred during 2022–2023, however, as prescribing criteria were updated in 2024, our results may not be reflective of current prescribing activity. At the time of writing, there was no off-label use for molnupiravir or nirmatrelvir–ritonavir which, combined with the large study population spanning over 18 months, strengthens the study findings.
Conclusion
General practice will have a continuing role in prescribing oral COVID-19 antivirals to eligible patients. Increases in prescribing activity in general practice have corresponded with periods of increased confirmed and probable COVID-19 case notifications. Telehealth utilisation has been important for prescribing oral COVID-19 antivirals, particularly given the time-sensitive need for actively infected patients to access treatment. Continued access to telehealth and a better understanding of barriers to treatment in lower socioeconomic groups will be important considerations in continuing to prescribe oral COVID-19 antivirals.
Data availability
The de-identified data used in this study are not publicly available and cannot be shared as the individual collaborator Primary Health Networks are the owners of their data.
Declaration of funding
This study was supported by a Digital Health CRC Limited (‘DHCRC’) grant (DHCRC-0202). DHCRC is funded under the Commonwealth’s Cooperative Research Centres (CRC) Program.
Acknowledgements
The authors acknowledge and thank Central and Eastern Sydney Primary Health Network (PHN), South Western Sydney PHN, South Eastern Melbourne PHN, and Eastern Melbourne PHN, for their collaboration and contributions to the research.
References
Al Naji H, Inglis JM, Tucker E, Rowett D, Larcombe R, Medlin S, Mangoni AA, Thynne T (2024) Prescribing of antivirals for COVID-19 in a South Australian local health network according to statewide guidelines. Internal Medicine Journal 54(1), 183-186.
| Crossref | Google Scholar |
Allard NL, Canevari J, Haslett N, Cowie BC (2023) Access to oral COVID-19 antivirals in the community: are eligibility criteria and systems ensuring equity? Medical Journal of Australia 218(10), 438-441.
| Crossref | Google Scholar | PubMed |
Andrews HS, Herman JD, Gandhi RT (2024) Treatments for COVID-19. Annual Review of Medicine 75, 145-157.
| Crossref | Google Scholar | PubMed |
Australian Bureau of Statistics (2021a) Search census data. Available at https://abs.gov.au/census/find-census-data/search-by-area
Australian Bureau of Statistics (2021b) Socio-Economic Indexes for Areas (SEIFA), Australia. Available at https://www.abs.gov.au/statistics/people/people-and-communities/socio-economic-indexes-areas-seifa-australia/latest-release
Australian Government Department of Health and Aged Care (2022a) Factsheet – Covid 19 treatment – Lagevrio® (molnupiravir) PBS listing (updated 6 March 2024). Available at https://www.pbs.gov.au/publication/factsheets/covid-19-treatments/PBS-Factsheet-lagevrio-molnupiravir-March-2024.pdf
Australian Government Department of Health and Aged Care (2022b) Factsheet – Covid 19 treatment – Paxlovid® (nirmatrelvir and ritonavir) PBS listing (updated 6 March 2024). Available at https://www.pbs.gov.au/publication/factsheets/covid-19-treatments/PBS-Factsheet-paxlovid-nirmatrelvir-and-ritonavir-March-2024.pdf
Australian Government Department of Health and Aged Care (2023) COVID-19 Oral Treatments – Factsheet. Available at https://www.health.gov.au/sites/default/files/2023-01/covid-19-oral-treatments-fact-sheet.pdf
Australian Government Department of Health and Aged Care (2024) COVID-19 reporting: COVID-19 treatments. Available at https://www.health.gov.au/topics/covid-19/reporting#covid19-treatments
Australian Government Department of Health and Aged Care – MBS Online (2022) Assessment for a COVID-19 oral anti-viral medication. Available at https://www.mbsonline.gov.au/internet/mbsonline/publishing.nsf/Content/Factsheet-Anti-Virals-C19
Australian Government – Services Australia (2024) Pharmaceutical benefits schedule item reports. Available at http://medicarestatistics.humanservices.gov.au/statistics/pbs_item.jsp
Clarke N, O’Keeffe J, Yerramilli A, Bartolo C, Mothobi N, Muleme M, McNamara B, O’Brien D, Athan E, Hussain MA (2024) Use of targeted SMS messaging to encourage COVID-19 oral antiviral uptake in South West Victoria. Public Health Research & Practice 34, e33342309.
| Crossref | Google Scholar |
COVID-19 Epidemiology and Surveillance Team (2023) COVID-19 Australia: epidemiology report 79: reporting period ending 24 September 2023. Communicable Diseases Intelligence 47, 1-22.
| Crossref | Google Scholar |
Imai C, Amin J, Prgomet M, Pearce C, Georgiou A (2022) An increase in antibiotic prescribing for respiratory tract infections through telehealth consultations: retrospective study in Australian general practice. Journal of Medical Internet Research 24(10), e40876.
| Crossref | Google Scholar |
Kaul CM, Cohen GM, Silverstein M, Wallach AB, Diago-Navarro E, Holzman RS, Foote MK (2023) Understanding the relationship between antiviral prescription data and COVID-19 Incidence in New York City: a retrospective cohort study. Open Forum Infectious Diseases 10(6), ofad281.
| Crossref | Google Scholar |
Lopez D, Pritchard D, Sanfilippo FM, Kelty E, Page A, Etherton-Beer C, Almeida OP, Preen DB (2024) Supply of nirmatrelvir/ritonavir and molnupiravir for patients with COVID-19 in the first eight months since listing on the Australian Pharmaceutical Benefits Scheme: a retrospective observational study. Infectious Diseases Now 54(6), 104953.
| Crossref | Google Scholar |
NSW Health (2022) Remember lessons learned as COVID-19 isolation ends. Available at https://www.health.nsw.gov.au/news/Pages/20221012_00.aspx
Paraskevis D, Gkova M, Mellou K, Gerolymatos G, Psalida N, Gkolfinopoulou K, Kostaki E-G, Loukides S, Kotanidou A, Skoutelis A, Thiraios E, Saroglou G, Zografopoulos D, Filippou D, Mossialos E, Zaoutis T, Gaga M, Tsiodras S, Antoniadou A (2023) Real-world effectiveness of Molnupiravir and Nirmatrelvir/Ritonavir as treatments for COVID-19 in patients at high risk. The Journal of Infectious Diseases 228(12), 1667-1674.
| Crossref | Google Scholar | PubMed |
Pearce C, McLeod A, Rinehart N, Ferrigi J, Shearer M (2019) What a comprehensive, integrated data strategy looks like: the Population Level Analysis and Reporting (POLAR) program. Studies in Health Technology and Informatics 264, 303-307.
| Crossref | Google Scholar | PubMed |
Sheppeard A (2022) Private antivirals for covid: what GPs need to know. The Medical Republic. Available at https://www.medicalrepublic.com.au/private-antivirals-for-covid-what-gps-need-to-know/7897
Snoswell CL, Caffery LJ, Taylor ML, Mendis R, Haydon HM, Thomas E, Smith AC, Centre for Online Health The University of Queensland (2024) Telehealth and coronavirus: Medicare Benefits Schedule (MBS) activity in Australia. Available at https://coh.centre.uq.edu.au/telehealth-and-coronavirus-medicare-benefits-schedule-mbs-activity-australia
The Hon Daniel Andrews MP Premier (2022) Media release – changes to pandemic management. Available at https://www.premier.vic.gov.au/sites/default/files/2022-10/221007%20-%20Changes%20To%20Pandemic%20Management.pdf
Van Heer C, Majumdar SS, Parta I, Martinie M, Dawson R, West D, Hewett L, Lister D, Sutton B, O’Brien DP, Cowie BC (2023) Effectiveness of community-based oral antiviral treatments against severe COVID-19 outcomes in people 70 years and over in Victoria, Australia, 2022: an observational study. The Lancet Regional Health–Western Pacific 41, 100917.
| Crossref | Google Scholar |
Wabe N, Thomas J, Sezgin G, Sheikh MK, Gault E, Georgiou A (2022) Medication prescribing in face-to-face versus telehealth consultations during the COVID-19 pandemic in Australian general practice: a retrospective observational study. BJGP Open 6(1), BJGPO.2021.0132.
| Crossref | Google Scholar |
World Health Organization (2023) Therapeutics and COVID-19: living guideline, 10 November 2023. Available at https://www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2023.2