A noodle in a haystack: determining the conservation status of the rare and Data Deficient Ravensthorpe Range slider, Lerista viduata
Luke R. Bonifacio A * , Jules E. Farquhar A , Arman N. Pili B , Jessica C. Walsh A and David G. Chapple
A * , Jules E. Farquhar A , Arman N. Pili B , Jessica C. Walsh A and David G. Chapple  A
A
A
B
Abstract
One-seventh of the ~157,000 species assessed by the IUCN Red List of Threatened Species are Data Deficient (DD), with insufficient information to assess their extinction risk. Such a statistic is concerning from a conservation perspective because more than half are predicted to be threatened by extinction, yet they are generally neglected from conservation priority.
Here, we aimed to improve ecological knowledge, and inform the conservation status of, the DD Ravensthorpe Range slider (Lerista viduata), a historically rare skink confined to Western Australia’s Ravensthorpe Range.
A detailed framework was developed to improve data on attributes integral to the species’ IUCN Red List assessment (e.g. distribution, threats, population size): collation of historical records, fieldwork within and around its known range, preserved specimen analysis, on-ground and spatial analysis of threats, and inference from ecologically similar species.
We found that L. viduata is threatened under multiple IUCN Red List criteria (B1ab[i,ii,iii,v], B2ab[i,ii,iii,v], C2ab[ii], D2), and overall should be considered Critically Endangered. This status is based on its Extent of Occurrence (32 km2) being <100 km2, occurrence at one location (defined by the threat of fire), and an inferred continuing decline in its distribution and habitat parameters. The species’ small estimated population size (3,514–9,276 mature individuals) also renders it extinction prone.
We demonstrate that L. viduata, long perceived as DD, should be reclassified as Critically Endangered, and is of the utmost conservation concern.
Our study reiterates the need for DD species to receive greater consideration in conservation research and action.
Keywords: Critically Endangered, ecological knowledge, extinction risk, IUCN Red List, reptiles, skink, threatened, Western Australia.
Introduction
A lack of ecological knowledge is often a major hinderance to undertaking effective conservation (Whittaker et al. 2005). Understanding a species’ distribution, ecology, population size, and threats is fundamental to evaluating its extinction risk and conservation status, and deficiencies in such information can make confident assignment to a threat category unattainable. In such situations, the species would be classified as Data Deficient (DD) (IUCN 2024a) by the IUCN Red List of Threatened Species (hereafter: IUCN Red List), the accepted leading global authority in assessing biodiversity extinction risk (Rodrigues et al. 2006; Mace et al. 2008). Currently, one in seven IUCN Red List assessed species, including 8,389 vertebrates, are classified as DD (IUCN 2024b). The IUCN recommends that DD species are afforded the same level of conservation attention as threatened classified taxa (i.e. those listed as Critically Endangered, Endangered or Vulnerable) because they may be threatened (IUCN 2024a); however, this guidance is rarely followed due to funding constraints and uncertainty about them being a worthy conservation investment, thus making them a lower management priority (Morais et al. 2013; Parsons 2016; Bland et al. 2017). For example, Target 4 of the Kunming-Montreal Global Biodiversity Framework in part seeks to ‘…halt human induced extinction of known threatened species…’ (i.e. not DD) (Convention on Biological Diversity 2022, p. 17), while the IUCN’s (2023) Global Species Action Plan, which supports implementation of the Framework, does not mention DD species as a conservation action priority. These are highly problematic from a conservation perspective, as more than half of DD species are predicted to be threatened by extinction (Borgelt et al. 2022). Furthermore, evidence suggests that they are more threatened than their data-sufficient counterparts (Bland et al. 2015; Gumbs et al. 2020; Caetano et al. 2022). Neglecting such species from conservation priority may mean they slip unnoticed towards extinction, or become so imperilled that recovery becomes time and resource intensive (Bland et al. 2015, 2017). Thus, improving ecological knowledge about DD species and assigning them to a specific conservation status, is of critical importance in current day conservation.
Of all terrestrial vertebrate groups, reptiles have the most DD classified species on the IUCN Red List (1,492 of 3,280 species; IUCN 2024b). Such a statistic reflects the relative paucity of ecological data available for reptiles generally (e.g. on population sizes, threats, distribution, life-history), the comparative lack of research attention they have received, and explains in part why many reptile species are typically neglected from global conservation prioritisation (Bland and Böhm 2016; Böhm et al. 2016; Tingley et al. 2016). Reptile diversity is highest in Australia, where it is predominantly made up by an enormous number of skinks (Squamata: Scincidae), 17 species of which are DD (Wilson and Swan 2021; IUCN 2024b). There are genuine concerns that numerous DD Australian skinks may indeed be threatened with extinction, with many being rare, range-restricted and not studied since their description multiple decades ago (Meiri et al. 2018; Chapple et al. 2019). Thus, it is essential that such species are afforded ample attention in conservation research. Recent field-based studies on two previously DD-listed Australian skinks, Lampropholis elongata (long sunskink) and Pseudemoia rawlinsoni (glossy grass skink), validate the importance of such a focus, with the resultant data invoking new conservation statuses for both species (Critically Endangered and Vulnerable, respectively) and increased attention from conservation authorities (DCCEEW 2023; Graham et al. 2023; Farquhar et al. 2024). However, far more species warrant similar consideration.
The Ravensthorpe Range slider (Lerista viduata) is one such species. This small (adult snout-vent length 47 mm), fossorial DD skink (Teale et al. 2017) has seldom received research interest despite being an accepted species since Storr’s (1991) description over 30 years ago. The species may well be threatened, having attributes that resemble those of threatened taxa; it is cryptic, rarely sighted, likely impacted by multiple threats and heavily range-restricted (Area of Occupancy [AOO] = 24 km2) (Teale et al. 2017; Chapple et al. 2019). Indeed, it has only been found at two sites, which are approximately 20 km apart, in the Ravensthorpe Range of southern Western Australia: (1) the south-western slopes of Mount McMahon; and (2) in the vicinity of the old Kundip township (Fig. 1). Only 15 specimens have ever been collected for the species, reflecting its rare nature; indeed, herpetologists and consultants typically report finding few, or sometimes no individuals after hours or even days of surveying (Biota Environmental Sciences 2004; B. Maryan and R. Lloyd pers. comm.). Such limited sightings have inhibited understandings of its ecology; current scientific knowledge does not extend beyond morphological descriptions of the type specimen (Storr 1991) and general notes on habitat preferences (i.e. under leaf litter in eucalypt or mallee woodlands on loam or clay loam soils; Teale et al. 2017; Chapple et al. 2019; Wilson and Swan 2021). Although threats have never been quantified, both fire and mining likely pose significant concern, with fires being prominent across the Ravensthorpe region over the past 50 years (DBCA 2023a) and an active mine currently operating atop the species’ historical range at Kundip (EPA 2020; Minister for Environment 2020). The species is considered a Priority 1 species in WA, meaning it occurs in areas not currently managed for conservation and urgently requires further survey (DBCA 2023b). Thus, it is unlikely to be receiving adequate consideration in conservation management. Ultimately, if sufficient data were available to assess the conservation status of L. viduata, it would quite possibly fit the criteria for a Threatened listing.
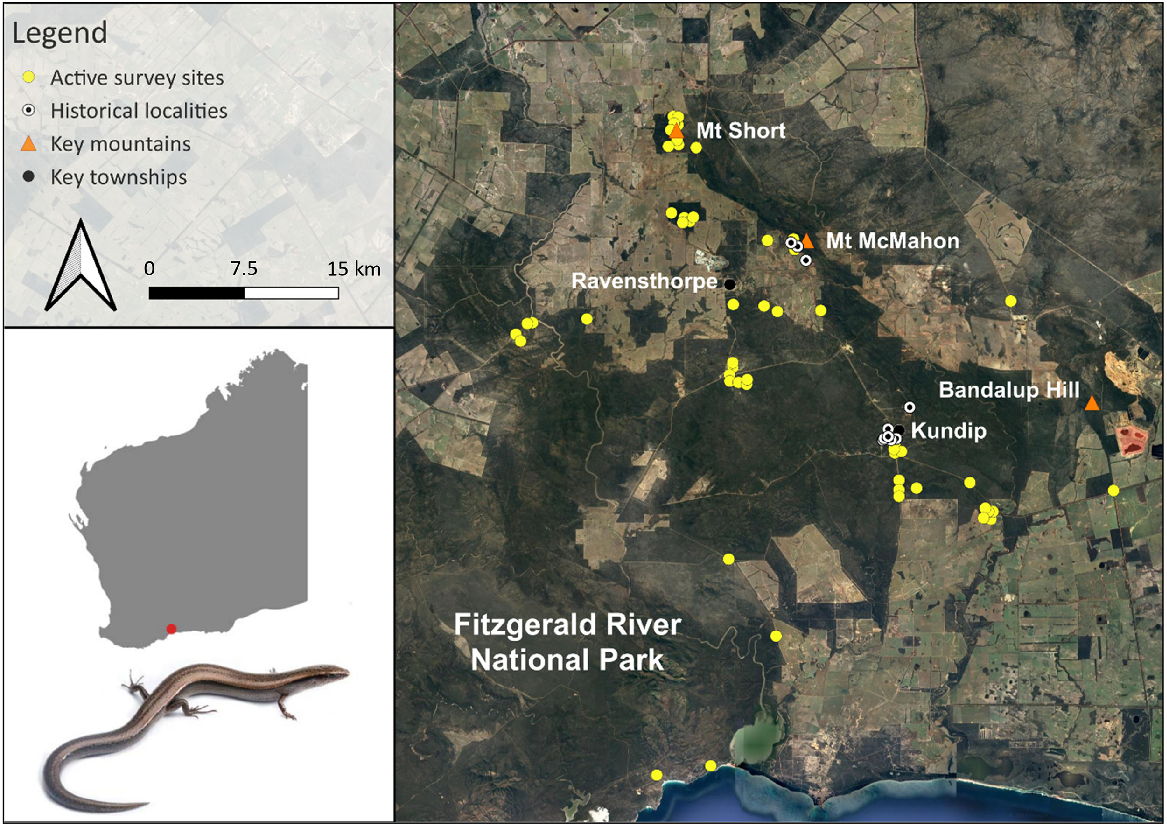
Locations of active survey sites during this study, historical sites for Lerista viduata and key mountains and townships within and around the Ravensthorpe area of Western Australia. Photograph of L. viduata by Jules Farquhar.
Here, new data are presented on L. viduata, with the aim of assigning the species to an IUCN Red List conservation status. Specifically, we provide new information on the species’:
Distribution, by conducting presence-absence surveys across the Ravensthorpe region, and collating new occurrences of the species with its historical vetted point records. Such an approach enables for an updated calculation of the species’ Extent of Occurrence (EOO) and AOO, which are essential metrics for its assessment under IUCN Red List Criterion B (Geographic Range Size). We expect that the species will be range-restricted and fit the EOO and AOO thresholds for threatened.
Habitat preferences, by assessing how several biotic and abiotic environmental variables differ between the species’ presence and absence sites. Aside from providing valuable insights into the species’ ecology, such information will assist in assessing L. viduata under IUCN Red List Criterion A (Population Size Reduction), B and E (Quantitative Analysis) by informing its potential threats (i.e. if a particular threat causes declines in its habitat, then it may also impact the species). We hypothesise that the species will prefer a specialised habitat within the Ravensthorpe Range.
Morphology, life-history and biology, by examining field-caught individuals, its 15 museum specimens and drawing inference from data on ecologically similar species. Life-history data will enable for calculations of the species’ generation length, which is needed for its assessment under Criterion A and E, while data on its morphology and biology contribute to enhancing scientific understandings of it. We expect the species will have a generation length of ~3 years, as is typical in small fossorial lizards.
Population size (i.e. number of mature individuals), by multiplying the estimated adult population density of the species by the area of its range. Such an estimate is integral to the species’ assessment under Criterion C (Small Population Size and Decline) and D (Very Small or Restricted Population). We anticipate the species will have a population size of 1,000–10,000 mature individuals, thus meeting the first sub-criterion threshold for threatened under Criterion C, but not Criterion D.
Threatening processes, by reviewing literature to assess the likely impact of several threats on the species, while also conducting on-ground assessments of whether fire degrades its habitat preference. Such information assists with the species’ assessment under all IUCN Red List criteria, but particularly Criterion B and D, by enabling calculation of how many locations L. viduata occurs at (i.e. the number of locations under the IUCN is based on the spatial extent of threats).
Materials and methods
Study area
The field-based component of this study was conducted between 28 May and 8 July 2023, which involved undertaking active surveys within and around the Ravensthorpe Range (within ~40 km) in Western Australia (Fig. 1). The Range is located ~550 km south-east of Perth and runs ~40 km in a narrow, linear shape from Mt Short in the north-west to Bandalup Hill in the south-east (Fig. 1). It is a floristically diverse region (1,418 plant species; Craig 2008) dominated generally by mallet eucalypt, mallee-heath and shrubland ecosystems (Beard 1973; Craig et al. 2008); however, mining and agricultural activities have cleared significant areas of vegetation since the early 1900s (Harris et al. 2008; Markey et al. 2012). Just two of the Range’s many reserves, Overshot Hill Nature Reserve and Kundip Nature Reserve, are protected (CAPAD 2022). The area is characterised by a warm Mediterranean climate with cool wet winters yet warm to hot summers (Harris et al. 2008), and receives an average of 426 mm annual rainfall (Bureau of Meteorology 2024). Permitted survey areas for this study covered varying topographies and habitat types across the region and partially encompassed both historical areas for the species (see Supplementary Fig. S1).
Distribution assessment
To better understand the species’ distribution, 53 time-constrained active surveys (i.e. presence-absence surveys), each at different sites, were conducted across the study area (Fig. 1). Six were within the accessible historical zones, so as to confirm the species’ persistence in those areas, while remaining survey sites encompassed the region’s various ecosystems over an extensive expanse of the landscape (Fig. 1). Such an approach enabled for a detailed assessment of whether the species occurs beyond its known range, which is critical for more accurately assessing its status under IUCN Red List Criterion B. All active surveys consisted of two people (LRB and either JEF, JFA or NPG; see ‘Acknowledgements’) traversing habitat for 2 h each, looking beneath rocks, logs, spoil heaps, dead mallee roots, metallic debris, within stick ant nests and raking leaf litter mats with 3-pronged cultivators. Time-constrained searches were favoured over area-constrained approaches because they typically increase the detectability of species and individuals at sites, particularly cryptic ones (i.e. they focus on careful, detailed searches of the available microhabitats and resources, whereas area-constrained searches occur in fixed spaces with potentially limited resources available for cryptic species. Thus, they may avoid detection; Kadlec et al. 2012; Jithin et al. 2023). Each active survey focused on one specific habitat type. Overall, presence data from our active surveys were combined with historical records for the species (Tables S1 and S2) to reassess its EOO (calculated from a minimum convex hull around all vetted point records) and AOO (4 km2 rectangular grids around occurrence points) using GeoCAT (Bachman et al. 2011). All other reptiles identified during our surveys were also recorded (Table S3).
We also attempted to predict the species’ distribution using the Maximum Entropy ENM algorithm (herein ‘MaxEnt’; Phillips et al. 2006). MaxEnt is a regression-based modelling algorithm that predicts suitable habitat for a species across a defined area, even with small sample sizes, by contrasting environmental conditions at occupied sites (i.e. presence locations) with potentially unoccupied areas (i.e. locations of the environmental background). We constrained our modelling domain to the area in Fig. 1, so as to estimate the species’ habitat suitability within and immediately surrounding the Ravensthorpe Range. Then, by examining several biotic and abiotic rasters relevant to L. viduata (Table S4) in a range of combinations, we generated several models of the species’ distribution at a ~1-arc second (30 m × 30 m) resolution, with MaxEnt parameters (i.e. randomisation multiplier, feature class) generated in R software ver. 4.3.1 (R Core Team 2023) using the ‘ENMevaluate’ function in the package ENMeval (ver. 2.0.3; Muscarella et al. 2014; Kass et al. 2021). All generated models suggested that the species was widespread across the modelling domain, including areas within and well beyond the Ravensthorpe Range, and thus that it occurs in most ecosystems across the landscape. However, such outcomes have been interpreted as major overpredictions, for the following reasons: (1) L. viduata prefers a highly specialised, dual habitat combination in the Ravensthorpe Range (see ‘Results’ section) for which specific spatial data were unavailable; thus, it could not be accounted for in modelling (i.e. models accounting for such data, if available, would likely predict a more restricted distribution for the species, given the specialised nature of the habitat and it not being widespread across the Ravensthorpe region). Indeed, insufficient data availability on biotic factors, including species’ habitat preferences, is known to be a key driver of distribution model overpredictions, and uncertain or inaccurate estimations (Peterson et al. 2011; Wisz et al. 2013; La Marca et al. 2019; De Kort et al. 2020; Velazco et al. 2020); and (2) we only recorded absences for the species in most areas predicted as suitable for it. As such, an ecological niche model for L. viduata was deemed unsuitable to include in this study.
Habitat assessments and analysis
Each active survey site was classified into one of six general habitat categories reflecting the different environments across the study area: (1) mallet woodlands; (2) burned woodland and shrubland (i.e. burned ~4 months prior); (3) mallee with open shrubland; (4) mallee with dense shrubland; (5) low shrubland; and (6) tall shrubland (Fig. S2). At each site, the following variables were recorded along a 100-m transect: ground-level cover (GLC) and vertical structure (both recorded every 1 m), litter depth (mm, every 10 m), and soil compactness, canopy height and canopy openness (all three every 20 m). GLC types included leaf litter, rocks, logs, coarse woody debris (sticks 0.3–1 cm width, bark), fine woody debris (sticks <0.3 cm width), bare ground, and shrubs. We classified vertical structure as the number of vegetation touches within three segments of a vertical pole: (1) 0–20 cm (low); (2) >20–100 cm (medium); and (3) >100–200 cm (high). Soil compactness (kg/cm2) was measured using an AMS G 281 E-280 Pocket Penetrometer (American Falls, United States), canopy height (m) with an extendable measuring pole, and canopy openness through rectangular photographs shot on an iPhone 12 Pro later processed using %Cover smartphone app (Mignanelli 2018). For each site, data for GLC and vertical structure were summed, with averages calculated for remaining variables. We also broadly categorised soil type at each site as sandy loam, sandy clay loam, or clay loam (Fig. S3). Data for all variables (Table S5) were then analysed using a factorial analysis of mixed data (FAMD) using the ‘FactoMineR’ package (v 2.9; Lê et al. 2008) implemented in R (R Core Team 2023). This enabled us to explore the species’ habitat preferences using continuous and categorical variables simultaneously. Dimensions with eigenvalues ≥1 were retained according to Kaiser’s criterion (Kaiser 1960). The presence or absence of stick ant nests at each site was also noted, given they are a key habitat for many fossorial Australian reptiles (Wilson and Swan 2021). Data for all aforementioned habitat variables were also collected from where we found an additional L. viduata individual outside of formal surveys, with this data also being included in the FAMD. Analysis of photographs taken from public roadsides and data from Biota Environmental Sciences (2004) enabled for classification of the general habitat category at unsurveyed historical sites.
To quantify the species’ microhabitat preferences, the following variables were recorded within a 1-m × 1-m quadrat centred around each capture point: elevation (m, asl); slope (°); aspect; soil compactness (kg/cm2, mean of the capture point and corners); dominant GLC type, substrate type and layer (at capture point); substrate temperature (°C, at capture point); and litter depth (mm, at capture point). Elevation, slope, and aspect were recorded using the following iPhone 12 Pro applications, respectively: Sightings (MacDonald 2017, coordinates also recorded), Clinometer + Bubble Level (Breitling 2019), and Compass ∞ (Camera LLC[TX] 2023), with slope measured from the most elevated point of the quadrat perimeter to the lowest point on the opposing side. Substrate temperature was measured using a Protech QM-1323 Digital Multimeter (Rydalmere, Australia). We also noted the nearest species of eucalyptus tree to each capture point.
Morphology, life-history and biology
Morphological attributes of the species were examined by measuring field-caught individuals and the 15 L. viduata specimens at the Western Australian Museum (Table S1). For each individual, external morphological measurements were taken as described in Table S6, with digital callipers (Mitutoyo 500–763–208”/200 mm Coolant Proof Digimatic Calipers; West Heidelberg, Australia). All field-caught individuals were weighed using a 100-g spring balance (Pesola; Chur, Switzerland), and sex determined by hemipenal eversion presence (male) or absence (female). For 11 of the 15 museum specimens, sex was determined by examining internal structures (presence of testes in males, ovaries in females) from existing ventral incisions. The remaining four could not be sexed due to their internal structures showing pre-existing damage. Only adult specimens were considered in calculating attribute means.
The following six life-history and biological attributes of the species were inferred from data on skinks exhibiting similar ecologies (e.g. comparable morphology, habitat, phylogeny): (1) size at sexual maturity; (2) clutch size; (3) reproductive mode; (4) diet; (5) age of first reproduction (females only); and (6) maximum age. Such inferences of core data are permitted by the IUCN where direct observation of the study species is limited (IUCN 2024a). For the initial four variables, a subset of skink species was deemed ecologically similar to L. viduata, based on two criteria: (1) morphological similarity (30–50 mm SVL; Wilson and Swan 2021); and (2) comparable distribution (in southern Western Australia, below −26° latitude, as per species’ IUCN (2024b) distribution shapefiles). The species’ closest phylogenetic relative, Lerista bougainvillii, was also included. Data for eligible species were then extracted from Greer (2022) (for size at sexual maturity), the database of Meiri (2018) (for the other three nominated variables) and our unpublished records (for Lerista christinae and Lerista distinguenda only; inclusive of the initial three variables). All eligible species and their nominated data are listed in Table S7. For maximum age and age of first reproduction, ecological similarity to L. viduata was defined solely by morphological comparability (≤55 mm SVL; Wilson and Swan 2021; Table S8), given that these variables lacked extensive data in literature (only Meiri (2018) contained data, albeit for few species). Averages for all continuous variables were then calculated and the most common categorical responses noted, interpreted as the outcomes for L. viduata. Using the averages for maximum age and age of first reproduction, we then calculated the generation length of the species through the following IUCN recommended formula: age at first reproduction + z × (maximum age is age at first reproduction), where z represents a constant dependent on survivorship and fecundity within a species (Fung and Waples 2017). We established a generalised z-value of 0.5 due to increased fecundity with size in lizards (Meiri et al. 2020) but increased mortality with age. Activity type (diurnal, crepuscular, cathemeral or nocturnal) for the species was identified through our own field observations.
Population size
The species’ population size, defined more specifically by the IUCN as its number of mature individuals (i.e. those known, estimated or inferred to be capable of reproduction), was calculated through the following IUCN endorsed formula: d × A × p, where d is an index of population density, A is an estimate of area, and p is the proportion of individuals that are mature (IUCN 2024a). We calculated single values for A (in km2) and p, but two for d (i.e. a minimum and maximum, in mature individuals/km2), to account for a non-constant population density across the species’ range and develop a range estimate. To calculate the values of d for the species, we referred to the mean minimum and maximum d data of species within Meiri’s (2024) comprehensive global database of squamate traits. From the Australian skink species with relevant data, ecological similarity to L. viduata was based on the following criteria: (1) SVL ≤ 70 mm (Wilson and Swan 2021); and (2) semi-restricted distribution (i.e. EOO ≤ 1,000,000 km2; Chapple et al. 2019). The applied criteria were simplified from those used for the species’ life-history and biology given that a limited number of Australian skinks had mean population density data within Meiri (2024); thus, using more generalised criteria enabled for a more sufficient sample size (n = 18) from which to denote d-values for the species. All eligible species were noted to occupy similar habitats to L. viduata (i.e. beneath leaf litter, within sandy soils, or in woodlands; Meiri 2018; Wilson and Swan 2021) and, given the vast majority of them are non-threatened and common within their ranges (Chapple et al. 2019; IUCN 2024b), their d-values are considered conservative approximations of d for the comparatively rarer L. viduata. One species, Carinascincus metallicus, was excluded, given its extremely common nature (Chapple et al. 2019) and inflated d value. All 18 eligible species are in Table S9. From the extracted data, averages for both measures of d were interpreted as the values for L. viduata. Given that d-values for species within Meiri (2024) were for mature individuals only, a default p-value of one was adopted.
To calculate A, we added the areas of two separate minimum convex hulls drawn around localised clusters of the species’ vetted points, with 200-m buffer zones applied, in QGIS 3.22.7 (QGIS Development Team 2022). Such an approach was preferred over using the species’ EOO or AOO because: (1) they cover large sections of cleared land upon which L. viduata is unlikely to occur; (2) historical surveys have not detected the species across large sections of its EOO and AOO, particularly areas intervening Mount McMahon and Kundip (Chapman and Newbey 1995; Harris et al. 2008); and (3) with 200-m buffer zones, it conservatively encompasses the species’ known vetted occurrence points. The applied 200-m buffer distance was based on a conservative inference of the species’ dispersal ability, taken from typical trends in small (i.e. ≤100 m SVL) Australian skinks (e.g. Robertson 1980; Olsson and Shine 2003; Atkins et al. 2007).
Threat analysis and conservation status assessment
To help facilitate the assessment of L. viduata under IUCN Red List Criterion B, we analysed the potential impact of fire on the species through three approaches. First, we examined geospatial fire data for the Ravensthorpe region (DBCA 2023a) in QGIS 3.22.7 (QGIS Development Team 2022), to determine what percentage of the species’ EOO and AOO has burned in the last 50 years. Second, by examining the same geospatial data, we compared the area and extent of the region’s 2023 fires with the size of the species’ EOO and AOO. Such an analysis enables calculation of how many locations L. viduata occurs at (given that species’ locations are tied to the extent of their threats; IUCN 2024a), and thus helps facilitate assessment of the species under both Criterion B and D. Finally, we examined how fire impacts features of the species’ preferred habitat, to understand whether fire correlates with poorer quality habitat for the species. To do this, we used our FAMD results to identify which continuous habitat variables were associated with the species’ occurrences, and for each of those variables, data were compared between sites that were burned (i.e. burned woodland and shrubland) and unburned (i.e. remaining habitat types) using two-sample t-tests (α = 0.05) in R. For variables with non-normal raw or transformed data (Shapiro–Wilk test P < 0.05), or non-equal variances (Bartlett’s test P < 0.05), the non-parametric Mann–Whitney U-test was used (α = 0.05). The potential impact of the following four additional threats to the species was also considered by reviewing existing literature: loss of climate niche, mining, feral cat and fox predation and dieback causing fungi (e.g. Phytophthora cinnamoni) (Tables S10 and S11). We then assessed the species’ conservation status against the five IUCN Red List criteria using all aforementioned data and calculations.
Results
Distribution
Four L. viduata individuals were detected during fieldwork, all within ~1 km of historical sites: one south-west of Mount McMahon; and three south of the old Kundip township. No more than one individual of the species was found per site. All individuals were found at elevations of 126–270 m. Based on the GeoCAT analyses of the species’ occurrence points, its EOO and AOO were calculated to be 32 km2 and 24 km2, respectively (Fig. 2).
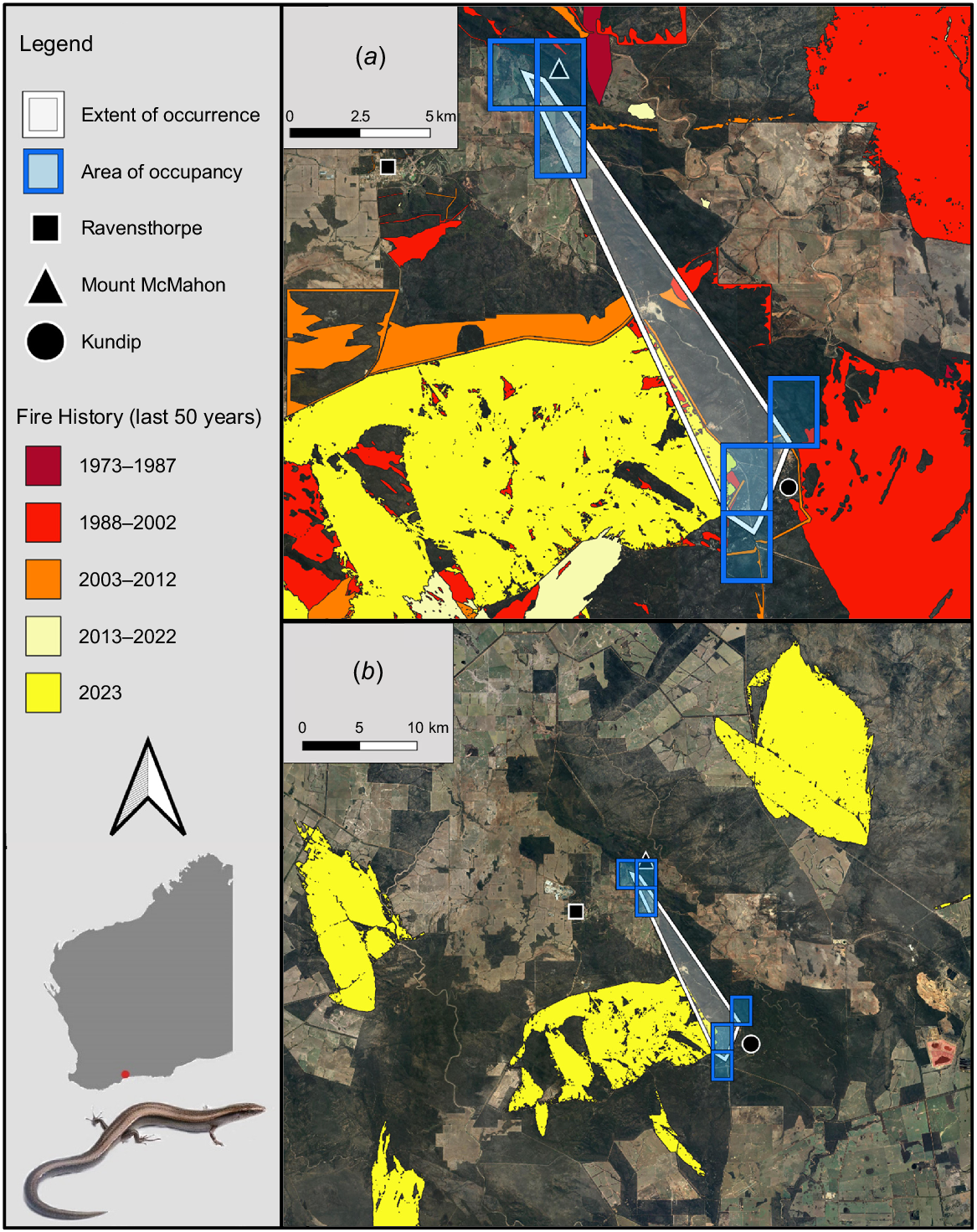
Locations of the Extent of Occurrence (32 km2) and Area of Occupancy (24 km2) for Lerista viduata within the Ravensthorpe Range, in relation to (a) fire-affected areas across the surrounding landscape over the last 50 years and (b) the extent and area of the region’s 2023 fires alone. The exact locations of the species’ occurrences are withheld from this figure, due to the sensitivity of such information at a fine scale. Photograph of L. viduata by Jules Farquhar.
Habitat
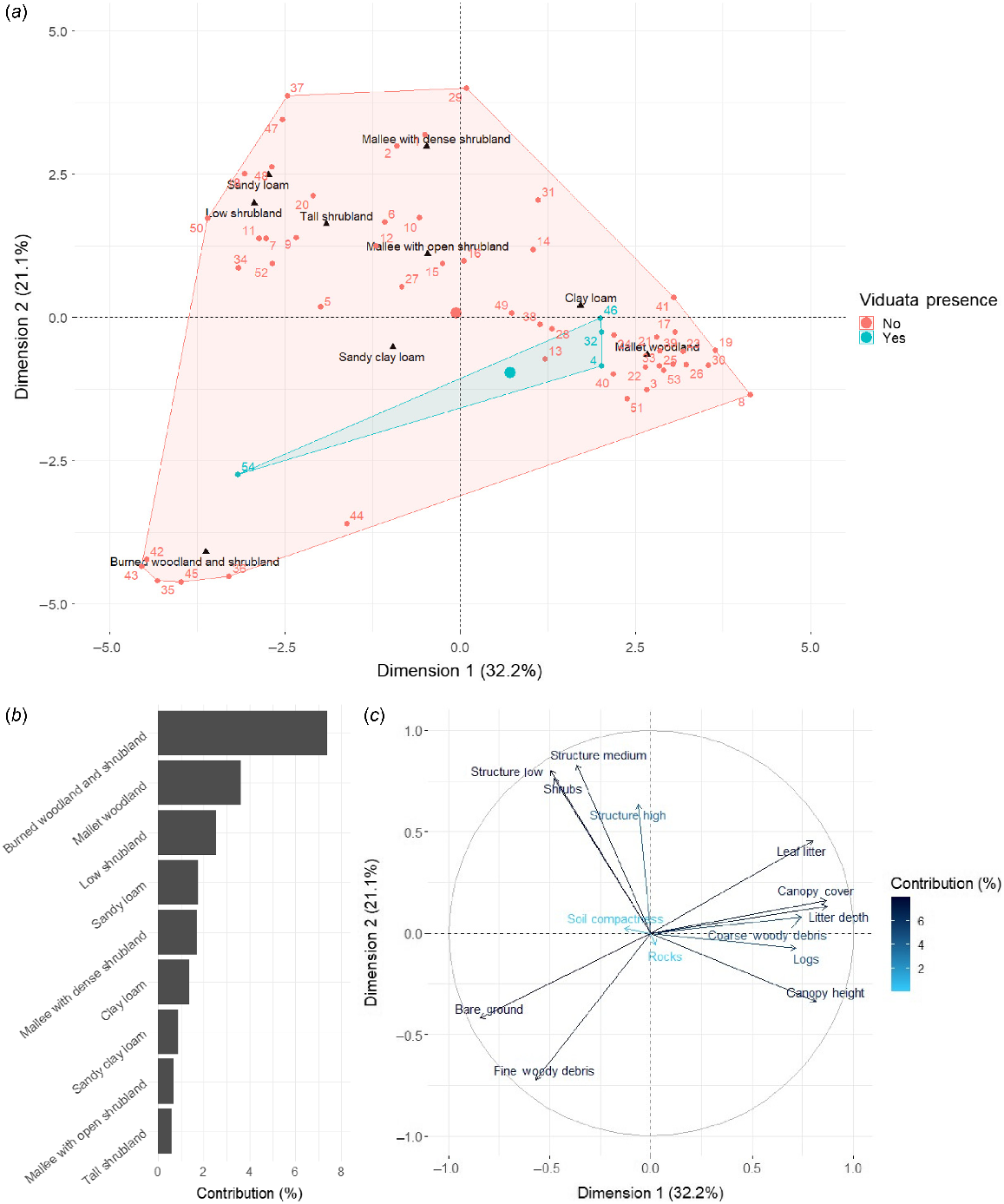
The first five dimensions of our FAMD met the retention criteria (eigenvalues ≥1), explaining 74.1% of the total inertia (Dimension [Dim] 1 = 32.2%, Dim 2 = 21.1%, Dim 3 = 8.1%, Dim 4 = 7.5%, Dim 5 = 5.3%). However, only Dims 1 and 2 were analysed further, given that they collectively explained a considerable proportion of variation (53.3%) and Dims 3 through 5 were dominated by few variables (i.e. negligible increases in explanatory power; Fig. S4). Analysis of Dims 1 and 2 showed that, of the examined general habitat categories, two were associated with L. viduata presences: (1) mallet woodlands (three of four occurrences), characterised by increasing values of leaf litter, litter depth, canopy cover, canopy height, coarse woody debris, and logs; and (2) burned woodland and shrubland (one occurrence), characterised by bare ground and fine woody debris (Fig. 3). Notably, the sole individual identified in burned woodland and shrubland was found within ~30 m of an unburned mallet woodland forest (Fig. 4). Mallet woodlands were identified as the general habitat at all except one of the examined unsurveyed historical sites (Fig. S5), with the sole exception featuring habitat described as ‘open mallee heathland with very dense proteaceous thicket’ (Biota Environmental Sciences 2004, p. 17). Notably though, mallet eucalypts occurred in close proximity to this area (Biota Environmental Sciences 2004). Regarding soil type, all four L. viduata occurrences from this study occurred on sandy clay loam grounds. Although soil type explained minimal variance for Dims 1 and 2 of our FAMD (Fig. 3b), sandy clay loam approximated the central area of the species’ four occurrences (Fig. 3a); thus, the FAMD provided some consolidated evidence of this variable’s importance. Overall, given the aforementioned habitat patterns, and that a reasonable proportion of sites with both mallet woodlands and sandy clay loam soils had L. viduata occurrences (i.e. four of eight sites; 50%), the combination of these two variables appears be the species’ main habitat preference.
Factorial analysis of mixed data (FAMD) results for site habitat characteristics (Dimensions 1 and 2 only). (a) Categorical variables and their relation to sites with Lerista viduata presences (light blue) and absences (red), (b) contribution (%) of each categorical variable to the FAMD, and (c) continuous variables and their contribution (%) to the FAMD. Factors that appear close to each other are considered to be correlated.
Locations and habitat from where the four Lerista viduata individuals were found during this study. (a) Mallet woodlands south-west of Mount McMahon, in the mulch layer of leaf litter at the base of a mature Eucalyptus megacornuta, (b) mallet woodlands of Kundip, in the loose dry sandy soil beneath the roots of a dead Eucalyptus cernua sapling, (c) mallet woodlands of Kundip, in the dry sandy soil under leaf litter at the base of a mature Eucalyptus platypus, and (d) recently burned woodland and shrubland of Kundip, within a stick ant nest ~30-m away from an unburned Eucalyptus merleae mallet woodland (i.e. opposite side of a public dirt road). Red circles denote the location of the individual when first sighted.

Regarding microhabitat, all four L. viduata individuals were found within substrates that Lerista spp. typically prefer: one was found in deep eucalyptus litter, one was within loose dry soil beneath a dead mallet eucalypt sapling, one was within loose soil covered by leaf litter, and one was within an abandoned stick ant nest (Fig. 4, Table 1). Further microhabitat data for the species are in Table 1.
| Date of capture | Location | General habitat | Microhabitat characteristic | Story of how found | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Elevation (m, ASL) | Slope (°) | Aspect | Soil compactness (kg/cm2, mean ± s.e.) | Dominant ground-level cover | Substrate | Substrate layer found in | Substrate temperature (°C) | Litter depth (mm) | Nearest species of eucalyptus | |||||
| 29 May 2023 | South-west of Mount McMahon | Mallet woodland | 264 | 2.8 | South-west | 1.35 (±0.29) | Leaf litter | Leaf litter | Mulch (deepest section of leaf litter) where the litter meets the dry sandy clay loam soil | 16.4 | 52.92 | Eucalyptus megacornuta | Raking in mulch layer of leaf litter at the base of a ~10 m tall E. megacornuta tree (Fig. 4a) | |
| 23 June 2023 | Kundip | Mallet woodland | 127 | 0 | NA | 1.36 (±0.37) | Bare ground | Soil | Loose dry sandy section of white sandy clay loam soil | 10.7 | NA | Eucalyptus cernua | Flipping a dead ~1 m tall E. cernua sapling, with the individual found in the loose dry sandy soil beneath the root (Fig. 4b) | |
| 03 July 2023 | Kundip | Mallet woodland | 126 | 3.1 | North | 0.95 (±0.25) | Leaf litter | Soil | Just below surface of dry sandy clay loam soil | 19.4 | 36.02 | Eucalyptus platypus | Raking in the sandy soil beneath leaf litter at the base of a ~8 m tall E. platypus tree (Fig. 4c) | |
| 03 July 2023 | Kundip | Burned woodland and shrubland (within ~30-m of unburned mallet woodland) | 149 | 0 | NA | 1.25 (±0.21) | Bare ground | Stick ant nest | Middle of stick ant nest (i.e. comprised of sand, small stones and sticks) | 10.3 | NA | Eucalyptus merleae | Raking a largely abandoned stick ant nest in recently burned habitat, adjacent a public dirt road and unburned E. merleae mallet woodland (Fig. 4d) | |
Associated images are in Fig. 4.
NA, not applicable due to lack of data.
Morphology, life-history and biology
Outcomes for the species’ snout-vent length, mass, life-history, and biological attributes are in Table 2. Females measured slightly larger than males for most morphological attributes assessed, though all weighed males were heavier than the lone weighed female (Table S12). By inputting the inferred values for age at first reproduction (0.87 years) and maximum age (3.35 years) into the Fung and Waples (2017) formula, the species’ generation length was calculated as 2.11 years (0.87 + 0.5 × (3.35–0.87)).
| Attribute | Outcome | |
|---|---|---|
| Morphology | ||
| SVL average (±s.d.) | 39.66 mm (±3.84) | |
| SVL range | 30.10–47.21 mm | |
| Mass average (±s.d.) | 0.76 g (±0.12) | |
| Mass range | 0.65–0.92 g | |
| Life-history | ||
| Age of first reproduction | 10.39 months (or 0.87 years) | |
| Clutch size average | 2.23 | |
| Clutch size range | 1–3 | |
| Generation length | 2.11 years | |
| Maximum age | 3.35 years | |
| Reproductive mode | Oviparous | |
| Size at sexual maturity | 36.03 mm (females), 32.64 mm (males) | |
| Biology | ||
| Activity type | Diurnal | |
| Diet | Insectivorous | |
Population size
The estimated minimum and maximum density (d-values) for L. viduata were 743 and 1,961 mature individuals per km2, respectively (Table S9). Area calculations from the separate minimum convex hulls drawn around buffered occurrences at Mount McMahon (A = 1.06) and Kundip (A = 3.67) resulted in a total A-value of 4.73 km2. By inputting these values into the d × A × p formula, along with the adopted p-value of 1, a minimum 3,514 (743 × 4.73 × 1) and maximum 9,276 (1961 × 4.73 × 1) mature individuals were calculated for the species.
Threat analysis
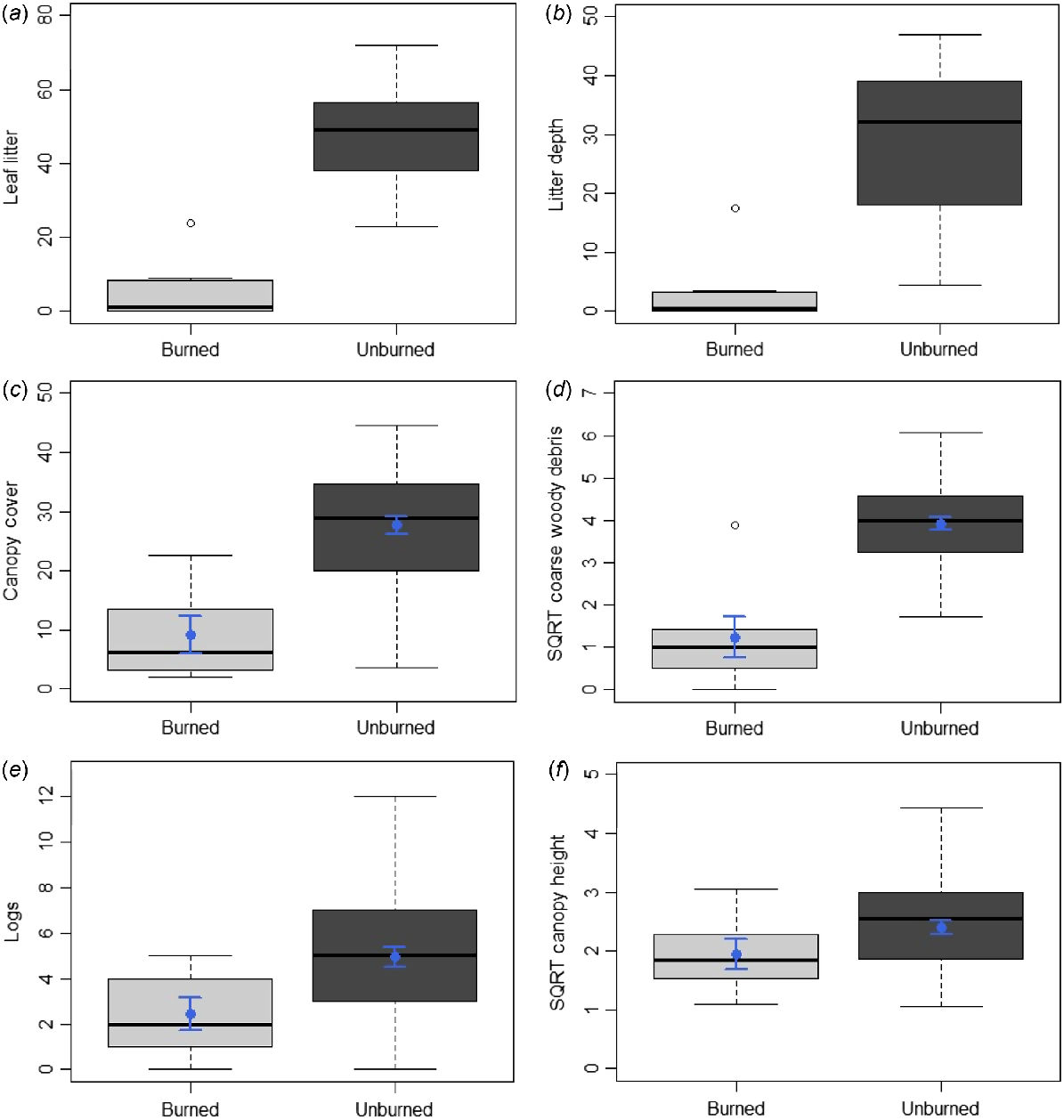
Fires were found to have collectively burned 17% of the species’ EOO, and 9% of its AOO, over the past 50 years, with these having occurred at consistent frequencies across this period (Fig. 2a). Habitat directly atop of, and close to, exact occurrence points of the species was among the vegetation affected (Fig. 2a). Concerningly, many such fires within the species’ EOO and AOO were prescribed burns (DBCA 2023a). Additionally, in 2023, three fires in the Ravensthorpe region individually burned areas over three to four times greater than the species’ EOO and AOO (Fig. 2b), meaning the species occurs at just one location under IUCN guidelines (IUCN 2024a). One such fire burned 115 km2 of vegetation ~15 km northeast of Mount McMahon, another covered 85 km2 of land ~10 km west of Ravensthorpe, while the third affected 100 km2 of flora just west of Kundip (Fig. 2b). The latter also burned atop of and around some of the species’ known occurrence points. Lastly, five of the six continuous variables associated with the species’ main biotic habitat preference (i.e. mallet woodlands), were significantly less abundant in burned compared to unburned sites: leaf litter (U = 131, P < 0.001), canopy cover (t = −4.48, d.f. = 52, P < 0.001), litter depth (U = 131, P < 0.001), coarse woody debris (t = −6.17, d.f. = 52, P < 0.001) and logs (t = −2.18, d.f. = 52, P < 0.033) (Fig. 5). Canopy height was also lower in burned compared to unburned habitat; however, the difference was not significant (t = −1.46, d.f. = 52, P = 0.151) (Fig. 5). Thus, under IUCN (2024a) guidelines, fire is inferred to cause declines in the species’ habitat quality (also area and extent, by inference), and regular fires within the species’ EOO and AOO over recent decades indicate that this decline is ‘continuing’. The concern regarding fire is further emphasised by climate change projections for southern Western Australia, including the Ravensthorpe Range, which predict harsher fire weather and increasing fire severity and frequency over time (Steffen et al. 2015; Canadell et al. 2021; DWER 2021). Further discussion regarding the potential impact of climate change on the species (i.e. through loss of climate niche), in addition to threats posed by mining, feral cats and foxes, and dieback-causing fungi are in Tables S10 and S11. Overall, these threats are likely having a lower impact on the species than fire; although notably, mining is also likely contributing to declines in the area, extent and quality of the species’ habitat (Table S10).
Boxplots of the differences between sites that were burned (i.e. burned woodland and shrubland, n = 7) and unburned (i.e. remaining habitat types, n = 47) for the six continuous habitat variables associated with mallet woodlands (i.e. the main biotic habitat preference of Lerista viduata): (a) leaf litter (count per 100 m), (b) litter depth (mm), (c) canopy cover (%), (d) coarse woody debris (count per 100 m); (e) logs (count per 100 m), and (f) canopy height (metres). SQRT = square root transformed. Boxplots show the median, first and third quartiles, the lower and upper limits and outliers for the data, with c−f also showing the mean (blue dot) and s.e. (blue line) given their data were analysed using t-tests. Differences were significant between burned and unburned sites for a−e.
Conservation status assessment
L. viduata was found to meet IUCN Red List criteria B1ab(i,ii,iii,v) for Critically Endangered, B2ab(i,ii,iii,v) for Endangered, and both C2ab(ii) and D2 for Vulnerable. Its suggested Criterion B statuses are based on combinations of the following data: EOO < 100 km2; AOO < 500 km2; one location relating to fire; and an inferred continuing decline in its EOO, AOO, habitat parameters (i.e. area, extent and quality), and number of mature individuals (the latter inferable from ongoing habitat decline; IUCN 2024a). Its listings under Criterion C and D are based on the following calculations, respectively: <10,000 mature individuals (all within one subpopulation); and ≤5 locations. All key assessment parameters for the species are in Table S13, and detailed assessment against all IUCN Red List criteria are in Table S14. Given that the IUCN classifies species as per their most severe status (IUCN 2024a), we recommend that L. viduata be listed as Critically Endangered.
Discussion
Our study addresses a key question about the rare and Data Deficient L. viduata: what is its IUCN Red List conservation status? We show that through improved understandings of its distribution, ecology, biology, population size, and threats, the species is threatened under multiple IUCN criteria, with its most severe status being Critically Endangered (i.e. the most extreme classification before the extinction categories; IUCN 2024a). Thus, we highlight that L. viduata should be a major conservation priority. We also uncover notable attributes on the species that will likely prove vital to conservation authorities involved in its management: it prefers a specialised habitat; only occurs at two sites and is under severe threat from changing fire regimes, with fire severity and frequency within its range predicted to increase with ongoing climate change. Altogether, our findings provide robust foundations on which to generate conservation actions for the species.
Many attributes and threats render L. viduata threatened
L. viduata meets threatened statuses under three of the five IUCN criteria and several different parameter combinations, indicating that many of its attributes, rather than a select few, render it susceptible to extinction. Amongst the most alarming of these is its heavily restricted distribution. Its EOO (32 km2) and AOO (24 km2) comfortably meet their respective thresholds for Critically Endangered (<100 km2) and Endangered (<500 km2), with its AOO falling marginally short of the <10 km2 AOO Critically Endangered level. Despite our widespread survey effort, which occurred within and around the species’ historic range and in key nature areas around Ravensthorpe (e.g. Mount Short, Overshot Hill Nature Reserve, Kundip Nature Reserve), we did not detect L. viduata outside its two known sites (i.e. south-west of Mount McMahon and near the old Kundip township). Historical fauna surveys across the region have consistently identified the same pattern (Chapman and Newbey 1995; Harris et al. 2008; Terrestrial Ecosystems 2015; Ninox Consulting and Biostat 2018). Our research team was vastly experienced in searching for fossorial lizards and, for this study, utilised typical Lerista survey methods (i.e. raking leaf litter and sand in winter, time-constrained searches; Gaikhorst 2015; Maryan and Gaikhorst 2017); thus, false negative surveys, although not impossible, are considered unlikely. They are especially unlikely when considering the region’s historical fauna surveys, which also typically found few, or no L. viduata individuals (Chapman and Newbey 1995; Terrestrial Ecosystems 2015; Ninox Consulting and Biostat 2018; Tables S1 and S2). Although our land access did not encompass some areas of survey interest (e.g. much land intervening Mount McMahon and Kundip), the species would need to persist at the far reaches of the Ravensthorpe Range (i.e. near Mount Short or Bandalup Hill, where surveys have not detected it) for its EOO to approach or exceed the Critically Endangered threshold, and even then, it would still comfortably fit the <5000 km2 EOO Endangered level and <20,000 km2 EOO threshold for Vulnerable. Thus, even if L. viduata was found at new locations within the Ravensthorpe Range in future, it would still highly likely meet the Critically Endangered criteria, or at least be Endangered or Vulnerable.
Our analyses demonstrate that changes in fire regimes are a key threat to L. viduata under IUCN Red List criteria. Fire significantly degrades key features of its preferred habitat, has occurred regularly within its EOO and AOO over the past 50 years and, based on 2023 Ravensthorpe fire data alone, a single burn could easily encompass its entire range. Climate projections for southern Western Australia, including the Ravensthorpe Range, predict increasing fire severity and frequency over time with intensifying climate change (Steffen et al. 2015; Canadell et al. 2021; DWER 2021); thus, harsher fire regimes within the species’ range are an ongoing expectation. Indeed, there are predictions of harsher fire weather across the region in future, with high confidence (DWER 2021; Climate Change in Australia 2024), and of severe fire rating days in the area increasing up to 12% by 2030, and 64% by 2090 (Steffen et al. 2015; DWER 2021). Adding to these concerns are predictions of 0.5–1.1°C temperature increases for the region by 2030, and up to 4°C by 2090, along with rainfall decreases of 15% by 2030 and up to 45% by 2090, which collectively will likely create drier, more fire-prone environments across the landscape (DWER 2021).
Although the fossorial nature of L. viduata would result in some individuals surviving fires initially (e.g. by burrowing underground, sheltering in stick ant nests), many would then likely perish from the lethal temperature, burns and smoke inhalation resulting from such events (Smith et al. 2012; Jordaan et al. 2020; Santos et al. 2022). Furthermore, remaining individuals would then be highly susceptible to post fire mortality given the challenges of living in degraded habitat (e.g. low prey densities, less shelter sites, higher exposure to predators; Santos et al. 2022). Further elevating concerns around fire is that the species’ main biotic habitat preference, mallet woodlands, are considered particularly fire sensitive, often being killed by low to moderate intensity fires given their thin bark relative to other eucalypts, and reliance on canopy-stored seeds for regeneration (Barrett et al. 2009). Furthermore, as serotinous obligate seeders, they are sensitive to fire frequencies under approximately 50 years (i.e. as they may not reach maturity to set seeds). Thus, ongoing increases in fire frequency are likely to render them vulnerable to degradation (Barrett et al. 2009; McQuoid and French 2021). Indeed, many mallet eucalypt species are listed as threatened by the IUCN Red List (e.g. Eucalyptus clivicola, E. megacornuta, E. platypus; IUCN 2024b), with altered fire regimes considered a key threat (Barrett et al. 2009; DEC 2012; McQuoid and French 2021), and they typically occupy very restricted areas (i.e. <1 ha), which may make them particularly vulnerable even to single burns (Barrett et al. 2009). It has even been suggested that mallet stands should be excluded from burnt strips (Barrett et al. 2009), given their sensitivity to individual, let alone repeat fires. Ultimately, fire-driven continuing declines in the species’ EOO, AOO, habitat parameters and mature population size are likely to intensify into the foreseeable future, and the effects are highly unlikely to be reversible. Thus, although the species has coexisted with fire historically, its heavily restricted distribution and vulnerable population size, combined with climate change projections of hotter, more frequent fires and the effects of fire on its habitat, mean that changing fire regimes currently constitutes a viable threat to its persistence under IUCN Red List criteria.
The threat of mining was not considered in our statistical analyses; however, its potential impact on the species should not be ignored. The Ravensthorpe Gold Project currently operates an active gold mine atop the species’ eastern most occurrence point at Kundip, and within ~2 km of several more of its vetted coordinate points in the area. Operations started in 2020, with approvals permitting clearance of 195.4 ha of native vegetation in the area over an 8-year period (EPA 2020; Minister for Environment 2020). Such clearings are almost certain to fall within the species’ EOO and AOO, given their proximity to the species’ occurrence points. Indeed, numerous mines (e.g. at Mount McMahon, Elverdton, Kundip) have operated historically atop, or close to, occurrence points for the species across several decades, and likely contributed to environmental degradation in these areas (e.g. through costeaning, trenching, construction of tunnels Harris et al. 2008). Further discussion on the potential impact of mining, and many other possible threats to the species are in Tables S10 and S11.
The species’ small calculated population size adds to concerns about its extinction vulnerability. The estimated 3,514–9,276 mature individuals for L. viduata, together with its persistence within a single subpopulation, suffice for a listing as Vulnerable under IUCN Red List Criterion C (IUCN 2024a), and contribute to mounting evidence that it is indeed a rare species. Aside from being heavily range restricted, it has also scarcely been detected within its range by herpetologists over long periods of survey effort (B. Maryan and R. Lloyd pers. comm.). Furthermore, consultancy surveys around Kundip have only ever recorded the species once, despite several days of search effort close to the species’ occurrences (Biota Environmental Sciences 2004). Our population size estimates were highly conservative given they considered the population density data of several skink species known to be common within their ranges, and accounted for the persistence of L. viduata in suitable habitat outside its known occurrences. Yet, the species still qualifies for Vulnerable based on population size alone. Of additional note, the most abundant species from our surveys show ecological similarity to L. viduata in terms of their fossoriality, habitat preference and distribution (i.e. Hemiergis initialis, Hemiergis peronii, L. distinguenda, Menetia greyii, Morethia obscura; Cogger 2018; Wilson and Swan 2021). Thus, the region does accommodate for the niches of small, litter-dwelling fossorial reptiles. However, L. viduata consistently proves to be scarce, and is therefore unlikely to be common within its range. Overall, the small population size of the species may render it sensitive to further natural drivers of extinction such as genetic drift, demographic stochasticity or inbreeding should its threats persist (Frankham et al. 2004). However, whether these will genuinely affect the species or not currently remains uncertain.
Habitat preferences
Lerista viduata appears to be a habitat specialist, with its occurrence areas featuring a unique habitat combination that was scarcely detected elsewhere during our surveys: mallet woodlands atop of sandy clay loam soils. Several of our survey sites close to the species’ occurrences, and at key landmarks across the region, typically featured just one of these habitat characteristics but not both (Table S5), indicating that each is of ecological importance to the species. As such, the two recorded occurrences of the species near, but not within mallet woodlands (i.e. open mallee heathland in Biota Environmental Sciences (2004), burned woodland and shrubland in this study) likely represent chance observations of it being just outside optimal habitat.
The species’ habitat preference is largely consistent with trends within its genus. Lerista is typically characterised by small, elongate and limb-reduced bodies, a shape superbly adapted to fossorial life swimming through loose sandy soil and inhabiting leaf litter mats atop the sand (Morinaga and Bergmann 2017, 2020; Camaiti et al. 2023). Its typically small, snake-like shape enables for fast penetration of sandy substrates when disturbance occurs at the surface, while still facilitating locomotion through the narrow spaces within leaf litter mats as it pursues invertebrate prey (Cogger 2018; Morinaga and Bergmann 2020). Given that mallet woodlands are characterised by deep, extensive leaf litter coverage at ground level, it makes sense that L. viduata would favour such areas of the Ravensthorpe Range for habitat, with the region’s mallee and heathland ecosystems featuring more shrubby, exposed grounds (i.e. Beard 1973; Craig et al. 2008) that are likely less optimal for movement and concealment. Furthermore, the loose, sandier soils within the Range likely provide a more penetrable substrate than the region’s more clay or gravel dominated grounds, which are typically more dense, compact, rocky, and hard-setting (Overheu 1995).
The unique geology of the Ravensthorpe Range may explain why L. viduata appears to be a micro-endemic to the region. The Range lies on the Ravensthorpe Greenstone Belt, a small, isolated geological formation that largely differs to other geologies found across southern Western Australia (Sofoulis 1958; Thom 1977; Witt 1998). The Belt comprises archaean greenstone, a very mineral rich rock formation, which has weathered over millions of years to produce mineral rich grounds with varying soils textures and compositions (Sofoulis 1958; Overheu 1995). Indeed, these unique features are regarded as key drivers of the region’s immense floristic diversity and long popularity as a mining hotspot (Chapman and Newbey 1995; Gibson et al. 2010; Markey et al. 2012). Given that Lerista spp. can adapt to unique, isolated soil types, it is possible that L. viduata has become adapted to the unique mineral rich grounds of the Ravensthorpe Range over time, and then maintains its typical Lerista behaviour by favouring the particularly sandy, leaf litter covered habitats. Although further research is encouraged to confirm this hypothesis, it is entirely plausible that the Range’s unique geology has driven not just the high floral endemism in the region, but also the evolution of its only known endemic vertebrate.
Conservation implications
With a recommended Critically Endangered status, L. viduata is now of the utmost and urgent conservation priority. Such efforts will require a concerted effort from conservation practitioners, with an obvious starting point to ensure appropriate fire management practices occur in the region. With many past fires within the species’ EOO and AOO being prescribed burns, including the 2023 fire near Kundip, which burned atop an occurrence point of the species (DBCA 2023a), strategic decisions around fire planning schemes, in addition to wildfire management, will be essential measures. Another key consideration pertains to the land tenure across the species’ range. None of the species’ occurrences fall into protected areas. Instead, most occur on unallocated crown land or in reserves that are not managed for conservation purposes. Proposals to establish more conservation reserves throughout the Range, including at Mount McMahon and Kundip, have been put forth many times (CTRC 1974; CALM 1992; Harris et al. 2008). However, due largely to future mining prospects in the region, these have not been implemented (EPA 1993; Chapman and Newbey 1995; Harris et al. 2008). As such, providing greater environmental protection of the species’ known areas by establishing additional conservation reserves, or liaising directly with existing land holders, will also be of critical importance. Furthermore, in addition to updating the species’ IUCN Red List conservation status, we suggest that L. viduata be listed as Critically Endangered under Australia’s Environment Protection and Biodiversity Conservation Act (1999) and Western Australia’s Biodiversity Conservation Act (2016). Such listings would create the legal requirement to protect the species and make it eligible for conservation action and funding, and thus are pivotal for its overall conservation. Data collected on the species’ life-history, morphology and microhabitat during this study should prove useful for conservation practitioners in any future on-ground works for the taxon.
Beyond these implications, our study likely has broader significance for tackling data deficiency in fossorial reptiles. Presently, 1,492 reptiles globally are DD (IUCN 2024b), with many being fossorial. Our framework of combining all the following approaches proved effective in achieving data sufficiency for the fossorial L. viduata under multiple IUCN Red List criteria: (1) collation of all its vetted point records; (2) fieldwork within its historical range, and across varying habitats surrounding it; (3) preserved specimen examination; (4) assessing threats via on-ground and desktop methods; and (5) drawing inference from ecologically comparable species. While data sufficiency may not always be achievable under such a framework (e.g. too few occurrences, fieldwork impracticalities), the sheer number of DD reptiles means it is likely to be effective in many cases, as evidenced by similar methods resulting in threatened listings for the previously DD L. elongata (DCCEEW 2023; Graham et al. 2023) and P. rawlinsoni (Farquhar et al. 2024). Ultimately, we suggest that this method may be particularly useful for researchers assessing fossorial reptiles under similar scenarios, whether they be DD, Not Evaluated or assessed using only one or two of the IUCN Red List criteria.
Conclusion
With a staggering 21,290 DD species globally (IUCN 2024b), and more than half of them predicted to be threatened by extinction (Borgelt et al. 2022), it is essential they are not discounted from conservation research priority. Here, we have demonstrated that an integrated process involving collation of existing data, fieldwork, museum specimen examination, threat analysis, and consideration for ecologically similar species was sufficient to overcome data deficiency in the Ravensthorpe Range slider, L. viduata. Most alarmingly, we show that this long DD species is actually Critically Endangered, and would likely remain Critically Endangered, or at least Endangered, if ever found elsewhere within the Ravensthorpe Range. As such, L. viduata has likely been edging towards extinction over an extended period of time. Ultimately, L. viduata represents the seemingly inevitable reality faced by so many DD species globally; it is actually severely threatened with extinction, yet its conservation status, lack of prior research attention, and minimal consideration in on-ground management means it has long been ignored from conservation priority. As such, changing this reality for the many DD species globally will be critical in efforts to curtail current rates of biodiversity extinction.
Data availability
Data and code associated with this study are provided in online supplementary material.
Declaration of funding
This project was supported by a grant from the Australian Research Council (FT200100108 to DGC) and Holsworth Wildlife Research Endowment (to LRB), the latter which was in partnership with the Ecological Society of Australia.
Acknowledgements
We acknowledge the Noongar people as the Traditional Custodians of the land upon which this study was conducted. Jessica Fenker Antunnes and Nicholas P. Gale provided fieldwork assistance. We thank the following individuals for their guidance as we prepared for and conducted fieldwork: Gregory Harold; Nathan McQuoid; Brad Maryan; Ray Lloyd; and Matthew Prophet. We also thank Paul Doughty and Kailah Thorn for providing access to specimens at the Western Australian Museum. Land access was generously granted by the Department of Biodiversity, Conservation and Attractions (DBCA), Department of Water and Environmental Regulation, the Water Corporation and numerous private property owners. All research was conducted under permission of the DBCA Wildlife Act 1975 Research Authorisation (Licence No: FO25000414-3), Department of Parks and Wildlife (Licence No: 08-005025-2) and was approved by the Monash University Animal Ethics Committee (Permit No: 32778).
References
Atkins N, Swain R, Wapstra E, Jones SM (2007) Late stage deferral of parturition in the viviparous lizard Niveoscincus ocellatus (Gray 1845): implications for offspring quality and survival. Biological Journal of the Linnean Society 90(4), 735-746.
| Crossref | Google Scholar |
Bachman S, Moat J, Hill AW, de la Torre J, Scott B (2011) Supporting Red List threat assessments with GeoCAT: geospatial conservation assessment tool. ZooKeys 150, 117-126.
| Crossref | Google Scholar |
Biota Environmental Sciences (2004) Fauna and fauna assemblages of the Kundip and Trilogy study sites. Fauna and fauna assemblages report. Unpublished report for Tectonic Resources NL, Perth. Available at https://www.epa.wa.gov.au/sites/default/files/Referral_Documentation/Appenix%20H%20Fauna%20studies%20and%20decision%20on%20MNES.pdf
Bland LM, Böhm M (2016) Overcoming data deficiency in reptiles. Biological Conservation 204, 16-22.
| Crossref | Google Scholar |
Bland LM, Collen B, Orme CDL, Bielby J (2015) Predicting the conservation status of data-deficient species. Conservation Biology 29(1), 250-259.
| Crossref | Google Scholar | PubMed |
Bland LM, Bielby J, Kearney S, Orme CDL, Watson JEM, Collen B (2017) Toward reassessing data-deficient species. Conservation Biology 31(3), 531-539.
| Crossref | Google Scholar | PubMed |
Böhm M, Williams R, Bramhall HR, McMillan KM, Davidson AD, García A, Bland LM, Bielby J, Collen B (2016) Correlates of extinction risk in squamate reptiles: the relative importance of biology, geography, threat and range size. Global Ecology and Biogeography 25(4), 391-405.
| Crossref | Google Scholar |
Borgelt J, Dorber M, Høiberg MA, Verones F (2022) More than half of data deficient species predicted to be threatened by extinction. Communications Biology 5, 679.
| Crossref | Google Scholar | PubMed |
Breitling P (2019) Clinometer + bubble level (Version 4.9.4) [Mobile App]. Available at https://www.apple.com/au/app-store/ [Downloaded 19 May 2023]
Bureau of Meteorology (2024) Climate statistics for Australian locations. Available at http://www.bom.gov.au/climate/averages/tables/cw_010633.shtml [accessed 22 February 2024]
Caetano GHdO, Chapple DG, Grenyer R, Raz T, Rosenblatt J, Tingley R, Böhm M, Meiri S, Roll U (2022) Automated assessment reveals that the extinction risk of reptiles is widely underestimated across space and phylogeny. PLoS Biology 20, e3001544.
| Crossref | Google Scholar | PubMed |
Camaiti M, Evans AR, Hipsley CA, Hutchinson MN, Meiri S, Anderson RdO, Slavenko A, Chapple DG (2023) Macroecological and biogeographical patterns of limb reduction in the world’s skinks. Journal of Biogeography 50(2), 428-440.
| Crossref | Google Scholar |
Camera LLC[TX] (2023) Compass ∞ (Version 3.4) [Mobile App]. Available at https://www.apple.com/au/app-store/ [Downloaded 19 May 2023].
Canadell JG, Meyer CP, Cook GD, Dowdy A, Briggs PR, Knauer J, Pepler A, Haverd V (2021) Multi-decadal increase of forest burned area in Australia is linked to climate change. Nature Communications 12, 6921.
| Crossref | Google Scholar |
CAPAD (2022) Collaborative Australian protected areas database. Available at https://www.dcceew.gov.au/environment/land/nrs/science/capad/2022 [accessed 20 December 2023]
Chapman A, Newbey KR (1995) A vertebrate fauna survey and some notes on the vegetation of the Ravensthorpe Range, Western Australia. CALMscience 1(4), 465-508.
| Google Scholar |
Climate Change in Australia (2024) Climate information, projections, tools and data. Available at https://www.climatechangeinaustralia.gov.au/en/ [accessed 22 February 2024]
DBCA (2023a) DBCA fire history (DBCA-060). Available at https://catalogue.data.wa.gov.au/dataset/dbca-fire-history [accessed 20 December 2023]
DBCA (2023b) List of threatened and priority fauna. Available at https://www.dbca.wa.gov.au/wildlife-and-ecosystems/animals/list-threatened-and-priority-fauna [accessed 11 January 2023]
De Kort H, Baguette M, Lenoir J, Stevens VM (2020) Toward reliable habitat suitability and accessibility models in an era of multiple environmental stressors. Ecology and Evolution 10(20), 10937-10952.
| Crossref | Google Scholar | PubMed |
Farquhar JE, Carlesso A, Pili A, Gale N, Chapple DG (2024) Capturing uncatalogued distribution records to improve conservation assessments of data-deficient species: a case study using the glossy grass skink. Animal Conservation 27(1), 124-137.
| Crossref | Google Scholar |
Fung HC, Waples RS (2017) Performance of IUCN proxies for generation length. Conservation Biology 31(4), 883-893.
| Crossref | Google Scholar | PubMed |
Gaikhorst G (2015) Ecology and distribution of the slider skink, Lerista nevinae. Journal of the Royal Society of Western Australia 98, 131-136.
| Google Scholar |
Gibson N, Yates CJ, Dillon R (2010) Plant communities of the ironstone ranges of South Western Australia: hotspots for plant diversity and mineral deposits. Biodiversity and Conservation 19, 3951-3962.
| Crossref | Google Scholar |
Graham KA, Mahony SV, Chapple DG, Farquhar JE (2023) The long unknown: rediscovery of the long sunskink, Lampropholis elongata (Squamata: Scincidae)—after almost a decade, and after 50 years of data deficiency. Austral Ecology 48(5), 877-884.
| Crossref | Google Scholar |
Gumbs R, Gray CL, Böhm M, Hoffmann M, Grenyer R, Jetz W, Meiri S, Roll U, Owen NR, Rosindell J (2020) Global priorities for conservation of reptilian phylogenetic diversity in the face of human impacts. Nature Communications 11, 2616.
| Crossref | Google Scholar | PubMed |
IUCN (2024a) Guidelines for using the IUCN Red List categories and criteria (version 16). Prepared by the Standards and Petitions Committee. Available at https://www.iucnredlist.org/documents/RedListGuidelines.pdf [accessed 25 April 2024]
IUCN (2024b) Summary Statistics. IUCN Red List. Available at https://www.iucnredlist.org/resources/summary-statistics [accessed 12 June 2024]
Jithin V, Rane M, Watve A, Giri VB, Naniwadekar R (2023) Between a rock and a hard place: comparing rock-dwelling animal prevalence across abandoned paddy, orchards, and rock outcrops in a biodiversity hotspot. Global Ecology and Conservation 46, e02582.
| Crossref | Google Scholar |
Jordaan PR, Steyl JCA, Hanekom CC, Combrink X (2020) Fire-associated reptile mortality in Tembe Elephant Park, South Africa. Fire Ecology 16, 3.
| Crossref | Google Scholar |
Kadlec T, Tropek R, Konvicka M (2012) Timed surveys and transect walks as comparable methods for monitoring butterflies in small plots. Journal of Insect Conservation 16, 275-280.
| Crossref | Google Scholar |
Kaiser HF (1960) The application of electronic computers to factor analysis. Educational and Psychological Measurement 20(1), 141-151.
| Crossref | Google Scholar |
Kass JM, Muscarella R, Galante PJ, Bohl CL, Pinilla-Buitrago GE, Boria RA, Soley-Guardia M, Anderson RP (2021) ENMeval 2.0: redesigned for customizable and reproducible modeling of species’ niches and distributions. Methods in Ecology and Evolution 12(9), 1602-1608.
| Crossref | Google Scholar |
La Marca W, Elith J, Firth RSC, Murphy BP, Regan TJ, Woinarski JCZ, Nicholson E (2019) The influence of data source and species distribution modelling method on spatial conservation priorities. Diversity and Distributions 25(7), 1060-1073.
| Crossref | Google Scholar |
Lê S, Josse J, Husson F (2008) FactoMineR: an R package for multivariate analysis. Journal of Statistical Software 25(1), 1-18.
| Crossref | Google Scholar |
MacDonald S (2017) Sightings: a field data collection app (Version 1.1) [Mobile App]. Available at https://www.apple.com/au/app-store/ [Downloaded 19 May 2023]
Mace GM, Collar NJ, Gaston KJ, Hilton-Taylor C, Akçakaya HR, Leader-Williams N, Milner-Gulland EJ, Stuart SN (2008) Quantification of extinction risk: IUCN’s system for classifying threatened species. Conservation Biology 22(6), 1424-1442.
| Crossref | Google Scholar | PubMed |
Markey A, Kern S, Gibson N (2012) Floristic communities of the ravensthorpe range, Western Australia. Conservation Science Western Australia 8(2), 187-239.
| Google Scholar |
Maryan B, Gaikhorst G (2017) Notes on the relative abundance of the Perth lined skink, Lerista lineata on Garden and Rottnest Islands: a hand-searching example. Western Australian Naturalist 30, 220-228.
| Google Scholar |
McQuoid NK, French ME (2021) Eucalyptus merleae (Myrtaceae), a new rare species endemic to Ravensthorpe Shire in south-west Australia. Nuytsia 32, 151-158.
| Crossref | Google Scholar |
Meiri S (2018) Traits of lizards of the world: variation around a successful evolutionary design. Global Ecology and Biogeography 27(10), 1168-1172.
| Crossref | Google Scholar |
Meiri S (2024) SquamBase – a database of squamate (Reptilia: Squamata) traits. Global Ecology and Biogeography 33(4), e13812.
| Crossref | Google Scholar |
Meiri S, Bauer AM, Allison A, Castro-Herrera F, Chirio L, Colli GR, Das I, Doan TM, Glaw F, Grismer LL, Hoogmoed M, Kraus F, LeBreton M, Meirte D, Nagy ZT, Nogueira CdC, Oliver P, Pauwels OSG, Pincheira-Donoso D, Shea G, Sindaco R, Tallowinb OJS, Torres-Carvajal O, Trape J-F, Uetz P, Wagner P, Wang Y, Ziegler T, Roll U (2018) Extinct, obscure or imaginary: the lizard species with the smallest ranges. Diversity and Distributions 24(2), 262-273.
| Crossref | Google Scholar |
Meiri S, Avila L, Bauer AM, Chapple DG, Das I, Doan TM, Doughty P, Ellis R, Grismer L, Kraus F, Morando M, Oliver P, Pincheira-Donoso D, Ribeiro-Junior MA, Shea G, Torres-Carvajal O, Slavenko A, Roll U, McGill B (2020) The global diversity and distribution of lizard clutch sizes. Global Ecology and Biogeography 29(9), 1515-1530.
| Crossref | Google Scholar |
Mignanelli M (2018) %Cover: canopy surveying (Version 1.0.4) [Mobile App]. Available at https://www.apple.com/au/app-store/ [Downloaded 19 May 2023]
Morais AR, Siqueira MN, Lemes P, Maciel NM, De Marco P, Jr, Brito D (2013) Unraveling the conservation status of Data Deficient species. Biological Conservation 166, 98-102.
| Crossref | Google Scholar |
Morinaga G, Bergmann PJ (2017) Convergent body shapes have evolved via deterministic and historically contingent pathways in Lerista lizards. Biological Journal of the Linnean Society 121(4), 858-875.
| Crossref | Google Scholar |
Morinaga G, Bergmann PJ (2020) Evolution of fossorial locomotion in the transition from tetrapod to snake-like in lizards. Proceedings of the Royal Society B 287(1923), 20200192.
| Crossref | Google Scholar |
Muscarella R, Galante PJ, Soley-Guardia M, Boria RA, Kass JM, Uriarte M, Anderson RP (2014) ENMeval: an R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods in Ecology and Evolution 5(11), 1198-1205.
| Crossref | Google Scholar |
Ninox Consulting and Biostat (2018) Desktop assessment of vertebrate fauna of the proposed ravensthorpe spodumene project, Western Australia. Ninox Consulting and Biostat, unpublished report for Galaxy Resources Ltd, Perth. Available at https://ftp.dwer.wa.gov.au/permit/9938/Appendix%20B_2018%20Ninox%20Vertebrate%20Fauna%20Assessment.pdf
Olsson M, Shine R (2003) Female-biased natal and breeding dispersal in an alpine lizard, Niveoscincus microlepidotus. Biological Journal of the Linnean Society 79(2), 277-283.
| Crossref | Google Scholar |
Parsons ECM (2016) Why IUCN should replace “Data Deficient” conservation status with a precautionary “Assume Threatened” status – a cetacean case study. Frontiers in Marine Science 3, 193.
| Crossref | Google Scholar |
Phillips SJ, Anderson RP, Schapire RE (2006) Maximum entropy modeling of species geographic distributions. Ecological Modelling 190(3–4), 231-259.
| Crossref | Google Scholar |
QGIS Development Team (2022) QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available at http://qgis.osgeo.org
R Core Team (2023) ‘R: a language and environment for statistical computing.’ (R Foundation for Statistical Computing: Vienna, Austria) Available at https://www.R-project.org/
Rodrigues ASL, Pilgrim JD, Lamoreux JF, Hoffmann M, Brooks TM (2006) The value of the IUCN Red List for conservation. Trends in Ecology & Evolution 21(2), 71-76.
| Crossref | Google Scholar | PubMed |
Santos JL, Sitters H, Keith DA, Geary WL, Tingley R, Kelly LT (2022) A demographic framework for understanding fire-driven reptile declines in the ‘land of the lizards’. Global Ecology and Biogeography 31(10), 2105-2119.
| Crossref | Google Scholar |
Smith A, Muelders B, Bull CM, Driscoll D (2012) Wildlfire-induced mortality of Australian reptiles. Herpetology Notes 5, 233-235.
| Google Scholar |
Storr GM (1991) Revision of Lerista microtis (Lacertilia: Scincidae). Records of the Western Australian Museum 15(2), 469-476.
| Google Scholar |
Teale R, Craig M, Valentine L (2017) Lerista viduata. The IUCN Red List of Threatened Species 2017: e.T109477698A109477721. Available at http://dx.doi.org/10.2305/IUCN.UK.2017-3.RLTS.T109477698A109477721.en [accessed 23 November 2022]
Tingley R, Meiri S, Chapple DG (2016) Addressing knowledge gaps in reptile conservation. Biological Conservation 204, 1-5.
| Crossref | Google Scholar |
Velazco SJE, Ribeiro BR, Laureto LMO, De Marco Júnior P (2020) Overprediction of species distribution models in conservation planning: a still neglected issue with strong effects. Biological Conservation 252, 108822.
| Crossref | Google Scholar |
Whittaker RJ, Araújo MB, Jepson P, Ladle RJ, Watson JEM, Willis KJ (2005) Conservation biogeography: assessment and prospect. Diversity and Distributions 11(1), 3-23.
| Crossref | Google Scholar |
Wisz MS, Pottier J, Kissling WD, Pellissier L, Lenoir J, Damgaard CF, Dormann CF, Forchhammer MC, Grytnes J-A, Guisan A, Heikkinen RK, Høye TT, Kühn I, Luoto M, Maiorano L, Nilsson M-C, Normand S, Öckinger E, Schmidt NM, Termansen M, Timmermann A, Wardle DA, Aastrup P, Svenning J-C (2013) The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biological Reviews 88(1), 15-30.
| Crossref | Google Scholar | PubMed |


