Artificial nesting hollows for the conservation of Carnaby’s cockatoo Calyptorhynchus latirostris: definitely not a case of erect and forget
Denis A. Saunders A , Rick Dawson B and Peter R. Mawson
A , Rick Dawson B and Peter R. Mawson  C *
C *
A Retired.
B Independent Consultant.
C Perth Zoo, Department of Biodiversity, Conservation and Attractions, WA 6151, Australia.
Pacific Conservation Biology 29(2) 119-129 https://doi.org/10.1071/PC21061
Submitted: 17 September 2021 Accepted: 18 January 2022 Published: 1 March 2022
© 2023 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Context: Loss of nest hollows in eucalypt woodlands is a major cause of decline for a range of Australian native birds including Carnaby’s cockatoo Calyptorhynchus latirostris, resulting in fewer nest sites for this species. Provision of artificial nesting hollows for Carnaby’s cockatoo is a recent part of approved environmental offsets under Western Australian and Australian government environment approvals processes.
Aims: We examined the continuing utility of natural and artificial nest hollows over time at Coomallo Creek, WA.
Methods: Data collected included the location of natural hollows, and their rates of utility and decay. We also installed artificial hollows and measured their use over time, as well as costs associated with their installation and maintenance.
Key results: Both nest types require repairs on average every 3–4 years. Repairs extend the working life of natural nest hollows and ensure that any artificial nest hollows established for conservation management purposes should continue to fulfil their purpose. Our results demonstrate the importance of regular maintenance to ensure hollows remain available for breeding cockatoos.
Conclusions: Artificial nest hollows provide a short-term solution to a larger problem of loss of native woodlands, but will only serve that purpose if current and future artificial nest hollows are monitored and repaired on a regular basis, and that adequate funds are provided to ensure that those nest hollows remain serviceable.
Implications: Provision and maintenance of large numbers of artificial hollows in association with restoration/replanting of woodlands in breeding areas is the only long-term solution to loss of breeding habitat.
Keywords: Calyptorhynchus, conservation offsets, cost of hollow maintenance, monitoring artificial hollows, monitoring natural hollows, natural hollow loss, nesting hollow repairs, Zanda latirostris.
Introduction
Loss and degradation of forests and woodlands have been occurring on every continent, except Antarctic, for a long time. The impacts of this destruction and degradation on biota that rely on tree hollows/cavities for breeding or roosting have been recognised for decades (Lack 1954; Saunders et al. 1982; Newton 1994). In addressing this loss of breeding and roosting habitat, there has been a long history of providing artificial installations such as nest boxes and hollows for many species on those continents (Newton 1994; Gibbons and Lindenmayer 2002; Vaughan et al. 2003; Cowan et al. 2021). Despite the large number of papers on nest/roost site augmentation, there has been a marked lack of rigour in the approach to installing and assessing the efficacy of artificial refuges. It is fair to state that in the majority of cases, installations were ad hoc, guided by the belief that ‘if you build them they will be used’ (Saunders et al. 2020; Cowan et al. 2021). Saunders et al. (2020) reviewed 40 nest site augmentation experiments, including those for parrots and cockatoos. They noted that few studies compared breeding success of those using artificial hollows with those using natural hollows. What was clear from the many studies of nest site augmentation was that the efficacy of artificial nesting sites is idiosyncratic and their conservation worth needs to be established for each species. Cowan et al. (2021) reviewed 224 studies of artificial wildlife refuges implemented in the field for mainly for birds and bats, from 1948 to 2018. They found that with few exceptions, there is little understanding of the science underpinning artificial refuges and what comprises best practice for artificial refuge design and implementation for wildlife conservation. They set out a series of steps in the design, implementation and monitoring of artificial refuges to prevent perverse outcomes and to maximise the chances of achieving useful conservation outcomes.
Saunders et al. (2020) presented the results of a rigorous experiment into the efficacy of artificial hollows for Carnaby’s cockatoo (Calyptorhyncus latirostris) at Coomallo Creek in Western Australia. The cockatoo is an obligate hollow-nesting species, using nest hollows found in large, mature eucalypt trees (Saunders 1979). The experiment demonstrated that the birds readily accepted artificial hollows and that breeding success and body condition of nestlings produced in artificial nest hollows was not significantly different from those breeding in natural hollows. They showed that lack of suitable natural tree hollows was limiting the breeding population at their study site. With the installation of artificial nesting hollows commencing halfway through the breeding season in 2011, the number of breeding attempts increased from 52 to 110 by 2018 (Saunders et al. 2020) and 134 by 2020 (D. A. Saunders, R. Dawson, P. R. Mawson, unpubl. data). Between 2011 and 2018, artificial hollows provided 45% of available hollows and 54% of breeding attempts were made in them. With the breeding pairs of Carnaby’s cockatoo at Coomallo Creek between 1970 and 2020 successfully fledging an average of 0.705 nestlings/year (D. A. Saunders, R. Dawson, P. R. Mawson, unpubl. data), the population does not have the capacity to more than double in 10 breeding seasons, so the provision of additional hollows must have allowed more of the population access to hollows. In addition, prior to 1996, no female under 4 years old was recorded breeding, yet with the provision of extra hollows, 3-year-old females attempted to breed, in some cases successfully (Saunders et al. 2020).
Carnaby’s cockatoo is a black cockatoo with a distinctive white tail-band endemic to south-western Australia. It was once common throughout its range, but development for broad-scale agriculture, towns and cities has resulted in extensive loss of breeding and foraging habitat resulting in major declines in the species’ range and abundance (Saunders 1982, 1990). The species has gone from being classified as vermin prior to the 1970s to being listed in Western Australia as a threatened species under the ranking of ‘Fauna that is rare or likely to become extinct’ in Schedule 1 of the Western Australian Wildlife Conservation Specially Protected Fauna Notice 2018; as endangered under the Australian Federal Government’s Environment Protection and Biodiversity Conservation Act 1999, and under IUCN’s Red List category and criteria (IUCN 2021). Recently the scientific name of the species was changed to Zanda latirostris (BirdLife International 2020), but we retain the name C. latirostris, as specified in the Western Australian Government notice.
As a result of its endangered status, Carnaby’s cockatoo is subject of a Recovery Plan (Department of Environment and Conservation 2012) that lists repair of damaged and sub-optimal natural hollows and supplementation of hollow availability by installing and maintaining artificial nest hollows as recovery management actions. The provision of artificial nesting hollows is a recent, but increasingly accepted as part of approved environmental offsets under Western Australian and Australian government environment approvals processes and there is a need for guidelines based on experimental field trials to maximise the effectiveness of artificial hollows for the species. Prior to 2016, artificial nest hollows had never been used as part of environmental offsets (Richards et al. 2020), but by 2020, 85 artificial nest hollows had been deployed as part of approved offsets associated with three development projects (P. R. Mawson, unpubl. data).
In this paper, we report on the change in status of natural and artificial hollows at Coomallo Creek, Western Australia over time, demonstrate the required frequency and importance of regular maintenance to ensure both natural and artificial hollows remain available for breeding cockatoos, and provide data on the costs associated with repair of derelict natural hollows and the installation and maintenance of artificial nest hollows. We offer the results of our study as a guide to improve the effectiveness of the installation of artificial hollows for the conservation of Carnaby’s cockatoo and potentially other black cockatoo species.
Materials and methods
Carnaby’s cockatoo
Carnaby’s cockatoo is a large (650 g), long-lived bird that commences breeding at 3 or 4 years of age, nests in large hollows in mature eucalypts, pairs for the life of their partner, and breeds every year in late Austral winter and spring in the same hollow if successful during the previous breeding attempt, or in a nearby hollow if unsuccessful. The usual clutch is two eggs, with the second egg laid on average 8 days after the first. Incubation begins with the first egg and takes 29 days for each egg. Both eggs usually hatch, but the second nestling usually dies within 48 h of hatching. At Coomallo Creek between 1969 and 2020, 70.5% of 2093 breeding attempts were successful, and in 5% of these breeding attempts both nestlings fledged 10–11 weeks after hatching (Saunders 1982; Saunders et al. 2014a; Saunders and Dawson 2018; D. A. Saunders, R. Dawson, P. R. Mawson, unpubl. data).
Natural nest hollows
One breeding population of Carnaby’s cockatoo has been studied intensively at Coomallo Creek in the northern wheatbelt of Western Australia. This study commenced in the breeding season of 1969 and continued until 1996, resumed in 2009 and continued to 2020. The breeding population was monitored during 22 of the breeding seasons from 1969 to 1996, and then every year from 2009, using the monitoring protocols described by Saunders and Ingram (1998). At Coomallo Creek, the cockatoos breed in hollows distributed along a 9 km belt of wandoo (Eucalyptus wandoo) woodland surrounded by cleared agricultural land and remnant native heathland vegetation (Saunders 1982; Saunders and Ingram 1998; Saunders and Dawson 2018).
During each breeding season, the study area was visited for up to 5 days in both September and November. Since 2009, the area was visited more often, including in January to record breeding success. During each visit in September and November, every natural hollow known to be used by the cockatoos was checked, contents noted, and any Carnaby’s cockatoo nestlings measured and banded with a unique numbered, stainless-steel Australian Bird and Bat Banding Scheme leg band. In addition, searches were made to find any breeding hollows not previously recorded by us.
From 2009, any natural hollows known to have been used for breeding that had become unusable by cockatoos were repaired, where possible. The reasons for hollows becoming unusable included trees being destroyed (blown or fallen over; pushed over during clearing of native vegetation; or burnt either deliberately, by wildfire or struck by lightning); the side or top of the hollow breaking off such that there is no hollow; the floor of the hollow falling through such that the floor becomes too narrow or jagged or the hollow becomes too deep; or removing branches that had fallen into the hollow, blocking access. Hollows where the side had fallen off were repaired by attaching sheet metal to cover the break and where the floor had fallen, it was raised to within 1000 mm of the entrance by filling with rocks, soil and finally 200–300 mm of woodchips. This nominal 1000 mm depth is the average depth of successful natural hollows used by the birds at Coomallo Creek (Saunders 1979).
Artificial nest hollows
In 2011, we commenced an experiment involving the installation of artificial hollows throughout the study area (Saunders et al. 2020). After some active adaptive experimentation, we found the most effective artificial hollows were black polyvinyl chloride tubes at least 375 mm in diameter and 1000 mm deep, fitted with galvanised steel access ladders, 50 × 100 mm eucalypt sacrificial chewing posts from the floor of the hollow to just above the entrance, and floors lined with eucalypt woodchips with the woodchip lining at least level with the base of the access ladder. The internal dimensions of the most effective artificial hollows were those of the average natural hollow (Saunders 1979; Saunders et al. 2020).
Complete descriptions of the Coomallo Creek study area, our experimental protocol, and the artificial hollows used can be found in Saunders et al. (2020). During each visit from 2011, each artificial hollow was checked, contents noted, any nestlings measured and banded, and any hollows needing repairs were attended to in the months following the end of the breeding season.
Costs associated with installation of artificial hollows and repairs of both natural and artificial hollows
Indicative costings for the supply and deployment of artificial nest hollows are provided, based on a sample size of 50 nest hollows, and our experience with such a program at the study site (800 km round trip from departure point). Estimates of per nest hollow repair costs are provided for the two nest hollow types, based on our experience of the number of each hollow type that can be repaired in a working day. Costs are based on considerable experience over a decade of installing and renovating natural and artificial hollows, not only at Coomallo Creek, but also at a number of other sites, throughout the breeding range of Carnaby’s cockatoo. Values are expressed in 2021 Australian dollars (AUD).
Statistical analyses
Summary statistics describing the number of natural and artificial nest hollows used in this study are provided, along with numbers and percentage remaining after specified dates (2009 and 2020). The causes of loss of natural nest hollows and the numbers of nest trees lost due to each cause are provided. The average (±s.d. and range (years)) interval between discovery of natural nest hollows and the date repairs were first required, and the interval between deployment of artificial nest hollows and the date repairs were first required were calculated. The average values calculated for the two nest hollow types were compared using Student’s t-test (unpaired sample size). The average interval for second and any subsequent repairs for both nest hollow types were also calculated. The average intervals between the date of discovery (natural nest hollows) or deployment (artificial nest hollows) and first repair were compared using a Student’s t-test.
Ethical approval
Field work and animal handling were conducted under appropriate ethics approvals (held by CSIRO staff for the period 1969–1996, and Western Australian Department of Biodiversity, Conservation and Attractions Animal Ethics Committee project approval numbers 2011/30 and 2014/23 for 2009–2020), and bird banding approvals for the same periods (Australian Bird and Bat Banding Authority #418 held by DAS and #1862 held by PRM).
Results
Natural hollows
Between 1969 and 1996, Carnaby’s cockatoos were recorded breeding in 188 nest hollows (Table 1) in 182 trees. The majority (n = 172) were in live wandoo trees, seven were in dead wandoo trees, and the remainder in three live powderbark wandoo (Eucalyptus accedens) trees. By 1996, 121 (64.4% of the original 188 nest hollows) of those nest hollows remained suitable for use by the cockatoos, by 2009 the total was 101 (53.7%) nest hollows, and by 2020 the total was 43 (22.9%) nest hollows (Table 2). Of the 182 trees containing the hollows, by 2020, 70 (38.5%) had been destroyed, an attrition rate over 51 years of 0.75% per annum.

|
Of the 101 natural hollows extant in 2009, 34 (33.6%) had been repaired at least once by 2020 to ensure they remained suitable for use (Table 1). Between 2009 and 2020, 68 newly discovered hollows (located in 67 trees) were recorded being used by the Carnaby’s cockatoos. By 2020, 52 (76.4%) of those remained suitable for use, and 29 (42.6%) had been repaired at least once (Table 1).
During the period 2009–2020, 62 natural nest hollows were removed from our list of those needing to be monitored as we no longer considered them suitable for use by Carnaby’s cockatoos based on the lack of evidence of interest being shown by the cockatoos or due to wildfire or catastrophic collapse of the tree/nest hollow. Forty four (71.0%) of those 62 hollows did not receive any repairs prior to being removed from the monitoring list. Thirty six of those 44 trees were first identified during the period prior to 1996 and the other eight were first identified in the period 2009–2020.
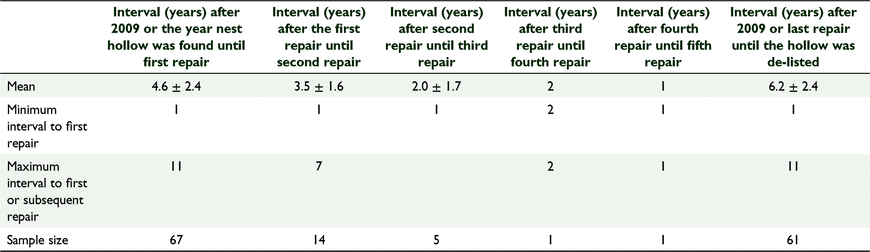
Between 2009 and 2020, 67 natural hollows were repaired and the average (±s.d.) interval before first (or only) repairs were required was 4.6 ± 2.4 years. Twenty one of these hollows required repairs on more than one occasion, and in those cases the average interval between the first and subsequent repairs shortened with each repair cycle until repairs were required annually (Table 3). Eighty three natural hollows did not require or receive any repairs.
The history of Tree #22 (Fig. 1) over the past 50 years provides an example of changes in hollow condition and the value of repairing natural hollows. The tree is forked with a hollow in each fork (22-high and 22-low) where the branches had broken off. In the 21 years, the area was monitored between 1970 and 1996, 22-high was used for 18 breeding attempts and 22-low for 10, with both nest hollows in use during eight of those years. In 2009, the tree still had the same form (Fig. 1) with two suitable nest hollows. Hollow 22-high was used by a pair of western corellas (Cacatua pastinator) from 2009 to 2014. This aggressive species would have discouraged use of 22-low by other birds. In 2017, the floor of 22-high collapsed into 22-low; the tree then had one hollow with two entrances. Repairs were carried out in January 2018 so that the tree again had two suitable hollows. Following the repairs, 22-high was used by Carnaby’s cockatoos in 2018, 2019 and 2020; however, 22-low was not used. In 2019, the floor of 22-low collapsed and the hollow base was too small for the cockatoos; woodchips and woodland litter were added to raise the floor so that the base of the hollow was large enough for the cockatoos to use again.
Artificial nest hollows
Between 2011 and 2020, 119 artificial nest hollows were installed at Coomallo Creek (Fig. 2). For reasons associated with an earlier study (Saunders et al. 2020), by 2020, 50 were intentionally removed after 3–7 years due to our better understanding of the cockatoos’ requirements and replaced with larger diameter artificial nest hollows (Table 4). Forty five (37.8%) artificial nest hollows were repaired during the period 2011–2020. The average (±s.d.) interval between installation of the hollow and the first repair was 3.7 ± 2.0 years (n = 45; range 1–7 years) and between the first and second repairs, 1.8 ± 0.5 years (n = 4; range 1–2 years). As with the natural nest hollows, for those artificial nest hollows that required more than one repair, the interval between successive repairs was on average of shorter duration than the interval from the time of installation to the first repairs. Repairs included replacing or reinforcing floors that had been chewed through by cockatoos; adding woodchips to ensure floors were level with the bottom steel access ladders (Fig. 2); repairing any attachments to the host tree; and replacing sacrificial chewing posts, some of which had been reduced to stumps within a single breeding season, but others were hardly touched (Fig. 3). A comparison of the average interval between the time of first recording a natural nest hollow and it being repaired (4.6 ± 2.4 years; n = 67) to that of an artificial nest hollow being erected and first repaired (3.7 ± 2.0 year; n = 49 s) was not significantly different (Student’s t-test: t = 1.946, d.f. = 114, P = 0.054).

|
Re-use of repaired hollows
Data were available on the re-use after repairs of natural and artificial nest hollows. The average interval between the date the hollows were repaired and their first use by any bird species was 1.0 ± 1.7 years (range 0–7 years; n = 45) for natural hollows and 0.4 ± 0.6 years (range 0–2 years; n = 26) for artificial hollows. There was no significant difference (Student’s t-test: t = 1.63, d.f. = 69, P = 0.11) between the average intervals for the two nest hollow types.
Costs associated with installation, and subsequent monitoring and maintenance
The total cost in Australian dollars associated with installing 50 artificial hollows at one location would be AUD39306, or AUD786 per artificial hollow (Table 5). These costs would vary with distance to the site where the artificial hollows were to be deployed and the time required for field staff transporting and installing the hollows. Once installed, hollows need to be monitored and maintained. The costs associated with these activities involving two people are labour, attention to occupational and health safety requirements, accommodation and meals, travel, and materials and total AUD1350 per day (two staff) plus AUD728 for travel (based on 800 km round trip).

|
Depending on the nature of the repairs required, the number of nest hollows that can be reasonably repaired in a day can range from 8 to 10 in situations where major repairs are carried out on natural hollows, and up to 30 where the hollow substrate is topped up or sacrificial chewing posts are replaced in artificial hollows. Our experience at Coomallo Creek indicated that a 3-day field trip can result in repairs to 16–20 natural nest hollows at a cost of AUD239–AUD299 per hollow and 60 artificial nest hollows at a cost of AUD79 per hollow. In areas where an elevated lift platform is required to install hollows beyond the reach of an extension ladder, extra costs for machinery hire would be incurred.
Given that a small number of both natural and artificial nest hollows required repairs within a year of installation or the previous repair, there are good reasons to monitor nesting hollows at least every 2 years to ensure that hollows, regardless of type remain serviceable and available to cockatoos for as long as possible.
Discussion
Loss of natural hollows
Saunders et al. (2003) demonstrated that in a remnant patch of salmon gum (Eucalyptus salmonophlioia) and York gum (Eucalyptus loxophleba) woodland at Nereeno Hill in the northern wheatbelt of Western Australia, in which four species of cockatoo (including Carnaby’s cockatoo) nested, over nearly 20 years there had been major degradation and loss of mature hollow-bearing trees with no regeneration since the 1920s. Saunders et al. (2014b) showed similar results for the wandoo woodland at Coomallo Creek from 1969 to 2013, a trend that continues to the present (Table 1). This phenomenon is not only common throughout woodland in agricultural areas of southern Australia (Commonwealth of Australia 1996), but most woodland the world over (Newton 1994).
As demonstrated by Valera et al. (2018), our results also show that repairing natural hollows can extend the life of hollows. However, for many of the older (i.e. first identified pre-1996) nest trees it only delays their inevitable demise (Tables 1 and 3) by a decade or so. It is worth noting that mature trees may be centuries old and having a workable capacity to prolong the life of critical resources such as nest hollows is important as it allows a long-lived species such as Carnaby’s cockatoo to remain faithful to a familiar breeding site and it ensures that a wider range of species (birds and potentially mammals) can also access the same hollows at other times of the year. However, given the annual rate of attrition of mature trees and the length of time it takes for a tree to grow large enough to support a hollow large enough for Carnaby’s cockatoo (Saunders 1979; Mawson and Long 1994), it also highlights the need for a long-term strategy of regeneration of breeding areas to replace the inevitable losses of mature hollow-bearing trees (Lindenmayer et al. 2009; Saunders et al. 2020).
Artificial nest hollows
Our results on the design, installation and efficacy of artificial hollows for Carnaby’s cockatoo are drawn from a rigorous experiment that conforms to the high standards laid out by Cowan et al. (2021). We acknowledge that it is based on only one site, Coomallo Creek in the northern wheatbelt of Western Australia, but our findings are also confirmed by installation of artificial nest hollows for Carnaby’s cockatoo in five other locations on private property over the range of the species. However, recommendations for the design, installation and maintenance of artificial nest hollows for Carnaby’s cockatoo can be applied with confidence elsewhere in the range of species. To be fit for purpose, artificial hollows should be 1000 mm deep, with a floor diameter of at least 375 mm, a base that cannot be destroyed by nesting birds, have a steel access ladder reaching to the bottom of the nest hollow, a floor lining of woodchips, and fixed with chain to a live tree affording the hollow shade during the middle of the day (Saunders et al. 2020).
Failure to follow these recommendations may render artificial hollows ineffective many years before the notional end of life of the construction materials used in the artificial nest hollows. For example, in 2013 a private landowner installed three artificial hollows and two more in 2018, all of which were at least 375 mm in diameter and 1200 mm deep with access ladders and sacrificial chewing posts. The property was within 3 km of an area where Carnaby’s cockatoos were known to breed. While all hollows showed signs that Carnaby’s cockatoos had inspected them (birds seen around the hollows and chipping of the sacrificial chewing post around the entrances), none were used by the birds between 2013 and 2020. In May 2021, RD was asked to inspect the hollows and found that the nesting substrate provided when the hollows were installed was poor quality and was well below the bottom rung of access ladder (Fig. 2). Jarrah (Eucalyptus marginata) woodchips were added to each hollow raising the floor to level with the bottom rung of the access ladder. On 3 September 2021, one hollow was in use by a pair of breeding Carnaby’s cockatoos with one egg, and the other four hollows were being inspected by other pairs.
Despite being new when installed, artificial hollows require repairs over time (Table 4), at an average frequency not significantly different from that recorded for natural hollows. Repairs to artificial nest hollows typically require less materials, labour and time than required for natural nest hollows. Cockatoos rapidly identified repaired hollows and re-occupied them, with no significant difference in the interval from repair to re-use between the two nest hollow types. The slightly longer, but not significantly different, period of time until reuse of natural hollows may reflect the need for Carnaby’s cockatoos (and other bird species) to include repaired hollows, especially those that had been out of service for a long time, in the suite of hollows prospected each year. More species of birds have been recorded using natural hollows than the artificial nest hollows we deployed at Coomallo Creek (Saunders et al. 2020), and artificial nest hollows are not used by European honeybees (Apis mellifera), which means that Carnaby’s cockatoos have less competition for artificial nest hollows than they do for natural nest hollows.
Monitoring and maintenance
Our results indicate that regardless of the type of nest hollow involved, some form of regular maintenance program is required to keep the supply of hollows stable. When the capital cost of supplying and deploying artificial hollows is considered (AUD786 per hollow), it would be false economy to consider artificial hollows to be a ‘set and forget’ option for conserving breeding populations of Carnaby’s cockatoos. If artificial hollows have been deployed as part of an environmental offset and then not maintained, the predictable failure of those same hollows would negate a key goal of offsets, in so much as they are meant to be an offset in perpetuity (Government of Western Australia 2011; Department of Sustainability, Environment, Water, Populations and Communities 2012; IUCN 2016). The materials that artificial hollows are constructed from could reasonably be expected to have a working life of 30–50 years, and possibly longer. This working life span can only be achieved if they are constructed and maintained in accordance with official guidelines (see https://www.dpaw.wa.gov.au/plants-and-animals/animals/208-saving-carnaby-s-cockatoo).
The erection of artificial nest hollows provides what can be termed a practical offset (Bull et al. 2013). Practical offsets include development proponent compliance with offset requirements, as well as measuring and monitoring ecological outcomes during and post-completion of the offset. Acceptable offset implementation relies heavily on enforcement (IUCN 2014). Given our findings in this study that artificial nest hollows on average require servicing every 3–4 years, it would seem prudent for regulators that approve the use of artificial nest hollows as environmental offsets for Carnaby’s cockatoos to also include a requirement that sufficient funds are set aside to cover the costs of monitoring and repair of those hollows (Lindenmayer et al. 2017). The monitoring serves two functions in that it provides important information to confirm that the artificial hollows are fulfilling their intended purpose (Richards et al. 2020), and it provides regular information on when and what type of repairs are required. This allows for cost-effective field planning to undertake the necessary repairs.
While our study of artificial and natural nest hollows was not part of any offset program, our results provide reason for concern about how well artificial hollows that have already been deployed at other sites in the recent past might be performing. In the absence of monitoring and or repairs, it is highly likely that a significant proportion (>50%) of them are no longer suitable for use by Carnaby’s cockatoos. If this is the case, then there is a clear opportunity to achieve significant gains for the species with only modest effort and investment via government (Department of Agriculture, Water and Environment 2021) and non-government (BirdLife Australia Western Australia 2021) conservation programs.
Artificial hollows provide an important short-term conservation action for breeding Carnaby’s cockatoos because without regeneration of natural woodland to provide a long-term solution to the loss of natural woodland, the future for mature hollow-bearing trees and for non-hollow-bearing trees needed to support artificial hollows is bleak. The concept of what constitutes ‘short-term’ is articulated by the findings from this study, which show that on average, artificial hollows required maintenance within 3–4 years of being deployed. In the absence of maintenance, there is the risk that artificial hollows will cease to provide any value to Carnaby’s cockatoos.
Conclusion
The results from this study show that natural nest hollows used by Carnaby’s cockatoos have a finite, and relatively short working life, although some of them may have been used for decades before 1969. Natural nest hollow life can be extended with regular (3–4 yearly) maintenance, but not indefinitely. Historically, this would have not been a problem because of the continuing natural hollows coming on-line, but habitat destruction and lack of regeneration for many decades has meant that this is no longer the case; so, the balance of new versus derelict nest hollows has been permanently altered. Repairing both natural and artificial nest hollows is rewarded with rapid re-use (<1 year) of both hollow types. Artificial hollows are a viable short-term alternative to the loss or attrition of natural nest hollows, but they too require regular repair every 3–4 years to remain functional. To assist landowners and consultants in developing budgets to ensure the viability of natural and artificial hollows for Carnaby’s cockatoos, indicative costs for the supply and deployment of artificial hollows are provided. The cost estimates will likely have application in other parts of Australia where the same type of artificial hollow has been or is being considered for use.
Data availability
The data used to generate the results in this paper are available on request from the senior author.
Conflicts of interest
We declare no conflicts of interest in relation to this research.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
We are grateful to the following: John Ingram for technical support 1972–1996; the Hayes, McAlpine, Paish, and Raffan families for supporting this research on their properties; the Raffan family for providing accommodation during the field work; Francis Schmidt of Serpentine-Jarrahdale Landcare who provided all artificial hollows; Palm Beach Rotary Club, and in particular Mr Desmond Mant, Mr Alan Guthrie, and Mr Neil Wall for their tireless assistance with installing/repairing and monitoring hollows; and Dr Manda Page for support. We are also grateful to Professor John Craig ONZM and Professor Ross Goldingay for critical comment on earlier drafts of our manuscript.
References
BirdLife Australia Western Australia (2021) Southwest Black-Cockatoo recovery program. Available at https://birdlife.org.au/projects/southwest-black-cockatoo-recovery [Accessed 16 September 2021]BirdLife International (2020) Species factsheet: Zanda latirostris. Available at http://datazone.birdlife.org/species/factsheet/short-billed-black-cockatoo-zanda-latirostris [Accessed 6 August 2021]
Bull, JW, Suttle, KB, Gordon, A, Singh, NJ, and Milner-Gulland, EJ (2013). Biodiversity offsets in theory and practice. Oryx 47, 369–380.
| Biodiversity offsets in theory and practice.Crossref | GoogleScholarGoogle Scholar |
Commonwealth of Australia (1996) ‘State of the environment: Australia 1996’. (CSIRO Publishing: Collingwood, Australia)
Cowan, MA, Callan, MN, Watson, MJ, Watson, DM, Doherty, TS, Michael, DR, Dunlop, JA, Turner, JM, Moore, HA, Watchorn, DJ, and Nimmo, DG (2021). Artificial refuges for wildlife conservation: what is the state of the science? Biological Reviews 96, 2735–2754.
| Artificial refuges for wildlife conservation: what is the state of the science?Crossref | GoogleScholarGoogle Scholar | 34269510PubMed |
Department of Agriculture, Water and Environment (2021) Threatened species strategy 2021–2031. Available at https://www.environment.gov.au/system/files/resources/e5a59d43-c034-4117-8dcb-3ffc5c465683/files/threatened-species-strategy-2021-2031.pdf. [Accessed 16 September 2021]
Department of Environment and Conservation (2012) ‘Carnaby’s Cockatoo (Calyptorhynchus latirostris) recovery plan’. (Department of Environment and Conservation: Perth, Western Australia). Available at http://www.environment.gov.au/biodiversity/threatened/recovery-plans/calyptorhynchus-latirostris-recovery-plan. [Accessed 16 September 2021]
Department of Sustainability, Environment, Water, Populations and Communities (2012) ‘Environmental Protection and Biodiversity Conservation Act 1999 Environmental Offsets Policy’. (Australian Department of Sustainability, Environment, Water, Population and Communities: Canberra). Available at https://www.awe.gov.au/environment/epbc/publications/epbc-act-environmental-offsets-policy [Accessed 25 November 2021]
Government of Western Australia (2011) ‘WA Environmental Offsets Policy’. (Government of Western Australia: Perth). Available at https://www.epa.wa.gov.au/policies-guidance/wa-environmental-offsets-policy-2011-and-guidelines [Accessed 25 November 2021]
Gibbons P, Lindenmayer D (2002) Tree Hollows and Wildlife Conservation in Australia. CSIRO Publishing, Melbourne.
IUCN (2014) ‘Biodiversity offsets technical study paper’. (International Union for Conservation of Nature: Switzerland). Available at https://portals.iucn.org/library/node/44900 [Accessed 16 September 2021]
IUCN (2016) ‘Policy on biodiversity offsets’. (International Union for Conservation of Nature: Switzerland). Available at https://www.iucn.org/downloads/iucn_biodiversity_offsets_policy_jan_29_2016.pdf [Accessed 25 November 2021]
IUCN (2021) The IUCN red list of threatened species. Version 2021-1. Available at https://www.iucnredlist.org [Accessed 31 August 2021]
Lack D (1954) ‘The natural regulation of animal numbers’. (Clarendon Press: Oxford)
Lindenmayer, DB, Welsh, A, Donnelly, C, Crane, M, Michael, D, Macgregor, C, McBurney, L, Montague-Drake, R, and Gibbons, P (2009). Are nest boxes a viable alternative source of cavities for hollow-dependent animals? Long-term monitoring of nest box occupancy, pest use and attrition. Biological Conservation 142, 33–42.
| Are nest boxes a viable alternative source of cavities for hollow-dependent animals? Long-term monitoring of nest box occupancy, pest use and attrition.Crossref | GoogleScholarGoogle Scholar |
Lindenmayer, DB, Crane, M, Evans, MC, Maron, M, Gibbons, P, Bekessy, S, and Blanchard, W (2017). The anatomy of a failed offset. Biological Conservation 210, 286–292.
| The anatomy of a failed offset.Crossref | GoogleScholarGoogle Scholar |
Mawson, PR, and Long, JL (1994). Size and age parameters of nest trees used by four species of parrot and one species of cockatoo in South-west Australia. Emu – Austral Ornithology 94, 149–155.
| Size and age parameters of nest trees used by four species of parrot and one species of cockatoo in South-west Australia.Crossref | GoogleScholarGoogle Scholar |
Newton, I (1994). The role of nest sites in limiting the numbers of hole-nesting birds: a review. Biological Conservation 70, 265–276.
| The role of nest sites in limiting the numbers of hole-nesting birds: a review.Crossref | GoogleScholarGoogle Scholar |
Richards, B, Sullivan, M, and Mawson, PR (2020). A case study of environmental offsets for the endangered Carnaby’s Cockatoo (Calyptorhynchus latirostris). Pacific Conservation Biology 26, 269–281.
| A case study of environmental offsets for the endangered Carnaby’s Cockatoo (Calyptorhynchus latirostris).Crossref | GoogleScholarGoogle Scholar |
Saunders, DA (1979). The availability of tree hollows for use as nest sites by White-tailed Black Cockatoos. Australian Wildlife Research 6, 205–216.
| The availability of tree hollows for use as nest sites by White-tailed Black Cockatoos.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA (1982). The breeding behaviour and biology of the short-billed form of the White-tailed Black Cockatoo Calyptorhynchus funereus. Ibis 124, 422–455.
| The breeding behaviour and biology of the short-billed form of the White-tailed Black Cockatoo Calyptorhynchus funereus.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA (1990). Problems of survival in an extensively cultivated landscape: the case of Carnaby’s cockatoo Calyptorhynchus funereus latirostris. Biological Conservation 54, 277–290.
| Problems of survival in an extensively cultivated landscape: the case of Carnaby’s cockatoo Calyptorhynchus funereus latirostris.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA, and Dawson, R (2018). Cumulative learnings and conservation implications of a long-term study of the endangered Carnaby’s Cockatoo Calyptorhynchus latirostris. Australian Zoologist 39, 591–609.
| Cumulative learnings and conservation implications of a long-term study of the endangered Carnaby’s Cockatoo Calyptorhynchus latirostris.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA, and Ingram, JA (1998). Twenty-eight years of monitoring a breeding population of Carnaby’s Cockatoo. Pacific Conservation Biology 4, 261–270.
| Twenty-eight years of monitoring a breeding population of Carnaby’s Cockatoo.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA, Smith, GT, and Rowley, I (1982). The availability and dimensions of tree hollows that provide nest sites for cockatoos (Psittaciformes) in Western Australia. Australian Wildlife Research 9, 541–556.
| The availability and dimensions of tree hollows that provide nest sites for cockatoos (Psittaciformes) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA, Smith, GT, Ingram, JA, and Forrester, RI (2003). Changes in a remnant of salmon gum Eucalyptus salmonophloia and York gum E. loxophleba woodland, 1978 to 1997. Implications for woodland conservation in the wheat-sheep regions of Australia. Biological Conservation 110, 245–256.
| Changes in a remnant of salmon gum Eucalyptus salmonophloia and York gum E. loxophleba woodland, 1978 to 1997. Implications for woodland conservation in the wheat-sheep regions of Australia.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA, Mawson, PR, and Dawson, R (2014a). One fledgling or two in the endangered Carnaby’s Cockatoo (Calyptorhynchus latirostris): a strategy for survival or legacy from a bygone era? Conservation Physiology 2, cou001.
| One fledgling or two in the endangered Carnaby’s Cockatoo (Calyptorhynchus latirostris): a strategy for survival or legacy from a bygone era?Crossref | GoogleScholarGoogle Scholar | 27293622PubMed |
Saunders, DA, Mawson, PR, and Dawson, R (2014b). Use of tree hollows by Carnaby’s Cockatoo and the fate of large hollow-bearing trees at Coomallo Creek, Western Australia 1969–2013. Biological Conservation 177, 185–193.
| Use of tree hollows by Carnaby’s Cockatoo and the fate of large hollow-bearing trees at Coomallo Creek, Western Australia 1969–2013.Crossref | GoogleScholarGoogle Scholar |
Saunders, DA, Dawson, R, Mawson, PR, and Cunningham, RB (2020). Artificial hollows provide an effective short-term solution to the loss of natural nesting hollows for Carnaby’s Cockatoo Calyptorhynchus latirostris. Biological Conservation 245, 108556.
| Artificial hollows provide an effective short-term solution to the loss of natural nesting hollows for Carnaby’s Cockatoo Calyptorhynchus latirostris.Crossref | GoogleScholarGoogle Scholar |
Valera, F, Václav, R, Calero-Torralbo, MÁ, Martínez, T, and Veiga, J (2018). Natural cavity restoration as an alternative to nest box supplementation. Restoration Ecology 27, 220–227.
| Natural cavity restoration as an alternative to nest box supplementation.Crossref | GoogleScholarGoogle Scholar |
Vaughan, C, Nemeth, N, and Marineros, L (2003). Ecology and management of natural and artificial scarlet macaw (Ara macao) nest cavities in Costa Rica. Ornitologia Neotropical 14, 381–396.