Whitebait conservation and protected areas at non-tidal rivermouths: integrating biogeography and environmental controls on īnanga (Galaxias maculatus) spawning grounds
Shane Orchard A B C and David R. Schiel A
A B C and David R. Schiel A
A Marine Ecology Research Group, University of Canterbury, Christchurch, New Zealand.
B Waterways Centre for Freshwater Management, University of Canterbury and Lincoln University, Christchurch, New Zealand.
C Corresponding author. Email: s.orchard@waterlink.nz
Pacific Conservation Biology 28(2) 140-153 https://doi.org/10.1071/PC21004
Submitted: 1 February 2021 Accepted: 29 March 2021 Published: 11 May 2021
Journal Compilation © CSIRO 2022 Open Access CC BY-NC
Abstract
Galaxias maculatus is a declining amphidromous fish that supports New Zealand’s culturally important whitebait fisheries targeting the migratory juvenile stage. Spawning ground protection and rehabilitation is required to reverse historical degradation and improve fisheries prospects alongside conservation goals. Although spawning habitat has been characterised in tidal rivers, there has been no previous study of spawning in non-tidal rivermouths that are open to the sea. We assessed seven non-tidal rivers over 4 months using census surveys to quantify spawning activity, identify environmental cues, and characterise fundamental aspects of the biogeography of spawning grounds. Results include the identification of compact spawning reaches near the rivermouths. Spawning events were triggered by periods of elevated water levels that were often of very short duration, suggesting that potential lunar cues were less important, and that rapid fish movements had likely occurred within the catchment prior to spawning events. Spawning grounds exhibited consistent vertical structuring above typical low-flow levels, with associated horizontal translation away from the river channel leading to increased exposure to anthropogenic stressors and associated management implications for protecting the areas concerned. These consistent patterns provide a sound basis for advancing protective management at non-tidal rivermouths. Attention to flood management, vegetation control, and bankside recreational activities is needed and may be assisted by elucidating the biogeography of spawning grounds. The identification of rapid responses to environmental cues deserves further research to assess floodplain connectivity aspects that enable fish movements in ephemeral flowpaths, and as a confounding factor in commonly used fish survey techniques.
Keywords: coastal lagoons, fisheries conservation, floodplain connectivity, migratory species, riparian zones, spatial planning.
Introduction
Galaxias maculatus is a small-bodied amphidromous fish that is widespread across the Pacific where it is known by various names including īnanga, common jollytail, puye and puyen (McDowall 1991; Zattara and Premoli 2005; Barile et al. 2015). Traditional ‘whitebait’ fisheries targeting juvenile fish have been established in several countries including New Zealand, Australia, and Chile, all of which have suffered historical declines that impact the viability of continued harvest and raise conservation concerns (Campos 1973; McDowall 1984; Fulton 2000; Mardones et al. 2008; Vega et al. 2013). These fisheries are situated near the coastal interface and exploit the postlarval stage of amphidromous populations as they migrate into freshwater systems following an oceanic development period of ~6 months (McDowall and Eldon 1980). At this stage, juvenile G. maculatus are largely colourless and typically in the 40–55 mm size range before undergoing rapid developmental changes soon after entering fresh water (McDowall 1968; McDowall et al. 1994). New Zealand’s fishery has considerable recreational, cultural and economic value that contributes to the wellbeing of local communities nationwide, and can be particularly important in small towns (McDowall 1984). In this paper, the management context of whitebait conservation recognises that G. maculatus is the most abundant species in the whitebait catch in the majority of New Zealand rivers (McDowall 1965; Yungnickel et al. 2020), while also being an ‘at risk – declining’ species (Dunn et al. 2018) that requires attention to all life stages.
The lifecycle of migratory galaxiids has important implications for their conservation and the sustainability of whitebait fisheries at a variety of scales (McDowall 2008). At the subglobal scale, the general pattern of amphidromy involves an oceanic development period in which larvae may be transported long distances and potentially colonise distant shores (McDowall 2002, 2007). This life history may be evolutionarily favourable in small island states otherwise depauperate in freshwater fish fauna by providing a mechanism for recruitment to new habitats (Berra et al. 1996; McDowall 2010a). At more local scales, the replenishment of larval sources remains critical to the maintenance of existing populations. In many cases this relies on the continued health of adult fish populations for egg production coupled with the bioregional partitioning of larval pools leading to recruitment pulses, or absences, as the case may be (McDowall 2010b).
In addressing these dynamics, there is considerable debate about the relative importance of oceanic dispersion pathways and larval retention effects, alongside the potential for natal homing, all of which may affect the biogeography of amphidromous larval pools (Waters et al. 2000; McDowall 2010a; Augspurger et al. 2017). Hickford and Schiel (2016) investigated natal homing in the South Island of New Zealand, and found no evidence of juvenile G. maculatus returning to the same rivers in which they hatched. However, some recruits were identified returning to rivers close to their natal origin on the same coast, and 15 juveniles originating from the west coast were identified in east coast whitebait samples (Hickford and Schiel 2016). These results are consistent with oceanic currents having an important role in the structuring of regional larval pools (Chiswell and Rickard 2011).
Several additional lines of evidence point to the importance of local recruitment sources for the maintenance of local populations at certain times. At one extreme, the capacity for a residential and largely non-migratory lifecycle has been identified through studies on land-locked water bodies in Australia (Pollard 1971; Chapman et al. 2006) and South America (Cussac et al. 2004; Barriga et al. 2002, 2007; Carrea et al. 2013). Inherent consequences include larval retainment such that all phases of the life cycle are completed within a significantly smaller spatial domain in comparison to a diadromous lifecycle (McDowall 2010c; Rojo et al. 2020). There is also evidence for a wide spectrum of life histories somewhere between these extremes, such as where larvae drift relatively slowly downstream and complete variable proportions of their growth and development in retentive freshwater bodies (Augspurger et al. 2017), or nearshore coastal environments such as river plumes (Sorensen and Hobson 2005; Shiao et al. 2015).
For G. maculatus, the potential effects of larval retention and bioregional partitioning are heightened in comparison to other whitebait species because of its shorter, generally annual, life cycle (McDowall 2010b). An additional piece of this puzzle is presented by variance in the biogeography of the spawning grounds themselves, with local egg production sources being disproportionately more important to the availability of postlarval recruits where partitioning occurs. This illustrates that effective management demands an understanding of spawning grounds in all regions and river types.
Although the biogeography of G. maculatus spawning grounds has been relatively well characterised in tidal rivers where they are strongly influenced by spring high tides (Benzie 1968; Taylor 2002; Orchard et al. 2018a, 2018b), many non-tidal rivers also support fish populations. In New Zealand, these rivers are particularly common on the east coast of the South Island and south-eastern North Island where they are often associated with mixed sand–gravel beaches at the coast (Kirk 1980, 1991). Many such rivers form perched lagoons, or hāpua, under the influence of high wave energies and associated barrier formation that promotes the establishment of non-estuarine conditions at the rivermouth (Kirk 1991; Hart 2009). Other examples are found in regions with significant alluvial transport from glaciated and paraglacial areas (e.g. Iceland, Russia, Alaska and Canada), and also on fine-grain shorelines in Israel and South Africa (Carter et al. 1989; Cooper 1994; Lichter and Klein 2011; Green et al. 2013; Smith et al. 2014; McSweeney et al. 2017). To date, however, there has been no comprehensive assessment of the location, timing and environmental controls on amphidromous G. maculatus spawning activity at non-tidal rivermouths.
The present study addresses this information gap in a region characterised by a high density of non-tidal rivermouths which facilitated both concurrent investigations at several study sites, and the generation of a wider landscape view. Specific hypotheses included that spawning grounds were likely to be found in rivermouth lagoon environments due to similarities with tidal lagoons, including their ability to support periodically inundated riparian vegetation, and that lunar cycles associated with spring high tide periods were unlikely to act as a trigger for spawning activity, as is the case in tidal rivers, due to the apparent lack of appreciable hydrodynamic effects. Instead, we considered that water level variations would be more likely to trigger spawning events, as has been reported in land-locked rivers (Pollard 1971), and tidal lakes (Richardson and Taylor 2002). Despite these expectations, the research was largely exploratory and involved the characterisation of previously unsurveyed rivers and their associated environments. In the remainder of the paper we report conclusive results that include strong spatiotemporal patterns likely to be transferable to other localities. These provide a sound basis for advancing the management of non-tidal rivermouths to support their role in whitebait conservation.
Methods
Study area
The Kaikōura coast is characterised by a predominance of non-tidal rivers, as is the case elsewhere in the Canterbury region of New Zealand (Supplementary Material, Fig. S1). Seven non-tidal waterways of varying character were chosen within a ~35 km section of coastline between Oaro and Mangamaunu Bay (Fig. 1). These study catchments included two relatively large rivers (Oaro and Kahutara) and five smaller streams. Within this area there were no previous records of G. maculatus spawning grounds in the National Īnanga Spawning Database (NISD) although Taylor and Marshall (2014) observed spawning shoals in Middle Creek. Records in the New Zealand Freshwater Fish Database (NZFFD) include G. maculatus observations in five of the seven study catchments, and further populations were expected to be present in other waterways (Supplementary Material, Fig. S2). Whitebait fishing occurs in at least three of these rivers (Oaro, Kahutara and Lyell Creek).
The study catchments range from 440 ha to over 23 000 ha in size, with the smallest (Blue Duck) representing a 2nd order stream originating in coastal hill-country, and the largest (Kahutara) being a 5th order river originating in the Seaward Kaikōura Range (Table 1). Predominant catchment geologies partly reflect the inland extent of each catchment in relation to hill-country and coastal outwash plains, with the latter being extensive either side of Kaikōura Peninsula where they form mixed sand–gravel beaches at the coast (Fig. 1). As is characteristic of many waterways in the region, five of the study sites featured non-estuarine lagoons at the rivermouth. At Blue Duck and Harnetts creeks no such lagoon was present during the study period, but they have been recorded in the past. All of the study sites, with the exception of Oaro, were affected by tectonic uplift of between 0.6 and 0.9 m during the 2016 Kaikōura earthquake (Clark et al. 2017; Schiel et al. 2019), which has generally increased the elevation of these lagoons and lower river reaches in relation to sea level. In the case of Lyell Creek and Middle Creek high spring tides would occasionally inundate the lower reaches pre-earthquake, but this no longer occurs since the uplift event (P. Adams, pers. comm.). All of the study sites were open to the sea during the study period. This partly reflects the effects of a flood event in February that cleared the rivermouths and also illustrates that these rivers are not land-locked. However, observations during low flow periods in other years have shown that at least four of these waterways (Blue Duck, Harnetts, Middle and Swan) may develop temporary closures associated with the development of semipermeable barriers at the coast (S. Orchard, pers. obs.).

|
Spawning ground surveys
Sampling design
In the first month of the study (February 2019) intensive eggs searches and environmental measurements were completed in all catchments by a team of three researchers in all catchments beginning at the rivermouth and working upstream, with the distribution of spawning sites (if any) being unknown. Although March and April were expected to be the months of peak spawning activity based on previous work in tidal waterways in the Canterbury region (Orchard et al. 2018a, 2018b), significant spawning activity was detected in February that was apparently triggered by a rain event. This finding helped to establish the general location of spawning in the study catchments and define areas for repeat surveys in the following months. This resulted in the delineation of survey reach lengths of between 400 and 700 m from the rivermouth, with the upstream limit marked by a prominent riffle or general increase of gradient in the riverbed. None of the spawning sites detected in the following months were located near the upstream limit of these survey areas, improving confidence that they were sufficient to detect the spawning activity that occurred. Data from permanent stage-height loggers operated by the Canterbury Regional Council were available for the Lyell and Middle Creek catchments (Environment Canterbury 2020). In both cases, these loggers were located several hundred metres upstream of the identified spawning reach but nonetheless provided a useful indication of water level fluctuations in relation to the timing of spawning events. Following discovery of the February spawning sites, additional water level loggers (Odyssey ODYWL, Dataflow Systems Ltd) were installed within the observed or potential/estimated spawning reach in the five remaining catchments to provide further information on water level changes in relation to subsequent spawning events. For all spawning sites detected, real-time kinematic (RTK) GPS surveys were conducted using a Trimble R8 GNSS receiver to measure the upper and lower elevation of the egg band. Geodetic benchmarks were included within all RTK-GPS surveys to achieve an estimated vertical accuracy of 3.5 mm + 0.4 ppm RMS. Heights were referenced to the New Zealand Vertical Datum (NZVD) (Land Information New Zealand 2016).
Detection and measurement of spawning sites
Field surveys
Spawning surveys were completed over four consecutive months (February–May). Following the standard approach in tidal waterways in which spawning events have an apparent relationship with the lunar cycle (Taylor 2002; Orchard and Hickford 2018), each round of surveys commenced a few days after the full moon of the month, which was associated with the largest monthly spring tides within the study period (Table 2). The field survey technique involved systematic searches for eggs laid in riparian vegetation by a team of three people. For every 5 m length of river bank three searches were made at random locations. Each search involved inspection of the stems and root mats along a transect line oriented perpendicular to the river bank. Typically, a 0.5 m wide swathe of vegetation was inspected on each transect, with these being of variable length depending on the bank slope and vegetation extent. In this case, the transect lengths were adjusted to include all vegetation within areas inundated by the February flood event as judged by the position of strand lines and silt deposition. As this was the highest water level recorded during the study period, the spatial extent established in February was applied to all subsequent surveys.

|
Area of occupancy
All egg detections were associated with a given location that was identified as a spawning site (Orchard and Hickford 2018). Confirmed identification of G. maculatus eggs was made on the basis of their characteristic size (~1 mm diameter), position on the riverbank, developmental attributes (i.e. development of ‘eyes’) and observations of adult fish in the vicinity of spawning sites. Individual sites were defined as continuous or semicontinuous patches of eggs with dimensions defined by the pattern of occupancy. In each case, the upstream and downstream extents of the patch were established in the field. Coordinates were recorded using a hand-held GPS and corrected in QGIS ver. 3.12 (QGIS Development Team 2020) with the assistance of site photographs and landmarks. For each spawning site, the length along the riverbank was measured along with the width of the egg band at the position of each of the original search transect, and additional transects where needed to provide a minimum of three measurements at all sites. Zero counts were recorded when they occurred within a spawning site as is common where the egg distribution is not a continuous band. The area of occupancy (AOO) was calculated as length × mean width.
Spawning site productivity
Egg production was assessed by direct egg counts using a subsampling method (Orchard and Hickford 2016, 2018). At each transect, as above, a 10 × 10 cm quadrat was placed in the centre of the egg band and all eggs within the quadrat counted. Egg numbers in quadrats with high egg densities (>200 per quadrat), were estimated by further subsampling using five randomly located 2 × 2 cm quadrats and the average egg density of these subunits used to calculate an egg density for the larger 10 × 10 cm quadrat. The mean egg density was calculated from all 10 × 10 cm quadrats sampled within the site, inclusive of zero counts. The productivity of each site was calculated as mean egg density × AOO.
Statistical analyses
Differences in AOO and egg production trends were tested using two-way ANOVAs with fixed factors of catchment and month, followed by post hoc Tukey HSD tests for significant main effects. Model assumptions were checked via Shapiro–Wilk and Levene tests using the car package in R (Fox and Weisberg 2011). Variation in AOO and egg production was also examined using robust one-way ANOVAs (Field and Wilcox 2017) to assess the effect of catchment and month separately using the robust package in R (Wang et al. 2020). These analyses considered catchment effects in relation to the pooled data from all months, and also for the two main spawning events treated separately (February and April). Similar tests were applied for the dependent variables of distance upstream, and vertical elevation based on the centre point of each spawning site in the horizontal and vertical dimensions, respectively. A regression analysis was used to explore the relationship between egg production and AOO using pooled data from all sites. All analyses were completed in R ver. 3.5.3 (R Core Team 2019).
Results
Occurrence, size and productivity of spawning grounds
Spawning sites were detected in all seven catchments, although at different times over the study period (Fig. 2). Two natural spawning events were responsible for the majority of spawning activity, the first occurring in February at the beginning of the study period, and the second in April. A total of 34 individual spawning sites were measured in these two events (14 and 20 sites, respectively). A single site was detected in March in one of the study catchments (Lyell Creek). However, this spawning event was triggered by the artificial raising of water levels as part of an ecological engineering experiment conducted with the local council, and was thus atypical of the wider regional trend. No spawning was detected in any of the study catchments in May.
Most of the spawning sites were relatively small though often dense patches of eggs. The total AOO recorded over all months (23.4 m2 ± 3.3, s.e.) was significantly different between rivers (d.f. = 6, F = 13.71, P < 0.001), and similar results were obtained when these analyses were restricted to results from February and April (Table 3). The mean egg density across all sites and months was 3.8 eggs cm−2. A regression on the combined data showed a significant relationship between the AOO and egg production of individual spawning sites (R2 = 0.86, F = 189.1, P < 0.001). Decomposition to the individual months showed that the February relationship was stronger (R2 = 0.85, F = 69.38, P < 0.001) than in April (R2 = 0.34, F = 9.28, P = 0.007), reflecting the influence of the large and highly productive Kahutara River sites in February, but also the observation of relatively few sparse sites with low egg densities in relation to AOO.
The total egg production across catchments and months was ~1.8 million eggs (Table 3). Egg production differed significantly between catchments over the study period (d.f. = 6, F = 20.42, P < 0.001) reflecting the influence of relatively high egg production at Kahutara in comparison to the other catchments, even though spawning was recorded there only in February (Table 3b). The between-month comparisons also showed significant differences for both AOO (d.f. = 1, F = 4.55, P = 0.03) and egg production (d.f. = 6, F = 8.89, P = 0.005) that is indicative of much greater spawning activity in February across the region as a whole. Two-way ANOVAs also showed significant differences between catchments (d.f. = 6, F = 15.34, P < 0.001) and between months (d.f. = 1, F = 7.80, P = 0.01), with no significant interaction between the two (d.f. = 4, F = 1.42, P = 0.26).
Catchment position
All of the spawning sites were located surprisingly close to the coast, despite the presence of apparently suitable habitat for spawning further upstream (Fig. 3). This pattern held in all catchments with the mean upstream limit of spawning being 248 m from the rivermouth. The maximum distance upstream of the rivermouth for any spawning site was 388 m in Lyell Creek, and the minimum distance ranged from 65 m to 275 m. This resulted in the identification of well-defined and relatively compact spawning reaches in all catchments by the end of the study period (Fig. 3). These spawning reaches ranged from 5 m in length (associated with a single spawning site) to 251 m at Lyell Creek, which also had the highest number of individual spawning sites (Table 3).
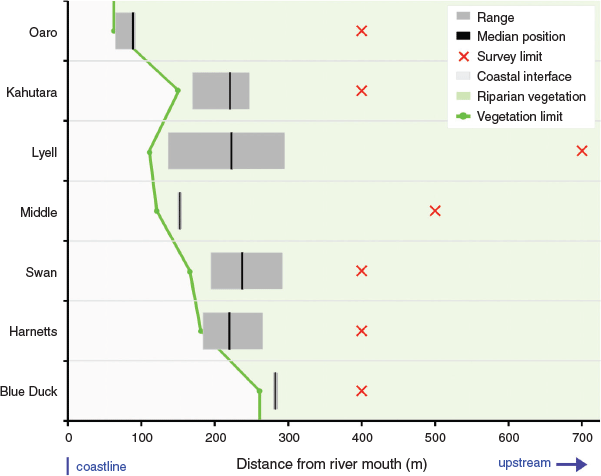
|
The downstream limit of spawning exhibited a close relationship with the downstream limit of riparian vegetation that was subject to at least periodic inundation during the study period (Fig. 3). This relationship was consistent between spawning events in the four study catchments where spawning was recorded more than once, as well as being common to all seven rivers despite marked differences in their morphology and size. The apparent high fidelity to a favoured spawning reach can be seen in the statistical comparisons that show a significant difference between catchments (d.f. = 6, F = 45.0, P < 0.001) for the mean position of spawning sites expressed as distance from the rivermouth (Table 3a). This fidelity is partially obscured in the seasonal totals visualised in Fig. 3, but can clearly be seen in the between-month comparisons for the two main spawning events (Table 3b). Differences in the distance inland were statistically significant between catchments (d.f. = 6, F = 53.3, P < 0.001), but not between months (d.f. = 1, F = 2.28, P = 0.124).
Vertical position
Significant differences were found between rivers in the vertical dimensions of spawning grounds. The highest elevation sites were in Harnetts Stream up to 4.5 m NZVD (New Zealand Vertical Datum 2016), and the lowest elevation sites (0.7 m NZVD) were in Lyell Creek (Fig. 4). In the other rivers spawning grounds fell within the range of 1.0–2.5 m NZVD, with the mean height across all individual spawning sites being 2.1 m ± 0.05 (s.e.). For comparison, the Mean High Water Springs (MHWS) elevation on the Kaikōura coast was ~0.4 m NZVD within this period (LINZ 2018a, 2018b). One-way ANOVAs showed that the mean elevation of spawning sites was significantly different between sites across all months (d.f. = 6, F = 273.3, P < 0.001), and similarly when only February and April were considered (Table 3). However, mean elevation was not significantly different between February and April (d.f. = 1, F = 0.18, P = 0.667). This reflects the occurrence of spawning sites in similar three-dimensional locations between months at Oaro, Lyell and Swan and Harnetts despite the addition of higher elevation sites at the latter in comparison to February (Fig. 4). These impressions are supported by two-way ANOVA results showing significant differences between catchments (d.f. = 6, F = 458.2, P < 0.001), but not months (d.f. = 1, F = 0.15, P = 0.669), and a significant interaction between the two (d.f. = 3, F = 0.19, P = 0.001).
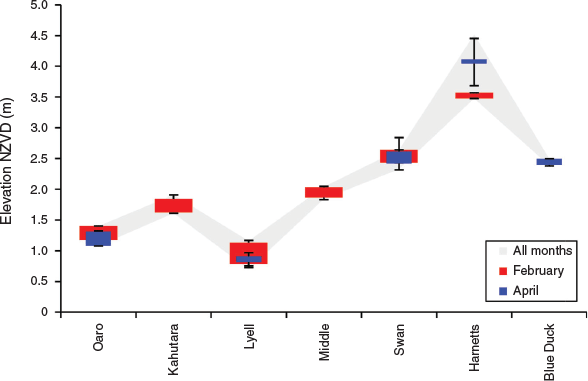
|
Role of environmental cues
There was a strong relationship between water level fluctuations and the timing of spawning events. The first of these occurred unexpectedly and involved an unseasonal heavy rain event in late February (Fig. 5a). Subsequent spawning surveys showed that this event triggered widespread spawning activity through the region that contributed 76% of the AOO and 95% of the egg production measured in the study period as a whole. Stage height data showed that the rain event on 23 February caused a rapid spike in water heights of 70 cm in Lyell Creek and 35 cm in Middle Creek (Fig. 5a), with this difference partly reflecting channel morphology and associated constriction effects at the logger positions as well as catchment-specific discharge rates. The apparent triggering effect of water level fluctuation had a considerable influence on the three-dimensional geography of the spawning grounds, with the lowest elevation eggs being located ~30 cm above typical low-flow levels in the spawning reach at all sites.
By late March, the continuing lack of rain suggested that the February eggs were unlikely to be reinundated naturally and an ecological engineering experiment was designed in collaboration with the regional council involving a temporary (4 h) closure of the Lyell Creek mouth using a gravel bund sufficient to raise water levels upstream by ~40 cm. In addition to facilitating the hatching of February eggs, the March spawning surveys showed that this artificial inundation event triggered spawning in the affected reach despite its short duration. In contrast, no March spawning was recorded in any of the other study catchments (Fig. 2). However, a series of moderate rain events occurred in April sufficient to raise water levels region-wide, and spawning was detected in five of the study catchments. Concurrent water level and spawning site elevation data from the April events provide conclusive evidence of the triggering effect of water level fluctuations (Fig. 5b). Despite marked differences in the hydrographs, all spawning sites were located in an elevated position relative to the water level during the previous period of low flows.
These observations also provide insights into the likely duration of spawning events as revealed by the time at which water levels dropped to below the minimum elevation of spawning grounds. For example, the developmental stage of eggs at Blue Duck Creek suggested that spawning occurred on the rain event on 7 April and the vertical position of eggs indicates that the spawning site was inundated for ~54 h (Fig. 5b). In contrast, the mean spawning site elevations at Lyell Creek were 10 cm lower than the February sites but still a minimum of 20 cm above typical low flow levels. Together with the obviously fresh (non-eyed) appearance of the eggs, the elevation range indicates that spawning occurred during the relatively sharp spike in water levels on 22 April, for which the period of sufficient inundation lasted only a few hours. Harnetts Creek showed a similar pattern, with spawning occurring close to the peak of the 22 April rain event within a short period. These temporal patterns suggest that the fish are both highly attuned to detecting water level fluctuations and likely to be highly mobile within the river at these times. The surprisingly short lag times between the onset of water level fluctuations and spawning events indicate very rapid responses to environmental cues.
Discussion
This study adds to previous evidence of plasticity in G. maculatus life histories (Augspurger et al. 2017), and is consistent with the prediction of Barbee et al. (2011) that the substantial differences in environmental characteristics found across the G. maculatus range could manifest as variability in spawning and migration cues. Moreover, the recognition of diverse life histories in several amphidromous species has also led to calls for reorientation of the term towards relationships between benthic and pelagic life stages rather than necessarily involving a marine–freshwater distinction (Closs et al. 2013; Closs and Warburton 2016). This highlights the need for further research on the characteristics of recruitment sources, and in turn emphasises the need for a solid understanding of the biogeography of spawning grounds, which are the ultimate larval source (McDowall 2010b).
In New Zealand, there is a noticeable information gap on the characteristics of non-tidal rivers despite a considerable body of work on G. maculatus spawning in estuarine locations (McDowall 1968, 1991; Hickford and Schiel 2011; Orchard et al. 2018a), and contrasts with numerous studies on landlocked G. maculatus populations in Australian and South American rivers and lakes (Pollard 1971; Cussac et al. 2004; Chapman et al. 2006; Barriga et al. 2007). Recent advances in New Zealand have included the identification of non-diadromous recruitment in amphidromous galaxiid species, including G. maculatus, where suitable freshwater pelagic habitat was present in rivers open to the sea (Hicks et al. 2017; David et al. 2019). However, despite confirming the potential for spawning in non-tidal environments, these studies provide only limited information on the specific location and relative importance of the associated spawning sites. This is, in part, due to the choice of sampling methodologies that take advantage of otolith microchemistry markers as an indicator of previous life histories such as exposure to marine environments or other chemically distinct water bodies such as lakes (Ruttenberg et al. 2005; Warner et al. 2005). Consequently, these approaches are well suited to identifying broad-scale phenomena such as the presence of non-diadromous individuals, but may lack the resolution needed to ascertain finer-grain aspects such the contribution of recruitment from different sources, and the associated biogeography of spawning grounds (Carson et al. 2013).
This study extends and complements the previous New Zealand work by applying a census survey approach with a focus on the characterisation of spawning grounds (Orchard and Hickford 2018). Although such surveys are resource-intensive in comparison to sparse sampling designs, they have the potential to elucidate fundamental spatial ecology aspects and generate information that is directly useful for site-based management needs. Findings from this study provide the first comprehensive account of spawning ecology in amphidromous G. maculatus populations at non-tidal rivermouths and include strong spatiotemporal patterns that were consistent across study catchments and months. The following sections provide a brief discussion of these aspects with a focus on transferable learning for conservation in other non-tidal rivers, and comparison with the existing knowledge base.
Position in catchment
The observed biogeographical pattern provides strong support for the prediction that spawning grounds would be found in non-estuarine lagoon environments due to similarities with known spawning areas in the lower reaches of tidal waterways. Five of the seven catchments exhibited a dynamic lagoon feature near the rivermouth, and in each case spawning was found within the lagoon or a short distance upstream. In the remaining two catchments (Blue Duck and Harnetts), no rivermouth lagoon feature was present during the study period but the spawning grounds were located in the first slow-flowing reach upstream. At the catchment scale, all spawning grounds occupy a relatively compact reach within the lower river and in all cases high-quality unused spawning habitat was present further upstream.
Although this pattern supports the notion of downstream fish movement to a preferred low-elevation and low-gradient reach as occurs in tidal situations (McDowall 1968), there was also some variation in the observed upstream limit of spawning between events. For example, in Lyell Creek in March, and Harnetts Creek in April, spawning was found further upstream of the February sites, which translated to a larger confirmed spawning reach than if these sites had not been observed. Consequently, different river conditions and potentially also fish-centric factors, such as the size of the spawning population, could introduce further variation and result in a larger reach being important at other times. Despite this, the downstream limit of spawning was relatively consistent and strongly related to the most downstream position of suitable vegetation in the periodically inundated riparian zone (Fig. 3). This suggests that fish are actively searching the lower reaches of the river during spawning events and are selecting appropriate spawning habitat in that vicinity in preference to similar habitat further upstream.
In comparison to other studies, these results show similarities with previous reports of G. maculatus spawning in tidal lowland lakes such as Te Waihora/Lake Ellesmere in Canterbury, and lakes Waihola and Waipori in Otago, where spawning sites have been reported near the confluence of inflowing streams (McDowall 1968; Richardson and Taylor 2002). In some cases, spawning has been recorded out-of-phase with the tidal influence in response to other sources of water level fluctuations that include wind-driven variations in water level height (Richardson and Taylor 2002). In addition, studies from lake environments in other countries have reported a variety of spawning behaviours including upstream migrations to spawning grounds in tributary streams (Pollard 1971), and lacustrine spawning in landlocked lakes (Barriga et al. 2002; Chapman et al. 2006). These variations demonstrate the need for further characterisation of spawning ecology across a range of environments and also the important role of adult fish migrations that are inherent in the selection of spawning sites.
Vertical dimension
Strong structuring in the vertical dimension was a common feature of all spawning sites (Fig. 4). At the reach scale, this resulted in the spawning grounds being 0.4–1.0 m above normal low-flow levels and translated to a wide variety of positional nuances in relation to human activities in, and adjacent to, the river bed. At Blue Duck, Harnetts, Swan and Middle creeks the spawning grounds are located on relatively steep banks close the river channel where they are relatively protected from nearby recreational activities and stock grazing in adjacent land. However, at Lyell Creek, which features well-defined banks in a more urban context, the spawning grounds overlap with areas subject to herbicide spraying and landscaping works. At Kahutara and Oaro rivers, which feature wider river beds and more extensive rivermouth lagoons, spawning grounds are exposed to river diversion earthworks for flood control, vegetation clearance and off-road vehicle use.
These results have important consequences for river management since many human activities are found in the same elevation zone as spawning grounds, as is the case in tidal waterways (Richardson and Taylor 2002; Orchard and Hickford 2018; Orchard et al. 2018a). In the non-tidal context, the major differences include the lack of a regular (i.e. tidal) inundation cycle. This is generally useful from a management perspective by providing a convenient indicator of the most likely elevation range in which spawning will be found, leading to planning approaches based on tidal height as a proxy for the confirmed location of spawning sites (Greer et al. 2015). Despite this, multimonth surveys in tidal river systems have also shown that high-discharge events can have the effect of raising spawning grounds above normal tidal levels, and this may be associated with appreciable horizontal translation away from the river channel into vulnerable areas such as recreation reserves (Orchard 2019). Consequently, attention to the role of discharge rates in the horizontal and vertical structuring of spawning grounds is important. This provides a sound basis for the integration of flood management, vegetation control, and bankside recreational activities to achieve effective conservation at all rivermouths where G. maculatus populations are found.
Timing of spawning, fish movement and environmental cues
The timing of spawning in all seven catchments provided additional support for the hypothesis of spawning events being triggered by rain-induced water level fluctuations. Multiple lines of evidence, including high-precision elevation data and concurrent water level data for independent spawning events, confirm the importance of this environmental cue with no exceptions across all 35 individual spawning sites. This functional relationship is consistent with G. maculatus ecology in tidal situations (McDowall 1968), and in landlocked riverine populations in which spawning was found in riparian vegetation inundated by freshes following rain events (Pollard 1971). Alongside these potentially widespread trends, it is evident that atypical variations are also possible such as in dry seasons where spawning in pools has been reported, and lacustrine spawning examples for which the spawning cues are poorly known (Chapman et al. 2006).
In this case, insights on the timing of spawning events obtained from fine scale spatiotemporal data suggest an important role for poorly understood aspects involving fish movement and migration triggers that precede the spawning event. The finding of a very short time lag between manifestation of the environmental cue and the onset of spawning deserves particular attention since this may be indicative of rapid fish movements at these times. Results from this study show general support for the hypothesis that lunar cycles associated with spring high tide periods would be unlikely to act as a trigger for non-tidal spawning migrations, as has been reported to be a potential cue in tidal waterways (McDowall 1968; Taylor 2002). However, the full moon period coincided with the heavy rain event of late February and could therefore have had a bearing on the position of fish within the wider catchment at that time (Table 2). In comparison, some of the April spawning events show divergence from the lunar cycle, and maintained an apparent synchrony with water level changes. Nonetheless, it remains difficult to disentangle the major factors influencing fish movement towards rivermouths prior to the timing of spawning events, especially on a seasonal basis. For example, lunar cues might still be functioning as an influence on fish movements at the catchment scale in connection with progression of the G. maculatus lifecycle within these waterways.
Questions surrounding the functional trigger for fish movements in these non-tidal rivers add intrigue to the already mysterious process by which tidal river fish initiate their migrations to estuarine spawning grounds to coincide with spring high tides (McDowall 1968, 1991). Further research on these aspects would be insightful for improving the understanding of migration triggers, as these have a fundamental influence on spawning behaviour and the associated biogeography of spawning grounds. Understanding these movements is also important for the design of sampling approaches that assume comparable study areas for size-class measurements (Ravn et al. 2018), or, in the case of mark–recapture techniques, require accounting for within-population detectability differences over time (MacKenzie et al. 2003; Lindberg and Lindberg 2012). For G. maculatus, the potential for variable dynamics between size or age classes combined with strong directional migration effects presents confounding factors that are difficult to control for, yet could directly influence size-frequency and catch-per-unit-effort measures over relatively short periods, including successive sampling days.
Protected area implications and concluding remarks
Spawning grounds provide an example of a geographically small but disproportionately important critical habitat that is vital for successful completion of the whitebait life cycle (Mitchell 1994). Protecting spawning grounds is therefore an important focus for management, and is indeed the subject of New Zealand legislation under the Conservation Act (1987) and the Resource Management Act (1991). Spatially explicit information on actual or likely spawning locations can assist their conservation by identifying areas for protection and enabling robust impact assessments to assess potential threats and determine management needs. For G. maculatus, spatial planning at relatively fine scales has the potential to help address the common occurrence of riparian and river management activities that are incompatible with habitat protection goals. Applications include improving the efficiency of area-based management tools through avoiding unnecessary trade-offs between competing demands for space (Faith and Walker 2002; Orchard and Hickford 2020). This study supports the quest for integrated management that embraces these aspects by providing the first comprehensive account of G. maculatus spawning ecology at non-tidal rivermouths and identifying biogeographical patterns that were consistent between rivers and over time. It is hoped that this may assist the development of both of regulatory measures and voluntary approaches to protect sensitive areas at the necessary times.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We thank the Ministry of Business, Innovation, and Employment (MBIE) and the New Zealand Ministry for Primary Industries (MPI) for funding support. Particular thanks to staff at the Canterbury Regional Council for helpful discussions and access to water level records, and to staff and students at the University of Canterbury who supported this work.
References
Augspurger, J. M., Warburton, M., and Closs, G. P. (2017). Life-history plasticity in amphidromous and catadromous fishes: a continuum of strategies. Reviews in Fish Biology and Fisheries 27, 177–192.| Life-history plasticity in amphidromous and catadromous fishes: a continuum of strategies.Crossref | GoogleScholarGoogle Scholar |
Barbee, N. C., Hale, R., Morrongiello, J., Hicks, A., Semmens, D., Downes, B. J., and Swearer, S. E. (2011). Large-scale variation in life history traits of the widespread diadromous fish, Galaxias maculatus, reflects geographic differences in local environmental conditions. Marine and Freshwater Research 62, 790–800.
| Large-scale variation in life history traits of the widespread diadromous fish, Galaxias maculatus, reflects geographic differences in local environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Barile, J., Escudero, M., Carreño, E., and Martín, D. S. (2015). Efecto de la salinidad en la supervivencia embrionaria de puye Galaxias maculatus (Jenyns, 1842)/Effect of salinity on survival of embryos of jollytail Galaxias maculatus (Jenyns, 1842). Latin American Journal of Aquatic Research 43, 282.
| Efecto de la salinidad en la supervivencia embrionaria de puye Galaxias maculatus (Jenyns, 1842)/Effect of salinity on survival of embryos of jollytail Galaxias maculatus (Jenyns, 1842).Crossref | GoogleScholarGoogle Scholar |
Barriga, J. P., Battini, M. A., Macchi, P. J., Milano, D., and Cussac, V. E. (2002). Spatial and temporal distribution of landlocked Galaxias maculatus and Galaxias platei (Pisces: Galaxiidae) in a lake in the South American Andes. New Zealand Journal of Marine and Freshwater Research 36, 345–359.
| Spatial and temporal distribution of landlocked Galaxias maculatus and Galaxias platei (Pisces: Galaxiidae) in a lake in the South American Andes.Crossref | GoogleScholarGoogle Scholar |
Barriga, J. P., Battini, M. A., and Cussac, V. E. (2007). Annual dynamics variation of a landlocked Galaxias maculatus (Jenyns 1842) population in a northern Patagonian river: occurrence of juvenile upstream migration. Journal of Applied Ichthyology 23, 128–135.
| Annual dynamics variation of a landlocked Galaxias maculatus (Jenyns 1842) population in a northern Patagonian river: occurrence of juvenile upstream migration.Crossref | GoogleScholarGoogle Scholar |
Benzie, V. (1968). Some ecological aspects of the spawning behaviour and early development of the common whitebait Galaxias maculatus attenuatus (Jenyns). Proceedings of the New Zealand Ecological Society 15, 31–39.
Berra, T. M., Crowley, L., Ivantsoff, W., and Fuerst, P. A. (1996). Galaxias maculatus: an explanation of its biogeography. Marine and Freshwater Research 47, 845–849.
| Galaxias maculatus: an explanation of its biogeography.Crossref | GoogleScholarGoogle Scholar |
Campos, H. (1973). Migration of Galaxias maculatus (Jenyns) (Galaxiidae, Pisces) in Valdivia Estuary, Chile. Hydrobiologia 43, 301–312.
| Migration of Galaxias maculatus (Jenyns) (Galaxiidae, Pisces) in Valdivia Estuary, Chile.Crossref | GoogleScholarGoogle Scholar |
Carrea, C., Cussac, V. E., and Ruzzante, D. E. (2013). Genetic and phenotypic variation among Galaxias maculatus populations reflects contrasting landscape effects between northern and southern Patagonia. Freshwater Biology 58, 36–49.
| Genetic and phenotypic variation among Galaxias maculatus populations reflects contrasting landscape effects between northern and southern Patagonia.Crossref | GoogleScholarGoogle Scholar |
Carson, H. S., López-Duarte, P. C., Cook, G. S., Fodrie, F. J., Becker, B. J., DiBacco, C., and Levin, L. A. (2013). Temporal, spatial, and interspecific variation in geochemical signatures within fish otoliths, bivalve larval shells, and crustacean larvae. Marine Ecology Progress Series 473, 133–148.
| Temporal, spatial, and interspecific variation in geochemical signatures within fish otoliths, bivalve larval shells, and crustacean larvae.Crossref | GoogleScholarGoogle Scholar |
Carter, R. W. G., Forbes, D. L., Jennings, S. C., Orford, J. D., Shaw, J., and Taylor, R. B. (1989). Barrier and lagoon coast evolution under differing relative sea-level regimes: examples from Ireland and Nova Scotia. Marine Geology 88, 221–242.
| Barrier and lagoon coast evolution under differing relative sea-level regimes: examples from Ireland and Nova Scotia.Crossref | GoogleScholarGoogle Scholar |
Chapman, A., Morgan, D. L., Beatty, S. J., and Gill, H. S. (2006). Variation in life history of land-locked lacustrine and riverine populations of Galaxias maculatus (Jenyns 1842) in Western Australia. Environmental Biology of Fishes 77, 21–37.
| Variation in life history of land-locked lacustrine and riverine populations of Galaxias maculatus (Jenyns 1842) in Western Australia.Crossref | GoogleScholarGoogle Scholar |
Chiswell, S. M., and Rickard, G. J. (2011). Larval connectivity of harbours via ocean currents: a New Zealand study. Continental Shelf Research 31, 1057–1074.
| Larval connectivity of harbours via ocean currents: a New Zealand study.Crossref | GoogleScholarGoogle Scholar |
Clark, K. J., Nissen, E. K., Howarth, J. D., Hamling, I. J., Mountjoy, J. J., Ries, W. F., et al. (2017). Highly variable coastal deformation in the 2016 MW7.8 Kaikōura earthquake reflects rupture complexity along a transpressional plate boundary. Earth and Planetary Science Letters 474, 334–344.
| Highly variable coastal deformation in the 2016 MW7.8 Kaikōura earthquake reflects rupture complexity along a transpressional plate boundary.Crossref | GoogleScholarGoogle Scholar |
Closs, G. P., and Warburton, M. (2016). Life histories of amphidromous fishes. In ‘An Introduction to Fish Migration’. (Eds P. Morais, and F. Daverat.) pp. 102–122. (CRC Press: Boca Raton.)
Closs, G. P., Hicks, A. S., and Jellyman, P. G. (2013). Life histories of closely related amphidromous and non-migratory fish species: a trade-off between egg size and fecundity. Freshwater Biology 58, 1162–1177.
| Life histories of closely related amphidromous and non-migratory fish species: a trade-off between egg size and fecundity.Crossref | GoogleScholarGoogle Scholar |
Cooper, J. A. G. (1994). Sedimentary processes in the river-dominated Mvoti estuary, South Africa. Geomorphology 9, 271–300.
| Sedimentary processes in the river-dominated Mvoti estuary, South Africa.Crossref | GoogleScholarGoogle Scholar |
Cussac, V., Ortubay, S., Iglesias, G., Milano, D., Lattuca, M. E., Barriga, J. P., et al. (2004). The distribution of South American galaxiid fishes: the role of biological traits and post-glacial history. Journal of Biogeography 31, 103–121.
| The distribution of South American galaxiid fishes: the role of biological traits and post-glacial history.Crossref | GoogleScholarGoogle Scholar |
David, B. O., Jarvis, M., Özkundakci, D., Collier, K. J., Hicks, A. S., and Reid, M. (2019). To sea or not to sea? Multiple lines of evidence reveal the contribution of non‐diadromous recruitment for supporting endemic fish populations within New Zealand’s longest river. Aquatic Conservation: Marine and Freshwater Ecosystems 29, 1409–1423.
| To sea or not to sea? Multiple lines of evidence reveal the contribution of non‐diadromous recruitment for supporting endemic fish populations within New Zealand’s longest river.Crossref | GoogleScholarGoogle Scholar |
Dunn, N. R., Allibone, R. M., Closs, G. P., Crow, S. K., David, B. O., Goodman, J. M., et al. (2018). Conservation status of New Zealand freshwater fishes, 2017. New Zealand Threat Classification Series 24. Department of Conservation, Wellington.
Environment Canterbury (2020). River flow data. Canterbury Regional Council. Available at https://www.ecan.govt.nz/data/riverflow/
Faith, D. P., and Walker, P. A. (2002). The role of trade-offs in biodiversity conservation planning: linking local management, regional planning and global conservation efforts. Journal of Biosciences 27, 393–407.
| The role of trade-offs in biodiversity conservation planning: linking local management, regional planning and global conservation efforts.Crossref | GoogleScholarGoogle Scholar | 12177537PubMed |
Field, A. P., and Wilcox, R. R. (2017). Robust statistical methods: a primer for clinical psychology and experimental psychopathology researchers. Behaviour Research and Therapy 98, 19–38.
| Robust statistical methods: a primer for clinical psychology and experimental psychopathology researchers.Crossref | GoogleScholarGoogle Scholar | 28577757PubMed |
Fox, J., and Weisberg, S. (2011). ‘An R Companion to Applied Regression.’ 2nd edn. (SAGE Publications: Thousand Oaks, CA.)
Fulton, W. (2000). Tasmanian Whitebait – a multispecies fishery targeting migrating fishes. In ‘Fish Movement and Migration. Australian Society for Fish Biology Workshop Proceedings, Bendigo, 28–29 September 1999’. (Eds D. A. Hancock, D. C. Smith, and J. D. Koehn.) pp. 256–260. (Australian Society for Fish Biology: Sydney.)
Green, A., Andrew, J., Cooper, G., and LeVieux, A. (2013). Unusual barrier/inlet behaviour associated with active coastal progradation and river-dominated estuaries. Journal of Coastal Research 69, 35–45.
| Unusual barrier/inlet behaviour associated with active coastal progradation and river-dominated estuaries.Crossref | GoogleScholarGoogle Scholar |
Greer, M., Gray, D., Duff, K., and Sykes, J. (2015). Predicting inanga/whitebait spawning habitat in Canterbury. Report No. R15/100. Environment Canterbury, Christchurch.
Hart, D. E. (2009). Morphodynamics of non-estuarine rivermouth lagoons on high-energy coasts. Journal of Coastal Research , 1355–1359.
Hickford, M. J. H., and Schiel, D. R. (2011). Population sinks resulting from degraded habitats of an obligate life-history pathway. Oecologia 166, 131–140.
| Population sinks resulting from degraded habitats of an obligate life-history pathway.Crossref | GoogleScholarGoogle Scholar |
Hickford, M. J. H., and Schiel, D. R. (2016). Otolith microchemistry of the amphidromous Galaxias maculatus shows recruitment to coastal rivers from unstructured larval pools. Marine Ecology Progress Series 548, 197–207.
| Otolith microchemistry of the amphidromous Galaxias maculatus shows recruitment to coastal rivers from unstructured larval pools.Crossref | GoogleScholarGoogle Scholar |
Hicks, A. S., Jarvis, M. G., David, B. O., Waters, J. M., Norman, M. D., and Closs, G. P. (2017). Lake and species specific patterns of non-diadromous recruitment in amphidromous fish: the importance of local recruitment and habitat requirements. Marine and Freshwater Research 68, 2315–2323.
| Lake and species specific patterns of non-diadromous recruitment in amphidromous fish: the importance of local recruitment and habitat requirements.Crossref | GoogleScholarGoogle Scholar |
Kirk, R. M. (1980). Mixed sand and gravel beaches: morphology, processes and sediments. Progress in Physical Geography 4, 189–210.
| Mixed sand and gravel beaches: morphology, processes and sediments.Crossref | GoogleScholarGoogle Scholar |
Kirk, R. M. (1991). River–beach interaction on mixed sand and gravel coasts: a geomorphic model for water resource planning. Applied Geography 11, 267–287.
| River–beach interaction on mixed sand and gravel coasts: a geomorphic model for water resource planning.Crossref | GoogleScholarGoogle Scholar |
Land Information New Zealand. (2016). LINZS25009 Standard for New Zealand Vertical Datum 2016. Land Information New Zealand, Wellington.
Lichter, M., and Klein, M. (2011). The effect of river floods on the morphology of small river mouths in the southeastern Mediterranean. Zeitschrift für Geomorphologie 55, 317–340.
| The effect of river floods on the morphology of small river mouths in the southeastern Mediterranean.Crossref | GoogleScholarGoogle Scholar |
Lindberg, M. S., and Lindberg, M. S. (2012). A review of designs for capture–mark–recapture studies in discrete time. Journal of Ornithology 152, 355–370.
| A review of designs for capture–mark–recapture studies in discrete time.Crossref | GoogleScholarGoogle Scholar |
LINZ. (2018a). Local mean sea level datums. Updated 22 March 2018. Available at http://www.linz.govt.nz/data/geodetic-system/datums-projections-and-heights/ [accessed 4 September 2020].
LINZ. (2018b). New Zealand Nautical Almanac, 2018/2019 edition, NZ 204. Land Information New Zealand, Wellington.
MacKenzie, D. I., Nichols, J. D., Hines, J. E., Knutson, M. G., and Franklin, A. B. (2003). Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly. Ecology 84, 2200–2207.
| Estimating site occupancy, colonization, and local extinction when a species is detected imperfectly.Crossref | GoogleScholarGoogle Scholar |
Mardones, A., Vega, R., and Encina, F. (2008). Cultivation of whitebait (Galaxias maculatus) in Chile. Aquaculture Research 39, 731–737.
| Cultivation of whitebait (Galaxias maculatus) in Chile.Crossref | GoogleScholarGoogle Scholar |
McDowall, R. M. (1965). The composition of the New Zealand whitebait catch, 1964. New Zealand Journal of Science 8, 285–300.
McDowall, R. M. (1968). Galaxias maculatus (Jenyns), the New Zealand whitebait. New Zealand Marine Department, Fisheries Research Bulletin 2, 1–83.
McDowall, R. M. (1984). ‘The New Zealand Whitebait Book.’ (Reed: Wellington.)
McDowall, R. M. (1991). Conservation and management of the whitebait fishery. Department of Conservation Science and Research Series 38, 1–18.
McDowall, R. M. (2002). Accumulating evidence for a dispersal biogeography of southern cool temperate freshwater fishes. Journal of Biogeography 29, 207–219.
| Accumulating evidence for a dispersal biogeography of southern cool temperate freshwater fishes.Crossref | GoogleScholarGoogle Scholar |
McDowall, R. M. (2007). On amphidromy, a distinct form of diadromy in aquatic organisms. Fish and Fisheries 8, 1–13.
| On amphidromy, a distinct form of diadromy in aquatic organisms.Crossref | GoogleScholarGoogle Scholar |
McDowall, R. M. (2008). Diadromy, history and ecology: a question of scale. Hydrobiologia 602, 5–14.
| Diadromy, history and ecology: a question of scale.Crossref | GoogleScholarGoogle Scholar |
McDowall, R. M. (2010a). Why be amphidromous: expatrial dispersal and the place of source and sink population dynamics? Reviews in Fish Biology and Fisheries 20, 87–100.
| Why be amphidromous: expatrial dispersal and the place of source and sink population dynamics?Crossref | GoogleScholarGoogle Scholar |
McDowall, R. M. (2010b). ‘New Zealand Freshwater Fishes: an Historical and Ecological Biogeography.’ (Vol. 32.) (Springer: New York.)
McDowall, R. M. (2010c). Historical and ecological context, pattern and process, in the derivation of New Zealand’s freshwater fish fauna. New Zealand Journal of Ecology 34, 185–194.
McDowall, R. M., and Eldon, G. A. (1980). The ecology of whitebait migrations (Galaxiidae: Galaxias spp.). New Zealand Ministry of Agriculture and Fisheries, Fisheries Research Bulletin 20, 1–172.
McDowall, R. M., Mitchell, C. P., and Brothers, E. B. (1994). Age at migration from the sea of juvenile Galaxias in New Zealand (Pisces, Galaxiidae). Bulletin of Marine Science 54, 385–402.
McSweeney, S. L., Kennedy, D. M., Rutherfurd, I. D., and Stout, J. C. (2017). Intermittently closed/open lakes and lagoons: their global distribution and boundary conditions. Geomorphology 292, 142–152.
| Intermittently closed/open lakes and lagoons: their global distribution and boundary conditions.Crossref | GoogleScholarGoogle Scholar |
Mitchell, C. P. (1994). Whitebait spawning ground management. New Zealand Department of Conservation, Science and Research Series 69, 1–23.
Orchard, S. (2019). River restoration opportunities in Amelia Rogers Reserve. Report prepared for Christchurch City Council, April 2019.
Orchard, S., and Hickford, M. J. H. (2016). Spatial effects of the Canterbury earthquakes on īnanga spawning habitat and implications for waterways management. Report prepared for IPENZ Rivers Group and Ngāi Tahu Research Centre. Waterways Centre for Freshwater Management and Marine Ecology Research Group. University of Canterbury, Christchurch.
Orchard, S., and Hickford, M. J. H. (2018). Census survey approach to quantifying īnanga spawning habitat for conservation and management. New Zealand Journal of Marine and Freshwater Research 52, 284–294.
| Census survey approach to quantifying īnanga spawning habitat for conservation and management.Crossref | GoogleScholarGoogle Scholar |
Orchard, S., and Hickford, M. J. H. (2020). Protected area effectiveness for fish spawning habitat in relation to earthquake-induced landscape change. In ‘Sustainable Bioresource Management: Climate Change Mitigation and Natural Resource Conservation’. (Eds R. Maiti, H. D. Rodríguez, C. A. Kumari, D. Mandal, and N. C. Sarkar.) Chapter 22. (Apple Academic Press.)
Orchard, S., Hickford, M. J. H., and Schiel, D. R. (2018a). Earthquake‐induced habitat migration in a riparian spawning fish has implications for conservation management. Aquatic Conservation: Marine and Freshwater Ecosystems 28, 702–712.
| Earthquake‐induced habitat migration in a riparian spawning fish has implications for conservation management.Crossref | GoogleScholarGoogle Scholar |
Orchard, S., Hickford, M. J. H., and Schiel, D. R. (2018b). Use of artificial habitats to detect spawning sites for the conservation of Galaxias maculatus, a riparian-spawning fish. Ecological Indicators 91, 617–625.
| Use of artificial habitats to detect spawning sites for the conservation of Galaxias maculatus, a riparian-spawning fish.Crossref | GoogleScholarGoogle Scholar |
Pollard, D. A. (1971). The biology of a landlocked form of the normally catadromous salmoniform fish Galaxias maculatus (Jenyns). I. Life cycle and origin. Australian Journal of Marine and Freshwater Research 22, 91–123.
| The biology of a landlocked form of the normally catadromous salmoniform fish Galaxias maculatus (Jenyns). I. Life cycle and origin.Crossref | GoogleScholarGoogle Scholar |
QGIS Development Team (2020). QGIS Geographic Information System. Open Source Geospatial Foundation Project. Available at http://qgis.org
Ravn, H. D., Lauridsen, T. L., Jepsen, N., Jeppesen, E., Hansen, P. G., Hansen, J. G., and Berg, S. (2018). A comparative study of three different methods for assessing fish communities in a small eutrophic lake. Ecology of Freshwater Fish 28, 341–352.
| A comparative study of three different methods for assessing fish communities in a small eutrophic lake.Crossref | GoogleScholarGoogle Scholar |
R Core Team. (2019). ‘R: A Language and Environment for Statistical Computing.’ (R Foundation for Statistical Computing: Vienna, Austria.)
Richardson, J., and Taylor, M. J. (2002). A guide to restoring inanga habitat. NIWA Science and Technology Series 50, 1–29.
Rojo, J. H., Fernández, D. A., Figueroa, D. E., and Boy, C. C. (2020). Phenotypic and genetic differentiation between diadromous and landlocked puyen Galaxias maculatus. Journal of Fish Biology 96, 956–967.
| Phenotypic and genetic differentiation between diadromous and landlocked puyen Galaxias maculatus.Crossref | GoogleScholarGoogle Scholar | 32048294PubMed |
Ruttenberg, B. I., Hamilton, S. L., Hickford, M. J. H., Paradis, G. L., Sheehy, M. S., Standish, J. D., et al. (2005). Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags. Marine Ecology Progress Series 297, 273–281.
| Elevated levels of trace elements in cores of otoliths and their potential for use as natural tags.Crossref | GoogleScholarGoogle Scholar |
Schiel, D. R., Alestra, T., Gerrity, S., Orchard, S., Dunmore, R., Pirker, J., et al. (2019). The Kaikōura earthquake in southern New Zealand: loss of connectivity of marine communities and the necessity of a cross‐ecosystem perspective. Aquatic Conservation: Marine and Freshwater Ecosystems 29, 1520–1534.
| The Kaikōura earthquake in southern New Zealand: loss of connectivity of marine communities and the necessity of a cross‐ecosystem perspective.Crossref | GoogleScholarGoogle Scholar |
Shiao, J.-C., Tzeng, C.-S., Li, P.-C., and Bell, K. N. I. (2015). Upstream migration and marine early life history of amphidromous gobies inferred from otolith increments and microchemistry. Environmental Biology of Fishes 98, 933–950.
| Upstream migration and marine early life history of amphidromous gobies inferred from otolith increments and microchemistry.Crossref | GoogleScholarGoogle Scholar |
Smith, A. M., Guastella, L. A., and Goble, B. J. (2014). Forecasting lagoon outlet erosion: KwaZulu-Natal, southeast Africa. Journal of Coastal Research 70, 151–155.
| Forecasting lagoon outlet erosion: KwaZulu-Natal, southeast Africa.Crossref | GoogleScholarGoogle Scholar |
Snelder, T. H., and Biggs, B. J. F. (2002). Multi-scale river environment classification for water resources management. Journal of the American Water Resources Association 38, 1225–1240.
| Multi-scale river environment classification for water resources management.Crossref | GoogleScholarGoogle Scholar |
Sorensen, P. W., and Hobson, K. A. (2005). Stable isotope analysis of amphidromous Hawaiian gobies suggests their larvae spend a substantial period of time in freshwater river plumes. Environmental Biology of Fishes 74, 31–42.
| Stable isotope analysis of amphidromous Hawaiian gobies suggests their larvae spend a substantial period of time in freshwater river plumes.Crossref | GoogleScholarGoogle Scholar |
Taylor, M. J. (2002). The national inanga spawning database: trends and implications for spawning site management. Science for Conservation 188. Department of Conservation, Wellington.
Taylor, M., and Marshall, W. (2014). Inanga spawning survey of the Canterbury Region. Report prepared for Environment Canterbury. 59pp.
Vega, R., Dantagnan, P., Mardones, A., Valdebenito, I., Zamorano, J., and Encina, F. (2013). Bases biológicas para el cultivo del puye Galaxias maculatus (Jenyns, 1842): una revisión/Biological bases for whitebait culture Galaxias maculatus (Jenyns, 1842): a review. Latin American Journal of Aquatic Research 41, 369.
| Bases biológicas para el cultivo del puye Galaxias maculatus (Jenyns, 1842): una revisión/Biological bases for whitebait culture Galaxias maculatus (Jenyns, 1842): a review.Crossref | GoogleScholarGoogle Scholar |
Wang, J., Zamar, R., Marazzi, A., Yohai, V., Salibian-Barrera, M., Maronna, R., et al. (2020). robust: Port of the S+ “Robust Library”. R package ver. 0.5-0.0. Available at https://CRAN.R-project.org/package=robust
Warner, R. R., Swearer, S. E., Caselle, J. E., Sheehy, M., and Paradis, G. (2005). Natal trace-elemental signatures in the otoliths of an open-coast fish. Limnology and Oceanography 50, 1529–1542.
| Natal trace-elemental signatures in the otoliths of an open-coast fish.Crossref | GoogleScholarGoogle Scholar |
Waters, J. M., Dijkstra, L. H., and Wallis, G. P. (2000). Biogeography of a southern hemisphere freshwater fish: how important is marine dispersal? Molecular Ecology 9, 1815–1821.
| Biogeography of a southern hemisphere freshwater fish: how important is marine dispersal?Crossref | GoogleScholarGoogle Scholar | 11091317PubMed |
Yungnickel, M. R., Hickford, M. J. H., and Schiel, D. R. (2020). Spatio-temporal variation in species composition of New Zealand’s whitebait fishery. New Zealand Journal of Marine and Freshwater Research 54, 679–694.
| Spatio-temporal variation in species composition of New Zealand’s whitebait fishery.Crossref | GoogleScholarGoogle Scholar |
Zattara, E. E., and Premoli, A. C. (2005). Genetic structuring in Andean landlocked populations of Galaxias maculatus: effects of biogeographic history. Journal of Biogeography 32, 5–14.
| Genetic structuring in Andean landlocked populations of Galaxias maculatus: effects of biogeographic history.Crossref | GoogleScholarGoogle Scholar |






