Using Samoan traditional ecological knowledge to identify calls of the critically endangered endemic tooth-billed pigeon (Didunculus strigirostris)
G. Serra A D , G. R. Wood B , S. A. Faiilagi C , S. T. Foliga C , M. Uili C and F. Enoka C
A D , G. R. Wood B , S. A. Faiilagi C , S. T. Foliga C , M. Uili C and F. Enoka C
A Freelance conservationist and ecologist, Florence, Italy.
B Department of Statistics, University of Otago, New Zealand.
C Ministry of Natural Resources and Environment, Samoa.
D Corresponding author. Email: ibiseremita@gmail.com
Pacific Conservation Biology - https://doi.org/10.1071/PC20052
Submitted: 11 June 2020 Accepted: 23 November 2020 Published online: 16 December 2020
Abstract
The tooth-billed pigeon (Didunculus strigirostris) is an endemic and highly cryptic bird of the rainforest canopy of Samoa. According to the recently released Tooth-billed Pigeon Recovery Plan (2020–2029), one of the greatest obstacles to conservation efforts is the inability of ornithologists to reliably separate its advertising coo call from that of the common and sympatric Pacific imperial pigeon (Ducula pacifica). Because tooth-billed pigeons are very rarely seen, acoustic methods of identifying them, which have been problematic for ornithologists, would be helpful for population surveys. Our study examines the traditional ecological knowledge and skill of Samoan hunters, peer selected for knowledge and reliability from six villages located on Upolu and Savaii islands, to determine whether they can consistently identify the species based on the calls. Through use of automatic devices, we recorded pigeon coo calls at nine forest sites recommended by reliable hunters within four key biodiversity areas of the islands of Upolu and Savaii from March to June 2016. We isolated and filtered 104 clear coo call sequences from these recordings. The two top hunters separately and confidently identified which of the two pigeon species were calling for 80 of the 104 sequences. On 54 of these 80 call sequences the hunters were in agreement, both assigning a call to the one species. We measured seven sonographic variables on each of the coo calls of each of these 54 sequences in order to investigate potential differences between the calls of Didunculus and Ducula. Two clear differences emerged: a strongly statistically significant difference in the highest frequency of the coo call, and the more regular spacing of the coo calls of Didunculus than of Ducula. Only the second rhythm outcome is consistent with a recent independent analysis by other authors. This regularity of the intervals between coo calls in a sequence may be the key to separation of the two species in the field.
Keywords: call overlap, Didunculus strigirostris, Ducula pacifica, local ecological knowledge, oceanic rainforest, Pacific imperial pigeon, Polynesia, Samoa, sympatric pigeons, tooth-billed pigeon, traditional ecological knowledge.
Introduction
The tooth-billed pigeon (Didunculus strigirostris), locally known as ‘Manumea’ (MNRE 2006, called ‘Didunculus’ hereafter), a Samoan endemic, is evolutionarily distinctive (Jetz et al. 2014). It has been assessed as Critically Endangered in the IUCN Red List since 2014 (BirdLife International 2015). Ecological and behavioural knowledge about this species is scant and scattered (Beichle 1982a, 1982b, 1987a, 1987b, 1989; Beichle and Baumann 2016; Pratt and Mittermeier 2016). All the available information on Didunculus was reviewed and collated recently by Collar (2015).
The precipitous decline of Didunculus was brought to the attention of the conservation community by Beichle (2006), who reported that only a ‘few hundred’ birds survived at the time, implying a 90% reduction in numbers since the mid-1980s (Stattersfield and Capper 2000; Beichle and Baumann 2016). The perilous conservation status of Didunculus was confirmed by a survey in 2012 of the species’ presumed stronghold in the remote uplands of Samoa’s largest island, Savaii, that found no Didunculus (Butler 2012). Didunculus has been listed as Endangered since 2000 (BirdLife 2015) and a recovery plan was approved for implementation in 2006 (MNRE 2006) although very few of its recommendations have been implemented (Serra 2017; U. Beichle, pers. comm.).
Didunculus is highly cryptic in rainforest habitat. Serra et al. (2017) found that medium to intensive surveys by Government or international experts produced one sighting every 3–5 years. The situation has been summarised by Collar (2015), who stated ‘The great difficulty throughout this century has simply been to find even a single representative of the species’, and Pratt and Mittermeier (2016) stated that Didunculus has turned into ‘an immediate conservation priority’ for Samoa.
Further complicating the issue of assessing the occurrence and conservation status of Didunculus is the extreme difficulty of reliably identifying its call in the field. The advertising coo call of Didunculus (sensu Baumann and Beichle 2020) is similar to a call of the relatively common sympatric Pacific imperial pigeon (Ducula pacifica, called ‘Ducula’ hereafter). The similarity in calls between the two species was first noted by Butler (2012) and then confirmed by Beichle and Baumann (2016), Pratt and Mittermeier (2016) and Serra (2016).
This ‘under-appreciated similarity’ (Pratt and Mittermeier 2016) was evidenced through use of traditional ecological knowledge (TEK): selected ‘reliable’ hunters (see definition in the Methods) showed an unexpected difficulty distinguishing the recorded vocalisations of the two species (Serra et al. 2017). Moreover, little consensus was found in terms of key differences between Didunculus and Ducula calls when consulting local hunters, and Government or international experts, including the Didunculus authorities (Ulf Beichle and Sabine Baumann) (Serra et al. 2017).
The distinguishing feature on which all agreed is that ~20% of Ducula coo call sequences present an introductory syllable and/or a brief modulation within the first third of the call (Beichle and Baumann 2016). The same authors indicated that Didunculus has shorter intervals between coo calls within a given sequence (Beichle and Baumann 2016).
Based on recordings of birds identified visually (9 Didunculus and 16 Ducula), Baumann and Beichle (2020) suggested that the coo call of Didunculus is higher pitched than that of Ducula and proposed a pitch higher than 400 Hz as a criterion for identifying the call of Didunculus. The same authors state that only Didunculus repeats the call more than 20 times, especially during the breeding season, presumed to be the dry season between April and August (Beichle and Baumann 2016; Baumann and Beichle 2020).
TEK mastered by the indigenous communities typically goes unrecorded and is often downplayed as ‘anecdotal information’ in the scientific literature (Blair 2005). The ‘observational value’, however, of TEK was underlined by Sinclair et al. (2010), while its relevance in relation to detecting rare birds was emphasised by Serra et al. (2004) and Blair (2005). Sourcing this type of knowledge requires spending a considerable amount of time establishing a trusting and viable working relationship with indigenous holders of TEK. By contrast, the ‘rapid’ survey methods popular nowadays such as the BIORAP (Conservation International 2016), reliant mainly on scientific ecological knowledge, are not ideal for the detection and assessment of the status of rare and elusive fauna (Powell 2008). In the present study, we regarded as TEK all the information we managed to collect, decode and verify from a rigorously selected sample of senior and experienced pigeon hunters based in the Samoan villages.
Given the present extreme challenge of detecting Didunculus visually in the field, this study adopts an indirect approach. We used TEK of experienced hunters to separate the coo calls of Didunculus and Ducula, based on automatically recorded forest sounds. We analysed sonographically independent sound recordings ascribed to the two species by hunters and identified spectral and temporal differences.
Materials and methods
Detailed information about this section is available as Supplementary Material online (refer to underlined words within the text).
Study areas
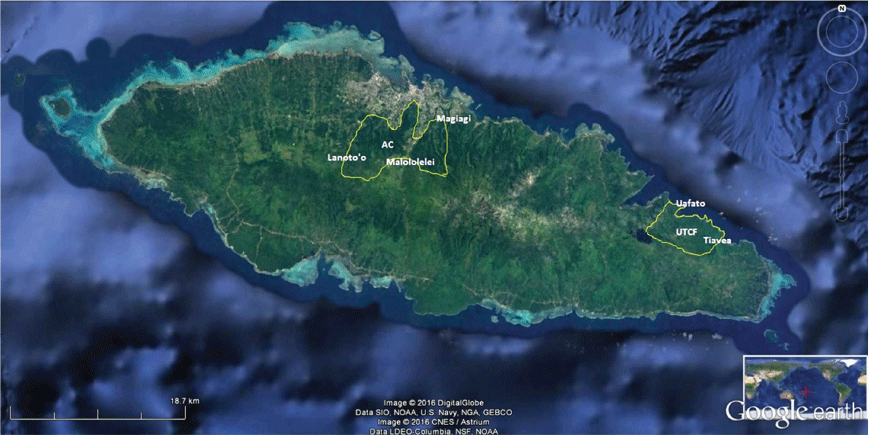
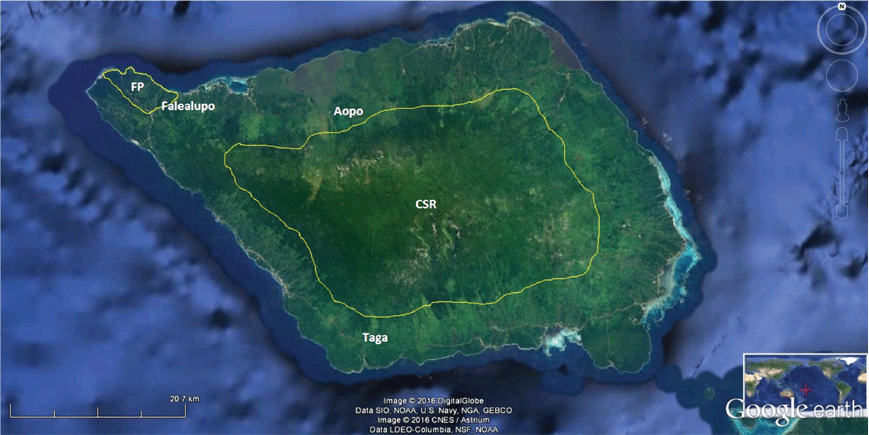
The study areas were in four of the eight designated terrestrial Key Biodiversity Areas (KBAs) of Samoa (Conservation International et al. 2010), namely Uafato-Tiavea Coastal Forest and Apia Catchments on Upolu island (Fig. 1) as well as Central Savaii Rainforest and Falealupo Peninsula on Savaii island (Fig. 2).
Selection of reliable pigeon hunters
Between November 2015 and February 2016 we designed a questionnaire to selected hunters holding reliable TEK of their ancestral forests (Serra 2016). The questionnaire was in the Samoan language and we followed customary protocols (Grattan 1985) before requesting to interview the hunters of the village. The respondents were not randomly selected (Text S1); they were identified by the village council of elders as ‘the most knowledgeable about native biodiversity and sincere’. We followed design recommendations to minimise response biases (White et al. 2005).
We used special care to test the ability of interviewees to identify birds (Text S2), the most biologically diversified taxonomic group targeted by hunters in Samoa, in an attempt to determine their credibility (Serra 2016). We aimed to obtain detailed anatomical, ecological and behavioural descriptions from interviewees, without influence from published images or use of local names.
The reliability and skills of each interviewee were independently assessed by three interviewers by scoring their replies on a scale of 1 (low) to 10 (high). The purpose of these assessments was to ensure that interviewees could consistently and accurately distinguish different bird species (with a focus on pigeons), and to gauge the quality of other information they related while answering the questionnaire.
We considered any interviewee who scored 6 or better to be a reliable source of TEK, hereafter indicated as ‘reliable hunter’. In total, 40 hunters from seven villages located within the mentioned four KBAs were interviewed and their bird identification skills assessed (refer to Serra et al. 2017, table 1). Based on their performance, we then selected 19 hunters whom we regarded as reliable (refer to Serra et al. 2017, table 2).
Automatic forest sound recording, in locations based on TEK
From the 19 selected hunters we chose one or two reliable hunters from each village. These hunters were then asked to lead field observations (Text S3) over 1 or 2 days in their ancestral forests (refer to Serra et al. 2017, table 1). During these field visits, we recorded all TEK about rare native species based on informal unhurried discussions; we double checked and ‘ground truthed’ any key information gathered during previous interviews and from questionnaires as much as feasible (refer to ‘consistency’ in Serra et al. 2017, table 2).
We deployed two autonomous recording units (ARU, Wildlife Acoustics SM3), each equipped with two non-directional microphones, for 7–15 days at two locations recommended by the reliable hunters within the forests of Uafato, Aopo, Taga and Falealupo (so across three different KBAs of Samoa) (Text S4).
Only in the Malolelei forest (Apia Catchments KBA) were two sets of ARUs set up at sites selected not based on TEK but on possible/probable identifications of Didunculus calls attempted by Government and international experts (Text S5). The ARUs were recording continuously, from sunrise to sunset, and recordings were saved as WAV files at a sampling frequency of 1 h.
Coo call sequence detection using Song Scope
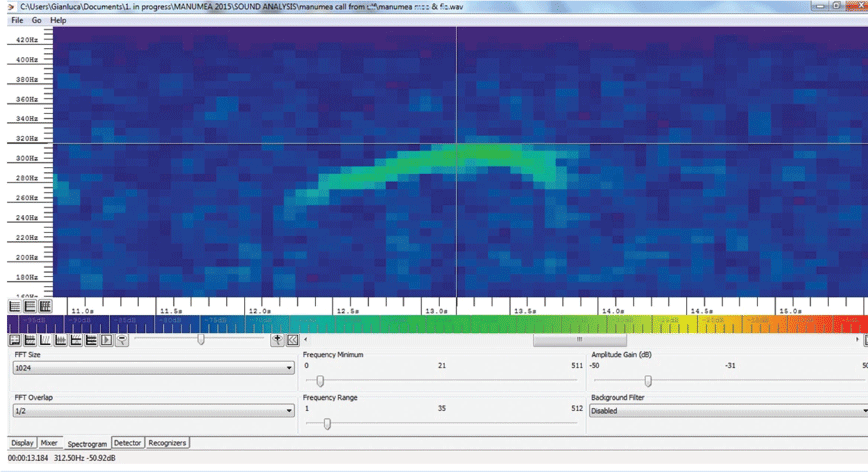
The advertising vocalisation of the two pigeon species in question, Didunculus and Ducula, consists of a sequence of repeated ‘coo calls’ (sensu Beichle and Baumann 2016). The two ARUs, deployed at different sites at different times, recorded 1290 h of forest sounds that were scanned using the program Song Scope 4.1.3 A (Wildlife Acoustics 2016) in order to capture Didunculus and Ducula coo call sequences (Fig. 3). We developed a coo call recogniser for the purpose using Song Scope functions, which isolated 201 unidentified coo call sequences, belonging to Didunculus or Ducula; from these, we selected 104 coo call sequences on the basis of favourable signal to noise ratio.
Testing the hunters on coo call sequences
During July–August 2017 10 hunters with the highest reliability and consistency scores (Serra et al. 2017, table 2), from five villages of Upolu and Savaii, attempted to identify (Text S6) each of the selected 104 coo call sequences automatically recorded at the forest sites. The order of presentation of sequences to each hunter was carefully shuffled before each session.
We obtained a matrix of 104 coo call sequences by 10 hunters, with entries recording the hunter’s identification of the call. During the process hunters were further assessed for reliability (Text S7).
Coo call sequence identification through TEK
We assessed the level of agreement for each pair of hunters using the following four-stage process:
In order to explore the degree of dependence between sequences recorded at the same location within a period of 3 days, we tested the 104 sequences for independence using a one-way analysis of variance. Both being frugivourous (Watling 2001), Didunculus and Ducula may be bound to a certain territory only over short-term periods, at least at certain times of the year, such as during the breeding season or the fruiting of certain trees. The analysis of variance tests did not provide consistent evidence of dependence within the same-location 3-day sets of sequences (Text S8).
We omitted from further analysis a row of the 104-row by 2-column matrix if either of the hunters was uncertain about the identification, or if the identification was other than Didunculus or Ducula (so leaving a ‘cleaned’ set).
We assembled the identifications (Didunculus or Ducula) for each pair of hunters in a 2 × 2 contingency table, as shown in the example of Table 1. Counts on the leading diagonal are numbers of sequences on which the classification of the two hunters agreed. The best agreement performance we obtained was for the pair of hunters Fiu and Afaese: specifically, they agreed that 32 of the calls were Didunculus and that 22 of the calls were Ducula.
We tested each table for independence using a Chi-square test; in this context, when most entries lie on the leading diagonal, evidence against independence is indicative of hunter agreement and so the presence of TEK.
The subset of the cleaned coo call sequences on which a pair of hunters were in agreement is termed their ‘TEK identified’ set; these are coo call sequences for which there is TEK evidence for the identification. With the aid of a cell colour coding (Text S9), the level of agreement in each matrix was identified. We then followed an analogous process for the three hunters Fiu, Afaese and Livingstone, considered all together. The most successful combination among all studied was the pair of Fiu and Afaese, with a TEK-identified set of size n = 54, seen in the total of leading diagonal entries of Table 1.
Data analysis
In order to improve statistical independence of the data, we grouped coo call sequences identified as the same species by a hunter combination and recorded within 3 days of each other at the same location. We retained the sequence in a group with highest signal-to-noise ratio. As a result, the Fiu and Afaese TEK-identified set was reduced from 54 to 30 coo call sequences (19 Didunculus and 11 Ducula).
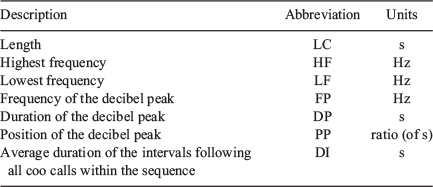
For each coo call of these 30 independent sequences, and the interval following, we used Song Scope to measure seven sonographic variables (Text S10), described in Table 2.

|
We measured the above-defined seven variables from a total of 73 and 56 coo calls, included in 19 and 11 sequences previously TEK-identified by the two mentioned selected hunters (F, A) as Didunculus and Ducula, respectively. We calculated the average for each variable for each sequence; we then determined the average and standard deviation across sequences of these within-sequence average values.
In order to evaluate the extent of the variation of the duration of the between-call intervals (DI), we measured the standard deviation, across sequences, of the standard deviation of DIs for each sequence. In order to do so, from the 30 sequences selected, we discarded those with ≤3 calls and ≤2 intervals: therefore we performed this exercise on 8 sequences of Didunculus and 5 of Ducula, TEK-identified by Fiu and Afaese (n = 13).
We used a two-sample t-test to detect differences between Didunculus and Ducula coo call sequences in the seven averaged sonographic variables. When the data were not normally distributed, we used a non-parametric Mann–Wilcoxon test.
Results
The 10 hunters tested did not agree on the classification of the 104 coo call sequences. Three, however, showed remarkable pairwise agreement ranging from 67% down to 51% over all their clearly identified coo call sequences. The analysis was thus narrowed down to these three reliable hunters: Fiu (F), Afaese (A) and Livingstone (L). Additionally, these three hunters scored as the top three amidst the selected 10, based on the reliability and consistency tests.
Fiu and Afaese showed highest identification agreement of coo call sequences: they agreed on the classification of 54 (67%) of the 80 sequences for which they both had clear identifications. Of the total of 80 sequences, note that the hunters agree on 32 Didunculus sequences and 22 Ducula sequences. We therefore largely focus on this pair (notated F, A) in the sequel to TEK-identify coo call sequences. Table 1, a contingency table, summarises the results of their identifications of Didunculus and Ducula.
|
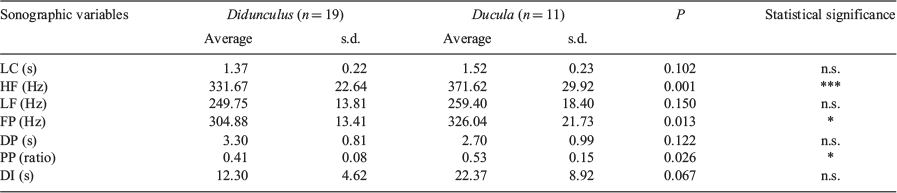
Table 3 reports the averages and standard deviations of the seven sonographic variables (already averaged across individual coo calls within each sequence) measured from the 30 independent coo call sequences on which F and A agree. The Didunculus and Ducula difference in highest frequency (HF) of the call is highly statistically significant (332 Hz vs 372 Hz, respectively, with P = 0.001). This difference corresponds to a full tone, roughly from E to F sharp above middle C. Supporting this finding, a t-test performed on the same seven variables based on the agreement coo call sequence sets of the other combinations of hunters (F, L; A, L; A, F, L) revealed consistent statistical significance (P < 0.05) for HF. Additionally, for F and A, the decibel peak of the call (FP) and its position within the call (PP) are also statistically significant (P-values of 0.013 and 0.026 respectively).
|
The final measure studied was DI, the average duration (in seconds) of intervals between coo calls within a sequence. For Didunculus these averaged (across sequences) 12.30 s and for Ducula, 22.37 s, mildly suggesting that the between-coo call interval duration is longer for Ducula than for Didunculus, but not quite reaching significance (P-value = 0.067). Table 4, however, reports that DI does vary significantly less in Didunculus than in Ducula. Simply stated, this says that the coo calls of Didunculus are more regularly spaced than those of Ducula.

|
In summary, the TEK-based sonographic analysis indicates that the coo calls of Didunculus are lower in pitch and more regularly spaced within a sequence than those of Ducula.
Finally, we tested two key criteria for Didunculus identification proposed by Baumann and Beichle (2020): namely, Didunculus’ HF is >400 Hz, and Didunculus’ repetitions can be >20. These two criteria were tested on the set of 104 unidentified coo call sequences recorded and used in the present study. Out of the only six sequences from our dataset with HF > 400 Hz five included either the introductory syllable or the brief modulation during the first third. These are the criteria used to identify Ducula by the same authors and by several other experts, local and international. On the other hand, the average HF of the only 10 sequences from our dataset with >20 repetitions of coo calls is 366 ± 26 Hz (and two of them, again, presented either the syllable or the modulation). Also noteworthy is that the average HF of the four coo call sequences identified as Didunculus by the same authors in the past upon request of the Samoa Ministry of Environment is 334 ± 16 Hz.
Discussion
Didunculus is a highly secretive and extremely rare bird living in the dense canopy of primary and secondary rainforests of Samoa. Relying on the identification of the call appears to be the only option available in order to survey and monitor this iconic and critically endangered species (MNRE and SCS 2020).
Results of the present study suggest that the coo call of Didunculus is lower in pitch and more regularly spaced within a sequence than that of Ducula: the sound analysis approach used in this study showed that the two top hunters, Fiu and Afaese, attribute these characteristics to Didunculus coo call sequences. These acoustic differences provide a preliminary step in the process of reliably separating the calls of these sympatric species in the field.
The complexity of the challenge is reflected by the fact that even the TEK shared by 10 reliable Samoan pigeon hunters, with an average rainforest pigeon hunting experience of over 28 years, appears only partially able to differentiate the recorded coo calls of the two sympatric rainforest pigeon species. This fact has an important conservation implication: Ducula hunting, still a common practice in Samoa, is confirmed to be a dire threat to survival of Didunculus, consistent with the findings of Serra et al. (2017) and Stirnemann et al. (2018).
The best TEK identification performance we obtained is the 67% agreement based on 80 sequences tested, with hunters Fiu and Afaese. While the analysis of variance tests on the original 104 sequences do show some hint of call homogeneity within same-location 3-day sets, this evidence is weak (refer to the table of Text S9). On the other hand, the Chi-square test P-value (Table 1) is highly significant, so we conclude that there is a satisfactory degree of agreement between F and A.
The TEK-based analysis of the acoustic parameters revealed that the call of Didunculus had lower HF than that of Ducula. Interestingly, this particular difference in HF was orally described and reported on two separate occasions by two other reliable hunters during the 2015–2016 TEK survey (Serra 2016; Serra et al. 2017). This 40 Hz difference may be hard to detect by the human ear in the forest. Hunters proficient at TEK must be using a combination of acoustic characteristics: for instance, they may be able to also pick and assess the variability of the intervals between coo calls within a sequence and possibly their length.
The difference in pitch between Didunculus and Ducula found in this study is inconsistent with results from Baumann and Beichle (2020). The mentioned inconsistency may be explained by the limited sample size of both analyses. These same authors report a difference of ~80 Hz between male and female Didunculus (where male shows a lower pitch). A bias of sampling toward male Didunculus individuals in the present analysis versus a bias by the Baumann and Beichle (2020) analysis toward female individuals would be sufficient to explain the inconsistency of results (especially if combined with an opposite bias by both analyses in relation to Ducula male and female calls).
The average and variation of the length of intervals between adjacent coo calls within a sequence were smaller in Didunculus than in Ducula, meaning that the intervals are shorter, more frequent and more regularly spaced in Didunculus. The shorter length of intervals in Didunculus is consistent with Beichle and Baumann (2016) and with statements by one of the reliable hunters (Serra et al. 2017). Also, the variation of the intervals is consistent with results from Baumann and Beichle (pers. comm.). This consistency of outcomes between two independent studies with very different approaches presents an opportunity for a conservation field application: the regularity of intervals separating coo calls in a sequence, if confirmed by further observations, holds the potential to be used as a simple and yet efficient method to identify Didunculus directly in the field, without need of a posteriori digital and spectrographic analysis.
Noteworthy, and unlike Baumann and Beichle (2020), the present study used ARU and non-directional microphones to automatically record forest sounds over periods of weeks. This method has substantial application potential as it could be used to design and implement standard surveys and monitoring programs over the whole Didunculus distribution range at reasonable cost.
Certainly, as noted also by Baumann and Beichle (2020), the two calls are extremely similar. If a set of coo call sequences is randomly recorded from the Samoan forest the chances are that only a portion with the more extreme sonographic values can be identified through TEK. Based on the present study, we believe that the two calls may be differentiated statistically only over a substantial sample size, since there probably exists considerable within-species variability.
On top of the extensive call overlap the possible reasons why TEK identification has proven not to be highly efficient in this specific field may include:
Some audio recordings we played may have not been of a sufficient quality and clarity for an elderly ear (10 hunters, 53 years old on average).
Pigeon coo call identification through listening to digitally recorded sounds, via a headset, may be challenging and partially confusing for a local hunter accustomed to direct acoustic identification in the field.
The challenging acoustic separation of the two species in the field may not be a critical need for the average Samoan hunter, as until relatively recently they were both targeted as food (Appleton 1871; U. Beichle, pers. comm.). A consensus, however, among reliable hunters interviewed in recent surveys (Serra et al. 2017) could not be reached regarding whether the meat of Didunculus tastes good.
The discrepancy between the estimated rate of visual detections of Didunculus by Government or international experts, mentioned in the Introduction (Serra et al. 2017), versus the rate of audio identifications of the same species by the hunters, from the sample of recorded sequences of the present study, can be explained by two possible factors. Firstly, it seems reasonable and likely that the visual detection rate of Didunculus by local hunters in the field may be higher than that by Government or international experts. Secondly, and more importantly, the visual versus audio sampling efforts are not comparable. Visual encounters were attempted during search efforts in the field lasting from a few hours to a few days maximum (on average, lasting from a few hours to a half/full day in the field). On the other hand, audio identifications were based on ARUs continuously recording from dawn to sunset for 7–15 days in a row, for a total of 90 full days or 1290 h of forest recordings. In addition, the audio recorders worked with zero noise in the forest: this is a very important aspect, taking into account that Didunculus is highly cryptic and actively hiding in the dense canopy when hearing distant noise associated with people moving through the forest.
We are aware that the sonographic differences found in this study may simply reflect what distinguishes Didunculus and Ducula in the minds of the hunters; this may be only their memory of the Didunculus call. It is also possible that a portion of the 54 (reduced to 30) TEK identifications were incorrectly identified (i.e. both hunters gave the same wrong identifications). We do not have the means to assess the proportion of possible TEK-based wrong identifications. Despite this ‘background noise’ (i.e. the possible hunters’ ‘interpretations’ and the possible wrong identifications), statistically significant differences emerged in relation to HF and to the average and variation of intrasequence coo call intervals. We regard these differences found in the present study to be interesting and unique TEK information and we strongly doubt that they are the result of pure coincidence.
Perfecting this knowledge through future more focused studies, either science-based or TEK-based (or, ideally, through a mix of the two approaches), may allow identification of forest areas with sufficient density of Didunculus to enable implementation of urgently needed conservation work (such as local community engagement, forest protection and restoration, invasive predator and hunting control) (sensu MNRE and SCS 2020). Additionally, it will allow the assessment of Didunculus population size and distribution across the whole historical range and allow population trend and conservation status to be monitored.
A recommended next stage would be to record coo call sequences of Ducula on Tutuila Island (American Samoa) where Didunculus has never been recorded, in order to better assess intraspecies variability. Also helpful would be to test, using the playback technique, samples of automatically recorded coo call sequences (including those of Didunculus) with different Ducula individuals in the field in Samoa in order to record their behavioural responses using already established protocols (Wolfenden et al. 2015).
In general, the advertising calls of sympatric species are not similar due to selection for species-specific signals (Wilkins et al. 2013). Exceptions to this general pattern are species that are not ecological competitors (Price 2008), rare cases where species have adapted to similar soundscapes (Cardoso and Price 2010), or situations where range expansion has led to recent sympatric occurrence of previously allopatric species (Johnson et al. 2001; Wolfenden et al. 2015).
Currently, there is insufficient ecological and zoogeographic information to assess the situation for Didunculus and Ducula in Samoa. It is conceivable that these two sympatric species are ecological competitors, although the former’s beak structure suggests a specialised diet (Beichle 1987a ). It is also possible that Ducula is a relatively recent arrival in Samoa, but there are no records to support this hypothesis. Thus why two sympatric pigeon species have such similar vocalisations remains a mystery.
Overall, the present study proposes an original approach, using traditional ecological knowledge to separate the calls of Didunculus and Ducula. Two main results emerge. First, the two identification criteria proposed by Baumann and Beichle (2020) (Didunculus indicated by HF > 400 Hz or >20 repetitions) appear not to be as definitive and clearcut as hoped. On the other hand, the study confirms that the regularity of the intervals between coo calls in a sequence may be the key to separation of the two species in the field.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We acknowledge and thank the Ministry of Natural Resources and Environment, the Strengthening Multi-sectoral Management of Critical Landscapes project and the United Nations Development Program (UNDP) Samoa multi-country office for their assistance. We are indebted to hunters Fiu Kilifi Ofa, Livingstone Siu, Tuaoi, Lohia, Mailata Tuaita, Mailata Onolua, Pili Falailo, Lupe Toilolo, Lupe Asimani, Afaese Alopopo and Siataoa Taliti Pepe, and all the other hunters interviewed at seven villages, for the valuable ecological knowledge they shared with us; we commit to always credit and acknowledge their considerable contribution. We also thank Sabine Baumann and Ulf Beichle for sharing their knowledge about the Didunculus call and Selvino de Kort for analysing the data using program Luscinia. The present work was made it possible thanks to a UNDP consultancy to GS (2015) and a Mohamed Bin Zayed conservation grant to the same author (2017).
References
Appleton, D. (1871). ‘Appletons’ journal: a magazine of general literature.’ (D. Appleton and Company: New York.) The Museum 5, 27–28.Baumann, S., and Beichle, U. (2020). Acoustical identification of Didunculus strigirostris, critically endangered tooth‐billed pigeon of Samoa. Journal of Ornithology 161, 439–446.
| Acoustical identification of Didunculus strigirostris, critically endangered tooth‐billed pigeon of Samoa.Crossref | GoogleScholarGoogle Scholar |
Beichle, U. (1982a). Untersuchungen zur Biologie und Systematik der Zahntaube, Didunculus strigirostris (Jardine, 1845). Dissertation zur Erlangung des Doktorgrades der Mathematisch-Naturwissenschaftlichen Fakultat der Christian-Albrechts Universitat zu Kiel
Beichle, U. (1982b). Zum Problem der Haltung von Zahntauben. Gefiederte Welt 106, 305–306.
Beichle, U. (1987a). Lebensraum, Bestand und Nahrungsaufnahme der Zahntaube, Didunculus strigirostris. Journal fur Ornithologie 128, 75–89.
| Lebensraum, Bestand und Nahrungsaufnahme der Zahntaube, Didunculus strigirostris.Crossref | GoogleScholarGoogle Scholar |
Beichle, U. (1987b). Zur Ernahrung der Zahntaube, Didunculus strigirostris (Jardine 1845). Trochilus 8, 83–86.
Beichle, U. (1989). Akustische Abgrenzung der Taubenarten der Samoa Inseln. Journal fur Ornithologie 130, 345–351.
| Akustische Abgrenzung der Taubenarten der Samoa Inseln.Crossref | GoogleScholarGoogle Scholar |
Beichle, U. (2006). Saving Samoa’s critically endangered maomao and Didunculus: report on field surveys on Upolu and Savaii from 5.10 to 25.10.2005 and from 3.5. to 22.5.2006. Unpublished report, Apia, Samoa, Ministry of Natural Resources and Environment
Beichle, U., and Baumann, S. (2016). ‘The Birds of Samoa.’ (Ulf Beichle, self- published: Germany.)
BirdLife International (2015). Species factsheet: Didunculus strigirostris. Available at: http://www.birdlife.org [accessed 31 December 2015].
Blair, M. (2005). Editorial. Sandgrouse 27, 2.
Butler, D. (2012). Report on the birds of upland Savaii. In ‘Rapid Biodiversity Assessment of Upland Savaii, Samoa’. (Eds J. Atherton, and B. Jefferies.) pp. 85–109. (SPREP: Apia, Samoa.)
Cardoso, G. C., and Price, T. D. (2010). Community convergence in bird song. Evolutionary Ecology 24, 447–461.
| Community convergence in bird song.Crossref | GoogleScholarGoogle Scholar |
Collar, N. J. (2015). Natural history and conservation biology of the tooth-billed pigeon (Didunculus strigirostris): a review. Pacific Conservation Biology 21, 186–199.
| Natural history and conservation biology of the tooth-billed pigeon (Didunculus strigirostris): a review.Crossref | GoogleScholarGoogle Scholar |
Conservational International (2016). Rapid Assessment Program. Available at http://www.conservation.org/projects/pages/rapid-assessment-program.aspx [accessed 31 May 2016].
Conservation International (Pacific Islands Programme), Ministry of Natural Resources and Environment, and Secretariat of the Pacific Regional Environment Programme (2010). Priority sites for conservation in Samoa: key biodiversity areas. (Apia, Samoa). 34 pp.
Grattan, F. J. H. (1985). An introduction to Samoan custom. NZ Electronic Text Centre, Victoria University, Auckland, New Zealand. Available at http://nzetc.victoria.ac.nz/tm/scholarly/tei-GraIntr.html [accessed 9 January 2017]
Jetz, W., Thomas, G. H., Joy, J. B., Redding, D. W., Hartmann, K., and Mooers, A. O. (2014). Global distribution and conservation of evolutionary distinctness in birds. Current Biology 24, 919–930.
| Global distribution and conservation of evolutionary distinctness in birds.Crossref | GoogleScholarGoogle Scholar | 24726155PubMed |
Johnson, K. P., De Kort, S., Dinwoodey, K., Mateman, A. C., Ten cate, C., and Lessells, C. M. (2001). A molecular phylogeny of the dove genera Streptopelia and Columba. Auk 118, 874–887.
| A molecular phylogeny of the dove genera Streptopelia and Columba.Crossref | GoogleScholarGoogle Scholar |
MNRE (2006). Recovery plan for the manumea or tooth-billed pigeon (Didunculus strigirostris), 2006–2016. Ministry of Natural Resources & Environment, Government of Samoa, Apia, Samoa. 41 pp
MNRE and SCS (2020). Recovery plan for the manumea or tooth-billed pigeon (Didunculus strigirostris), 2020–2029. Ministry of Natural Resources & Environment, Government of Samoa, and Samoa Conservation Society. Apia, Samoa. 23 pp
Powell, A. (2008). ‘The Race to Save the World’s Rarest Bird: The Discovery and Death of the Po’ouli.’ (Stackpole Books: Mechanicsburg, PA, USA.)
Pratt, H. D., and Mittermeier, J. C. (2016). Notes on the natural history, taxonomy, and conservation of the endemic avifauna of the Samoan archipelago. The Wilson Journal of Ornithology 128, 217–241.
| Notes on the natural history, taxonomy, and conservation of the endemic avifauna of the Samoan archipelago.Crossref | GoogleScholarGoogle Scholar |
Price, T. (2008). ‘Speciation in Birds.’ (Roberts & Company Publishers: Greenwood Village, CO.)
Serra, G. (2016). Biodiversity surveying of four KBAs in Samoa through traditional ecological knowledge: Uafato-Tiavea Coastal Forest, Apia Catchments, Central Savaii Rainforest and Falealupo Peninsula. Unpublished report, Apia, Samoa, MNRE and UNDP. 83 pp
Serra, G. (2017). Review of implementation of Manumea Recovery Plan 2006–2016. Technical report to the Ministry of Natural Resources & Environment, Department of Environment and Conservation, Apia, Samoa. 49 pp
Serra, G., Abdallah, M., Assaed, A., Abdallah, A., Al qaim, G., Fayad, T., and Williamson, D. (2004). Discovery of a relict breeding colony of northern bald ibis Geronticus eremita in Syria. Oryx 38, 1–7.
| Discovery of a relict breeding colony of northern bald ibis Geronticus eremita in Syria.Crossref | GoogleScholarGoogle Scholar |
Serra, G., Sherley, G., Failagi, S. A., Foliga, S. T., Uili, M., Enoka, F., and Suaesi, T. (2018). Traditional ecological knowledge of the Critically Endangered Tooth-billed Pigeon Didunculus strigirostris, endemic to Samoa. Bird Conservation International 28, 620–642.
| Traditional ecological knowledge of the Critically Endangered Tooth-billed Pigeon Didunculus strigirostris, endemic to Samoa.Crossref | GoogleScholarGoogle Scholar |
Sinclair, J. R., Tuke, L., and Opiang, M. D. (2010). What the locals know: comparing traditional and scientific knowledge of megapodes in Melanesia. In ‘Ethno-ornithology: Global Studies in Indigenous Ornithology: Culture, Society and Conservation’. (Eds S. Tidemann, A. Gosler, and R. Gosford.) pp. 115–137. (Earthscan: London, UK.)
Stattersfield, A. J., and Capper, D. R. (2000). ‘Threatened Birds of the World.’ (BirdLife International: Cambridge, UK, and Lynx Ediciones: Barcelona, Spain.)
Stirnemann, R. L., Stirnemann, I. A., Abbot, D., Biggs, D., and Heinsohn, R. (2018). Interactive impacts of by-catch take and elite consumption of illegal wildlife. Biodiversity and Conservation 27, 931–946.
| Interactive impacts of by-catch take and elite consumption of illegal wildlife.Crossref | GoogleScholarGoogle Scholar |
Watling, D. (2001). ‘A Guide to Birds of Fiji & Western Polynesia.’ (Environmental Consultants: Suva, Fiji.)
White, P. C. L., Jennings, N. V., Renwick, A. R., and Barker, N. H. L. (2005). REVIEW: Questionnaires in ecology: a review of past use and recommendations for best practice: Questionnaires in ecology. Journal of Applied Ecology 42, 421–430.
| REVIEW: Questionnaires in ecology: a review of past use and recommendations for best practice: Questionnaires in ecology.Crossref | GoogleScholarGoogle Scholar |
Wildlife Acoustics (2016). Bioacoustics software version 4.0 documentation. Available at https://www.wildlifeacoustics.com/images/documentation/Song-Scope-Users-Manual.pdf [accessed 10 December 2020].
Wilkins, M. R., Seddon, N., and Safran, R. J. (2013). Evolutionary divergence in acoustic signals: causes and consequences. Trends in Ecology & Evolution 28, 156–166.
| Evolutionary divergence in acoustic signals: causes and consequences.Crossref | GoogleScholarGoogle Scholar |
Wolfenden, A., Jones, C. G., Tatayah, V., Züel, N., and de Kort, S. R. (2015). Endangered pink pigeons treat calls of the ubiquitous Madagascan turtle dove as conspecific. Animal Behaviour 99, 83–88.
| Endangered pink pigeons treat calls of the ubiquitous Madagascan turtle dove as conspecific.Crossref | GoogleScholarGoogle Scholar |