Nesting success and productivity of Tucuman Parrots (Amazona tucumana) in high-altitude forests of Argentina: do they differ from lowland Amazona parrots?
Luis Rivera A D , Natalia Politi A , Enrique H. Bucher B and Anna Pidgeon CA Facultad de Ciencias Agrarias, Universidad Nacional de Jujuy, CONICET, Alberdi 47, S. S. de Jujuy (4600), Jujuy, Argentina.
B Centro de Zoología Aplicada, Universidad Nacional de Córdoba, CONICET, Casilla de Correo 122, Córdoba (5000), Argentina.
C Department of Forest and Wildlife Ecology, University of Wisconsin – Madison, 120 Russell Laboratories, 1630 Linden Drive, Madison, Wisconsin, WI 53706-1598, USA.
D Corresponding author. Email: luosvriv@yahoo.com
Emu 114(1) 41-49 https://doi.org/10.1071/MU12062
Submitted: 1 October 2012 Accepted: 10 May 2013 Published: 20 September 2013
Abstract
Most of our knowledge of reproduction of wild parrots in the Neotropics comes from studies of tropical lowland species, with few studies addressing species of high-altitude forests. We studied the reproductive biology of Tucuman Parrots (Amazona tucumana) in north-western Argentina between 2004 and 2009. We obtained data on reproductive output for 86 nests and on causes of mortality for 94 nests. Mean clutch-size per nesting attempt was 3.6 eggs ± 1.0 (s.d.). Hatching success (proportion of eggs laid that hatch) was 0.77 ± 0.17. Fledging success (proportion of nestlings that fledge) was 0.83 ± 0.13. The overall breeding success (mean number of fledglings per laying female per year) was 2.3 ± 0.8. Overall finite nesting success (daily survival rate to the power of the nesting length) was 0.53 ± 0.27, and chick finite nesting success rate was 0.74 ± 0.22. We did not find differences in reproductive rate between Tucuman Parrots and other species of Amazona parrot from lowland habitats. Productivity and nesting success of Tucuman Parrots had high values in some years and low values in others. This was probably related to fruiting events of Podocarpus parlatorei – a critical food item. The main causes of nesting failure were predation (16%) and abandonment (12%). Our results suggest that for several species of Amazona in lowland habitats, predation and poaching may be the main limiting factors whereas climatic factors and food availability may contribute most to nesting failure at higher altitudes.
Additional keywords: breeding biology, mortality factors, reproductive output, subtropical cloud forests.
Introduction
Detailed knowledge of breeding biology is necessary for understanding variation in avian reproductive strategies, because it provides critical natural history data that are useful for generating new hypotheses and testing old ones (Auer et al. 2007). The lack of information on the natural history, ecology and demography of many parrot species precludes an assessment of the mechanisms that regulate population dynamics (Koenig 2001). There have been a number of studies of the nesting and reproductive behaviour of several lowland tropical forest species of Amazona parrot (Gnam 1991; Rojas Suárez 1991; Enkerlin-Hoeflich 1995; Koenig 2001; Fernandes Seixas and Mourao 2002; Renton and Salinas Melgoza 2004; Sanz and Rodríguez Ferraro 2006; Rodríguez Castillo and Eberhard 2006; Berkunsky and Reboreda 2009). However, the behaviour and reproductive rates of Amazona parrots that nest in temperate and high-altitude forests (above 1500 m above sea level), specifically the Scaly-naped Parrot (A. mercenaria) and the Tucuman Parrot (A. tucumana), are not known (Juniper and Parr 1998). Of the other two parrots that occur in high-altitude forests, i.e. the Thick-billed Parrot (Rhynchopsitta pachyrhyncha) and the Indigo-winged Parrot (Hapalopsittaca fuertesi), there has been only one study that has described reproductive rates (Thick-billed: Monterrubio et al. 2002) and another on nestling growth and plumage development (Indigo-winged: Tovar-Martinez 2009).
There is a well-established pattern of variation in bird life-history traits along latitudinal gradients in which life-history strategies of tropical and southern hemisphere birds differ markedly from those of northern temperate birds (Martin 2004). Elevation has long been considered an important factor in shaping the evolution of life-history traits (Cody 1966; Badyaev 1997). High-elevation species have been thought to have higher reproductive rates, potentially compensating for the less predictable climate, and lower population density and to experience generally more limited availability of food (Cody 1966; Badyaev 1997). Congeners whose young are altricial have larger clutches at high elevation than at lower elevations (Stewart et al. 1977; Weathers et al. 2002; Johnson et al. 2006). However, there is also evidence suggesting that birds shift to a slower life-history strategy with increasing elevation, investing less in reproduction and producing smaller clutches than birds at lower altitudes (Krementz and Handford 1984; Badyaev 1997; Badyaev and Ghalambor 2001).
Some reproductive parameters of Amazona parrots from lowland tropical forest are well known. Most Amazona parrots have rates of nesting success of 35–57% (Gnam and Rockwell 1991; Enkerlin-Hoeflich 1995; Koenig 2001; Fernandes Seixas and Mourao 2002; Renton and Salinas Melgoza 2004; Sanz and Rodríguez Ferraro 2006). However, island species tend to have higher rates of success, of 46–82% (Snyder et al. 1987; Gnam and Rockwell 1991) and in studies of two Amazona species with the lowest documented rates of nesting success (i.e. 0–13%), rates of nest poaching were high (Martuscelli 1995; Rodríguez Castillo and Eberhard 2006). Most Amazona species have a mean clutch-size of 2.6–3.6 eggs and produce 0.3–2.5 fledglings per female (Snyder et al. 1987; Gnam and Rockwell 1991; Enkerlin-Hoeflich 1995; Martuscelli 1995; Koenig 2001; Renton and Salinas Melgoza 2004; Rodríguez Castillo and Eberhard 2006; Sanz and Rodríguez Ferraro 2006).
In contrast to many lowland forest species of Amazona, the Tucuman Parrot has a small geographical range in the narrow strip of cloud forest (1400–2200 m above sea level) of the Southern Yungas on the eastern slopes of the Andes from south-eastern Bolivia to north-western Argentina (Fjeldså and Krabbe 1990). The species is categorised as Vulnerable on the IUCN Red List, and is included in CITES Appendix I prohibiting international trade (BirdLife International 2011). A recent assessment of the status of the species estimated that current population is probably ~33% smaller than in the 1980s (Rivera et al. 2007, 2010).
Food availability is a major factor affecting annual productivity of parrots (Renton and Salinas Melgoza 2004; Díaz et al. 2012). The Tucuman Parrot relies heavily on a single food item, the seeds of Podocarpus parlatorei (Pinales : Podocarpaceae), for feeding nestlings (Rivera 2011). The reproductive biology of the Tucuman Parrot is virtually unknown, with only one record of a nest, from January in the 1940s in Chuquisaca Department, Bolivia (Bond and Meyer de Schauensee 1943).
An understanding of the reproductive biology of the Tucuman Parrot may provide insights into the mechanisms that regulate population dynamics of this species and the associated limiting factors that apparently continue to limit its recovery. Here we present novel information on nesting success and productivity of the Tucuman Parrot in the wild, gathered over a 5-year period. In addition, we compare our results with published data for other Psittaciformes, mainly Amazona parrots from lowland forests of the Neotropics and on the Thick-billed Parrot from comparable high-altitude forests. Through this comparison we aim to evaluate the differences and similarities of reproductive ecology and factors that limit the populations of highland and lowland species of parrot.
Material and methods
Study area
The study was conducted in the central sector of the Southern Yungas of north-western Argentina, on the eastern slopes of the Sierras Subandinas Centrales or Sistema de Santa Bárbara – a mountain range ~100 km long, between the Cordillera Oriental to the west and the Chaco plain to the east. The Southern Yungas supports a semi-evergreen subtropical montane cloud forest that forms the southernmost part of the Andean tropical forest (Cabrera 1976). The cloud forest is dominated by Podocarpus parlatorei, Alnus acuminata and trees of the family Myrtaceae. The local climate has a marked dry season from April to October and a rainy season from November to March. Annual rainfall is 800–1500 mm and mean annual temperature is 11.7°C (Mendoza 2005).
Within the central sector of the Southern Yungas, we focussed on two areas where we knew of active nests: (1) El Rey National Park (24°43′S, 64°38′W, 44 000 ha) and (2) Portal de Piedra Private Reserve (24°05′S, 64°26′W, 400 ha). Both areas are strictly protected and controlled, no people live within the reserves and, to the best of our knowledge, there is no poaching (in 5 years of field-work we did not detect any signs or tracks of people in the nesting areas). The study was conducted within accessible areas only. These comprised ~170 ha in Portal de Piedra Private Reserve and 50 ha in El Rey National Park. Elevation of these areas is between 1450 and 2100 m above sea level.
Data collection
Field-work was conducted from November to March over five breeding seasons between 2004 and 2009. We recorded pairs of Tucuman Parrots prospecting for nesting cavities in November, laying eggs from early to mid-December, and young fledging from mid- to late February. Daily searches for active nests were conducted for 35–40 days each breeding season during the laying and incubation periods (December–mid-January). We used a hand-held GPS unit (Garmin Etrex HCx, Garmin International Inc., Olathe, KS, USA) to record the boundaries of the area surveyed, which were later imported into a geographical information system (ArcGIS 9, ESRI, Redlands, CA, USA). Active nests were found by following males to the nesting area and locating the cavity when the female left to be fed by the male (González Elizondo 1998). Nests were found throughout the incubation period and the early nestling period, when females were incubating or brooding. We did not find nests later in the nesting cycle because it becomes difficult to detect nests after females stop brooding. We identified a total of 94 breeding attempts for which we recorded nesting success or causes of nesting failure. Data on reproductive output were gathered from 86 nests in which complete clutches were laid (defined as consecutive visits to active nests during which the number of eggs did not change, but the nest remained active). We were unable to record the number of eggs for eight active nests that were found in the early nestling period. Each active nest was monitored on average every 9.4 days ± 3.9 (s.e.) (less often during incubation and more often during the nestling period) to determine status and to record contents of the nests. Most nests <15 m above the ground were inspected with a mini-camera system (miniature black-and-white CMOS camera, Model MCC-340EH , and a 10.16-cm Portable TFT LCD Monitor, Model VM-2003, EPCOM, El Paso, TX, USA) assembled by us and attached to a pole, known as a tree-peeper, that could be extended to 15 m (Crain fibreglass telescoping measuring rod, Crain Entreprises, Mound City, IL, USA) (Richardson et al. 1999). To avoid excessive disturbance, we restricted our time at each nest to ≤10 min; if that was insufficient time to complete our observations, we returned to the nest on a subsequent day. Nests >15 m above the ground were visually inspected using climbing equipment to reach the nest (Perry 1978). During the incubation period nests above 15 m were inspected once only. We monitored contents of nests when females left the nests to be fed by males.
When possible, we recorded the date clutches were initiated, and determined incubation period and length of the entire reproductive effort for each nest. We estimated the following parameters: clutch-size (i.e. number of eggs laid per nesting attempt), brood-size (i.e. number of chicks per nest), hatching success (i.e. proportion of eggs present in the nest at the end of incubation that hatch), fledging success (i.e. proportion of nestlings that fledge), and nesting success (i.e. proportion of nests producing at least one fledgling). To allow comparison with other studies, reproductive output was expressed as fledglings per successful nest and fledglings per laying female (including successful and unsuccessful females). We considered a nest successful when it produced at least one fledgling. We considered a nest abandoned when it contained eggs but no adults were recorded in more than 1 h of observation on two successive visits. A nest was considered depredated when all of the eggs disappeared before hatching, nestlings disappeared before reaching 47 days of age, or remains of eggs or nestlings were found in an otherwise empty nest. We used 47 days as the cut-off age because fledglings attain maximum primary feather length at ~50 days and we estimate that before 47 days wings are too short to sustain flight (Rivera 2011). Nests were considered lost by starvation when nestlings were found dead with an empty crop. We examined productivity at different stages of the nesting cycle on the basis of all laying pairs.
Statistical analyses
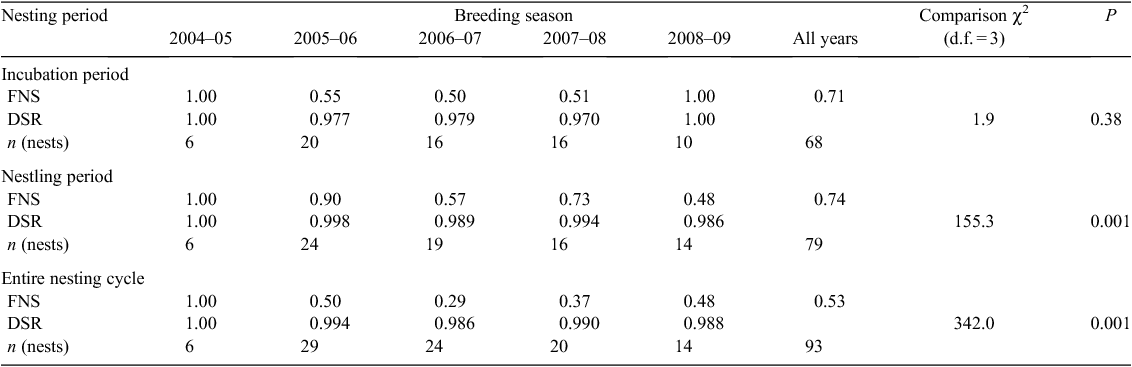
Data were not normally distributed (modified Shapiro–Wilk test) so we used Kruskal–Wallis non-parametric analyses of variance (ANOVA) to examine differences among years. When differences were significant we performed a test of critical difference of the mean ranks (Conover 1999). We used Mayfield’s (1975) method to calculate daily nest-survival rate because this method avoids the bias introduced when nests are found at different stages of the nesting cycle. Because intervals between visits to nests varied we used a maximum-likelihood estimate modification of the Mayfield method (Johnson 1979; Krebs 1989). We calculated the variance of the Mayfield estimator according to Johnson (1979) to make comparisons with the program Contrast (see below). We estimated the daily nest-survival rate (DSR) during the incubation (28 days) and nestling (1–50 days after hatching) stages and multiplied the DSR of nests during incubation with the DSR of nests during the nestling stage to obtain the finite survival rate. The program Contrast (Hines and Sauer 1989) was used to compare DSR among periods of the nesting cycle and among breeding seasons. We were not able to use dataset from the 2004–05 breeding season because DSR had no associated variance. We assumed that a juvenile had fledged when it was absent from a nest at ≥47 days after hatching. Disappearances at earlier ages were considered to be deaths. We used P < 0.05 for statistical significance, and all values are presented as mean ± standard deviation, unless otherwise stated. Statistical analysis were performed with INFOSTAT Software (Di Rienzo et al. 2011).
We conducted a literature review to obtain information on productivity parameters for other species of Amazona parrot and for other parrot species of highland temperate forest. In order to understand whether clutch-size of Tucuman Parrots is higher or lower than expected for the body-size of the species, we assessed whether adult Tucuman Parrot body mass (~280 g, Low 2005) and clutch-size followed the allometric equation (y = 2.2 + 5.5 exp [–0.006x]) developed by Masello and Quillfeldt (2002) for other species of parrots, where y is clutch-size is and x is body-mass. Additionally, we performed a regression analysis of clutch-size as a function of body-mass for all species of Amazona parrot for which we could obtain data, to test if clutch-size of Tucuman Parrots lies inside the 95% confidence interval of the allometric relationship.
Results
Productivity and breeding success
The length of the incubation period (mean 28.33 ± 0.58 days, range = 28–29), nestling period (49.7 ± 1.1 days, range = 49–51) and overall nesting period of Tucuman Parrots were determined from data from three nests for which date of laying of the first egg, date of hatching of the first egg, and date of fledging of the first chick were known with certainty. Clutch-size ranged from one to five eggs (mean 3.6 ± 1.0, mode = 4, n = 86; Fig. 1). Clutches of one are most likely to be complete clutches because we did not find any evidence of partial loss or predation. The smallest overall clutch-size was recorded during the 2008–09 breeding season, and differed significantly from mean annual clutch-size of three other breeding seasons (H4 = 9.6, d.f. = 4, P = 0.03; Table 1). Overall hatching success (proportion of eggs that hatch; see Methods) was 0.77, with the lowest rate (0.54), recorded in 2006–07 (Table 1). The number of fledglings per successful nest (overall mean 3.2 ± 0.2, n = 51) differed among years with lowest values in 2005–06 and 2006–07 (H4 = 8.3, d.f. = 4, P = 0.05; Table 1). The number of fledglings per laying female was 2.3 ± 0.8 (n = 86) and differed significantly among years, with the lowest value during the 2006–07 breeding season (H4 = 11.9, d.f. = 4, P = 0.01; Table 1).

|
The daily survival rate during the incubation period did not differ significantly among years (Table 2). The daily survival rate during the nestling period and the entire nesting cycle differed significantly among breeding seasons (Table 2). The highest finite survival rate for the nestling period and the nesting cycle was in the 2005–06 breeding season and the lowest finite survival rate was in 2006–07 (Table 2).
Causes of nesting failure
Over the nesting cycle, the primary causes of nest-loss were predation (16%) and abandonment (12%). Nine nests were depredated during the incubation period and six during the nestling period (Fig. 2). Abandonment was the main cause of failure during the incubation period, followed by predation, whereas predation was the main cause of failure during the nestling period (Fig. 2). Nesting failure was higher during incubation: 22 nests were lost during this period (63% of all losses), whereas 13 nests were lost during the nestling period (37%). Eleven nests were abandoned during incubation whereas none was abandoned during the nestling period. In two breeding seasons (2006–07 and 2008–09) predation was the primary cause of nest-loss, in one breeding season (2005–06) the main cause was abandonment, and in one season (2007–08) predation and abandonment were equal causes. Predation of four nests <100 m apart might be attributed to Black-capped Capuchin monkeys (Cebus apella) that were observed on a tree in which we had confirmed active nests the previous day and found empty the day after the monkeys were observed. In two nests with advanced nestlings we found bundles of plucked feathers at the entrance to the nesting cavity and on the ground, suggesting predation by mammals. Most nesting failures (n = 13) occurred during the 2006–07 breeding season, with fewer failed attempts per year in the other breeding seasons.

|
Allometry relative to other parrots
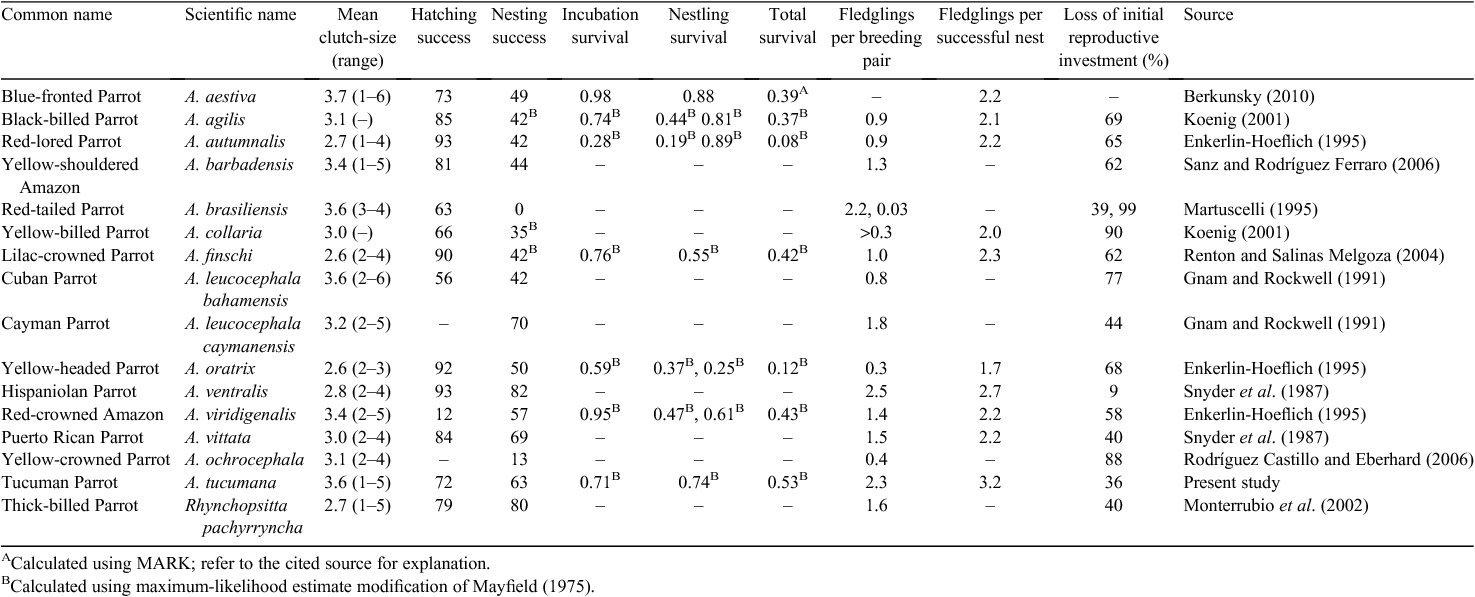
We obtained published and unpublished information on the reproductive ecology of 14 species of Amazona parrot from lowland habitats and one species from high-altitude habitat (Table 3). With a mean body-mass of 280 g, the predicted clutch-size of Tucuman Parrots would be 3.2 eggs based on the allometric equation of Masello and Quillfeldt (2002; see Methods). The 95% confidence interval for the expected clutch-size of the allometric equation for 14 other species of Amazona parrots is 2.94–3.43, which is significantly lower than the mean clutch-size of 3.6 that we observed for the Tucuman Parrot. Nesting success of the Tucuman Parrot is higher than that of other mainland species of Amazona, but lower than that of island species (Table 3). The Tucuman Parrot has one of the highest fledging success per pair, exceeded only by the Hispaniolan Parrot (Amazona ventralis) (Tables 2, 3).
Discussion
Reproductive output
The Tucuman Parrot has high rates of nesting success, large clutches and a large number of fledglings per laying female. These results differ from trends observed in other bird species that tend to shift to a slower life-history strategy with increased elevation (Sandercock et al. 2005a, 2005b). There are several alternative explanations for our finding that the Tucuman Parrot has the second largest clutch-size among Amazona parrots. Firstly, the health (or nutritional state) of female birds, which is strongly related to availability of food, might affect both the number and quality of the eggs laid (Lack 1954; Martin 1987); secondly, birds with higher rates of nesting success may be expected to lay larger clutches (Skutch 1985); thirdly, clutch-size is inversely related to population density (Ricklefs 1980), which, in the case of nesting Tucuman Parrots, may be low; and lastly, the large clutch-size may be evidence of a life-history trade-off in which increased productivity is compensated for by lower survival of juveniles or adults (Bears et al. 2009).
In comparing the Tucuman Parrot and Thick-billed Parrot from high-altitude forests of Mexico (Monterrubio et al. 2002), both species have breeding success (percentage of fledglings per egg laid) of 60%, and both species also have rather high rates of nesting success. However, clutch-size and overall mean number of fledglings per breeding female is higher for the Tucuman Parrot than for the Thick-billed Parrot. It also appears that there is less variation in the breeding parameters between years for the Thick-billed Parrot than for the Tucuman Parrot. The overall loss of the initial reproductive investment is similar for both species.
The overall mean number of fledglings per breeding female and the overall nesting success for Tucuman Parrots calculated as the maximum likelihood estimator are the second highest values reported for Amazona parrots. Likewise, the Tucuman Parrot has the lowest loss of initial reproductive investment among Amazona parrots that have been studied. The probability of nesting success varied significantly among years at the combined nestling stages, similar to the pattern of the Lilac-crowned Parrot (Amazona finschi) (Renton and Salinas Melgoza 2004). However, unlike Lilac-crowned Parrots, Tucuman Parrots showed significant differences among years in nesting success over the entire nesting cycle.
Inter-annual variability in the availability of key food items may influence productivity and nesting survival of Tucuman Parrots, as is the case for the Lilac-crowned Parrot (Renton and Salinas Melgoza 2004), the Austral Parakeet (Enicognathus ferrugineus) (Díaz et al. 2012) and for many other bird species (Newton 1980). The extremely low productivity of the 2006–07 breeding season coincided with low seed production of Podocarpus parlatorei (Rivera 2011). The high nesting success and productivity of the 2005–06 breeding season coincided with mast-fruiting of Podocarpus parlatorei (Rivera 2011). Assuming that the availability of suitable cavities was not limiting at the population levels in our study area, the recorded variation in number of nests among years likely reflects true variation in the number of nesting pairs among years.
Factors potentially influencing reproductive output
We recorded 11 nests with intact eggs that were abandoned, although we did not check for infertility or diseases. Low temperatures (e.g. 8°C minimum on rainy days) in the cloud forest might make Tucuman Parrots more susceptible to loss of eggs through embryonic chilling (Stoodley and Stoodley 1990), especially when incubating adults are disturbed. A decrease in attendance at nests and an increase in the duration of incubation recesses are associated with abandonment of nests in the Puerto Rican Parrot (Amazona vittata) (Wilson et al. 1997). Nest abandonment has been reported as the main cause of total nest failure in the Thick-billed Parrot (Monterrubio et al. 2002), Red-crowned Amazon (A. viridigenalis), Red-lored Parrot (A. autumnalis), and Yellow-headed Parrot (A. oratrix) (Enkerlin-Hoeflich 1995), but it is infrequent among other lowland Amazona species. Other factors that could explain abandonment are predation of incubating adults or food scarcity, which might lead females to end incubation because they cannot fulfil the high energy requirements of incubation.
Predation and abandonment of nests accounted for 28% of all losses of nests in the Tucuman Parrot. Predation is the main cause of nest losses for lowland species of Amazona (Enkerlin-Hoeflich 1995; Koenig 2001; Fernandes Seixas and Mourao 2002; Renton and Salinas Melgoza 2004; Sanz and Rodríguez Ferraro 2006) and for most birds (Ricklefs 1969; Skutch 1985; Newton 1998). Because Tucuman Parrots inhabit the cloud forest between 1400 and 2200 m above sea level, where richness and abundance of predators is expected to be lower than at lower elevations (Skutch 1985), we expected to observe a lower rate of nest predation than for other Amazona species of lowlands. Although there are no snakes – one of the most important predators of other Amazona parrots (Enkerlin-Hoeflich 1995; Koenig 2001; Renton and Salinas Melgoza 2004; Rodríguez Castillo and Eberhard 2006; Berkunsky et al. 2011) – in cloud forests of the Southern Yungas, other predators present include Black-capped Capuchins, at least four species of raptor (Barred Forest-Falcon, Micrastur ruficollis; White-rumped Hawk, Parabuteo leucorrhous; Roadside Hawk; Buteo magnirostris: Bicoloured Hawk, Accipiter bicolor) and three other potential nest predators: two mammals, the Tayra (Eira barbara) and the Lesser Grison (Galictis cuja), and the Plush-crested Jay (Cyanocorax chrysops). We frequently observed all of these species in the breeding habitat of Tucuman Parrots. Predation was the third-most frequent cause of total nesting failure for the Thick-billed Parrot in the high-altitude forests of Mexico (i.e. above 2200 m above sea level) (Monterrubio et al. 2002). A reduction in abundance and richness of predators in forests can influence rates of nesting failure at elevations >2200 m above sea level, as shown by the low rates of predation reported for the Thick-billed Parrot. The Tucuman Parrot breeds during the rainy season and, therefore, we expected to find a high number of flooded nests. However, we found few flooded nests in extremely rainy years (i.e. 2006–07 and 2007–08).
A factor contributing to the high reproductive output of Tucuman Parrots that we observed may be the lack of poaching in the study area. High rates of nesting success for Thick-billed Parrots have also been attributed in part to low rates of poaching (1 of 187 nests) (Monterrubio et al. 2002). Many studies reporting low reproductive output for parrots were conducted in areas where poaching affects productivity and nesting success (Martuscelli 1995; Wright et al. 2001; Rodríguez Castillo and Eberhard 2006; Sanz and Rodríguez Ferraro 2006). However for some species, low productivity can be explained not by the influence of poaching but by high levels of predation and low food availability (Renton and Salinas Melgoza 2004). Predation and poaching may be the main limiting factors in lowland habitats, whereas parrots nesting at higher altitudes may be more affected by climatic factors and food availability. Abandonment of nests was the main cause of nesting failure for the Thick-billed Parrot (Monterrubio et al. 2002) and the second-most frequent cause of nest failure for Tucuman Parrot, but it seems that this is not a significant cause of nesting failure for species nesting in the lowlands.
Acknowledgements
This study was supported by Loro Parque Fundación, Conservation Leadership Program, Rufford Small Grants, Wildlife Conservation Society Research Fellowships, Scott Neotropical Fund, Overbrook Fellowships for Career Development, Idea Wild and YPF Foundation through a doctoral fellowship to L. Rivera at Córdoba University in Argentina. National Park Administration and Direccion de Recursos Naturales of Jujuy Province granted the research permits. We are very grateful to Carlos and Silvia Cuñado, owners of Portal de Piedra Private Reserve, and El Rey National Park personnel for their support during field-work. We thank the many field assistants and students that collaborated in field-work, and to Katherine Buchanan and three anonymous reviewers that helped to improve this manuscript substantially.
References
Auer, S. K., Bassar, R. D., Fontaine, J. J., and Martin, T. E. (2007). Breeding biology of passerines in a subtropical montane forest in northwestern Argentina. Condor 109, 321–333.| Breeding biology of passerines in a subtropical montane forest in northwestern Argentina.Crossref | GoogleScholarGoogle Scholar |
Badyaev, A. V. (1997). Avian life history variation along altitudinal gradients: an example with cardueline finches. Oecologia 111, 365–374.
| Avian life history variation along altitudinal gradients: an example with cardueline finches.Crossref | GoogleScholarGoogle Scholar |
Badyaev, A. V., and Ghalambor, C. K. (2001). Evolution of life histories along elevational gradients: trade-off between parental care and fecundity. Ecology 82, 2948–2960.
| Evolution of life histories along elevational gradients: trade-off between parental care and fecundity.Crossref | GoogleScholarGoogle Scholar |
Bears, H., Martin, K., and White, G. C. (2009). Breeding in high-elevation habitat results in shift to slower life-history strategy within a single species. Journal of Animal Ecology 78, 365–375.
| Breeding in high-elevation habitat results in shift to slower life-history strategy within a single species.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M3it1Glsg%3D%3D&md5=07eadb66178628bc7429908093780181CAS | 19007385PubMed |
Berkunsky, I. (2010). Ecología reproductiva del Loro Hablador (Amazona aestiva) en el Chaco Argentino. Ph.D. Thesis, Universidad Nacional de La Plata, La Plata, Argentina.
Berkunsky, I., and Reboreda, J. C. (2009). Nest-site fidelity and cavity reoccupation by Blue-fronted Parrots Amazona aestiva in the dry chaco of Argentina. Ibis 151, 145–150.
| Nest-site fidelity and cavity reoccupation by Blue-fronted Parrots Amazona aestiva in the dry chaco of Argentina.Crossref | GoogleScholarGoogle Scholar |
Berkunsky, I., Kacoliris, F., Faegre, S., Ruggera, R., Carrera, J., and Aramburú, R. (2011). Nest predation by tree-snakes on cavity nesting birds in dry chaco woodlands. Ornitologia Neotropical 22, 459–464.
BirdLife International (2011). Species factsheet: Tucuman Amazon Amazona tucumana. Available at www.birdlife.org/datazone/speciesfactsheet.php?id=1667 [Verified 5 August 2013].
Bond, J., and Meyer de Schauensee, R. (1943). The birds of Bolivia. Part II. Proceedings of the Academy of Natural Sciences of Philadelphia 95, 167–221.
Cabrera, A. L. (1976). Regiones fitogeográficas Argentinas, In ‘Enciclopedia Argentina de Agricultura y Jardines’. (Ed. W. F. Kugler.) pp. 1–85. (Editorial ACME S.A.C.I.: Buenos Aires.)
Cody, M. L. (1966). A general theory of clutch size. Evolution 20, 174–184.
| A general theory of clutch size.Crossref | GoogleScholarGoogle Scholar |
Conover, W. J. (1999). ‘Practical Nonparametric Statistics.’ (Wiley: New York.)
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., Gonzalez, L., Tablada, M., and Robledo, C. W. (2011). InfoStat versión 2011. InfoStat, FCA, Universidad Nacional de Córdoba, Argentina.
Díaz, S., Kitzberger, T., and Peris, S. (2012). Food resources and reproductive output of the Austral Parakeet (Enicognathus ferrugineus) in forests of northern Patagonia. Emu 112, 234–243.
| Food resources and reproductive output of the Austral Parakeet (Enicognathus ferrugineus) in forests of northern Patagonia.Crossref | GoogleScholarGoogle Scholar |
Enkerlin-Hoeflich, E. C. (1995). Comparative ecology and reproductive biology of three species of Amazona parrots in northeastern Mexico. Ph.D. Thesis, Texas A&M University, Austin.
Fernandes Seixas, G., and Mourao, G. M. (2002). Nesting success and hatching survival of the Blue-fronted Amazon (Amazona aestiva) in the Pantanal of Mato Grosso do Sul, Brazil. Journal of Field Ornithology 73, 399–409.
Fjeldså, J., and Krabbe, N. (1990). ‘Birds of the High Andes.’ (Apollo Books: Stenstrup, Denmark.)
Gnam, R. S. (1991). Nesting behaviour of the Bahama Parrot Amazona leucocephala bahamensis on Abaco Island, Bahamas. Proceedings of the International Ornithological Congress 20, 673–680.
Gnam, R. S., and Rockwell, R. F. (1991). Reproductive potential and output of the Bahama Parrot Amazona leucocephala bahamensis. Ibis 133, 400–405.
| Reproductive potential and output of the Bahama Parrot Amazona leucocephala bahamensis.Crossref | GoogleScholarGoogle Scholar |
González Elizondo, J. J. (1998). Productividad, causas de mortalidad en nidos y dieta de los polluelos de tres especies de loro del género Amazona en el sur de Tamaulipas. Ph.D. Thesis, Universidad del Noroeste, Tampico, México.
Hines, J. E., and Sauer, J. R. (1989). Program CONTRAST: a general program for the analysis of several survival or recovery rate estimates. Fish and Wildlife Technical Report 24, 1–7.
Johnson, D. H. (1979). Estimating nest success: the Mayfield method and an alternative. Auk 96, 651–661.
Johnson, L. S., Ostlind, E., Brubaker, J. L., and Balenger, S. L. (2006). Changes in egg size and clutch size with elevation in a Wyoming population of Mountain Bluebirds. Condor 108, 591–600.
| Changes in egg size and clutch size with elevation in a Wyoming population of Mountain Bluebirds.Crossref | GoogleScholarGoogle Scholar |
Juniper, P., and Parr, M. (1998). ‘Parrots: A Guide to Parrots of the World.’ (Yale University Press: New Haven, CT.)
Koenig, S. E. (2001). The breeding biology of Black-billed Parrot Amazona agilis and Yellow-billed Parrot Amazona collaria in Cockpit Country, Jamaica. Bird Conservation International 11, 205–225.
| The breeding biology of Black-billed Parrot Amazona agilis and Yellow-billed Parrot Amazona collaria in Cockpit Country, Jamaica.Crossref | GoogleScholarGoogle Scholar |
Krebs, C. J. (1989). ‘Ecological Methodology.’ (Harper Collins: New York.)
Krementz, D. G., and Handford, P. (1984). Does avian clutch size increase with altitude? Oikos 43, 256–259.
| Does avian clutch size increase with altitude?Crossref | GoogleScholarGoogle Scholar |
Lack, D. (1954). ‘The Natural Regulation of Animal Numbers.’ (Clarendon Press: Oxford, UK.)
Low, R. (2005). ‘Amazon Parrots, Avicultura, Trade, and Conservation.’ (Dona/Insignis Publications: České Budějovice, Czech Republic.)
Martin, T. E. (1987). Food as a limit on breeding birds: a life-history perspective. Annual Review of Ecology and Systematics 18, 453–487.
| Food as a limit on breeding birds: a life-history perspective.Crossref | GoogleScholarGoogle Scholar |
Martin, T. E. (2004). Avian life-history evolution has an eminent past: does it have a bright future? Auk 121, 289–301.
Martuscelli, P. (1995). Ecology and conservation of the Red-tailed Amazon Amazona brasiliensis in southeastern Brazil. Bird Conservation International 5, 405–420.
| Ecology and conservation of the Red-tailed Amazon Amazona brasiliensis in southeastern Brazil.Crossref | GoogleScholarGoogle Scholar |
Masello, J., and Quillfeldt, P. (2002). Chick growth and breeding success of the Burrowing Parrot. Condor 104, 574–586.
| Chick growth and breeding success of the Burrowing Parrot.Crossref | GoogleScholarGoogle Scholar |
Mayfield, H. (1975). Suggestions for calculating nest success. Wilson Bulletin 84, 456–466.
Mendoza, E. A. (2005). El clima y la vegetación natural. In ‘El clima del Noroeste Argentino’. (Ed. J. L. Minetti.) pp. 233–279. (Editorial Magna: Tucumán, Argentina.)
Monterrubio, T., Enkerlin-Hoeflich, E., and Hamilton, R. B. (2002). Productivity and nest success of Thick-billed Parrots. Condor 104, 788–794.
| Productivity and nest success of Thick-billed Parrots.Crossref | GoogleScholarGoogle Scholar |
Newton, I. (1980). The role of food in limiting bird numbers. Ardea 68, 11–30.
Newton, I. (1998). ‘Population Limitation in Birds.’ (Academic Press: San Diego, CA.)
Perry, D. R. (1978). A method of access into the crowns of emergent and canopy trees. Biotropica 10, 155–156.
| A method of access into the crowns of emergent and canopy trees.Crossref | GoogleScholarGoogle Scholar |
Renton, K., and Salinas Melgoza, A. (2004). Climatic variability, nest predation, and reproductive output of Lilac-crowned Parrots (Amazona finschi) in tropical dry forest of western Mexico. Auk 121, 1214–1225.
Richardson, D. M., Bradford, J. W., Range, P. G., and Christensen, J. (1999). A video probe system to inspect Red-cockaded Woodpecker cavities. Wildlife Society Bulletin 27, 353–356.
Ricklefs, R. E. (1969). An analysis of nesting mortality in birds. Smithsonian Contributions to Zoology 9, 1–48.
| An analysis of nesting mortality in birds.Crossref | GoogleScholarGoogle Scholar |
Ricklefs, R. E. (1980). Geographical variation in clutch size among passerines birds: Ashmole’s hypothesis. Auk 97, 38–49.
Rivera, L. (2011). Ecología, biología reproductiva y conservación del Loro alisero (Amazona tucumana) en Argentina. Ph.D. Thesis, Universidad Nacional de Córdoba, Córdoba, Argentina.
Rivera, L., Politi, N., and Bucher, E. H. (2007). Decline of the Tucuman Parrot Amazona tucumana in Argentina: present status and conservation needs. Oryx 41, 101–105.
| Decline of the Tucuman Parrot Amazona tucumana in Argentina: present status and conservation needs.Crossref | GoogleScholarGoogle Scholar |
Rivera, L., Rojas Llanos, R., Politi, N., Hennessey, B., and Bucher, E. H. (2010). The Near Threatened Tucumán Parrot Amazona tucumana in Bolivia: insights for a global assessment. Oryx 44, 110–113.
| The Near Threatened Tucumán Parrot Amazona tucumana in Bolivia: insights for a global assessment.Crossref | GoogleScholarGoogle Scholar |
Rodríguez Castillo, A. M., and Eberhard, J. R. (2006). Reproductive behavior of the Yellow-crowned Parrot (Amazona ochrocephala) in western Panama. Wilson Journal of Ornithology 118, 225–236.
| Reproductive behavior of the Yellow-crowned Parrot (Amazona ochrocephala) in western Panama.Crossref | GoogleScholarGoogle Scholar |
Rojas Suárez, F. (1991). Biología reproductiva de la cotorra Amazona barbadensis en la Península de Macanao. Ph.D. Thesis, Universidad Central de Venezuela, Caracas.
Sandercock, B. K., Martin, K., and Hannon, S. J. (2005a). Life history strategies in extreme environments: comparative demography of arctic and alpine ptarmigan. Ecology 86, 2176–2186.
| Life history strategies in extreme environments: comparative demography of arctic and alpine ptarmigan.Crossref | GoogleScholarGoogle Scholar |
Sandercock, B. K., Martin, K., and Hannon, S. J. (2005b). Demographic consequences of age-structure in extreme environments: population models for arctic and alpine ptarmigan. Oecologia 146, 13–24.
| Demographic consequences of age-structure in extreme environments: population models for arctic and alpine ptarmigan.Crossref | GoogleScholarGoogle Scholar | 16010534PubMed |
Sanz, V., and Rodríguez Ferraro, A. (2006). Reproductive parameters and productivity of the Yellow-shouldered Parrot on Margarita Island, Venezuela: a long-term study. Condor 108, 178–192.
| Reproductive parameters and productivity of the Yellow-shouldered Parrot on Margarita Island, Venezuela: a long-term study.Crossref | GoogleScholarGoogle Scholar |
Skutch, A. E. (1985). Clutch size, nesting success, and predation on nests of Neotropical birds, reviewed. Ornithological Monographs 36, 575–594.
| Clutch size, nesting success, and predation on nests of Neotropical birds, reviewed.Crossref | GoogleScholarGoogle Scholar |
Snyder, N. F. R., Wiley, J. W., and Kepler, C. B. (1987). ‘The Parrots of Luquillo: Natural History and Conservation of the Puerto Rican Parrot.’ (Western Foundation of Vertebrate Zoology: Los Angeles, CA.)
Stewart, R. M., Henderson, R. P., and Darling, K. (1977). Breeding ecology of the Wilson’s Warbler in the high Sierra Nevada, California. Living Bird 16, 83–102.
Stoodley, J., and Stoodley, P. (1990). ‘Genus Amazona.’ (Avian Publications: Altoona, WI.)
Tovar-Martinez, A. (2009). Crecimiento y desarrollo del plumaje en pichones de la cotorra aliazul (Hapalopsittca fuertesi) en la Cordillera Central Colombiana. Ornitología Colombiana 8, 5–18.
Weathers, W. W., Davidson, C. L., Olson, C. R., Morton, M. L., Nur, N., and Famula, T. R. (2002). Altitudinal variation in parental energy expenditure by White-crowned Sparrows. Journal of Experimental Biology 205, 2915–2924.
| 12177156PubMed |
Wilson, K., Wilson, A., Wilson, M. H., and Fields, R. (1997). Behavior of Puerto Rican Parrots during failed nesting attempts. Wilson Bulletin 109, 490–503.
Wright, T. F., Toft, C. A., Enkerlin-Hoeflich, E., Gonzalez-Elizondo, J., Albonoz, M., Rodríguez Ferraro, A., Rojas Suarez, F., Sanz, V., Trujillo, A., Beissinger, S. R., Berovides, V., Galvez, X., Brice, A. T., Joyner, K., Eberhard, J., Gilardi, J., Koenig, S. E., Stoleson, S., Martuscelli, P., Meyer, J. M., Renton, K., Rodríguez, A. M., Sosa-Asanza, A.C., Vilella, F. J., and Wiley, J. W. (2001). Nest poaching in Neotropical parrots. Conservation Biology 15, 710–720.
| Nest poaching in Neotropical parrots.Crossref | GoogleScholarGoogle Scholar |