Aerial drones and recreational fish finders: evaluating a low-cost method for surveying fish aggregations
B. Scoulding A * , D. V. Fairclough B , C. Devine A , G. Jackson B , P. Lewis B , D. Waltrick
A * , D. V. Fairclough B , C. Devine A , G. Jackson B , P. Lewis B , D. Waltrick  B , L. West B , C. Skepper B , J. Briggs B , E. Lek B , D. E. Yeoh B , B. M. Crisafulli B , E. A. Fisher B , A. Denham B , P. J. Mitchell B and S. Gastauer C
B , L. West B , C. Skepper B , J. Briggs B , E. Lek B , D. E. Yeoh B , B. M. Crisafulli B , E. A. Fisher B , A. Denham B , P. J. Mitchell B and S. Gastauer C
A
B
C
Abstract
Globally, anthropogenic activities such as fishing and industrial development have affected fish abundance. Cockburn Sound, a crucial spawning site for the sparid Chrysophrys auratus near Perth, Western Australia, faces potential impacts from ongoing infrastructure development, such as ports.
To inform environmental-impact assessment, innovative non-extractive methodologies are needed to quantify C. auratus aggregation abundance.
This study evaluated an ‘aerial–acoustic survey method’ that combines data from aerial drones and recreational fish finders. We investigated the ability of both methods to identify C. auratus aggregations across known spawning sites, types of proxies of abundance obtained, the practicality of each method in ambient environmental conditions and survey costs.
By integrating drones and fish finders, surface and subsurface aggregations of C. auratus were documented over two spawning periods (September–November in 2022 and 2023), capturing important parameters related to aggregation frequency, distribution and size.
Although initial equipment investments were modest, expenses for survey planning, operations and data analysis were substantial. Nevertheless, this approach offers a cost-effective alternative to using more expensive scientific-grade equipment.
The study presents a promising methodology for assessing distribution and abundance as part of environmental impacts in infrastructure developments and managing resources, in ecologically sensitive areas such as Cockburn Sound.
Keywords: anthropogenic impacts, Chrysophrys auratus, Cockburn Sound, marine monitoring, pink snapper, predator–prey interactions, recreational, spawning.
Introduction
Anthropogenic activities, such as fishing and industrial development, have influenced the abundance of fishes globally (Halpern et al. 2008; Brown et al. 2018; Brooker et al. 2020). This is often intensified in sheltered or partially smooth and easily accessible coastal waters, where marine industries such as fishing, aquaculture, ports, energy, mining and tourism operate (van Putten et al. 2016). These activities have led to habitat modification, pollution, hypoxia and over-exploitation (Nordhaus et al. 2018). Together, these can have cumulative effects on ecosystem services such as fisheries, which is inconsistent with the UN Sustainable Development Goal 14 to conserve and sustainably use the oceans, seas and marine resources for sustainable development (Ward and Jacoby 1992; Pillay et al. 2008; Cloern et al. 2016; Nordhaus et al. 2018; United Nations, Ocean & seas, see https://sustainabledevelopment.un.org/topics/oceanandseas).
In Australia, most of the human population lives on its coastline (van Putten et al. 2016). Many fishes also use coastal environments to provide food, nurseries and adult spawning grounds and are important in fisheries, for example, sparids, sillaginids and platycephalids (Roelofs et al. 2024). The sparid Chrysophrys auratus (locally known as pink snapper) uses protected habitats such as estuarine basins, inlets and embayments as nurseries across temperate Australia and its adults also aggregate to spawn in some of those environments (Gillanders 2002; Wakefield et al. 2011; Jackson and Moran 2012). Ease of human access to these environments has led to aggregations being targeted by commercial and recreational fisheries, contributing to over-exploitation of some stocks (Jackson and Moran 2012; Roelofs et al. 2024).
On the lower western coast of Australia, the adjacent embayments of Cockburn Sound and Warnbro Sound and the shallow coastal habitats of Owen Anchorage are the only locations along several hundred kilometres where adult C. auratus migrates to, and aggregates to spawn (Crisafulli et al. 2019; Bertram et al. 2022). The aggregations and the resultant juveniles that use those habitats as nurseries provide an important contribution to the broader coastal stocks (Wakefield 2010). These environments are also close to Perth, the main population centre of Western Australia, leading to substantial historical fishing of the aggregations and resultant management in the past 25 years to protect them. The sheltered nature of those waters, particularly Cockburn Sound, has led to significant development for petroleum, ship building, desalination plants and defence industries (Wakefield 2010; Molony et al. 2020). A new, large port facility is also proposed for Cockburn Sound to support projected increases in container ship size and trade activity (see https://westport.wa.gov.au/). Thus, the question has arisen as to the potential impacts of increasing development on the Cockburn Sound ecosystem and important fishes, such as C. auratus, and how they may be monitored.
Estimation of the spawning biomass of C. auratus at the biological stock scale across the entire lower western coast of Australia is important for its management (e.g. Fairclough et al. 2021; Bertram et al. 2022). However, C. auratus aggregations in Cockburn Sound are transient and represent only part of that broader spawning biomass (Crisafulli et al. 2019). Thus, their abundance in the Sound may not relate directly to the overall breeding stock, but could be used as a tuning index and could represent a useful metric for quantifying the impact of industrial development on C. auratus.
Methods for estimating spawning biomass of C. auratus in Cockburn Sound, such as the daily egg-production method (DEPM), are possible, but can be imprecise (low spatial and temporal resolution), affecting the ability to detect change (Zeldis and Francis 1998; Jackson et al. 2012). The method requires that the eggs of the target species are able to be accurately identified and quantified in plankton samples. This has traditionally been undertaken using microscopy; however; this is challenging, because other species co-occur in Cockburn Sound and spawn at the same time, for example, other sparids and some platycephalids, have eggs with similar visual characteristics and can therefore be mistaken for C. auratus (Dias et al. 2016). Genetic species-validation methods have been developed to overcome this issue, and although the laboratory work is time consuming (Dias et al. 2016; Oxley et al. 2017), it has led to further development and application of the method for assessment of C. auratus elsewhere in Australia (Steer et al. 2017).
Many surveys employ multiple methods to develop a full picture of fish species composition or abundances (e.g. Connell et al. 1998, Cappo et al. 2004; Christiansen et al. 2020) and frequently use non-invasive visual and video techniques (e.g. Langlois et al. 2020; Fairclough 2021). A combined acoustic and optical method was developed for estimating biomass of demersal C. auratus spawning aggregations on the upper western coast of Australia, using scientific single-beam echosounders and unbaited remote underwater video (Scoulding et al. 2023). These fish aggregated near the seabed in fairly clear, deep (~40 m) waters, allowing the use of downward-looking echosounders and seafloor video to identify C. auratus. By contrast, aggregations in the shallow (~5–20 m deep) and often turbid (mean monthly turbidity range January 2021–July 2023: 0.11–3.08 NTU, see https://estuaries.dwer.wa.gov.au/cockburn-sound/) Cockburn Sound are observed at and below the surface (Fig. 1), which may require alternative methods to maximise the success of surveys.
Aggregations of Chrysophrys auratus at and below the surface in Cockburn Sound, Western Australia.

Identifying whether increasing industrial activities are negatively affecting spawning aggregations of C. auratus in Cockburn Sound requires the development of novel and non-extractive methods. Rapid technological advancement has produced affordable and powerful downward- and side-scanning recreational fish finders, which can acquire data and imagery useful for surveying aquatic organisms (e.g. Bollinger and Kline 2017; Wolfenkoehler et al. 2023). Similarly, recreational aerial drones are now cheap and are increasingly being used in surveys of aquatic environments (e.g. Schofield et al. 2019; Raoult et al. 2020). In this study, we designed and evaluated an ‘aerial–acoustic survey method’ that combines observations from recreationally available drones and fish finders to survey C. auratus aggregations in Cockburn Sound. Specifically, we evaluated (1) the ability of the two methods separately, and in combination, to identify C. auratus aggregations along pre-defined transects across known spawning areas, (2) the types of proxies of abundance that could be obtained, for example, frequency of occurrence and aggregation size, (3) the practicality of each method in ambient environmental conditions, and (4) the cost of conducting surveys for the duration of the spawning period.
Methods
Study site
Cockburn Sound is a sheltered, marine embayment 22 km south of Perth, Western Australia (Fig. 2). It is ~16 km long and 9 km wide (103 km2). Its basin ranges from 9 to 23 m deep and comprises fairly homogenous soft sediment seabed. The basin is bordered by shallow banks (<10 m) consisting of seagrass meadows, small outcrops of limestone and unvegetated sand (Kendrick et al. 2002; Wakefield et al. 2013). Commercial shipping in Cockburn Sound is served by deep-water channels excavated into the eastern and northern banks and designated anchorage areas for existing industrial operations. The eastern shoreline of the Sound is heavily industrialised and is the location of a proposed new port (Fig. 2). The western side of the Sound is bounded by Garden Island, which hosts a large naval base (HMAS Stirling) that is serviced by a 3.3-km rock-filled causeway connecting the southern end of the island to the mainland. A key feature of the Cockburn Sound benthos is the D9 dredge shipwreck, recognised as an important site for C. auratus spawning aggregations.
Map of Cockburn Sound, showing Garden Island, Carnac Island and the mainland south of Perth, Western Australia (see inset). The left panel shows the eight 1-km2 cells sampled using fish finders and drones in 2022: Woodman Channel (WC, average depth, z = 12.5 m), Lumps Top (LT, z = 11.8 m), Lumps Bottom (LB, z = 11.5 m), D9 (z = 10.2 m), South Basin (SB, z = 20.6 m), Ammo Jetty (AJ, z = 19.5 m), Harding Rock (HR, z = 18.5 m) and Inside Rock (IR, z = 8.8 m). The right panel shows the three larger cells sampled in 2023. The light blue circles show ship anchorages, the yellow polygons show military exclusion zones, the red circles show no-fly zones, and the purple polygon shows the footprint of the Westport development and proposed dredged channels.
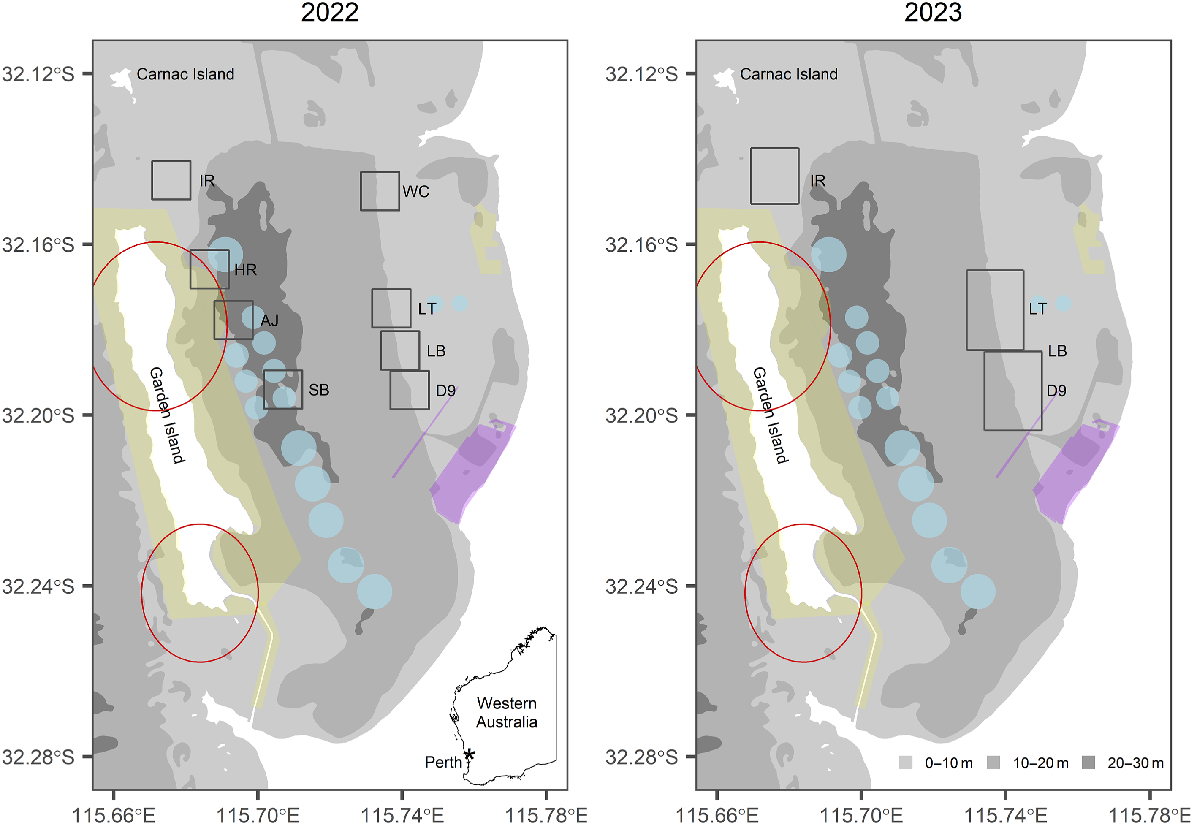
Because Cockburn Sound is quite large and has ship anchorages, military-exclusion zones and no-fly zones (Fig. 2), it was not feasible to conduct an aerial–acoustic survey of the entire Sound. Therefore, we selected eight subregions (referred to as cells, each 1 km2), which were: (1) Woodman Channel (WC), (2) Lumps Top (LT), (3) Lumps Bottom (LB), (4) D9 shipwreck, (5) South Basin (SB), (6) Ammo Jetty (AJ), (7) Harding Rock (HR) and (8) Inside Rock (IR) (Fig. 2). These sites were selected on the basis of C. auratus aggregations identified during previous mark–recapture studies and plankton surveys (Wakefield 2010) and are positioned around features such as limestone outcrops and the D9 shipwreck.
Peak spawning of C. auratus in Cockburn Sound is observed within a narrow temperature range of 19–21°C (Wakefield 2010), which typically occurs during spring to early summer (October–December; Wakefield 2010; Wakefield et al. 2015). From approximately August each year, C. auratus individuals migrate into Cockburn Sound via the shallow waters between Carnac Island and Garden Island (Fig. 2, Crisafulli et al. 2019). They then remain in the Sound to spawn before leaving via the same route by January (Crisafulli et al. 2019).
Survey design
Aerial–acoustic surveys were conducted in Cockburn Sound during peak spawning periods in 2022 and 2023. In 2022, surveys were repeated over 3 consecutive days in 3 months around the new moon (a period when C. auratus is known to spawn, Wakefield 2010), i.e. 28–30 September, 26–28 October and 22–24 November. IR was added as a new survey site in November 2022, following C. auratus sightings.
In 2023, the survey strategy was refined to concentrate on two cells on the eastern bank (1500-m longitude and 2000-m latitude and encompassing LT, LB and D9) and one cell in the north-east (1200-m longitude and 1500-m latitude and encompassing IR) (Fig. 2). This change to survey design was informed by the absence of C. auratus sightings in the other regions (WC, SB, AJ and HR) in 2022, juxtaposed with C. auratus observations slightly outside the LT, LB and D9 cells during the same period. In 2023, the surveys were conducted close to the time of the new moon on 10–12 September, 15–17 October and 13–15 November.
A two-stage survey design was implemented in both years to minimise any vessel impact on fish behaviour, comprising aerial surveys followed by acoustic surveys.
Stage 1: aerial surveys – data collection and analysis
A DJI Mavic II drone, equipped with a Hasselblad L1D-20c camera and 1-inch CMOS sensor, was used for aerial surveys. A polariser four-lens filter was fitted to decrease water reflection and glare. The drone flew at 12 m s−1 at an altitude of ~100 m, capturing 20-megapixel still JPEG photos every 3 s. Surveys were conducted using pre-programmed north-to-south parallel transects with 100-m spacing, resulting in ~20% lateral overlap and 60% forward overlap between images. The drone was piloted from a dedicated vessel and took ~20 min to map the 1-km2 cells in 2022 and ~60 min to survey the larger cells in 2023. Aerial surveys commenced at c. 07:00 hours daily (~1 h before acoustic surveys), took 3–4 h to complete and were scheduled to finish before the onset of the late-morning sea breeze (Pattiaratchi et al. 1997). To avoid disturbing C. auratus aggregations, the drone vessel operated outside surveyed areas. After each day, exchangeable image file format (EXIF) data were downloaded from the drone, and images were labelled according to altitude, longitude, latitude, time and species of interest, namely C. auratus.
Longitude, latitude, altitude (h, m) and camera focal length (Lf, cm) were extracted from the image EXIF data by using the Pillow python library (Clark 2015). The image extent (m, the spatial dimensions or geographic area covered by an image) and pixel size, including physical dimensions of a single pixel in the x (xres) and y (yres) direction as:
were computed on the basis of the camera sensor width (ws, cm), sensor height (hs, cm), h, Lf and the image width () and height (, pixels). Polygons bounding C. auratus aggregations were manually drawn on images and masks were created in ImageJ (ver. 1.54, see https://imagej.nih.gov/ij/; Rueden et al. 2017). OpenCV (see https://opencv.org/; Bradski 2000) contour detection was then applied, and the contour area was computed. The pixel resolution was applied to convert the computed contour area from pixels into metres. Key metrics, namely surface area (m2, the total visible extent of the aggregation measured in a two-dimensional plane) and maximum width (m, the longest visible straight-line dimension as observed from the aerial perspective) were calculated.
To assess the quality of the imagery data, a custom script using the Python OpenCV library was employed to detect glare and evaluate the proportion of ‘good-quality’ v. ‘bad-quality’ pixels in each image collected during the 2022 surveys. Images were converted from a red–green–blue (RGB) into a hue–saturation–value (or intensity) (HSV) colour space. Saturation and intensity values surpassing a threshold of 225 were considered saturated. The expanded saturated pixels were identified as glare or regions of poor quality, whereas the eroded non-saturated pixels were deemed non-glare or regions of good quality. The percentage of glare and non-glare pixels in each image was subsequently calculated.
Stage 2: recreational acoustic surveys – data collection and analysis
Lowrance HDS LIVE fish finders (Navico, Norway) connected to Lowrance Active-Imaging transducers, combining sidescan sonar (SSS; 800 kHz and 120° beam width) and CHIRP single-beam echosounder (SBES) (200 kHz, 38° beam width) capabilities, were used for the acoustic surveys. Other than frequency, all other settings were controlled by the HDS. Beam-shape information, needed for accurate calibration and the quantitative integration of acoustic density information, is not publicly available for the Lowrance Active-Imaging transducer. As a result, we evaluated the Lowrance HDS primarily for its detection capabilities, rather than its ability to provide quantitative insights into fish density within an aggregation. The SSS covered 30 m on either side of the vessel and the SBES provided vertical coverage to the seabed. Note that we did not consider the transducers Downscan Imaging SBES capability, nor the additional operating frequencies of the SSS (400 kHz) and SBES (83 kHz) because it was beyond the scope of this study to describe the operating characteristics of the HDS.
Four research vessels, namely, Snipe, Haliotis, FD60 and Puerulus, were fitted with Lowrance HDS fish finders and used for synchronised surveys of the designated cells. In September 2022, two vessels, Snipe and FD60, were deployed, with Haliotis becoming available in October 2022. In October 2023, Puerulus replaced the Haliotis. Transducers on the Snipe, Haliotis and Puerulus were mounted on the starboard side of the transoms, ~0.5 m below the water line, away from the starboard engine. The transducer on the FD60, a catamaran, was mounted on the inside of the starboard hull ~0.5 m below the water line. The vessels surveyed east–west parallel transects 50 m apart within each cell. At an average speed of 6 knots (~11.1 km h−1), surveying of the smaller 1-km2 cells (2022) took ~50 min, and the larger 1500- × 2000-m cells (2023) required ~130 min. To validate acoustic observations, a GoPro video camera was lowered into all acoustically detected aggregation considered to be C. auratus in 2022 and most acoustically detected aggregations in 2023. Other than C. auratus, no other fish species were seen.
SSS and CHIRP SBES acoustic data were recorded as sl3 sonar files and analysed using Echoview (ver. 14.0.189, Echoview Software Pty Ltd, Hobart, Tas., Australia, see https://echoview.com/). Chrysophrys auratus aggregations were manually identified and subsequently detected using Echoview’s school-detection algorithm (minimum total school height 2 m, minimum candidate length 5 m, minimum candidate height 2 m, maximum vertical linking distance 2 m, maximum horizontal linking distance 3 m, minimum total school length 5 m) so that metrics, including position (latitude and longitude), maximum corrected width (m, the maximum horizontal distance in the plane of the echogram), maximum corrected heights (m, the maximum vertical extent in the plane of the echogram) and corrected cross-sectional area (m2, in the plane of the echogram) could be exported for analysis (note that height could not be calculated for SSS data). Morphological metrics (width, height and cross-sectional area) were corrected on the basis of the attack angle derived from the 3-dB beam angle and the intensity of volume backscatter values above the data threshold. The cross-sectional area, as measured from a vertical perspective by using fish finders, cannot be directly compared with the surface area observed from drones. The surface area represents the total area of an aggregation’s surface as seen from above, whereas the acoustic cross-sectional area refers to the area of a slice through the aggregation, captured during an acoustic survey.
To assess acoustic-data quality, a signal-to-noise ratio algorithm, as described by De Robertis and Higginbottom (2007), was applied in the 2022 data. Python routines were then developed to determine the number of good and bad samples (between the acoustically detected seabed and surface noise layer, Jech et al. 2021) and calculate the percentage of good data on the bais of the various noise detections for each ping.
Environmental data
Sea height (combined wave and swell height, metres) data recorded at 30-min intervals were obtained from the nearest open-water site (Cottesloe, 31.98°S, 115.69°E) for the 2022 survey dates by request from the Department of Transport Western Australia. These were used as a proxy for sea condition because sea level data specifically for within Cockburn Sound are not available. Wind strength (km h−1) data recorded every minute on Garden Island at Colpoy’s Point (32.227°S, 115.699°E) were obtained for the same dates in 2022 by request from the Bureau of Meteorology. Altitude of the sun was also determined at the time as when each drone image was captured. Spearman rank correlations were determined for drone-image glare (using the bad-glare variable) v. sun altitude, wind strength and sea height and for acoustic quality with wind strength and sea height to evaluate any relationships with environmental variables. Drone-image glare, acoustic quality, wind strength and altitude were averaged to 30-min time intervals to match the frequency at which sea height was recorded. As correlations were mostly weak (p < ~0.3; see Results) and not all possible conditions, for example, altitude, were experienced during the survey, influencing factors were not evaluated further, for example, with general additive models.
Survey costings
Costings (A$) were determined for purchasing equipment (fish finders and drone), installation, survey planning, fieldwork and data processing during 2022. Although different models of Lowrance HDS fish finders were used (differing by screen size only), costs are provided for the HDS LIVE 7 model (see https://www.lowrance.com/en-au, accessed 19 June 2023), which are less than the costs for models with a larger screen. The cost of the drone is based on the current model available, the DJI Mavic 3 Pro (Mavic Pro – DJI, date accessed 19 June 2023). To simplify the estimate of the cost of fuel for aerial and acoustic surveys, each vessel was assumed to consume the same volume of fuel per day (80 L), on the basis of the amount required to fill the Snipe at the end of Day 1 of sampling. This was considered reasonable because each vessel covered the same distance at approximately the same speed each day. The cost of fuel was based on the average price per litre in Perth in September, October and November 2022 derived from www.fuelwatch.wa.gov.au (accessed 17 June 2023).
Individual scientific staff conducting surveys varied over the 3 months and were employed at different salary levels. Costs per person per day were based on the Agency Specific Agreement of the Department of Primary Industries and Regional Development, Western Australia, applicable from June 2022. Each vessel required a skipper (assumed to be a Level 4 Technical Officer (TO)). The lead acoustics vessel also required two scientists with acoustics and fisheries experience (at Specified Callings Levels 3 and 1 respectively), the drone vessel required a scientist with drone operation experience and a Level 2 TO deckhand, whereas the remaining acoustic vessels each required a Level 2 TO deckhand. Laboratory analyses of drone footage and acoustic data were conducted at the Commonwealth Scientific and Industrial Research Organisation (CSIRO) and the cost per day of those analyses was based on CSIRO salary schedules.
Ethics statement
Ethical review and approval were not required for the animal study because videos analysed in this article were collected by Western Australian Department of Primary Industries and Regional Development (DPIRD, authors D. V. Fairclough and G. Jackson). In Western Australia, the Animal Welfare Act 2002 does not require the DPIRD to obtain a permit to use animals (fish) for scientific purposes unless the species are outside the provisions of the governing legislation (i.e. Fish Resources Management Act 1994 and Fish Resources Management Regulations 1995). Nonetheless, all sampling was undertaken in strict adherence to the DPIRD policy for the handling, use and care of marine fauna for research purposes. No marine fauna was collected, injured or required to be euthanased for the purposes of this study.
Results
Aerial surveys 2022
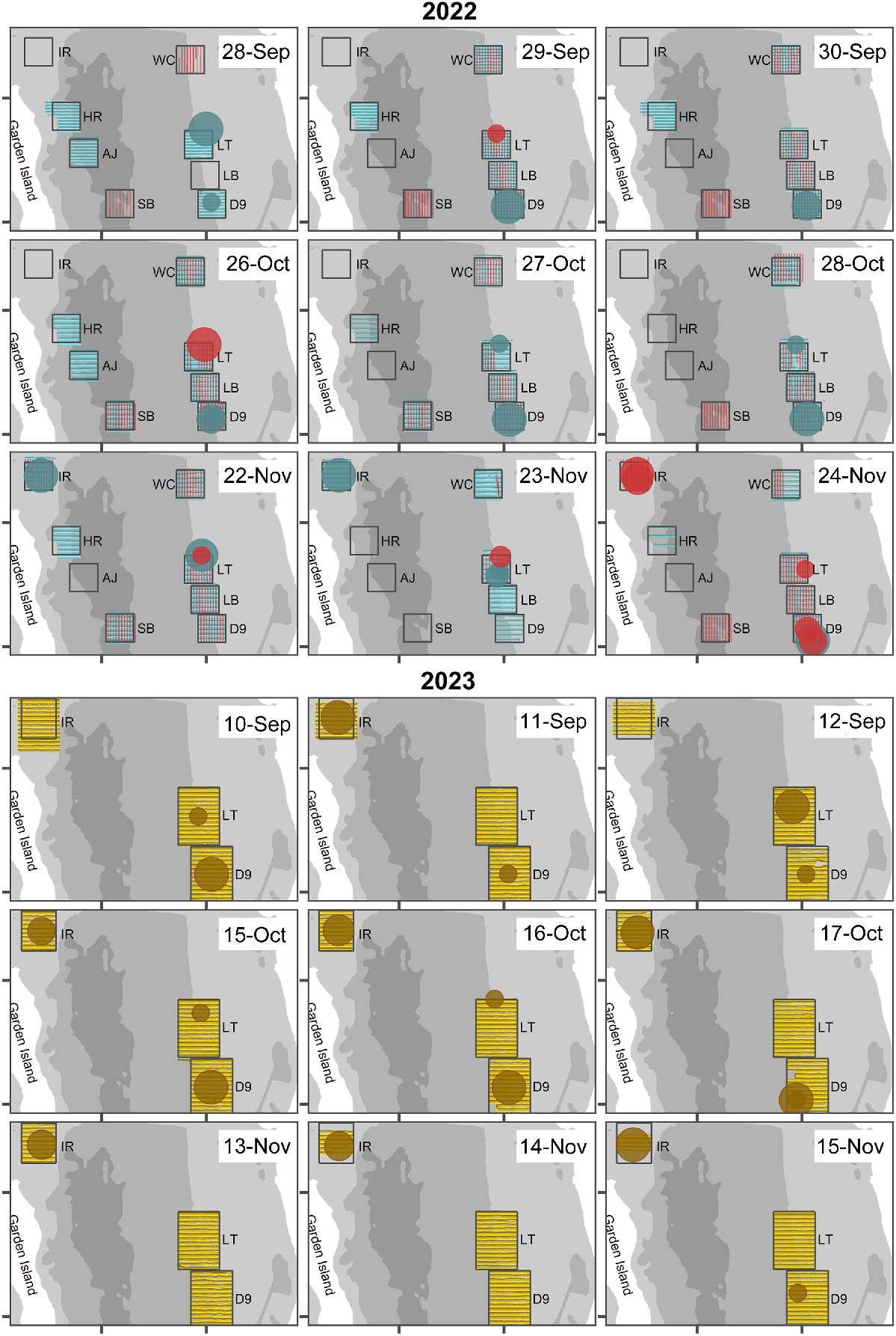
Forty-two aerial surveys were conducted in 2022, producing 12,625 images. The WC, LT, LB, D9 and SB cells were surveyed on all days except for 28 September (technical issues) and 23 November (winds above the maximum wind-strength resistance of the drone – 27.8 km h−1; Fig. 3). In 2022, surface aggregations of C. auratus were observed nine times over 5 of the 9 survey days between September and October (Fig. 3). These aggregations, identified by their round shape and pink colour at or just below the surface (e.g. Fig. 4), ranged in surface area from 10 to 822 m2, with maximum widths between 3.6 and 32.4 m. Most observations occurred at LT (n = 5), with additional sightings at D9 (n = 2) and IR (n = 2). No aggregations were seen at WC, LB or SB. Notably surface aggregations were observed five times on 24 November, coinciding with the fewest (n = 1) below-surface aggregations detected by the acoustics (Fig. 3).
Top three rows of panels show location and date of Chrysophrys auratus aggregations detected during aerial (red circles) and acoustic (blue circles) surveys in Cockburn Sound in 2022. Horizontal blue lines show the acoustic survey tracks and red vertical lines show aerial survey tracks. Bottom three rows of panels show location and date of C. auratus aggregations observed during acoustic (gold circles) surveys in Cockburn Sound in 2023. Horizontal yellow lines show the acoustic survey tracks. Note the aerial survey tracks are not shown because no surface aggregations were observed in 2023. Cells surveyed are Woodman Channel (WC), Lumps Top (LT), Lumps Bottom (LB), D9, South Basin (SB), Ammo Jetty (AJ), Harding Rock (HR) and Inside Rock (IR). Values are square-root scaled to make the bubble area proportional to the recorded size and the largest observation of 49.1 m.
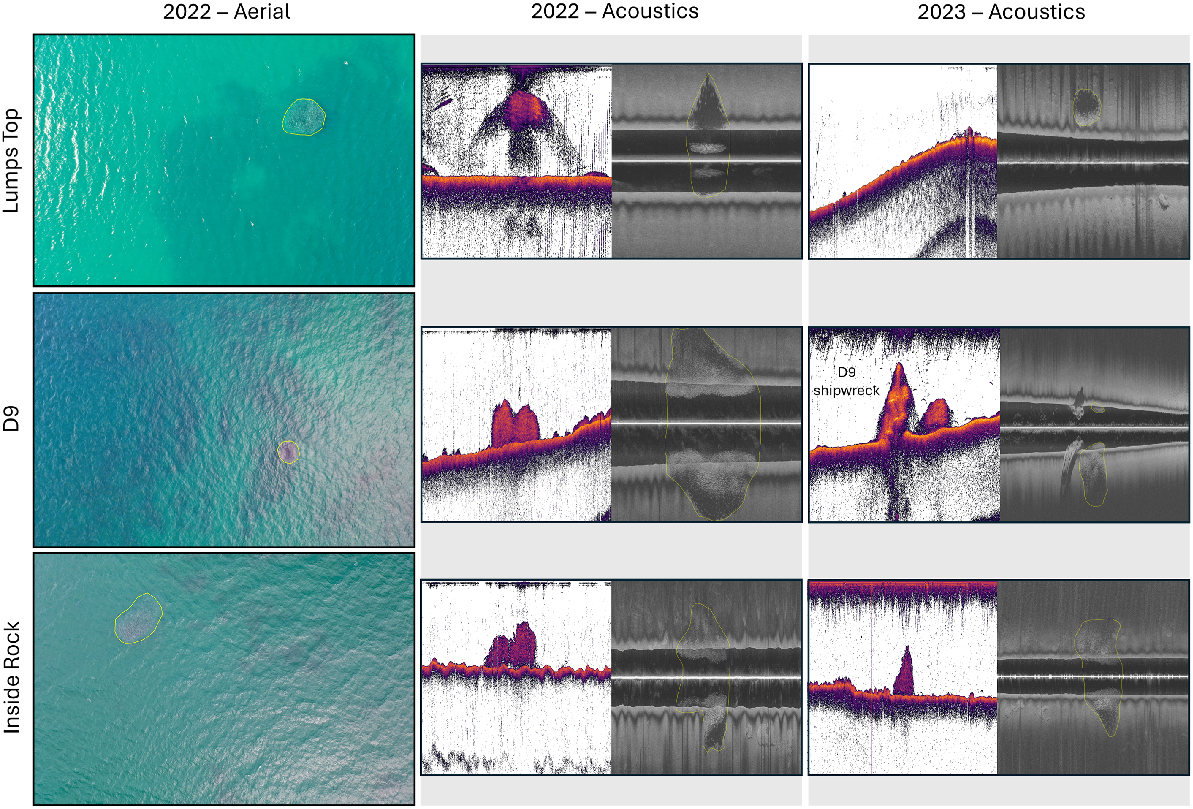
Left-hand panels show an example of Chrysophrys auratus aggregations (highlighted by yellow polygons) in Cockburn Sound observed at Lumps Top, D9 and Inside Rock by the drone (2022) and Lowrance HDS fish finder (2022 and 2023). Middle panels show the CHIRP SBES acoustic data and right-hand panels show sidescan sonar acoustic data.
Acoustic surveys 2022
Acoustic coverage of the 1-km2 cells in 2022 varied by day and month (Fig. 3). The eastern cells (LT, LB, D9, WC) were surveyed on all days except 28 September for LB and WC because of poor weather. Coverage of HR, AJ and SB varied, with HR surveyed seven times, AJ twice and SB three times. IR was introduced in November but was only partially covered on 24 November owing to time restraints and recording issues. We prioritised the eastern cells and IR, anticipating more C. auratus aggregations, which proved accurate because no aggregations were observed at SB, AJ or HR. They were also not recorded at WC and LB on the eastern bank.
Below-surface C. auratus aggregations were detected 16 times on both CHIRP SBES and SSS echograms (examples shown in Fig. 4), except for the 22 November aggregation at IR, visible only on the SSS echogram. Consistent detections of below-surface aggregations were recorded in each survey month, including at LT on 5 of the 9 survey days and at D9 on 7 of the 9 survey days (Fig. 3). A third aggregation was observed at IR on consecutive days in November. The C. auratus aggregations varied in maximum corrected width (9.6–46.8 m, mean 26.9 m), maximum corrected height (1.6–7.1 m, mean 4.1 m) and corrected cross-sectional area (10–230 m2, mean 83 m2) (Fig. 5).
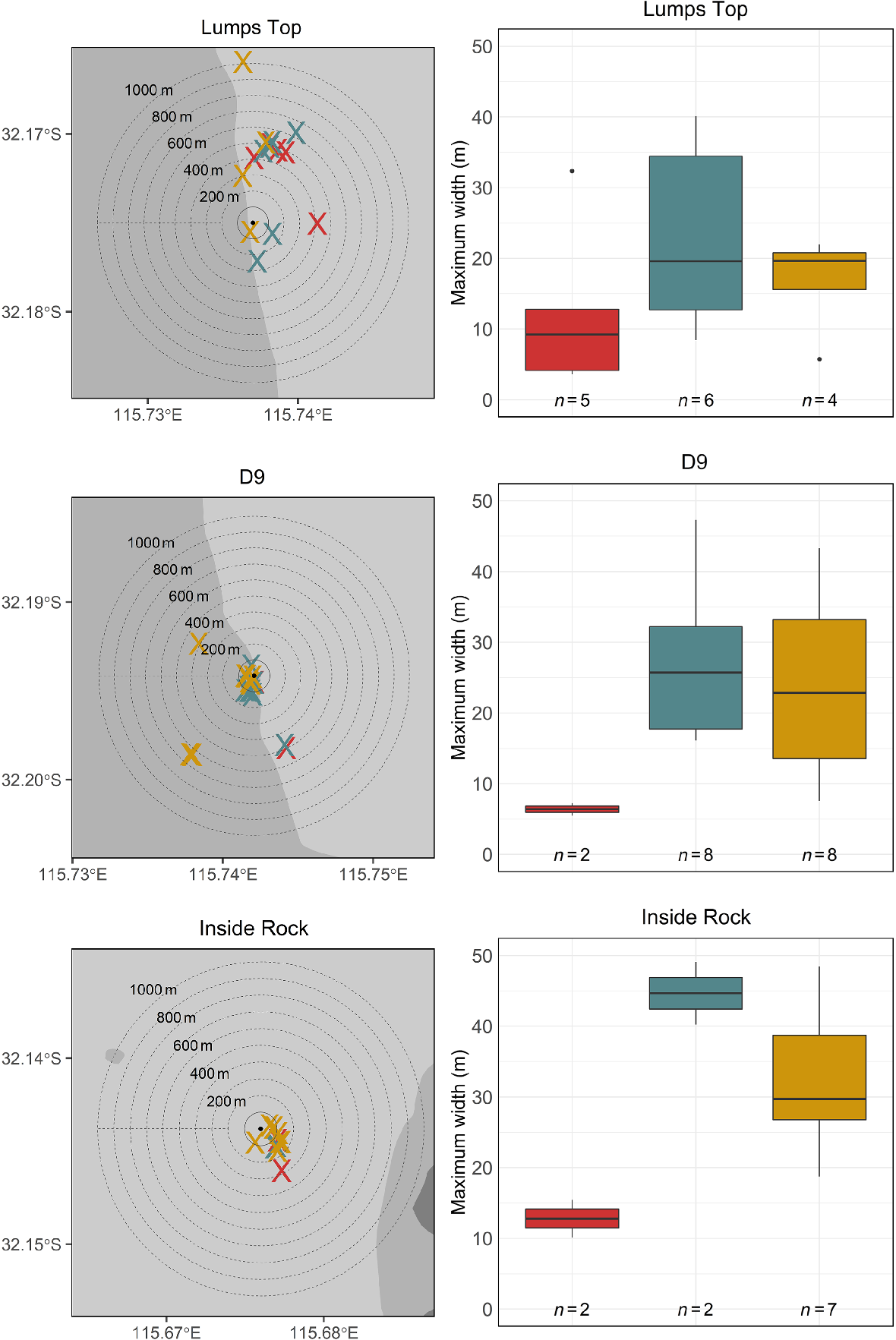
Locations (left-hand side) and maximum size (right-hand side) of Chrysophrys auratus aggregations at Lumps Top, D9 and Inside Rock as detected during the aerial survey in 2022 (red), and the acoustic surveys in 2022 (blue) and 2023 (gold). The dashed rings on the left panels represent 100-m intervals, progressively expanding away from the feature (black dot). The black horizontal line on each box plot indicates the median, the lower and upper edge of the box indicate the 25th and 75th percentile respectively. Whiskers extend to the most extreme data points that are not considered to be outliers. Outliers (black circles) are plotted individually. N is number of times an aggregation was observed.
In 2022, surface and below-surface aggregations were detected on the same day at LT and D9. Nearly concurrent sightings within 40 min suggest that these were separate aggregations, not repeat observations of the same group moving below the surface.
Aerial surveys 2023
In 2023, 22 aerial surveys were conducted, generating 20,278 images. All cells were surveyed on all days except for three of the cells, which could not be surveyed when winds were too strong to fly (>27.8 km h−1) and for another two cells when the drone failed mid-flight. That resulted in loss of the drone and data for that day. No surface aggregations were observed by the drone in 2023. However, the acoustic teams did report surface aggregations on three occasions, namely, a small (estimated to be <50 fish) aggregation at LT on 15 October, and large and small aggregations at D9 and LT respectively, on 13 November.
Acoustic surveys 2023
In 2023, the LT, D9 and IR cells were surveyed on all days. However, the overall coverage of IR did vary because of poor weather. In total, below-surface C. auratus aggregations were detected on 19 occasions (Fig. 3), five of which were seen only on the SSS echograms (example shown in Fig. 4). Large aggregations were observed seven times at D9 and IR, whereas a smaller aggregation was observed at LT five times. On 17 October, a second smaller aggregation was observed close to the main aggregation at D9. The C. auratus aggregations varied in maximum corrected width (5.7–48.4 m, mean 25.1 m), maximum corrected height (1.6–9.3 m, mean 4.1 m) and corrected cross-sectional area (13.3–212.6 m2, mean 84.6 m2) and are comparable to the observations made in 2022 (Fig. 5).
Spatial distribution, aggregation size and predator–prey interactions
Throughout 2022 and 2023, the C. auratus aggregations at LT were mostly observed north of the centre mark and along the shallower eastern bank (Fig. 5). A total of 12 of the 17 detections of the C. auratus aggregation at D9 were within 200 m of the D9 wreck, with the remaining five detections being made 400–700 m away (Fig. 5). Similarly, at IR, all but one (a surface detection in 2022) of the C. auratus aggregation detections were made within a 200-m radius of the centre of the cell (Fig. 5).
Except for a large surface aggregation detected at LT on 26 October 2022 (maximum width 32.4 m), surface aggregations were generally smaller than those recorded below the surface during acoustic surveys (Fig. 5). Surface aggregations detected during aerial surveys at LT and IR in 2022 were similar in size, with mean maximum widths of 12.4 and 12.8 m respectively, whereas the surface aggregation detected at IR was smaller, with a mean maximum width of 6.4 m (Fig. 5). The largest below-surface aggregations were detected at IR (mean maximum corrected width 35.3 m), followed by D9 (mean maximum corrected width 25.6 m) and LT (mean maximum width 20.5 m). Maximum corrected widths of below-surface aggregations detected at LT and D9 were similar in 2022 and 2023, but the C. auratus aggregation detected at IR was larger in 2022 than 2023 (Fig. 5).
To understand reasons why C. auratus aggregations appear at the surface in Cockburn Sound, we examined their interactions with Tursiops aduncus (bottlenose dolphins). In 2022, Tursiops aduncus (one to six individuals) were regularly observed near C. auratus surface aggregations. The drone captured T. aduncus encircling C. auratus twice in 2022 (LT on 29 September, D9 on 26 October) (Fig. 6), and the acoustic teams reported T. aduncus within ~10 m of C. auratus three times (LT on 28 September, LT on 29 September, D9 on 24 November). Tursiops aduncus and C. auratus were also recorded together below the surface during an acoustic survey of LT on 28 September (Fig. 6). On 29 September, the drone photographed T. aduncus three times (twice at LT, once at LB) without C. auratus. Tursiops aduncus was observed at HR, but never with C. auratus.
Example of Tursiops aduncus (bottlenose dolphin) and Chrysophrys auratus observed together in Cockburn Sound at Lumps Top, highlighted by yellow polygons, as detected by both drone and Lowrance HDS fish finder in September 2022. The left panel of the acoustic data displays CHIRP SBES data, whereas the right panel shows sidescan sonar imagery. Note: The aerial photograph was taken at a different time than the acoustic data were collected.
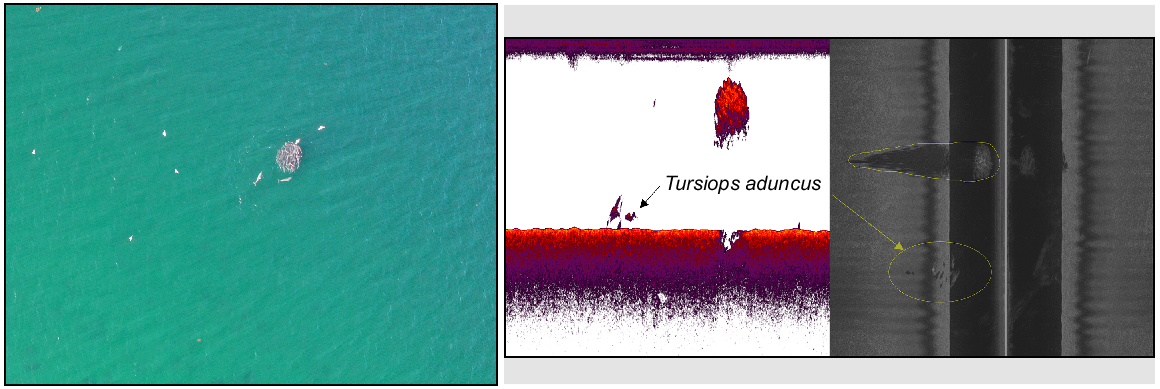
In 2023, the drone did not detect any T. aduncus within the survey area during its operations. However, the acoustic team did report sightings of T. aduncus and C. auratus together on two occasions, namely, 15 October and 13 November, both at LT. On 13 November, the drone team also reported surface C. auratus at D9 with T. aduncus; however, adverse weather prevented the drone from conducting the survey. Tursiops aduncus was observed on four additional occasions without C. auratus, namely, once at LT (13 November), twice at D9 (14 November and 15 November) and once at LT (15 November). Notably, the 15 November sighting at D9 was accompanied with a ‘surface slick’, which is commonly observed when an aggregation is present, although no C. auratus were visible.
Relationships between environmental characteristics and quality of both drone images and acoustic data
Maximum wind strength (gusts lasting 1 min) in 2022 was below the threshold for flying of 15 knots (~27.8 km h−1) on most mornings when drone surveys were planned to be conducted. However, on 26 and 28 October and 23 November 2022, wind gusts were close to or above the threshold during at least the morning.
Glare in each drone image was influenced by breaking waves creating white water surface and by sun reflection and comprised less than 10% of most images (range 0.001–17%) in 2022. Chrysophrys auratus aggregations were recorded in 30 drone images in 2022, when glare covered 0–4.4% of the image. Some of those images were taken consecutively within a site and thus recorded the same aggregation in similar conditions. Image glare was most strongly and positively correlated with sun altitude (r = 0.72) but was only weakly positively correlated with wind strength and negatively correlated with sea height (0.25, −0.29 respectively; Fig. 7).
Correlations among drone-image glare (Glare) and altitude, wind strength and sea height (left column) and between acoustic data quality (Quality) for each vessel (FD60, Haliotis, Snipe) and both wind strength and sea height in 2022. Spearman rank correlation values are shown on each plot.
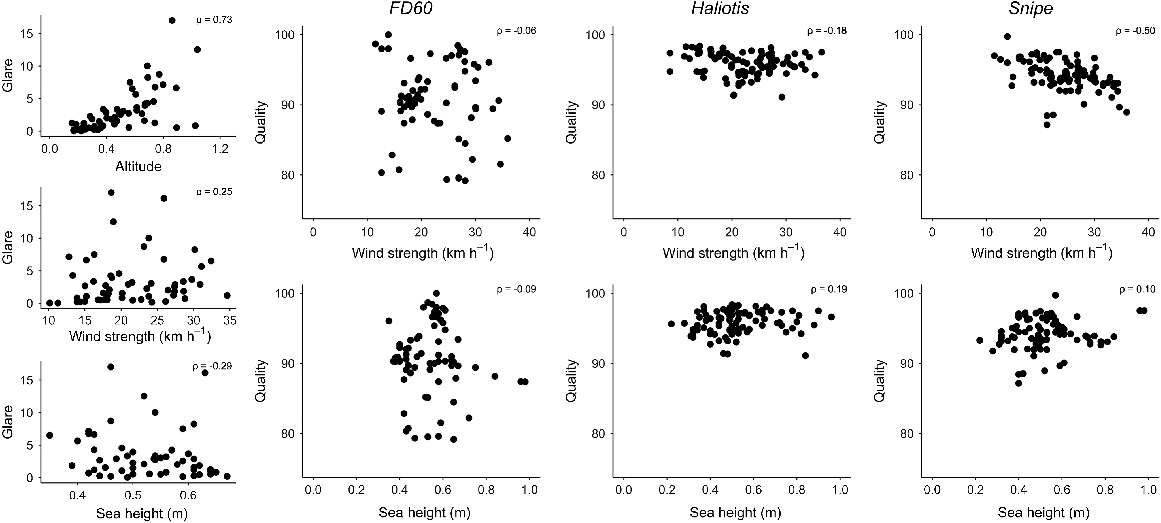
Acoustic-data quality was typically above 80% for each of the three vessels, the FD60, Haliotis and Snipe (Fig. 7), used in 2022. However, a greater frequency of lower-quality data was recorded by the Lowrance HDS on the FD60. Correlations between acoustic-data quality on each vessel and environmental variables (wind strength and sea height) were mostly low (Fig. 7). However, there was a negative relationship with wind strength for the Snipe, r = −0.50 (Fig. 7).
Cost breakdown
The initial cost for purchasing equipment and planning surveys in 2022 was estimated to be A$27,937, comprising three Lowrance HDS7 fish finders (A$1950 each) with C-Map software (A$329 each), plus installation over 5 days at A$800 day−1, i.e. A$4000; a DJI-Mavic 3 Pro drone (A$3100); and the 10 working days it took two scientists to plan the survey at A$700 day−1 each, i.e. A$14,000.
Conducting the combined aerial–acoustic survey over 9 days (3 days in each month) was costed at A$55,564 (Table 1). The cost per month increased because of the increasing cost of unleaded fuel during the survey period, (i.e. averages of A$1.65, A$1.81 and A$1.85 per litre in September, October and November 2022 respectively).
| Activity | Item | September | October | November | Total | |
|---|---|---|---|---|---|---|
| Field | Number of days | 3 | 3 | 3 | 9 | |
| Number of vessel days (four boats) | 12 | 12 | 12 | 36 | ||
| Total cost of vessel days (A$) | 1584 | 1738 | 1776 | 5098 | ||
| Drone – number of person days (3 per day) | 9 | 9 | 9 | 27 | ||
| Acoustics – number of person days | 21 | 21 | 21 | 63 | ||
| Total cost of person days (A$) | 16,822 | 16,822 | 16,822 | 50,466 | ||
| Laboratory | Acoustic and drone data management and analysis person days | 15 | 15 | 15 | 45 | |
| Total cost of data analysis (A$) | 14,799 | 14,799 | 14,799 | 44,397 | ||
| Total cost (A$) | 33,205 | 33,359 | 33,397 | 99,961 |
The cost of management and analysis of drone and acoustic data was estimated to be A$44,397 (Table 1). This comprised the cost of time (15 person days per month) spent on data management (image and acoustic files), quality control and statistical analyses. Thus, the total cost of conducting the surveys over 3 months in 2022, including equipment outlay, planning, field and laboratory costs was A$127,898.
Discussion
In this study, we have successfully demonstrated that recreational drones and fish finders can be used to detect C. auratus spawning aggregations in the shallow, coastal embayment of Cockburn Sound, Western Australia. We employed a two-stage survey design consisting of aerial surveys followed by acoustic surveys. Both methods utilised a parallel transect design, ensuring a comparable coverage of the survey area. By integrating drones and fish finders in a single survey program, we were able to observe C. auratus aggregations above and below the surface in a turbid environment and record data on parameters related to location, number and size of aggregations (Fig. 5). The outlay for a drone and the purchase and installation of fish finders were low (~A$14,000), compared with the cost of running the vessels and staff employed to plan surveys and conduct data analyses (~A$113,000) (Table 1).
Performance of recreational drones and fish finders
Surface aggregations were observed nine times during aerial surveys of Cockburn Sound in 2022 and zero times in 2023, while below-surface aggregations were observed 16 times in 2022 and 19 times in 2023 during acoustic surveys (Fig. 5). To the authors’ knowledge, surface aggregations of C. auratus do not occur elsewhere in Australia, including Shark Bay in Western Australia, and Gulf Saint Vincent and Spencer Gulf in South Australia. Together, the two methods identified different C. auratus aggregations at three key sites (LT, D9 and IR) on or adjacent to the shallow northern and eastern banks of Cockburn Sound (Fig. 5). These findings confirmed those sites as being important for aggregations of C. auratus (Wakefield 2010; Crisafulli et al. 2019) and should be considered for any ongoing monitoring programs in Cockburn Sound.
The differences in size between surface aggregations observed in drone imagery and those recorded at depth during acoustic surveys (Fig. 5) may be attributed to several factors, including variations in sampling volume between optics and acoustics, as well as changes in schooling behaviour with depth or in response to predation. Drone imagery measures only the visible extent from above, and may underestimate size, particularly when schools extend below the surface or visibility is poor. By contrast, acoustics provide more accurate measurements at depth but depend on knowledge of the acoustic beam shape and that the vessel passes over the centre of the aggregation. These factors highlight the complexity of comparing optical and acoustic methods, suggesting the need for combined approaches in future research.
For the aerial component of the survey, the low-cost drone enabled rapid and repeatable coverage of survey cells. All C. auratus aggregations detected by the drone were close to the surface or were found over sandy bottoms in the shallow eastern and northern bank sites (i.e. LT, D9 and IR; Fig. 3). The often turbid waters of Cockburn Sound would likely make detecting aggregations more than a few metres below the surface difficult. Furthermore, seagrass beds within Cockburn Sound (Kendrick et al. 2002) could be mistaken in aerial imagery for below-surface aggregations in turbid water because some were circular in shape and similar in size to C. auratus aggregations. Owing to their lightweight nature and power limitations, drones are often restricted to being used in calm and dry conditions (Casella et al. 2016; Cao et al. 2018). On several occasions, wind strength or wind gusts restricted the ability to conduct or complete the aerial surveys and lead to the loss of a single drone during the project. This is an issue if surveys need to be conducted within short timeframes, as is the case for surveying C. auratus aggregations around new moons when spawning activity peaks. The prevailing strong southerly winds on the lower western coast of Australia in spring and summer (Pattiaratchi et al. 1997), when C. auratus is aggregating, dictated the choice to conduct aerial surveys in the morning (c. 06:20–11:00 hours). This helped minimise glare associated with wind-driven waves forming foam and bubbles or white water on the surface that would occur later in the day (Jaud et al. 2019) and therefore increase the useful portion of drone images. Although the percentage of glare on drone images was mostly low in 2022, it increased with sun altitude, further demonstrating that early morning surveys were preferable. Because few surveys occurred beyond late morning, it is unclear what the effect of high sun altitude and white water from strong prevailing winds would have had on drone image glare later in the day.
Recreational fish finders do not allow for accurate measurements of fish density and biomass, because, unlike scientific-grade echosounders, they cannot be calibrated. However, they can be used to quantify the relative abundance of fish, school numbers and dimensions and map their temporal and spatial distribution (McInnes et al. 2015; Brough et al. 2019). In this study, we do not evaluate the dynamic range of the Lowrance HDS Live fish finder – the range of sound intensities the system can detect and accurately process targets. However, owing to having a large gas-filled swim bladder and high schooling density (Fig. 4), we do not consider the lower dynamic range of the HDS Live, than that of a scientific echosounder, to be a significant factor in the detection of C. auratus aggregations and that it has sufficient sensitivity for this purpose. We have shown that C. auratus aggregations are easily detected using the Lowrance HDS, despite several other aggregating or schooling fish species, such as silver trevally (Pseudocaranx spp.), western butterfish (Pentapodus vitta), scaly mackerel (Sardinella lemuru), Australian sardines (Sardinops sagax) and yellowtail scad (Trachurus novaezelandiae), co-occurring in Cockburn Sound (Wakefield et al. 2013; Bearham et al. 2020). However, these bentho-pelagic or pelagic species have much smaller body sizes than C. auratus and typically form aggregations with different characteristics, for example, with respect to their size, shape, density, texture and position in the water column. Even though acoustic methodologies can distinguish between certain fish species, especially when their scattering characteristics differ, they are typically combined with other methods such as video, trawl or hook-and-line sampling to enhance identification and accuracy (Fernandes et al. 2016). In this study, we routinely deployed a GoPro video camera to validate the acoustic observation and consistently recorded only C. auratus.
The quality of acoustic data varied among vessels in 2022, with the catamaran FD60 having the greatest frequency of lower-quality recordings. This was likely to be influenced by the placement of its transducer between the hulls, because the motor fittings leave limited space on the transoms. Although the quality of data recorded on the Snipe appeared to deteriorate in stronger winds, this was more likely to be due to wind-driven seas. Sea-height data were derived from a buoy located outside Cockburn Sound, which may not have reflected conditions within the Sound and thus the lack of apparent relationship with acoustic-data quality. Wind or sea did not appear to affect the acoustic-data quality on the Haliotis, which could be related to factors such as a deeper V-shaped hull. This information highlights the fact that the choice of vessel design could be an important factor in conducting acoustic surveys from small vessels using recreational equipment.
In the shallow depths of Cockburn Sound, avoidance owing to vessel noise and presence may lead to underestimations of C. auratus aggregations, because fish could move outside the detectable range of a fish finder. Personal observations indicated that C. auratus exhibits avoidance behaviour in response to repeated acoustic sampling aimed at mapping aggregations. However, this avoidance is less pronounced during a single pass as part of a systematic acoustic survey, although it cannot be entirely eliminated. While we acknowledge the potential to underestimate the number of aggregations because of avoidance, we consider its impact to be minimal, given the slow survey speed, the systematic design that typically results in a single pass over an aggregation, and the capability of three boats to simultaneously survey a large section of the water column.
Opportunistic observations of other fauna
Bottlenose dolphins (Tursiops aduncus) were recorded by the drone or fish finders near the surface and below the surface close to C. auratus aggregations on six occasions (two drone images, three observer sightings and one acoustic detection) in 2022 (Fig. 6). Another three observations were made by the drone and acoustic teams in 2023. Tursiops aduncus is a known predator of C. auratus in Cockburn Sound (Finn and Calver 2008) and may be responsible for driving C. auratus aggregations to the surface. Many fish change their schooling behaviour, including alterations in density, size and shape, in response to predation pressure (e.g. Pitcher and Wyche 1983; Axelsen et al. 2001; Romenskyy et al. 2020), and dolphins are well known for herding prey in a horizontal or vertical direction (e.g. Benoit-Bird and Au 2009; Heithaus and Dill 2009). Although dolphins are also abundant in the shallow, inner gulfs of Shark Bay, where C. auratus also aggregates to spawn, such herding has not been reported.
Our observations also suggest that predation pressure may cause C. auratus aggregations to split, as recorded in other fish (e.g. Pitcher and Wyche 1983). This is supported by drone footage taken at IR on 24 November 2022, which identified two C. auratus aggregations, distinguishable by their size and density (Fig. 3). During the preceding 2 days (22 and 23 November), the drone did not observe any surface aggregations. However, a single large below-surface aggregation was detected during the acoustic surveys. Although no T. aduncus was observed at IR in November 2022, it is possible that this change in aggregating behaviour was in direct response to predation pressure from T. aduncus or other predators, such as carcharinid sharks, which are known to frequent Cockburn Sound. These behavioural responses to predation (surfacing and splitting) confirm the value of an integrated aerial–acoustic survey method for C. auratus in Cockburn Sound. Furthermore, the different survey methods may also help understand the spatial distribution of megafauna in Cockburn Sound in relation to current or proposed industrial activity (Pirotta et al. 2013).
Cost
An important objective of this study was to develop a low-cost survey method capable of detecting long-term changes in the occurrence of C. auratus aggregations. Thus, we employed a low-cost, consumer-grade drone and recreational fish finders because these systems have become widely available and have improved dramatically in performance. They are also growing in popularity among scientists working in aquatic environments (e.g. Westley and Mcneary 2017; Sánchez-Carnero et al. 2018; Schofield et al. 2019; Raoult et al. 2020; Dainys et al. 2022) because of their affordability (typically several thousand dollars per unit), small size, portability, ease of installation and operation, and their ability to record directly to SD cards. By contrast, scientific echosounders and specialised transducers can cost at least A$50,000 and often require dedicated computers and software to record acoustic data. However, providing quantitative estimates of fish abundance in aggregations would be beneficial and should be considered in future monitoring strategies. For example, low-cost spatial aerial–acoustic surveys could be used to inform targeted surveys that deploy a scientific echosounder aimed at estimating the biomass of aggregating C. auratus in Cockburn Sound.
Small research vessels (5–10 m) offer the ideal platforms for conducting coastal research (e.g. Dawson et al. 2008; Williams and Thomas 2009; Williams et al. 2017) and are often used for this purpose in Cockburn Sound because they are easy to transport, cheap to run (consume small amounts of fuel) and have onboard power supplies. They are also easy to manoeuvre, travel quickly between sample sites, can access shallow areas and can be operated by two or three people. These attributes are also shared by many recreational vessels popular among Western Australian residents (~53,000 recreational vessels in the Perth area in 2019; Ryan et al. 2022). We had intended to involve volunteer recreational vessels and their owners to assist in the acoustic component of this study because citizen-science approaches can reduce costs, provide effective marine research and encourage greater stewardship of the science and resource (Fairclough et al. 2014; Earp and Liconti 2020). The participation of volunteer vessel owners may have allowed broader coverage of Cockburn Sound and increased data-collection opportunities. However, despite several willing volunteers, using recreational vessels to conduct pre-designed scientific research in Australia is challenging. The Australian Maritime Safety Authority (www.amsa.gov.au) required recreational vessels to register for temporary, short-term exemptions from commercial vessel licences to allow such participation. These cost several hundred dollars each and require renewal if work occurs over >90 days, as such exemptions are typically used as an interim measure, prior to a vessel becoming commercially licensed indefinitely. During this period, the vessel cannot be used recreationally, and it was considered unreasonable for vessel owners to forfeit recreational activities during this period. However, safety regulations may differ in other parts of the world, making recreational-vessel involvement more feasible.
Throughout this study, we have emphasised the significance of cost. Although the terms ‘low cost’ and ‘cost effective’ are commonly used in aquatic research (e.g. Williams and Thomas 2009; Marre et al. 2019) and many studies have compared different scientific methods and their cost effectiveness (e.g. Bosch et al. 2017; Cundy et al. 2017), it is less common to provide specific dollar values, especially regarding the time invested by researchers (cf. Langlois et al. 2010; Fairclough et al. 2014). However, without such an evaluation, it becomes difficult for readers to ascertain whether a method is genuinely ‘low-cost’ and thus suitable for their own applications. The instruments used in this study were inexpensive, costing several thousand dollars per unit, but the staff time and resources required for data collection and analysis was large, dramatically increasing overall cost. Therefore, it is important to explore strategies to minimise costs. The costs associated with conducting a survey, including staff time and vessel operations, are generally fixed, as a minimum number of crew are required for specific vessels. Without fully autonomous alternatives, these costs may remain unchanged or potentially increase. However, operating an autonomous vessel in a busy multi-use environment such as Cockburn Sound and restrictions on how far a drone is allowed to fly from its operator may render this option unfeasible.
Instead, advancements in data analysis are more likely to bring about improvements and therefore reduce overall costs. In this study, the processing of drone data using third-party software, custom scripts and manual annotation proved a time-consuming practice (Chabot et al. 2018; Fairley et al. 2018). This can also potentially limit the amount of useful information that can be gathered. Instead, machine-learning techniques could be used for object detection of species of interest, particularly when there are many objects of interest, and has been successfully tested for identifying and counting C. auratus in baited-video surveys (Connolly et al. 2021; Xu et al. 2023). In addition, automated algorithms for acoustic school detection, or utilising well-scripted open-source functions in popular programming languages, are more likely to bring about improvements and reduce overall costs. Assessing the value of time savings and enhanced analysis efficiency will help determine whether the initial investment is worthwhile.
Conclusions and recommendations
In this study, we have successfully demonstrated an effective, combined aerial–acoustic survey for detecting aggregations of C. auratus, both at and below the surface, within a limited number of survey cells within Cockburn Sound. To apply this integrated method to the overarching objective of long-term monitoring of C. auratus aggregations in Cockburn Sound during the development and ongoing operation of a port, future surveys should consider the costs and benefits associated with expanding the survey effort beyond the cells covered in this study or consider refining the existing survey design to focus on priority areas. Therefore, it is necessary to consider the scalability of the method in terms of number and size of survey cells, as well as the associated costs of conducting additional sampling, including using a scientific echosounder to provide estimates of schooling biomass. Furthermore, if the port development goes ahead, it is important to know whether the method needs to be expanded to other areas beyond Cockburn Sound (e.g. the adjacent Warnbro Sound and Owen Anchorage) either as a control site or to identify any potential larger spatial-scale effects. In this study, we applied the method to a single fish species; however, it is also applicable to other species exhibiting similar schooling behaviours in comparable environments. Although aerial drones and recreational fish finders are effective as standalone tools, their combined use is particularly advantageous when a species forms both surface and subsurface aggregations in shallow waters. Together, these methods can serve as a viable approach for detecting changes in schooling abundance in response to anthropogenic activities.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author. Echoview templates and Python code used within this study are made available upon request.
Declaration of funding
We express our gratitude to the Western Australian State Government, Westport, Department of Transport WA and the Western Australian Marine Science Institution (WAMSI) for funding the project.
Author contributions
B. Scoulding, D. V. Fairclough and G. Jackson conceptualised the project and raised funds for its completion. B. Scoulding, D. V. Fairclough, G. Jackson, P. Lewis, D. Waltrick, L. West, C. Skepper, J. Briggs, E. Lek, D. Yeoh, B. Crisafulli, E. A. Fisher and P. J. Mitchell implemented the surveys. B. Scoulding, D. V. Fairclough, C. Devine and S. Gastauer conducted the analysis and interpreted the results. B. Scoulding, D. V. Fairclough and S. Gastauer wrote the first draft of the article. All co-authors engaged in editing the final draft.
Acknowledgements
We are also grateful to the WAMSI–Department of Primary Industries and Regional Development (DPIRD) partnership developed through the Westport program. The authors thank numerous staff of DPIRD for assisting with boating operations. Nick Mortimer and Chris Jackett from CSIRO are thanked for assisting with the analysis of the drone images. Jeff Cordell is thanked for supplying the Lowrance HDS fish finders and for installing them on the vessels.
References
Axelsen BE, Anker-Nilssen T, Fossum P, Kvamme C, Nøttestad L (2001) Pretty patterns but a simple strategy: predator–prey interactions between juvenile herring and Atlantic puffins observed with multibeam sonar. Canadian Journal of Zoology 79, 1586-1596.
| Crossref | Google Scholar |
Bearham D, Robert M, Chaplin JA, Moore GI, Fairclough DV, Bertram A (2020) Molecular evidence of three species in the Pseudocaranx dentex complex (Carangidae) in Australian waters. Marine and Freshwater Research 71, 518-531.
| Crossref | Google Scholar |
Benoit-Bird KJ, Au WWL (2009) Cooperative prey herding by the pelagic dolphin, Stenella longirostris. The Journal of the Acoustical Society of America 125, 125-137.
| Crossref | Google Scholar | PubMed |
Bertram A, Fairclough D, Sandoval-Castillo J, Brauer C, Fowler A, Wellenreuther M, Beheregaray LB (2022) Fisheries genomics of snapper (Chrysophrys auratus) along the west Australian coast. Evolutionary Applications 15, 1099-1114.
| Crossref | Google Scholar | PubMed |
Bollinger MA, Kline RJ (2017) Validating sidescan sonar as a fish survey tool over artificial reefs. Journal of Coastal Research 33, 1397-1407.
| Crossref | Google Scholar |
Bosch NE, Gonçalves JMS, Erzini K, Tuya F (2017) ‘How’ and ‘what’ matters: sampling method affects biodiversity estimates of reef fishes. Ecology and Evolution 7, 4891-4906.
| Crossref | Google Scholar | PubMed |
Bradski G (2000) The OpenCV library. Dr Dobb’s Journal of Software Tools 120, 122-125.
| Google Scholar |
Brooker MA, de Lestang S, Fairclough DV, McLean D, Slawinski D, Pember MB, Langlois TJ (2020) Environmental and anthropogenic factors affect fish abundance: relationships revealed by automated cameras deployed by fishers. Frontiers in Marine Science 7, 1-12.
| Crossref | Google Scholar |
Brough T, Rayment W, Dawson S (2019) Using a recreational grade echosounder to quantify the potential prey field of coastal predators. PLoS ONE 14, e0217013.
| Crossref | Google Scholar | PubMed |
Brown EJ, Vasconcelos RP, Wennhage H, Bergström U, Stottrup JG, Van De Wolfshaar K, Millisenda G, Colloca F, Le Pape O (2018) Conflicts in the coastal zone: human impacts on commercially important fish species utilizing coastal habitat. ICES Journal of Marine Science 75, 1203-1213.
| Crossref | Google Scholar |
Cao J, Leng W, Liu K, Liu L, He Z, Zhu Y (2018) Object-Based mangrove species classification using unmanned aerial vehicle hyperspectral images and digital surface models. Remote Sensing 10, 89.
| Crossref | Google Scholar |
Cappo M, Speare P, De’Ath G (2004) Comparison of baited remote underwater video stations (BRUVS) and prawn (shrimp) trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. Journal of Experimental Marine Biology and Ecology 302, 123-152.
| Crossref | Google Scholar |
Casella E, Rovere A, Pedroncini A, Stark CP, Casella M, Ferrari M, Firpo M (2016) Drones as tools for monitoring beach topography changes in the Ligurian Sea (NW Mediterranean). Geo-Marine Letters 36, 151-163.
| Crossref | Google Scholar |
Chabot D, Dillon C, Shemrock A, Weissflog N, Sager EPS (2018) An object-based image analysis workflow for monitoring shallow-water aquatic vegetation in multispectral drone imagery. ISPRS International Journal of Geo-Information 7, 294.
| Crossref | Google Scholar |
Christiansen HM, Switzer TS, Keenan SF, Tyler-Jedlund AJ, Winner BL (2020) Assessing the relative selectivity of multiple sampling gears for managed reef fishes in the eastern Gulf of Mexico. Marine and Coastal Fisheries 12, 322-338.
| Crossref | Google Scholar |
Clark A (2015) Pillow (pil fork) documentation. Release 11.1.0.dev0. Available at https://readthedocs.org/projects/pillow/downloads/pdf/latest/
Cloern JE, Abreu PC, Carstensen J, Chauvaud L, Elmgren R, Grall J, Greening H, Johansson JOR, Kahru M, Sherwood ET, et al. (2016) Human activities and climate variability drive fast-paced change across the world’s estuarine – coastal ecosystems. Global Change Biology 22, 513-529.
| Crossref | Google Scholar | PubMed |
Connell SD, Samoilys MA, Lincoln Smith MP, Leqata J (1998) Comparisons of abundance of coral-reef fish: catch and effort surveys vs visual census. Australian Journal of Ecology 23, 579-586.
| Crossref | Google Scholar |
Connolly RM, Fairclough DV, Jinks EL, Ditria EM, Jackson G, Lopez-Marcano S, Olds AD, Jinks KI (2021) Improved accuracy for automated counting of a fish in baited underwater videos for stock assessment. Frontiers in Marine Science 8, 658135.
| Crossref | Google Scholar |
Crisafulli BM, Fairclough DV, Keay IS, Lewis P, How JR, Ryan KL, Taylor SM, Wakefield CB (2019) Does a spatiotemporal closure to fishing Chrysophrys auratus (Sparidae) spawning aggregations also protect individuals during migration? Canadian Journal of Fisheries and Aquatic Sciences 76, 1171-1185.
| Crossref | Google Scholar |
Cundy ME, Santana-Garcon J, Ferguson AM, Fairclough DV, Jennings P, Harvey ES (2017) Baited remote underwater stereo-video outperforms baited downward-facing single-video for assessments of fish diversity, abundance and size composition. Journal of Experimental Marine Biology and Ecology 497, 19-32.
| Crossref | Google Scholar |
Dainys J, Gorfine H, Mateos-González F, Skov C, Urbanavičius R, Audzijonyte A (2022) Angling counts: harnessing the power of technological advances for recreational fishing surveys. Fisheries Research 254, 106410.
| Crossref | Google Scholar |
Dawson S, Wade P, Slooten E, Barlow J (2008) Design and field methods for sighting surveys of cetaceans in coastal and riverine habitats. Mammal Review 38, 19-49.
| Crossref | Google Scholar |
De Robertis A, Higginbottom I (2007) A post-processing technique to estimate the signal-to-noise ratio and remove echosounder background noise. ICES Journal of Marine Science 64, 1282-1291.
| Crossref | Google Scholar |
Dias PJ, Wakefield CB, Fairclough DV, Jackson G, Travers MJ, Snow M (2016) Real-time PCR validation of visually identified snapper Chrysophrys auratus (Sparidae) eggs. Journal of Fish Biology 88, 811-819.
| Crossref | Google Scholar | PubMed |
Earp HS, Liconti A (2020) Science for the future: the use of citizen science in marine research and conservation. In ‘YOUMARES 9 – the oceans: our research, our future’. (Eds S Jungblut, V Liebich, M Bode-Dalby) pp. 1–19. (Springer) 10.1007/978-3-030-20389-4_1
Fairclough DV (2021) Partitioning of marine transition zone reefs among temperate, sub-tropical and tropical fishes is related more to depth and habitat than temperature. Marine Ecology Progress Series 672, 175-192.
| Crossref | Google Scholar |
Fairclough DV, Brown JI, Carlish BJ, Crisafulli BM, Keay IS (2014) Breathing life into fisheries stock assessments with citizen science. Scientific Reports 4, 7249.
| Crossref | Google Scholar |
Fairclough DV, Hesp Sybrand A, Denham AM, Fisher EA, Marks R, Ryan KL, Lek E, Allen R, Crisafulli BM (2021) 2021 assessment of the status of the West Coast Demersal Scalefish Resource. Fisheries research report number 316. (Department of Primary Industries and Regional Development: Perth, WA, Australia) Available at https://www.fish.wa.gov.au/Documents/research_reports/frr316.pdf
Fairley I, Mendzil A, Togneri M, Reeve DE (2018) The use of unmanned aerial systems to map intertidal sediment. Remote Sensing 10, 1918.
| Crossref | Google Scholar |
Fernandes PG, Copland P, Garcia R, Nicosevici T, Scoulding B (2016) Additional evidence for fisheries acoustics: small cameras and angling gear provide tilt angle distributions and other relevant data for mackerel surveys. ICES Journal of Marine Science 73, 2009-2019.
| Crossref | Google Scholar |
Finn H, Calver MC (2008) Feeding aggregations of bottlenose dolphins and seabirds in Cockburn Sound, Western Australia. The Western Australian Naturalist 26, 157-172.
| Google Scholar |
Gillanders BM (2002) Connectivity between juvenile and adult fish populations: do adults remain near their recruitment estuaries? Marine Ecology Progress Series 240, 215-223.
| Crossref | Google Scholar |
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D’Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319, 948-952.
| Crossref | Google Scholar |
Heithaus M, Dill L (2009) Feeding tactics and strategies. In ‘Encyclopedia of marine mammals’. (Eds WF Perrin, B Würsig, JGM Thewissen) pp. 414–423. (Academic Press) 10.1016/B978-0-12-373553-9.00099-7
Jackson G, Moran M (2012) Recovery of inner Shark Bay snapper (Pagrus auratus) stocks: relevant research and adaptive recreational fisheries management in a World Heritage Property. Marine and Freshwater Research 63, 1180-1190.
| Crossref | Google Scholar |
Jackson G, Cheng YW, Wakefield CB (2012) An evaluation of the daily egg production method to estimate spawning biomass of snapper (Pagrus auratus) in inner Shark Bay, Western Australia, following more than a decade of surveys 1997–2007. Fisheries Research 117–118, 22-34.
| Crossref | Google Scholar |
Jaud M, Delacourt C, Le Dantec N, Allemand P, Ammann J, Grandjean P, Nouaille H, Prunier C, Cuq V, Augereau E, Cocquempot L, Floc’h F (2019) Diachronic UAV photogrammetry of a sandy beach in Brittany (France) for a long-term coastal observatory. ISPRS International Journal of Geo-Information 8, 267.
| Crossref | Google Scholar |
Jech JM, Schaber M, Cox M, Escobar-Flores P, Gastauer S, Haris K, Horne J, Jarvis T, Ladroit Y, O’Driscoll R, Pedersen G, Peña M, Ryan T, Sakinan S, Thomas R, Viehman H, Wall C, Whitton TT, O’Driscoll R, Pedersen G, Pena M, Ryan T, Sakinan S, Thomas R, Viehman H, Whitton TT (2021) Collecting quality echosounder data in inclement weather. ICES Cooperative Research Report 352. (International Council for the Exploration of the Sea–Conseil International pour l’Exploration de la Mer: Copenhagen, Denmark) Available at https://doi.org/10.17895/ices.pub.7539
Kendrick GA, Aylward MJ, Hegge BJ, Cambridge ML, Hillman K, Wyllie A, Lord DA (2002) Changes in seagrass coverage in Cockburn Sound, Western Australia between 1967 and 1999. Aquatic Botany 73, 75-87.
| Crossref | Google Scholar |
Langlois TJ, Harvey ES, Fitzpatrick B, Meeuwig JJ, Shedrawi G, Watson DL (2010) Cost-efficient sampling of fish assemblages: comparison of baited video stations and diver video transects. Aquatic Biology 9, 155-168.
| Crossref | Google Scholar |
Langlois T, Goetze J, Bond T, Monk J, Abesamis RA, Asher J, Barrett N, Bernard ATF, Bouchet PJ, Birt MJ, Cappo M, Currey-Randall LM, Driessen D, Fairclough DV, Fullwood LAF, Gibbons BA, Harasti D, Heupel MR, Hicks J, Holmes TH, Huveneers C, Ierodiaconou D, Jordan A, Knott NA, Lindfield S, Malcolm HA, McLean D, Meekan M, Miller D, Mitchell PJ, Newman SJ, Radford B, Rolim FA, Saunders BJ, Stowar M, Smith ANH, Travers MJ, Wakefield CB, Whitmarsh SK, Williams J, Harvey ES (2020) A field and video annotation guide for baited remote underwater stereo-video surveys of demersal fish assemblages. Methods in Ecology and Evolution 11, 1401-1409.
| Crossref | Google Scholar |
Marre G, Holon F, Luque S, Boissery P, Deter J (2019) Monitoring marine habitats with photogrammetry: a cost-effective, accurate, precise and high-resolution reconstruction method. Frontiers in Marine Science 6, 276.
| Crossref | Google Scholar |
McInnes AM, Khoosal A, Murrell B, Merkle D, Lacerda M, Nyengera R, Coetzee JC, Edwards LC, Ryan PG, Rademan J, Van Der Westhuizen JJ, Pichegru L (2015) Recreational fish-finders: an inexpensive alternative to scientific echo-sounders for unravelling the links between marine top predators and their prey. PLoS ONE 10, e0140936.
| Crossref | Google Scholar | PubMed |
Molony BW, Kemps H, Lockwood N (2020) Coastal marine managed areas. Journal of Ocean Technology 15, 34-47.
| Google Scholar |
Nordhaus I, Roelke DL, Vaquer-Sunyer R, Winter C (2018) Coastal systems in transition: from a ‘natural’ to an ‘anthropogenically modified’ state. Estuarine, Coastal and Shelf Science 211, 1-5.
| Crossref | Google Scholar |
Oxley APA, Catalano SR, Wos-Oxley ML, Westlake EL, Grammer GL, Steer MA (2017) Using in situ hybridization to expand the daily egg production method to new fish species. Molecular Ecology Resources 17, 1108-1121.
| Crossref | Google Scholar | PubMed |
Pattiaratchi C, Hegge B, Gould J, Eliot I (1997) Impact of sea-breeze activity on nearshore and foreshore processes in southwestern Australia. Continental Shelf Research 17, 1539-1560.
| Crossref | Google Scholar |
Pillay D, Branch GM, Forbes AT (2008) Habitat change in an estuarine embayment: anthropogenic influences and a regime shift in biotic interactions. Marine Ecology Progress Series 370, 19-31.
| Crossref | Google Scholar |
Pirotta E, Laesser BE, Hardaker A, Riddoch N, Marcoux M, Lusseau D (2013) Dredging displaces bottlenose dolphins from an urbanised foraging patch. Marine Pollution Bulletin 74, 396-402.
| Crossref | Google Scholar | PubMed |
Raoult V, Colefax AP, Allan BM, Cagnazzi D, Castelblanco-Martínez N, Ierodiaconou D, Johnston DW, Landeo-Yauri S, Lyons M, Pirotta V, Schofield G, Butcher PA (2020) Operational protocols for the use of drones in marine animal research. Drones 4, 64.
| Crossref | Google Scholar |
Romenskyy M, Herbert-Read JE, Ioannou CC, Szorkovszky A, Ward AJW, Sumpter DJT (2020) Quantifying the structure and dynamics of fish shoals under predation threat in three dimensions. Behavioral Ecology 31, 311-321.
| Crossref | Google Scholar |
Rueden CT, Schindelin J, Hiner MC, DeZonia BE, Walter AE, Arena ET, Eliceiri KW (2017) ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinformatics 18, 529.
| Crossref | Google Scholar |
Sánchez-Carnero N, Rodríguez-Pérez D, Irigoyen A, Trobbiani G, Parma AM (2018) What can a low-cost fish-finder tell us about the seabed? Estuarine, Coastal and Shelf Science 215, 94-99.
| Crossref | Google Scholar |
Schofield G, Esteban N, Katselidis KA, Hays GC (2019) Drones for research on sea turtles and other marine vertebrates – a review. Biological Conservation 238, 108214.
| Crossref | Google Scholar |
Scoulding B, Gastauer S, Taylor JC, Boswell KM, Fairclough DV, Jackson G, Sullivan P, Shertzer K, Campanella F, Bacheler N, Campbell M, Domokos R, Schobernd Z, Switzer TS, Jarvis N, Crisafulli BM, Untiedt C, Fernandes PG (2023) Estimating abundance of fish associated with structured habitats by combining acoustics and optics. Journal of Applied Ecology 60, 1274-1285.
| Crossref | Google Scholar |
Steer MA, McGarvey R, Oxley A, Fowler AJ, Grammer G, Ward TM, Westlake E, Matthews D, Matthews J (2017) Developing a fishery independent estimate of biomass for snapper (Chrysophrys auratus). FRDC Project Number 2014/019. (Fisheries Research and Development Corporation and South Australian Research and Development Institute: Adelaide, SA, Australia) Available at https://pir.sa.gov.au/__data/assets/pdf_file/0008/294785/Developing_a_fishery_independent_estimate_of_biomass_for_Snapper_Chrysophrys_auratus.pdf
van Putten I, Cvitanovic C, Fulton EA (2016) A changing marine sector in Australian coastal communities: an analysis of inter and intra sectoral industry connections and employment. Ocean & Coastal Management 131, 1-12.
| Crossref | Google Scholar |
Wakefield CB (2010) Annual, lunar and diel reproductive periodicity of a spawning aggregation of snapper Pagrus auratus (Sparidae) in a marine embayment on the lower west coast of Australia. Journal of Fish Biology 77, 1359-1378.
| Crossref | Google Scholar | PubMed |
Wakefield CB, Fairclough DV, Lenanton RCJ, Potter IC (2011) Spawning and nursery habitat partitioning and movement patterns of Pagrus auratus (Sparidae) on the lower west coast of Australia. Fisheries Research 109, 243-251.
| Crossref | Google Scholar |
Wakefield CB, Lewis PD, Coutts TB, Fairclough DV, Langlois TJ (2013) Fish assemblages associated with natural and anthropogenically modified habitats in a marine embayment: comparison of baited videos and opera-house traps. PLoS ONE 8, e59959.
| Crossref | Google Scholar | PubMed |
Wakefield CB, Potter IC, Hall NG, Lenanton RCJ, Hesp SA (2015) Marked variations in reproductive characteristics of snapper (Chrysophrys auratus, Sparidae) and their relationship with temperature over a wide latitudinal range. ICES Journal of Marine Science 72, 2341-2349.
| Crossref | Google Scholar |
Ward TJ, Jacoby CA (1992) A strategy for assessment and management of marine ecosystems: baseline and monitoring studies in Jervis Bay, a temperate Australian embayment. Marine Pollution Bulletin 25, 163-171.
| Crossref | Google Scholar |
Westley K, Mcneary R (2017) Archaeological applications of low-cost integrated sidescan sonar/single-beam echosounder systems in irish inland waterways. Archaeological Prospection 24, 37-57.
| Crossref | Google Scholar |
Williams R, Thomas L (2009) Cost-effective abundance estimation of rare animals: testing performance of small-boat surveys for killer whales in British Columbia. Biological Conservation 142, 1542-1547.
| Crossref | Google Scholar |
Williams R, Ashe E, Gaut K, Gryba R, Moore JE, Rexstad E, Sandilands D, Steventon J, Reeves RR (2017) Animal counting toolkit: a practical guide to small-boat surveys for estimating abundance of coastal marine mammals. Endangered Species Research 34, 149-165.
| Crossref | Google Scholar |
Wolfenkoehler W, Long JM, Gary R, Snow RA, Schooley JD, Bruckerhoff LA, Lonsinger RC (2023) Viability of side-scan sonar to enumerate Paddlefish, a large pelagic freshwater fish, in rivers and reservoirs. Fisheries Research 261, 106639.
| Crossref | Google Scholar |
Xu S, Zhang M, Song W, Mei H, He Q, Liotta A (2023) A systematic review and analysis of deep learning-based underwater object detection. Neurocomputing 527, 204-232.
| Crossref | Google Scholar |
Zeldis JR, Francis RICC (1998) A daily egg production method estimate of snapper biomass in Hauraki Gulf, New Zealand. ICES Journal of Marine Science 55, 522-534.
| Crossref | Google Scholar |


