Dietary overlap of carcharhinid sharks in the Gulf of Papua
Leontine Baje A B * , Andrew Chin B , William T. White C D and Colin A. Simpfendorfer B
A B * , Andrew Chin B , William T. White C D and Colin A. Simpfendorfer B
A National Fisheries Authority, National Capital District, Port Moresby, Papua New Guinea.
B Centre for Sustainable Tropical Fisheries and Aquaculture & College of Science and Engineering, James Cook University, Townsville, Qld, Australia.
C CSIRO Oceans & Atmosphere, Hobart, Tas., Australia.
D CSIRO Australian National Fish Collection, National Research Collections Australia, Hobart, Tas., Australia.
Marine and Freshwater Research 73(5) 605-614 https://doi.org/10.1071/MF21212
Submitted: 9 June 2021 Accepted: 8 January 2022 Published: 11 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Assessing the feeding patterns of sharks provides insight into ecological interactions. Three coastal sharks are common by-catch in the Gulf of Papua prawn fishery in Papua New Guinea. The diets of Carcharhinus coatesi (n = 122), Rhizoprionodon acutus (n = 83) and Rhizoprionodon taylori (n = 177) were assessed using stomach content analysis. Teleosts, crustaceans and molluscs were the main prey. Percentage frequency of occurrence (%FO) and percentage frequency by number (%N) were computed to describe dietary compositions. Non-metric multidimensional scaling and Morisita Index determined the level of feeding overlap. Rhizoprionodon taylori was a generalist feeder having the broadest diet, R. acutus was the most selective feeder, preying predominantly on teleosts and C. coatesi consumed the greatest proportion of crustaceans that increased with size. The pairwise ANOSIM tests showed significant difference in dietary compositions of R. acutus and R. taylori (P = 0.1%, R = 0.318) and R. acutus and C. coatesi (P = 0.1%, R = 0.589), which indicate potential resource partitioning. Further work should aim to adequately characterise diets, improve prey identification and investigate spatial and temporal resource use patterns. Understanding ecological processes informs ecosystem approaches fisheries management.
Keywords: carcharhinid sharks, Carcharhinus coatesi, coastal shark diet, fisheries management, Gulf of Papua Prawn Fishery, GoPPF, Rhizoprionodon acutus, Rhizoprionodon taylori, shark by-catch.
Introduction
Fisheries are a major contributor to the decline of shark populations (Dulvy et al. 2014) that function mainly as top and middle order predators in marine ecosystems (Heupel et al. 2014). Concern for the survival of these populations has also highlighted that the flow on effects of low predator abundance on the ecosystem remain largely unknown, partly due to the paucity of ecological information for specific regions (Ferretti et al. 2010). Therefore, establishing an understanding of the ecology of species, and their contributions to ecosystem processes (Bornatowski et al. 2014a), is a crucial element in predicting the outcomes of population declines and potential species loss. Assessing the ecosystem impacts of fisheries in order to set appropriate management and conservation guidelines requires information from both target and non-target (by-catch) species (Pikitch et al. 2004).
Characterising the dietary traits of sharks from stomach content analysis provides empirical evidence of the trophic linkages in the food chain (Cortes 1999). Such information can be incorporated into ecosystem models to support fisheries management (Rogers et al. 2012). In addition, knowledge of feeding patterns may reveal diet specialisation, which highlights the vulnerability of predators and the impacts on the ecosystem from their decline. Specialised feeders have a narrow range of prey and may be more vulnerable to perturbations that may directly affect food availability whereas generalist feeders are more resilient to environmental changes (Simpfendorfer et al. 2001; Munroe et al. 2014). The level of diet overlap among similar sympatric species is an indirect measure of competition when food resources are limited (Wetherbee et al. 2012). Diet studies show that elasmobranchs reduce competition through partitioning of food resources by consuming different proportions of available prey (Platell et al. 1998; White et al. 2004), specialisation on a specific set of prey (Sommerville et al. 2011; Bornatowski et al. 2014b) and differential spatial distribution of species (Papastamatiou et al. 2006). Dietary investigations, therefore, provide some insight into complex and dynamic ecological interactions.
Small-bodied coastal sharks are generally considered to be meso-predators that connect the lower and top trophic levels of the food chain (Heupel et al. 2014) and are also common in coastal fisheries by-catch (Stobutzki et al. 2002). The Australian blackspot shark (Carcharhinus coatesi), the milk shark (Rhizoprionodon acutus) and the Australian sharpnose shark (Rhizoprionodon taylori) are all small-bodied coastal sharks that are commonly caught as by-catch in the Gulf of Papua Prawn Fishery (GoPPF) in Papua New Guinea, making up 9, 7 and 29% respectively of the total elasmobranch by-catch sampled by number (White et al. 2019). The life histories of C. coatesi and R. taylori indicate that the populations of each species may be affected differently by the fishery based on growth and biological productivity (Baje et al. 2018; Baje et al. 2019). However, the ecology of these sympatric sharks has not been investigated in the Gulf of Papua, and their ecological roles are not well understood. Using samples collected from the by-catch of this fishery, this study aimed to characterise the diets of C. coatesi, R. acutus and R. taylori and estimate the level of dietary overlap to assess if competition and partitioning of food resources occurs among these species in the Gulf of Papua. We hypothesise that R. taylori will have the broadest diet range to support rapid growth in early life stages, whereas R. acutus, attaining a larger size and greater depth range, will have the most distinct diet and potentially partition food resources.
Methods
Study site
The Gulf of Papua, situated on the south coast of Papua New Guinea (PNG) (Fig. 1), is a region composed of extensive mangrove and estuarine areas with high riverine input. Waterways from high-altitude areas of PNG drain into the Gulf forming several major river systems, the largest of which is the Fly River in the West. North Eastward of the Fly River are the Kikori and Purari rivers along with several other systems. These areas provide major nursery grounds for penaeid prawn species that eventually recruit into the Gulf of Papua prawn trawl fishery that mainly targets banana prawns (Penaeus merguiensis) (Evans et al. 1997). The region experiences two main seasons: the north-west monsoon from November to March each year and the south-east monsoon winds that occur from April to October (Moore and MacFarlane 1984). The GoPPF is only open to domestic based vessels offering 15 licenses annually. However, only 6–9 twin- or quad-rigged trawlers are active at any one time due to the old age of vessels that constantly face operational problems (Kompas and Kuk 2008). Fishing operations have remained relatively unchanged over the past 20 years where trawling typically occurs over soft muddy continental shelf areas. The fishery is open throughout the year although sections of the fishing zone in the east of the Gulf of Papua are closed off to allow for recruitment from December to March each year (National Fisheries Authority 2008).

|
Sampling and sample preservation
Fishery observers were deployed on seven prawn trawl fishing trips between June 2014 and August 2015 to collect shark by-catch samples. No shark was intentionally harmed for the study, all samples collected had suffered mortality during the fishing activity due to the length of trawls that last on average for 4–5 h. Samples were kept whole and frozen on board. In a laboratory, sharks were thawed, total length (TL) measured to the nearest ±1 cm, sex recorded, and stomachs excised. Contents from each stomach were removed, fixed in 10% formalin and transferred to 70% ethanol for preservation. Each set of stomach contents were weighed and examined to identify the number and type(s) of prey to the lowest possible taxa. In order to detect if the sample size was sufficient to adequately describe diets, a cumulative prey curve was produced using the specaccum function of the ‘vegan’ package (ver. 2.4.3, J. Oksanen, F. G. Blanchet, R. Kindt, P. Legendre, P. R. Minchin, R. O’Hara, G. L. Simpson, P. Solymos, M. H. H. Stevens, and H. Wagner, see https://cran.r-project.org/web/packages/vegan/index.html) in R (ver. 3.2.2, R Foundation for Statistical Computing, Vienna, Austria, see https://www.R-project.org/).
Fisheries observers of the National Fisheries Authority (NFA) collected sharks that were caught as by-catch and discarded on prawn trawl vessels. All sampling procedures were allowed by the NFA and in line with James Cook University, Animal Ethics approval A2310 that was obtained before the commencement of the study. Sampling did not involve endangered or protected species. No further permits were required by authorities in PNG.
Dietary indices
To assess the importance of each prey item in the diet of the three shark species the percentage frequency of occurrence (%FO) and the percentage by number (%N) were calculated. The former is the number of times a prey category is present in one or more stomachs expressed as a percentage of the total number of stomachs containing food, whereas the latter is the number of each prey category found in each stomach expressed as a proportion of the total number of prey for all stomachs of a particular species (Hyslop 1980). The advanced state of digestion and mastication in most of the samples meant that prey items could not be adequately identified and separated, therefore, volumetric and gravimetric methods were not carried out.
Dietary overlap
Dietary overlap, which is a measure of the level of similarity in the diets between shark species, was measured using the simplified Morisita index (Krebs 1989):

where CH is the Simplified Morisita index of overlap between two species with values ranging from 0 (no overlap) to 1 (complete overlap); pij is the proportion prey in species i that is of the total prey categories used by species j; pik is the proportion prey i is of the total prey categories used by species k; and n is the total number of prey categories.
To avoid confounding results all teleost both identified and unidentified were grouped and unidentified prey that could not be allocated to a specific prey category were omitted from the analysis.
Multivariate analysis
Samples were randomised and pooled according to species, sex, season and size (10-cm size classes) to minimise the large number of zeros and improve the effectiveness of the analyses (Sommerville et al. 2011). The resulting new samples for each factor comprised stomach contents from four to five individuals randomly pooled together. Grouping of prey items for the analyses was the same as for the diet overlap above. The percentage by number (%N) was calculated for each prey item in each sample and entered into Primer-E (ver. 7.0.13, Plymouth Routines in Multivariate Ecological Research, Quest Research Limited, Auckland, New Zealand). Prior to further analysis the data were subject to square-root transformation followed by creation of a Bray–Curtis resemblance matrix. To test for differences in dietary composition a one-way Analysis of Similarities (ANOSIM) was conducted, in this context the R statistic computed does not infer multifactorial tests and interactions that are also available in Primer. Similarities of Percentages (SIMPER) was used to identify the components that typified the diets of each shark species. Non-metric Multidimensional Scaling (nMDS) ordination was used to produce plots to visualise the similarities in the diets. To test for the multivariate variability in the diet of each species Multivariate Dispersion (MVDISP) was conducted. To support the nMDS plot for size classes a column graph was constructed to assess if the diets of the three shark species undergo changes with respect to growth.
Results
Size ranges and sample size
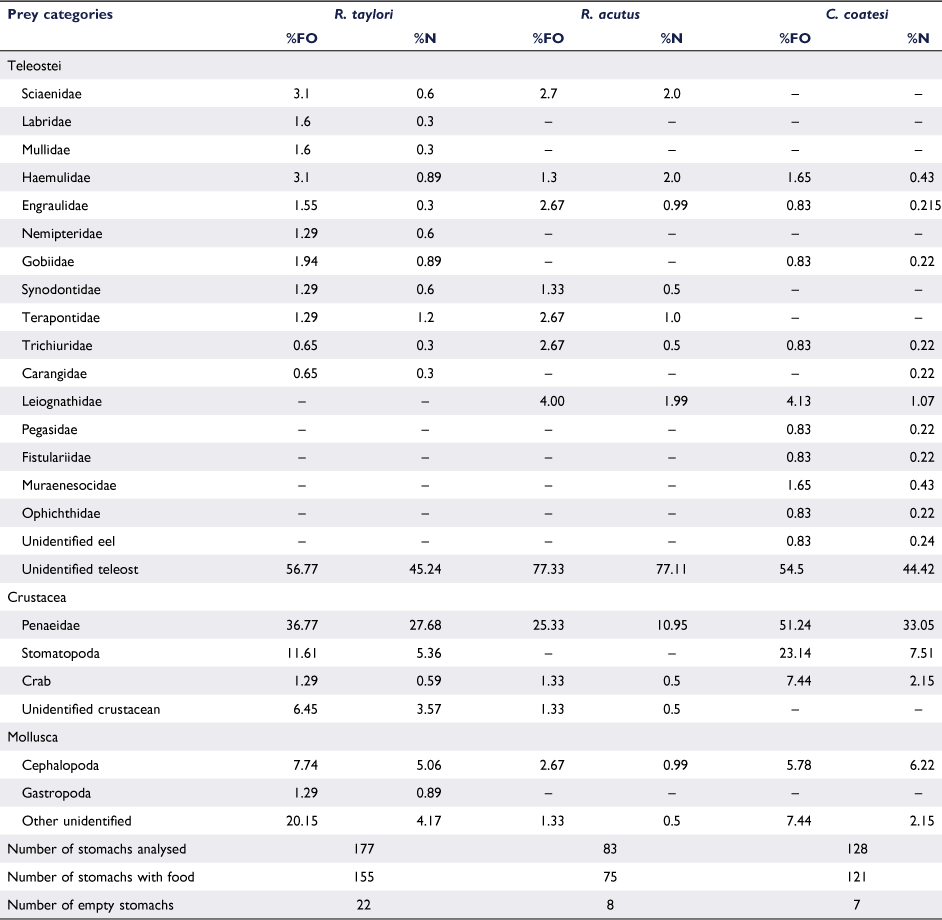
Total lengths recorded were similar among species and ranged from 31 to 76 cm TL for R. taylori; 31–84 cm TL for R. acutus; and 35–79 cm TL for C. coatesi (Fig. 2). A total of 177 stomachs were sampled of R. taylori, 83 of R. acutus, and 122 of C. coatesi. The cumulative prey curve for all three species did not appear to reach asymptote, indicating a larger sample size would be required to fully characterise the diets (Fig. 3). The number of stomachs containing prey was high with few empty stomachs encountered for each species (Table 1).

|

|
Main prey types and proportions in diet
Teleosts, crustaceans and molluscs were observed as the main prey groups, with sixteen teleost families, three crustacean families and two families of molluscs identified. The proportion of individual %FO and %N of each teleost family was low, not exceeding 5% owing to mastication and the process of digestion that resulted in only a small number of individual fishes being identified. Of the 16 families of teleosts observed only 3 families: Haemulidae, Engraulidae and Trichiuridae appeared in the diet of all 3 shark species. Other families were only shared between two of the species, for example Leiognathidae was only present in the stomach contents of R. acutus and C. coatesi. However, distinctively the families Pegasidae, Fistulariidae and the eel families Muraenesocidae and Ophichthidae, were only present in the diet of C. coatesi. The proportion of unidentified teleosts was high for all species (Table 1).
The presence of crustaceans in the diet, %FO and %N of Penaeidae was high for all species, but particularly prevalent in the diet of C. coatesi (54.5%FO). Stomatopoda were also more common in the diet of C. coatesi (23.14%FO and 7.51%N) compared to R. taylori (11.61%FO and 5.36%N) but were absent in the diet of R. acutus. Similarly, with respect to crabs there was a higher %FO and %N in the diet of C. coatesi (7.44%FO and 2.15%N) compared to R. taylori (1.29%FO and 0.59%N) and R. acutus (1.33%FO and 0.5%N). Molluscs played a lesser role in the diet of the all three species; R. acutus (2.67%FO and 0.99%N) consumed fewer cephalopods than R. taylori (7.74%FO and 5.06%N) and C. coatesi (5.78%FO and 6.22%N), whereas Gastropoda were only found in the stomach contents of R. taylori (Table 1).
Diet overlap
The Morisita Index of Similarity calculated for each pair of species showed high overlap for all species. The highest overlap was between R. taylori and C. coatesi (CH = 0.99) with less overlap between the diets of R. taylori and R. acutus (CH = 0.85) and R. acutus and C. coatesi (CH = 0.82). Langton (1982) prescribes a ranking of the similarity index as 0–0.29 as low overlap, 0.30–0.59 as medium and >0.6 as high.
Multivariate analyses
Intraspecific dietary comparison
Dietary data for males and females were pooled for subsequent analysis as there was no significant difference between sexes (P = 0.4, R = 0.039). A one-way ANOSIM indicated a significant difference among the diets of R. taylori, R. acutus and C. coatesi (P = 0.1%, R = 0.259). The pairwise tests between species showed a significant difference in dietary compositions of R. acutus and R. taylori (P = 0.1%, R = 0.318) and R. actus and C. coatesi (P = 0.1%, R = 0.589). However, there was no significant difference in dietary compositions between R. taylori and C. coatesi (P > 0.1%, R = 0.05). The multivariate dispersion (MVDISP) analysis showed that R. taylori had the highest dispersion of 1.13, followed by C. coatesi with 0.89 and R. actus with 0.67. Similarities of percentages (SIMPERs) showed that the main groups that typified the diets of R. taylori and C. coatesi were teleosts and penaeid prawns, whereas teleosts typified the diet of R. acutus. Between pairs of species teleosts (41.8%), followed by penaeids (20.5%) and stomatopods (15.5%) contributed most to the dissimilarity in prey consumption of R. taylori and R. acutus. Between R. taylori and C. coatesi, the dissimilarity resulted from penaeids (22.7%), teleosts (20.31%), stomatopods (17.37%) and cephalopods (15.27%). Although the dissimilarity between R. acutus and C. coatesi was characterised by teleosts (31.3%), penaeids (24%) and stomatopods (18.2%).
The nMDS ordination plot of the dietary compositions of the three shark species showed that R. taylori has a broad diet that overlaps with C. coatesi and R. acutus. Samples of R. acutus appeared in the bottom left of the plot and did not overlap with C. coatesi (Fig. 4).

|
Dietary comparison by season
A one-way ANOSIM testing between north-west monsoon and south-east monsoon periods did not detect a significant result (P > 0.1%, R = 0.017) indicating there was no difference in the diets of all three species between seasons. The nMDS ordination of diets sampled in different seasons showed that most south-east monsoon samples overlapped with north-west monsoon indicating similarity (Fig. 5).

|
Dietary composition among size classes
Comparison of diet composition among size classes for each species showed that R. taylori has a relatively consistent diet with respect to proportions of different dietary components. Cephalopods were not consumed by the smallest size class and there may be a reduction in the consumption of penaeid prawns in the largest sizes class with a possible increase in the consumption of teleosts. Rhizoprionodon acutus consumes large proportions of teleosts in all size classes and may consume less crustaceans and cephalopods with increasing size. Carcharhinus coatesi had a marked decrease in teleost consumption with increasing size accompanied by an increase in the consumption of crustaceans particularly penaeid prawns (Fig. 6).

|
The nMDS ordination plot of size classes showed both similarities and differences particularly in the smallest and largest size classes (Fig. 7). Among the R. taylori samples there is a general similarity across all size classes except for the largest size class 61–70 cm, samples in this size class were the furthest right on the plot of all R. taylori samples. Similarly, diets of mainly larger size C. coatesi in the 71–80-cm category were clustered together towards the lower section of the plot whereas some of the smallest R. acutus, 31–40 cm also showed a larger dissimilarity to other size classes of this species.

|
Discussion
Many shark species are considered to be generalist feeders (Munroe et al. 2014) and have been observed to feed in a density-dependent manner (Salini et al. 1992). The small-bodied carcharhinids studied here feed at similar trophic levels (Cortes 1999), therefore where they co-occur competition for food resources can arise if prey are limited. This study shows that teleosts, crustaceans and molluscs make up most of the prey of C. coatesi, R. acutus and R. taylori. Teleosts and greater proportions of crustaceans were found in the diet of R. taylori and C. coatesi whereas the diet of R. actus consisted predominantly of teleost with other prey categories being much less important. Stevens and McLoughlin (1991) found similar predominant prey types for all three species in northern Australia, however the relative amounts of prey differed from this study. The findings of this study align with the classification of R. taylori as dietary generalist due to its broad diet breath (Munroe et al. 2015) and additionally a predominance of teleosts in the diet of R. acutus (White et al. 2004; Ba et al. 2013).
The level of diet similarity as an indicator of competition for food resources was high between species, however, differences in individual diets exist and may facilitate co-occurrence. Significant dietary differences and the lower level of similarity in the diet of R. acutus indicate this species may partition food resources by feeding predominantly on teleosts and consuming the lowest levels of crustaceans, molluscs and other prey groups among all three species. Rhizoprionodon acutus occupies a broader depth range (White et al. 2017), therefore, characterisation of diets alone is insufficient to detect temporal and spatial partitioning that may also be occurring (Bornatowski et al. 2014b; Lear et al. 2021). Future work should incorporate datasets from all resource use axes (White et al. 2004) to draw a clearer picture of the food web and ecosystem use.
High similarity in diets of sympatric species may indicate that prey is not limited (Heithaus et al. 2013) although prey availability may fluctuate with temporal seasonality (Nunn et al. 2020). Despite the high diet similarity between R. taylori and C. coatesi a few differences were observed. Some prey found in the diet of C. coatesi were not detected in the R. taylori diet. Carcharhinius coatesi also fed on more crustaceans overall increasing its intake with size. Intra-specific dietary change with growth is widely detected in elasmobranchs (Sommerville et al. 2011; Barbini and Lucifora 2012). Morphological traits such as dentition and gape size develop with growth and enable capture of larger prey (Powter et al. 2010), in this instance a preference for crustaceans in older C. coatesi could potentially be a means to avoid competition. In particular, C. coatesi consumed a greater amount of crab compared to R. taylori. One criticism of stomach content analysis is the predominance of hard parts such as cephalopod beaks and crustacean exoskeleton, which may overestimate the presence of these groups (Kim et al. 2012). Since the cumulative prey curves show that the sample size was not sufficient to fully describe diets further sampling and proper identification of prey will be required to adequately characterise diets and investigate the extent of these preliminary observed differences. Both R. taylori and C. coatesi are morphologically similar but appear to be reproductively different, R. taylori breeds annually and has a rapid growth rate reaching maturity in less than 1 year (Baje et al. 2018) compared to aseasonal reproduction in C. coatesi, which reaches maturity at 5 years of age (Baje et al. 2019) these factors may also influence feeding behaviour at various life stages such as the broad diet of R. taylori is needed to support rapid growth in the first year of life to reach maturity.
Volumetric and gravimetric (bulk) descriptions of diets have been consistently included with other measures to produce compound indices and have been the preferred measure on which to conduct multivariate analysis. However, practically assessing stomach contents to achieve bulk measures of diets is associated with the difficulty of sorting through masticated and partially digested prey items that are separated into many pieces or loose tissue, which makes it impossible to know which prey item they belong to or if they are part of a separate prey item altogether. Thus, the inclusion of bulk dietary measures introduces inherent errors linked to the difficulty in identifying and quantifying prey items (Baker et al. 2014). The absence of a bulk measure of the diet meant that a compound index (e.g. percentage index of relative importance) was not calculated for this study. Compound indices have been recommended as a standard practice (Hyslop 1980; Cortés 1997; Brown et al. 2012), however, they have been found to have little significance, as opposed to considering separate dietary measures individually (Baker et al. 2014), particularly for demersal species (Macdonald and Green 1983).
This study is the first attempt to investigage the diets of inshore meso‐predator sharks frequently caught as by‐catch in the GoPPF that focuses on a component of the ecological system that fishing activities constantly interact with. The results are in agreement with the stated hypotheses, however, this work is limited in the use of methodology to identify prey. Future studies should incorporate molecular techniques and stable isotope analysis to improve prey identification (Matley et al. 2018). Stable isotope analysis is non-lethal and can also detect longer-term habitat use and diet preferences (Kinney et al. 2011; Shiffman et al. 2012). Defining the ecological niche of a species is a multivariate exercise requiring empirical evidence from multiple sources (Munroe et al. 2014), therefore, future research should complement dietary information with fishery independent surveys, tag–recapture and acoustic tracking to investigate the ecology of species across time and space (Wiley and Simpfendorfer 2007; Donaldson et al. 2014) if possible. The Gulf of Papua is a hot spot for species diversity (Pernetta and Hill 1981) including a large proportion of elasmobranchs that are encountered in the GoPPF, some of which are endemic (White et al. 2017; Baje et al. 2021). Ecological data and information from this region are therefore important to support ecosystem approaches to fisheries management and conservation of vulnerable species.
Data availability
The data that support this study are available in the article.
Conflicts of interest
Colin Simpfendorfer is an Associate Editor of Marine and Freshwater Research but did not at any stage have editor-level access to this manuscript while in peer review, as is the standard practice when handling manuscripts submitted by an editor to this journal. Marine and Freshwater Research encourages its editors to publish in the journal and they are kept totally separate from the decision-making processes for their manuscripts. The authors have no further conflicts of interest to declare.
Declaration of funding
Funding for the study (sample collection, logistics and processing) was provided by the Australian Centre for International Agricultural Research (ACIAR), the National Fisheries Authority (NFA) of Papua New Guinea and CSIRO for the ACIAR project FIS-2012–102: Sustainable management of shark resources of Papua New Guinea: socio economic and biological characteristics of the fishery. The lead author was funded by the John Allwright Fellowship Award administered by ACIAR and the Schlumberger Foundation Faculty for the Fellowship program.
Acknowledgements
The authors thank Dr Chris Barlow, Dr Ann Fleming and Dr Jes Sammut for their support. In addition, the authors also thank Ludwig Kumoru, Leban Gisawa, Luanah Yaman, Brian Kumasi, Thomas Usu and Benthly Sabub of the NFA. Special thanks are also accorded to Fishery Observers, Baera Nawia, Siwen Ohuesaho, Ronald Wala, Sarea Tova and Ian Tony for data collection on board fishing vessels; and to Dr Ralph Mana at the University of PNG for providing laboratory space for processing specimens. Sincere thanks to John Pogonoski of CSIRO who helped with the identification of diet samples and Noel Baje for helping with figures.
References
Ba, A, Diop, MS, Diatta, Y, Justine, D, and Ba, CT (2013). Diet of the milk shark, Rhizoprionodon acutus (Ruppel, 1837) (Chondrichthyes: Carcharhinidae), from the Senegalese coast. Journal of Applied Ichthyology 29, 789–795.| Diet of the milk shark, Rhizoprionodon acutus (Ruppel, 1837) (Chondrichthyes: Carcharhinidae), from the Senegalese coast.Crossref | GoogleScholarGoogle Scholar |
Baje, L, Smart, JJ, Chin, A, White, WT, and Simpfendorfer, CA (2018). Age, growth and maturity of the Australian sharpnose shark Rhizoprionodon taylori from the gulf of Papua. PLoS One 13, e0206581.
| Age, growth and maturity of the Australian sharpnose shark Rhizoprionodon taylori from the gulf of Papua.Crossref | GoogleScholarGoogle Scholar | 30379918PubMed |
Baje, L, Smart, JJ, Grant, MI, Chin, A, White, WT, and Simpfendorfer, CA (2019). Age, growth and maturity of the Australian blackspot shark (Carcharhinus coatesi) in the gulf of Papua. Pacific Conservation Biology 25, 403–412.
| Age, growth and maturity of the Australian blackspot shark (Carcharhinus coatesi) in the gulf of Papua.Crossref | GoogleScholarGoogle Scholar |
Baje, L, Chin, A, White, WT, and Simpfendorfer, CA (2021). Ecological risk assessment of elasmobranchs caught in the gulf of Papua prawn fishery. Aquatic Conservation 31, 3100–3110.
| Ecological risk assessment of elasmobranchs caught in the gulf of Papua prawn fishery.Crossref | GoogleScholarGoogle Scholar |
Baker, R, Buckland, A, and Sheaves, M (2014). Fish gut content analysis: robust measures of diet composition. Fish and Fisheries 15, 170–177.
| Fish gut content analysis: robust measures of diet composition.Crossref | GoogleScholarGoogle Scholar |
Barbini, SA, and Lucifora, LO (2012). Ontogenetic diet shifts and food partitioning between two small sympatric skates (Chondrichthyes, Rajidae) in the southwestern Atlantic. Marine and Freshwater Research 63, 905–913.
| Ontogenetic diet shifts and food partitioning between two small sympatric skates (Chondrichthyes, Rajidae) in the southwestern Atlantic.Crossref | GoogleScholarGoogle Scholar |
Bornatowski, H, Navia, AF, Braga, RR, Abilhoa, V, and Corrêa, MFM (2014a). Ecological importance of sharks and rays in a structural foodweb analysis in southern brazil. ICES Journal of Marine Science 71, 1586–1592.
| Ecological importance of sharks and rays in a structural foodweb analysis in southern brazil.Crossref | GoogleScholarGoogle Scholar |
Bornatowski, H, Wosnick, N, David Do Carmo, WP, Maia Correa, MF, and Abilhoa, V (2014b). Feeding comparisons of four batoids (Elasmobranchii) in coastal waters of southern brazil. Journal of the Marine Biological Association of the United Kingdom 94, 1491–1499.
| Feeding comparisons of four batoids (Elasmobranchii) in coastal waters of southern brazil.Crossref | GoogleScholarGoogle Scholar |
Brown, SC, Bizzarro, JJ, Cailliet, GM, and Ebert, DA (2012). Breaking with tradition: redefining measures for diet description with a case study of the Aleutian skate Bathyraja aleutica (Gilbert 1896). Environmental Biology of Fishes 95, 3–20.
| Breaking with tradition: redefining measures for diet description with a case study of the Aleutian skate Bathyraja aleutica (Gilbert 1896).Crossref | GoogleScholarGoogle Scholar |
Cortés, E (1997). A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Canadian Journal of Fisheries and Aquatic Sciences 54, 726–738.
| A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes.Crossref | GoogleScholarGoogle Scholar |
Cortes, E (1999). Standardized diet compositions and trophic levels of sharks. ICES Journal of Marine Science 56, 707–717.
| Standardized diet compositions and trophic levels of sharks.Crossref | GoogleScholarGoogle Scholar |
Donaldson, MR, Hinch, SG, Suski, CD, Fisk, AT, Heupel, MR, and Cooke, SJ (2014). Making connections in aquatic ecosystems with acoustic telemetry monitoring. Frontiers in Ecology and the Environment 12, 565–573.
| Making connections in aquatic ecosystems with acoustic telemetry monitoring.Crossref | GoogleScholarGoogle Scholar |
Dulvy, NK, Fowler, SL, Musick, JA, Cavanagh, RD, Kyne, PM, Harrison, LR, Carlson, JK, Davidson, LNK, Fordham, SV, Francis, MP, Pollock, CM, Simpfendorfer, CA, Burgess, GH, Carpenter, KE, Compagno, LJV, Ebert, DA, Gibson, C, Heupel, MR, Livingstone, SR, Sanciangco, JC, Stevens, JD, Valenti, S, and White, WT (2014). Extinction risk and conservation of the world’s sharks and rays. eLife 3, e00590.
| Extinction risk and conservation of the world’s sharks and rays.Crossref | GoogleScholarGoogle Scholar | 24448405PubMed |
Evans, CR, Opnai, LJ, and Kare, BD (1997). Fishery ecology and oceanography of the prawn Penaeus merguiensis (De Man) in the gulf of Papua: estimation of maximum sustainable yield and modelling of yield, effort and rainfall. Marine and Freshwater Research 48, 219–228.
| Fishery ecology and oceanography of the prawn Penaeus merguiensis (De Man) in the gulf of Papua: estimation of maximum sustainable yield and modelling of yield, effort and rainfall.Crossref | GoogleScholarGoogle Scholar |
Ferretti, F, Worm, B, Britten, GL, Heithaus, MR, and Lotze, HK (2010). Patterns and ecosystem consequences of shark declines in the ocean. Ecology Letters 13, 1055–1071.
| Patterns and ecosystem consequences of shark declines in the ocean.Crossref | GoogleScholarGoogle Scholar | 20528897PubMed |
Heithaus, MR, Vaudo, JJ, Kreicker, S, Layman, CA, Kruetzen, M, Burkholder, DA, Gastrich, K, Bessey, C, Sarabia, R, Cameron, K, Wirsing, A, Thomson, JA, and Dunphy-Daly, MM (2013). Apparent resource partitioning and trophic structure of large-bodied marine predators in a relatively pristine seagrass ecosystem. Marine Ecology Progress Series 481, 225–237.
| Apparent resource partitioning and trophic structure of large-bodied marine predators in a relatively pristine seagrass ecosystem.Crossref | GoogleScholarGoogle Scholar |
Heupel, MR, Knip, DM, Simpfendorfer, CA, and Dulvy, NK (2014). Sizing up the ecological role of sharks as predators. Marine Ecology Progress Series 495, 291–298.
| Sizing up the ecological role of sharks as predators.Crossref | GoogleScholarGoogle Scholar |
Hyslop, EJ (1980). Stomach contents analysis – a review of methods and their application. Journal of Fish Biology 17, 411–429.
| Stomach contents analysis – a review of methods and their application.Crossref | GoogleScholarGoogle Scholar |
Kim, SL, Casper, DR, Galván-Magaña, F, Ochoa-Díaz, R, Hernández-Aguilar, SB, and Koch, PL (2012). Carbon and nitrogen discrimination factors for elasmobranch soft tissues based on a long-term controlled feeding study. Environmental Biology of Fishes 95, 37–52.
| Carbon and nitrogen discrimination factors for elasmobranch soft tissues based on a long-term controlled feeding study.Crossref | GoogleScholarGoogle Scholar |
Kinney, MJ, Hussey, NE, Fisk, AT, Tobin, AJ, and Simpfendorfer, CA (2011). Communal or competitive? Stable isotope analysis provides evidence of resource partitioning within a communal shark nursery. Marine Ecology Progress Series 439, 263–276.
| Communal or competitive? Stable isotope analysis provides evidence of resource partitioning within a communal shark nursery.Crossref | GoogleScholarGoogle Scholar |
Kompas, T, and Kuk, R (2008). Managing the gulf of Papua prawn fishery: sustainability, maximum returns and cooperation between commercial fishing and indigenous fishing communities. Pacific Economic Bulletin 23, 29–38.
Krebs CJ (1989) ‘Ecology methodology.’ (Harper Collins: New York, NY, USA)
Langton, RW (1982). Diet overlap between Atlantic cod, Gadus morhua, silver hake, Merluccius bilinearis, and fifteen other Northwest Atlantic finfish. Fishery Bulletin 80, 745–759.
Lear, KO, Whitney, NM, Morris, JJ, and Gleiss, AC (2021). Temporal niche partitioning as a novel mechanism promoting co-existence of sympatric predators in marine systems. Proceedings of the Royal Society – B. Biological Sciences 288, 20210816.
| Temporal niche partitioning as a novel mechanism promoting co-existence of sympatric predators in marine systems.Crossref | GoogleScholarGoogle Scholar |
Macdonald, JS, and Green, R (1983). Redundancy of variables used to describe importance of prey species in fish diets. Canadian Journal of Fisheries and Aquatic Sciences 40, 635–637.
| Redundancy of variables used to describe importance of prey species in fish diets.Crossref | GoogleScholarGoogle Scholar |
Matley, JK, Maes, GE, Devloo‐Delva, F, Huerlimann, R, Chua, G, Tobin, AJ, Fisk, AT, Simpfendorfer, CA, and Heupel, MR (2018). Integrating complementary methods to improve diet analysis in fishery‐targeted species. Ecology and Evolution 8, 9503–9515.
| Integrating complementary methods to improve diet analysis in fishery‐targeted species.Crossref | GoogleScholarGoogle Scholar | 30377518PubMed |
Moore, R, and MacFarlane, J (1984). Migration of the ornate rock lobster, Panulirus ornatus (Fabricius), in Papua New Guinea. Marine and Freshwater Research 35, 197–212.
| Migration of the ornate rock lobster, Panulirus ornatus (Fabricius), in Papua New Guinea.Crossref | GoogleScholarGoogle Scholar |
Munroe, SEM, Simpfendorfer, CA, and Heupel, MR (2014). Defining shark ecological specialisation: concepts, context, and examples. Reviews in Fish Biology and Fisheries 24, 317–331.
| Defining shark ecological specialisation: concepts, context, and examples.Crossref | GoogleScholarGoogle Scholar |
Munroe, SEM, Heupel, MR, Fisk, AT, and Simpfendorfer, CA (2015). Geographic and temporal variation in the trophic ecology of a small-bodied shark: evidence of resilience to environmental change. Canadian Journal of Fisheries and Aquatic Sciences 72, 343–351.
| Geographic and temporal variation in the trophic ecology of a small-bodied shark: evidence of resilience to environmental change.Crossref | GoogleScholarGoogle Scholar |
National Fisheries Authority (2008) ‘Gulf of Papua Prawn Management Plan.’ (National Gazette: Port Moresby, Papua New Guinea)
Nunn, A, Vickers, L, Mazik, K, Bolland, J, Peirson, G, Axford, S, Henshaw, A, and Cowx, I (2020). Dynamic competition and resource partitioning during the early life of two widespread, abundant and ecologically similar fishes. Hydrobiologia 847, 2211–2224.
| Dynamic competition and resource partitioning during the early life of two widespread, abundant and ecologically similar fishes.Crossref | GoogleScholarGoogle Scholar |
Papastamatiou, YP, Wetherbee, BM, Lowe, CG, and Crow, GL (2006). Distribution and diet of four species of carcharhinid shark in the Hawaiian Islands: evidence for resource partitioning and competitive exclusion. Marine Ecology Progress Series 320, 239–251.
| Distribution and diet of four species of carcharhinid shark in the Hawaiian Islands: evidence for resource partitioning and competitive exclusion.Crossref | GoogleScholarGoogle Scholar |
Pernetta, JC, and Hill, L (1981). A review of marine resource use in coastal Papua. Journal de la Société des Océanistes 37, 175–191.
| A review of marine resource use in coastal Papua.Crossref | GoogleScholarGoogle Scholar |
Pikitch, EK, Santora, C, Babcock, EA, Bakun, A, Bonfil, R, Conover, DO, Dayton, P, Doukakis, P, Fluharty, D, Heneman, B, Houde, ED, Link, J, Livingston, PA, Mangel, M, McAllister, MK, Pope, J, and Sainsbury, KJ (2004). Ecosystem-based fishery management. Science 305, 346–347.
| Ecosystem-based fishery management.Crossref | GoogleScholarGoogle Scholar | 15256658PubMed |
Platell, ME, Potter, IC, and Clark, KR (1998). Resource partitioning by four species of elasmobranchs (Batoidea: Urolophidae) in coastal waters of temperate Australia. Marine Biology 131, 719–734.
| Resource partitioning by four species of elasmobranchs (Batoidea: Urolophidae) in coastal waters of temperate Australia.Crossref | GoogleScholarGoogle Scholar |
Powter, DM, Gladstone, W, and Platell, M (2010). The influence of sex and maturity on the diet, mouth morphology and dentition of the Port Jackson shark, Heterodontus portusjacksoni. Marine and Freshwater Research 61, 74–85.
| The influence of sex and maturity on the diet, mouth morphology and dentition of the Port Jackson shark, Heterodontus portusjacksoni.Crossref | GoogleScholarGoogle Scholar |
Rogers, PJ, Huveneers, C, Page, B, Hamer, DJ, Goldsworthy, SD, Mitchell, JG, and Seuront, L (2012). A quantitative comparison of the diets of sympatric pelagic sharks in gulf and shelf ecosystems off southern Australia. ICES Journal of Marine Science 69, 1382–1393.
| A quantitative comparison of the diets of sympatric pelagic sharks in gulf and shelf ecosystems off southern Australia.Crossref | GoogleScholarGoogle Scholar |
Salini, J, Blaber, S, and Brewer, D (1992). Diets of sharks from estuaries and adjacent waters of the north-eastern Gulf of Carpentaria, Australia. Marine and Freshwater Research 43, 87–96.
| Diets of sharks from estuaries and adjacent waters of the north-eastern Gulf of Carpentaria, Australia.Crossref | GoogleScholarGoogle Scholar |
Shiffman, DS, Gallagher, AJ, Boyle, MD, Hammerschlag-Peyer, CM, and Hammerschlag, N (2012). Stable isotope analysis as a tool for elasmobranch conservation research: a primer for non-specialists. Marine and Freshwater Research 63, 635–643.
| Stable isotope analysis as a tool for elasmobranch conservation research: a primer for non-specialists.Crossref | GoogleScholarGoogle Scholar |
Simpfendorfer, CA, Goodreid, A, and McAuley, RB (2001). Diet of three commercially important shark species from Western Australian waters. Marine and Freshwater Research 52, 975–985.
| Diet of three commercially important shark species from Western Australian waters.Crossref | GoogleScholarGoogle Scholar |
Sommerville, E, Platell, ME, White, WT, Jones, AA, and Potter, IC (2011). Partitioning of food resources by four abundant, co-occurring elasmobranch species: relationships between diet and both body size and season. Marine and Freshwater Research 62, 54–65.
| Partitioning of food resources by four abundant, co-occurring elasmobranch species: relationships between diet and both body size and season.Crossref | GoogleScholarGoogle Scholar |
Stevens, JD, and McLoughlin, KJ (1991). Distribution, size and sex composition, reproductive biology and diet of sharks from northern Australia. Australian Journal of Marine and Freshwater Research 42, 151–199.
| Distribution, size and sex composition, reproductive biology and diet of sharks from northern Australia.Crossref | GoogleScholarGoogle Scholar |
Stobutzki, IC, Miller, MJ, Heales, DS, and Brewer, DT (2002). Sustainability of elasmobranchs caught as bycatch in a tropical prawn (shrimp) trawl fishery. Fishery Bulletin 100, 800–821.
Wetherbee BM, Cortés E, Bizzarro JJ (2012) Food consumption and feeding habits. In ‘Biology of Sharks and their Relatives’. pp. 239–263. (CRC Press)
White, WT, Platell, ME, and Potter, IC (2004). Comparisons between the diets of four abundant species of elasmobranchs in a subtropical embayment: implications for resource partitioning. Marine Biology 144, 439–448.
| Comparisons between the diets of four abundant species of elasmobranchs in a subtropical embayment: implications for resource partitioning.Crossref | GoogleScholarGoogle Scholar |
White WT, Baje L, Sabub B, Appleyard SA, Pogonoski JJ, Mana RR (2017) ‘Sharks and rays of Papua New Guinea.’ (Australian Centre for International Agricultural Research: Canberra, ACT, Australia)
White, WT, Baje, L, Simpfendorfer, CA, Appleyard, SA, Chin, A, Sabub, B, Rochel, E, and Naylor, GJP (2019). Elasmobranch bycatch in the demersal prawn trawl fishery in the Gulf of Papua, Papua New Guinea. Scientific Reports 9, 9254.
| Elasmobranch bycatch in the demersal prawn trawl fishery in the Gulf of Papua, Papua New Guinea.Crossref | GoogleScholarGoogle Scholar | 31239504PubMed |
Wiley, TR, and Simpfendorfer, CA (2007). The ecology of elasmobranchs occurring in the Everglades National Park, Florida: implications for conservation and management. Bulletin of Marine Science 80, 171–189.