Oceanographic influence on coastal zooplankton assemblages at three IMOS National Reference Stations in Western Australia
Erin McCosker A C , Claire H. Davies B and Lynnath E. Beckley
A C , Claire H. Davies B and Lynnath E. Beckley  A
A
A Environmental & Conservation Sciences, Murdoch University, 90 South Street, Murdoch, WA 6150, Australia.
B CSIRO Oceans and Atmosphere, Castray Esplanade, Hobart, Tas. 7000, Australia.
C Corresponding author. Present address: Centre for Marine Science and Innovation, Evolution & Ecology Research Centre, School of Biological, Earth and Environmental Sciences, University of New South Wales, Sydney, NSW 2052, Australia. Email: erin.mccosker@student.unsw.edu.au
Marine and Freshwater Research 71(12) 1672-1685 https://doi.org/10.1071/MF19397
Submitted: 4 February 2020 Accepted: 30 June 2020 Published: 7 August 2020
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Knowledge about the coastal zooplankton of the south-eastern Indian Ocean is limited, with few studies having compared assemblages across the latitudinal range of the western seaboard of Australia. The dominant oceanographic feature in this region is the Leeuwin Current, which transports warm, lower-salinity, tropical waters southward along the shelf-edge. This study examined data collected by Australia’s Integrated Marine Observing System at three coastal National Reference Stations located at 22°S 114°E, 32°S 115°E and 34°S 122°E. Spatial and temporal patterns in zooplankton abundance, composition and diversity were investigated, and differences in assemblage structure, particularly with respect to copepods, were related to oceanographic conditions. Clear dissimilarities among copepod assemblages were observed, becoming weaker in winter owing to enhanced connectivity of species driven by alongshore and cross-shelf transport in the Leeuwin Current. Both physical and biogeochemical factors were significant in structuring copepod assemblages, with seawater density, incorporating temperature and salinity, exerting the greatest influence. The results suggest that both broad-scale latitudinal gradients and mesoscale events contribute to variation in zooplankton assemblages in these waters. This study provides the first detailed comparison of zooplankton assemblages among the north-west, south-west and southern coastal waters of Western Australia, and enhances understanding of the processes influencing zooplankton distribution and structure.
Additional keywords: mesozooplankton, oceanography.
Introduction
Zooplankton are generally short-lived, widely distributed and make very good indicators of changing ocean conditions (Hays et al. 2005; Richardson 2008). Changes in zooplankton distribution and population sizes can be driven by physical processes or biological interactions, with the rate of change being rapid enough to track seasonal and interannual variation in environmental conditions (Mackas et al. 2012). Within the zooplankton, copepods are the dominant and most diverse component and are, therefore, particularly useful to examine how changes in zooplankton assemblage structure reflect the underlying oceanography (Longhurst 1985; Dias et al. 2015).
Various zooplankton studies have demonstrated that plankton communities respond to the physical and chemical environment, as well as large- and small-scale ocean processes (e.g. Verheye and Richardson 1998; Mackas et al. 2001; Muhling and Beckley 2007; Richardson 2008). Specific zooplankton patterns of distribution and productivity have been related to ocean currents (Beaugrand et al. 2002a) and, particularly, boundary currents, such as the Benguela (Verheye et al. 2016), Humboldt (Ayón et al. 2008) and East Australian Currents (Johnson et al. 2011; Kelly et al. 2016). Further studies have identified relationships between zooplankton and climate systems such as the El Niño Southern Oscillation (ENSO; Fromentin and Planque 1996; McKinnon et al. 2008), while recently, studies are increasingly providing evidence of climate change-mediated shifts in zooplankton geography and structure (Beaugrand et al. 2002b; Chiba et al. 2006; Steinberg et al. 2012; Brun et al. 2019).
Many of the valuable findings of zooplankton studies are a result of long-term ocean monitoring programs. Early significant interdisciplinary ocean surveys, such as the Discovery Investigations in the Southern Ocean (1950s; Hardy 1967) and the International Indian Ocean Expedition (1960s), were critical to establishing a baseline knowledge of zooplankton distribution patterns (Tranter and Kerr 1969; Tranter and Kerr 1977). The acknowledged importance and support for continued ocean monitoring is demonstrated by >30 countries holding multi-decadal zooplankton time-series (Mackas et al. 2012). On a global scale, continuous plankton recorder (CPR) surveys operate across all oceans and have been used to highlight variation in ocean conditions and differing biological responses to ocean change (Richardson and Schoeman 2004; Richardson et al. 2015). Numerous sustained zooplankton observation programs in the northern hemisphere, such as the California Cooperative Oceanic Fisheries Investigations (CalCOFI), the Hawaii Ocean Time-series and the Bermuda Atlantic Time-series Study, have been significant in detecting region-specific seasonal physical processes, ocean warming, changing biogeochemical dynamics, and in tracing ocean–climate perturbations (Mackas et al. 2006; Jiang et al. 2007; Valencia et al. 2016).
In comparison, significant long-term zooplankton time-series are rare for the southern hemisphere. In Australia, the Integrated Marine Observing System (IMOS) began systematic monitoring of the physical and biogeochemical properties of coastal waters via a network of National Reference Stations (NRS) in 2006, with the aim of understanding changes in Australian waters (Lynch et al. 2014; Lara-Lopez et al. 2016). The IMOS zooplankton sampling program commenced in 2008 and continues to operate on a continental scale. The recent establishment of a comprehensive zooplankton monitoring program means that little is still known about the distribution, abundance, diversity, and long-term trends in zooplankton in these waters.
In Australia, with a mainland coastline that spans >35 000 km, the geographical isolation of study locations and logistical constraints have created challenges for sustained ocean monitoring (Lynch et al. 2014; Eriksen et al. 2019). This is particularly true of the western coast, with its unique and complex oceanography. The dominant oceanographic feature off Western Australia is the poleward flowing Leeuwin Current (LC), which originates near the North West Shelf (21°S) and transports warm, lower salinity, oligotrophic, tropical water southward along the shelf break and around Cape Leeuwin at 35°S, before turning eastward to flow towards Tasmania, a distance of almost 5500 km (Cresswell and Golding 1980; Ridgway and Condie 2004; Cresswell and Domingues 2009). The temperature–salinity profile of the LC is modified along its southward trajectory. As it flows south, the LC undergoes a gradual decrease in temperature and an increase in salinity, and, consequently, surface waters flowing in the LC are of greater density in the south than in the north (Waite et al. 2007; Weller et al. 2011). Seasonal and inter-annual variation in LC intensity occurs; the LC flow is stronger in the austral autumn–winter (Godfrey and Ridgway 1985; Cresswell 1991) and during La Niña years (Huang and Feng 2015). Further complexity is added to the environment by the opposing, wind-driven, cooler, Ningaloo (22°S–24°S) and Capes Currents (32°S–35°S; Gersbach et al. 1999; Woo et al. 2006), and by localised physical processes, such as coastal upwelling, eddies and meanders (Pearce 1991; Waite et al. 2007).
Although numerous studies have examined the physical and chemical properties of the LC system, few have elucidated its influence on zooplankton. Studies of zooplankton assemblages in Western Australian waters include those in the Kimberley region (Holliday et al. 2011; McKinnon et al. 2015), the North West Shelf (McKinnon et al. 2003a, 2008; Wilson et al. 2003), Shark Bay (Kimmerer et al. 1985), south-western eddy system (Muhling et al. 2008; Säwström et al. 2014) and southern shelf (Gaughan and Fletcher 1997). Contributing to our understanding of the region’s zoogeography are studies that have examined the influence of the LC on the distribution and composition of macrozooplankton and larval fishes (Muhling and Beckley 2007; Muhling et al. 2008; Beckley et al. 2009; Gaughan et al. 2009; Holliday et al. 2012). More recently, studies of chaetognaths and euphausiids in the LC have shown relationships between assemblage structures and latitudinal changes in LC water properties (Buchanan and Beckley 2016; Sutton and Beckley 2016). However, the mechanisms of the LC that structure mesozooplankton assemblages in Western Australian coastal waters remain largely unresolved.
This study is the first to present a comprehensive account of spatial and temporal variation in zooplankton assemblages at three IMOS NRS located in coastal Western Australian waters, at Ningaloo (22°S), Rottnest (32°S) and Esperance (34°S), and to relate the variation to oceanographic conditions. The time period of focus for the study was the 2-year period during which all three NRS were simultaneously functional (2011–2012). The specific research questions addressed by the study were as follows: (1) what are the oceanographic conditions at the three NRS, (2) what are the zooplankton assemblages at the three NRS, (3) do the copepod assemblages at the three NRS differ and (4) which oceanographic factors explain the variability in copepod assemblage structure among the three NRS?
Materials and methods
Study locations
The Ningaloo (NIN), Rottnest (ROT) and Esperance (ESP) NRS are located in Western Australian coastal waters and are adjacent to the path of the Leeuwin Current. The characteristics of the three NRS are summarised in Table 1.

|
Data sources and sampling methods
Data used in this study were sourced from the IMOS Australian Ocean Data Network Portal (AODN; https://portal.aodn.org.au/, accessed 3 October 2016). Data were derived from a combination of in situ moored sensors and vessel-based sampling. Statistical analyses were performed using data from 2011–2012, the period during which the three Western Australian NRS were simultaneously operational. This time period provided seasonal samples from the three NRS, but resulted in uneven sample numbers because of differences in sampling frequency (Ningaloo and Esperance, n = 8; Rottnest, n = 22; Table 1). Seasons were considered by grouping data from December to February (summer), March to May (autumn), June to August (winter) and September to November (spring).
Physical and chemical data
National Reference Station mooring-based in situ water quality monitors (WQM) (WETLabs) deployed in shallow (18–25 m) and deep (48–55 m) water collected continuous measurements of temperature (°C), salinity (psu) and oxygen (µmol kg–1; Seabird Model SBE 39). Data were collected at 1-s intervals across 1 min of continual sampling every 15 min and logged internally. WQM data harvesting was concurrent with mooring servicing, which occurred three times per year at Esperance and Rottnest, and twice annually at Ningaloo (Lynch et al. 2014).
Vessel-based water column profile sampling at the NRS involved conducting vertical conductivity, temperature and depth (CTD) casts using a Seabird Model SBE 19+ instrument. Casts were taken within 1 h of zooplankton sampling. Temperature (°C), salinity (psu), density (kg m–3) and dissolved oxygen concentration (µmol kg–1) were measured at 1-s intervals through the water column to near the seabed (~50 m), and the resulting data were binned to 1-m measurements (Davies and Sommerville 2017).
Water samples were collected in 5-L Niskin bottles at 10-m depth intervals from the surface to 50 m. Triplicate samples were collected into 10-mL tubes for nitrate, phosphate and silicate (µmol L–1) analysis via flow injection analysis on a LachatTM system. A composite water column sample consisting of equal parts of water from each 10-m depth sample was prepared on board, and from this mixed water sample, 4 L was used for phytoplankton pigment analysis using an established high performance liquid chromatography procedure (Davies and Sommerville 2017), and the determined chlorophyll a concentration (mg m–3) was recorded.
Monthly mean Fremantle sea level and Southern Oscillation Index (SOI) data obtained from the Bureau of Meteorology (http://www.bom.gov.au/ntc/IDO70000/IDO70000_62230_SLD.shtml, accessed 24 September 2016) were examined to evaluate variation in the velocity and intensity of the Leeuwin Current.
Zooplankton data
Vessel-based sampling of mesozooplankton (0.2–20 mm) at the NRS was conducted using a weighted 100 µm mesh drop net (Heron 1982). The drop net collected a water column sample from the surface to near the seabed (~50 m). The zooplankton sample was preserved in 10% formalin before transfer to a CSIRO laboratory for composition analysis by members of the IMOS Plankton Ecology group. Composition analysis was performed using a Leica M165C microscope with ×120 magnification and data were recorded as number of individuals per cubic metre of water. Identification of zooplankton was guided by an assembled taxonomic library that is verified by the World Register of Marine Species (WoRMS; www.marinespecies.org), and performed to the lowest taxonomic level possible. Generally, adult copepods were identified to species level and juveniles to genus level, and thus, data for this zooplankton group formed the core biological data source for the analyses.
Data analyses
Water column profiles for temperature–salinity (TS) and nutrients (nitrate, phosphate and silicate) were examined using the vessel-based sampling data. High temporal resolution temperature and salinity data measured by the WQMs were used for finer detail time-series analysis of these water properties.
Copepod assemblage structure was examined using the software package PRIMER v6 PERMANOVA+ (Clarke and Gorley 2006; Anderson et al. 2008). Abundances were square-root transformed to reduce the weight of contribution by dominant taxa and a Bray–Curtis resemblance matrix was constructed. Analyses were performed on a reduced dataset of copepods identified to at least genus level. Cluster analysis was applied to identify similarities in copepod assemblages in terms of abundance and composition among NRS. The results of the cluster analysis were verified using a fixed, three-factor permutational multivariate analysis of variance (PERMANOVA; Anderson 2001), which was used to test for significant differences in copepod assemblages among the a priori factors of NRS, year and season, and any interaction among these factors. The similarity percentage (SIMPER) procedure was used to identify the copepod taxa most responsible for assemblage dissimilarities.
Distance-based linear modelling (DistLM; Anderson 2001) was used to examine the relationship between water column properties (predictor variables) and copepod assemblages (response matrix) and determine the set of predictor variables that best explained variation in copepod assemblage structure. Prior to the analysis, examination of draftsman plots showed that temperature and salinity were covariates, and seawater density was included in the DistLM as a combination of these variables. Dissolved oxygen and silicate were subsequently removed from the analysis because of their strong positive and negative correlations (r = 0.92, r = –0.82) respectively, with seawater density. The remaining data were standardised (by square-root transformation) to reduce skewness and normalised. A stepwise regression selection procedure with the adjusted R2 selection criterion was used in the DistLM. Results were presented in a distance-based redundancy (dbRDA) bi-plot (Legendre and Anderson 1999).
Results
Physical oceanography
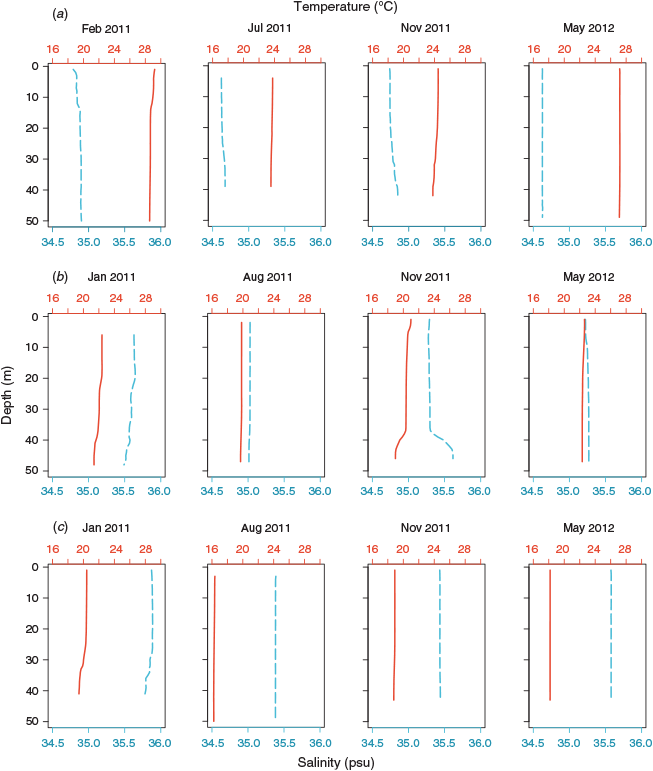
TS profiles of the water columns showed each NRS site to have distinct water column properties. Increasing latitude corresponded to a decrease in temperature (~8°C), and an increase in salinity (~0.7 psu) and, hence, seawater density (~3 kg m–3) across the 12° latitudinal range (Fig. 1, Table 2). Water columns at the three NRS were well mixed, indicating the presence of a single water mass at each site. The TS profiles and WQM time-series data indicated the presence of warm (22–29°C), lower salinity (34.5–35.0 psu) surface waters at Ningaloo that were characteristic of Tropical Surface Water (TSW; >22°C, <35 psu, <1024 kg m–3; Woo and Pattiaratchi 2008; Fig. 2, Table 2). The TSW was modified as it was transported south by the LC, with waters at Rottnest being, on average, cooler (21.1°C), more saline (35.3 psu), and, hence, denser (1025 kg m–3). Further south at Esperance, cool (16–21°C), higher salinity (>35 psu), dense (>1025 kg m–3) waters indicated that the dominant water mass was Sub Tropical Surface Water (STSW; <21°C, >35.4 psu, >1025 kg m–3; Pearce et al. 2006; Fig. 2, Table 2). Despite its southern location, waters at Esperance remained warm in autumn, maintaining a mean temperature of 20.2°C, as LC water penetrated around Cape Leeuwin and along the southern coast (Fig. 3).

|

|
|

|
All three NRS presented well defined seasonal patterns of water temperature and salinity, with maxima in summer–early autumn and minima in late winter–early spring (Fig. 3). Elevated water temperatures at the NRS in 2011 relative to 2012 reflected the strong La Niña event that affected the Western Australian coast in the austral summer of 2010–2011 (Feng et al. 2013). The La Niña event caused rapid warming of ocean temperatures that exceeded long-term monthly means and peaked in February–March along much of the coastline (Pearce and Feng 2013). Accordingly, waters were, on average, >1°C warmer at Ningaloo in summer 2011, and >0.7°C warmer at Rottnest and Esperance in autumn 2011, than in 2012.
Chemical oceanography
Generally, NRS waters were nutrient poor, and became more depleted as the latitude increased. Overall, silicate had concentrations exceeding 4 µmol L–1 at Ningaloo, but declined to a mean of 1.96 µmol L–1 at Rottnest and 0.3 µmol L–1 at Esperance (Table 2). Nitrate concentrations rarely exceeded 0.5 µmol L–1 at the three NRS, the exception being Ningaloo waters in spring 2012, which recorded a concentration of 1.2 µmol L–1. Phosphate concentrations were similar across the three NRS and ranged from 0.01 to 0.39 µmol L–1.
Chlorophyll a concentrations were generally <0.4 mg m–3 and increased from north to south along the coast (Table 2). Higher chlorophyll a concentrations were associated with autumn–winter at Rottnest and Esperance, with an increase of ~0.2–0.4 mg m–3 from summer values (Fig. 4). Ningaloo did not present a consistent seasonal signal for chlorophyll a.

|
Zooplankton diversity, abundance and distribution
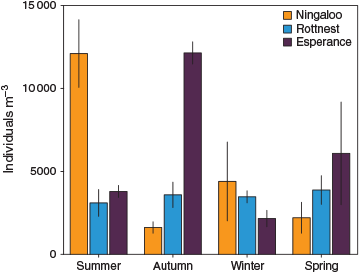
The total abundance of zooplankton organisms caught was highly variable across the NRS (893 individuals (ind) m–3 to 14 131 ind m–3) and there was no discernible effect of latitude. Variability was mainly attributed to sporadically high densities of copepodites, which reached >5100 ind m–3 and contributed >50% of abundance in some samples. On average, Esperance recorded the highest abundance (mean ± s.e., 6043 ± 1552 ind m–3, n = 8), which was slightly higher than that for Ningaloo (5084 ± 1696 SE ind m–3, n = 8), and Rottnest had the lowest abundance (3512 ± 355 ind m–3, n = 22). A strong seasonal cycle in abundance was observed at Ningaloo and Esperance, with maxima in summer and autumn respectively, whereas this was not obvious at Rottnest (Fig. 5).
|
In total, 226 zooplankton taxa representing 12 phyla and 76 genera were identified at the three NRS (see Supplementary material Table S1 available at the journal’s website). Copepods were the most numerous group in the zooplankton assemblages and comprised 66–77% of the abundance and 149 of the identified taxa. The relative proportions of other zooplankton groups to assemblages varied. From north to south, contributions of Appendicularia (family Oikopleuridae) and Chaetognatha (family Sagittidae) to the overall zooplankton assemblages declined, whereas the proportion of Mollusca increased. Assemblage compositions varied throughout the year, with seasonal blooms of larval Bivalvia and Gastropoda at all three NRS, in addition to blooms of Oikopleuridae (larvaceans) and Cladocera (mostly Penilia avirostris) at Ningaloo and Esperance respectively. The zooplankton also comprised lesser numbers of meroplanktonic Polychaeta, Bryozoa, Decapoda and Echinodermata.
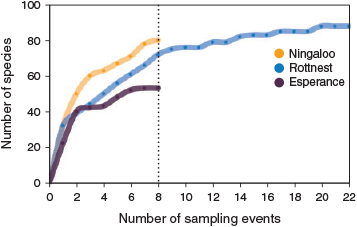
Copepods comprised 116 identified species, of which 34 were distributed across all three NRS. On average, a greater number of copepod species was identified per sampling event at Ningaloo (27), than at Rottnest (23) and Esperance (22), indicating a decline in species richness with latitude. This was supported by examination of species accumulation curves constructed by combining data on copepod species occurrences obtained during the 2011–2012 sampling period. The species accumulation curves demonstrated a levelling off of species richness at Rottnest and Esperance, which contrasted with a continued upward trajectory of an increasing species richness at Ningaloo (Fig. 6). This indicated underestimation of copepod species richness at Ningaloo, which had not become saturated at the completion of the 2-year sampling period at this NRS. Species richness varied throughout the year, being greatest in autumn at Ningaloo and Esperance, and in winter at Rottnest (data not shown).
|
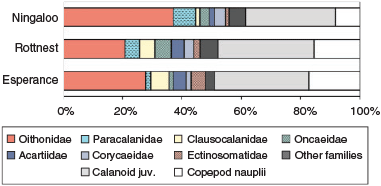
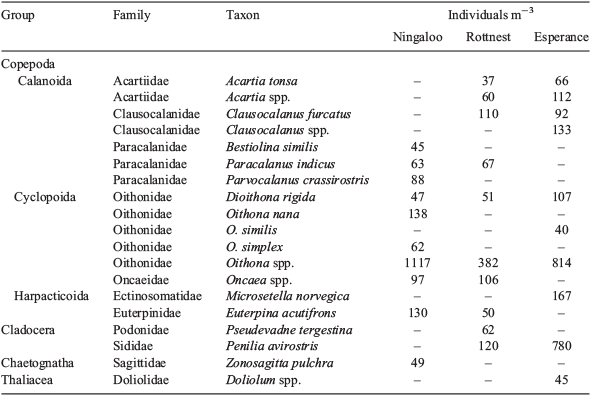
Copepod assemblages were dominated by Calanoida (48–63%), most of which were copepodites (>70%), with Cyclopoida comprising 31–47%, and Harpacticoida 5–6%. Among the calanoid copepods, the family Paracalanidae was dominant at Ningaloo (16%), where high abundances, generally >60 ind m–3, of species that typically dominate tropical copepod assemblages, such as Paracalanus indicus and Parvocalanus crassirostris (McKinnon et al. 2003b; McKinnon and Duggan 2014), were recorded (Fig. 7, Table 3). At Rottnest and Esperance, two calanoid families associated with cooler water, namely, Clausocalanidae (9–10%) and Acartidae (7%; Frost and Fleminger 1968; Brylinski 1981), were important; the mean abundance of taxa of these families exceeded 100 ind m–3. The majority of the cyclopoid copepods were of the family Oithonidae and formed a key component of assemblages (22–38%). In particular, Oithona spp., O. nana, Dioithona rigida and O. similis were ubiquitous and numerous, and reached mean abundances of >1100 ind m–3.
|
|
Copepod assemblage structure
Cluster analysis of copepod assemblages showed distinct groupings that were closely related to NRS (Fig. 8). Esperance assemblages all were grouped together and, in the SIMPER analysis, were shown to be similar (61%; Table 4). Rottnest assemblages were relatively well clustered together and were found to have an average similarity of 54%. Ningaloo assemblages were less closely related (44%). The observed clustering pattern was supported by PERMANOVA, which showed that there were significant dissimilarities in copepod assemblage structures in relation to NRS (F = 4.44, P = 0.001) and that NRS explained 18% of the variability in assemblage structure. SIMPER analysis identified Oithona spp. as the copepod taxon that most strongly typified the copepod assemblage at each NRS (Table 4).

|

|
The degree of dissimilarity among copepod assemblages corresponded to their spatial distribution, being greatest between Ningaloo and Esperance (58%) and least between Rottnest and Esperance (49%; Table 4). Dissimilarities between the assemblages at Ningaloo and Esperance were mostly due to the greater dominance of the tropical species Oithona nana (McKinnon and Duggan 2014), and comparatively low abundances of Clausocalanus spp. and C. furcatus at Ningaloo, relative to Esperance. Clausocalanus furcatus also distinguished Ningaloo and Rottnest assemblages, being 10 times more abundant, on average, at Rottnest. The tropical affinity of Euterpina acutifrons and Paracalanus crassirostris was apparent, with these two species being rarely recorded at the two southern NRS, but occurring at abundances of >80 ind m–3, on average, at Ningaloo, and being important discriminators for this site. Rottnest and Esperance were differentiated by an order of magnitude greater dominance of Clausocalanus spp. at Esperance, together with exclusivity of some particular taxa, namely, Oncaea media at Rottnest, and Dioithona oculata at Esperance (Table 4).
Dissimilarities among NRS assemblages varied temporally, being more pronounced in summer than in winter. A corresponding increase in the proportion of copepod taxa distributed across all three NRS occurred in winter (27%), relative to summer (20%). PERMANOVA indicated that there were significant differences in copepod assemblage structure among seasons (F = 1.54, P = 0.04), and an interaction between the two factors of NRS and season (F = 1.67, P = 0.008). The NRS–season interaction effect explained 15.8% of the variability in copepod assemblages, while season alone explained a further 7% of the variability.
Influence of oceanography on copepod assemblages
The distance-based linear model (DistLM) for copepod assemblages selected mean seawater density, depth-integrated chlorophyll a, and depth-integrated nitrate for inclusion in the best model explaining the variance in assemblage structures (adjusted R2 = 0.17). The model explained 17% of the overall variation, with the two significant explanatory variables, namely, seawater density and chlorophyll a, accounting for 9.7% (F = 3.5, P = 0.0001) and 4.8% (F = 1.7, P = 0.0346) respectively. There was no significant correlation of nitrate concentration with copepod assemblage structure (F = 1.5, P = 0.0817). For the fitted model, the dbRDA bi-plot showed the first and second axes to significantly explain 87% of the variation in copepod assemblage structure (Fig. 9).

|
Discussion
The three NRS sites examined in this study had clearly distinct water mass properties and oceanographic characteristics across the 12° latitudinal range. Ningaloo was generally characterised by TSW, which was modified as it was transported south in the LC; at Rottnest, the modified TSW was cooler and more saline, owing to the inflow of more dense offshore water, air–sea heat flux and evaporation (Weller et al. 2011). Despite its location in the temperate zone, waters at Esperance remained warm (>18°C) throughout much of the year, with a stronger STSW signature of cooler, more dense water evident in winter because of wind-forcing and the subsequent heat loss that occurs along this coast (Middleton and Bye 2007). Waters were generally depleted of nutrients and, with typically low chlorophyll a concentrations (<1 mg m–3), were representative of the oligotrophic nature of Western Australian coastal waters, which is consequence of the downwelling-favourable LC suppressing nutrient enrichment (Hanson et al. 2005; Thompson et al. 2011). Chlorophyll a enriched waters in autumn–winter at Rottnest and Esperance were indicative of the annual shelf-wide phytoplankton bloom known to occur at that time (Koslow et al. 2008; Thompson et al. 2009; Lourey et al. 2013).
Temporal oceanographic change occurred at the sites as they were exposed to physical forcing and the strong La Niña event that occurred in early 2011. At Ningaloo, the sporadic presence of denser, nitrate-enriched water provided an indication of spring upwelling (Rossi et al. 2013). Similar conditions at Rottnest were likely to be an indication of localised upwelling that was driven by the Capes Current (Gersbach et al. 1999; Lourey et al. 2013). Associated with the La Niña event were exceptionally high positive SOI values and high sea levels at Fremantle (Bureau of Meteorology 2012), indicative of an intensified LC. Driven by the strong La Niña conditions, the surging LC frequently flooded the coastal shelf (Feng et al. 2013) and was sufficient to suppress the northward-flowing, summer coastal Ningaloo and Capes Currents. Sustained elevated water temperatures along the Western Australian coast associated with the La Niña (Pearce and Feng 2013; Benthuysen et al. 2014) were demonstrated at all three sites, but were particularly marked at Esperance, where unseasonably warm water (>20°C) persisted throughout summer and autumn in 2011.
Zooplankton abundance at the NRS was highly variable and largely driven by fluctuations in numbers of copepodites and nauplii, with the substantial abundances of these small organisms captured in the zooplankton samples being a result of sampling using a 100-µm net. However, domination of the zooplankton by small copepods is typical of marine systems (Longhurst 1985). NRS copepod assemblages were clearly distinct, and the strength of dissimilarity corresponded to the degree of spatial separation and variation in oceanographic conditions among sites. The composition of the Ningaloo assemblage resembled that of previous studies of Western Australian tropical, coastal waters, particularly its dominance by the copepod families Paracalanidae and Oithonidae, that conferred an Indo-Pacific influence (Kimmerer et al. 1985; McKinnon et al. 2008; McKinnon et al. 2015). The location of the Rottnest NRS in a marine overlap zone (Commonwealth of Australia 2006) was demonstrated by its copepod assemblage, which resembled both the Ningaloo and Esperance assemblages. This included proportions of Corycaeidae and Oncaeaidae copepods similar to those at Ningaloo, and proportions of Acartidae and Clausocalanidae copepods similar to those at Esperance. Greater dominance of taxa from the latter two families in the Esperance assemblage distinguished this site and reflected its warm-temperate bioregion.
Observations at the three NRS showed that there were no significant differences in overall compositions of copepod assemblages between years, despite a shift from La Niña to more stable conditions in 2012. However, substantially greater densities of some copepod species common in tropical waters such as Dioithona oculata, D. rigida and Microsetella norvegica (McKinnon and Duggan 2014) in mid- and higher-latitude coastal waters in 2011 than in 2012, may reflect the increased water temperatures associated with La Niña.
A decline in copepod species richness with an increasing latitude along the Western Australian coast was found, similar to the pattern observed for marine crustaceans and fishes along both the western and eastern coasts of Australia (O’Hara and Poore 2000; Fox and Beckley 2005). Species accumulation curves suggested an incomplete inventory of the copepod species pool at Ningaloo. This is supported, for example, by a study of copepods of the inshore waters at the North West Cape by McKinnon et al. (2008), which used a 73-µm plankton net and found >120 species to occur, compared with the 80 species identified here. These findings are relevant for the sampling designs of future zooplankton studies in these waters, which could, through extended sampling, yield a more accurate measure of zooplankton species diversity at Ningaloo.
Plankton assemblages have been linked with the physical and biogeochemical properties of water masses (Hopcroft et al. 2010; Holliday et al. 2011; Beckley et al. 2019). Discrete water masses on the basis of the water column properties were identified at the NRS sites. Examination of the relationship between oceanography and copepod assemblages using distance-based linear modelling showed that seawater density, indicative of the underlying water mass, was the most significant environmental variable shaping copepod assemblage structure, although it explained only a small proportion of the underlying variation in the assemblage structure. Seawater density has similarly been found to be important in structuring plankton assemblages in other studies of LC plankton, including larval fishes (Muhling et al. 2008), euphausiids (Sutton and Beckley 2016) and chaetognaths (Buchanan and Beckley 2016).
The latitudinal modification in water mass from lower density TSW to denser STSW corresponded to a change in assemblage structure, from primarily tropical copepod taxa at Ningaloo, to a mix of tropical–subtropical–temperate taxa in the transition zone at Rottnest, to mainly temperate-associated taxa at Esperance. At Ningaloo, the dominant TSW provides conditions favourable for Indo-Pacific copepods, whereas, in contrast, at Esperance, lower tropical species richness and abundance suggest exceedance of species temperature tolerance limits. The varied assemblage at Rottnest is likely to be influenced by both LC intrusion (Weller et al. 2011) and an opposing inshore Capes Current from the south, resulting in an intersection of entrained pelagic species. A similar overlap of plankton of varied bioregional affinity occurs as a result of the convergence of waters in the East Australian Current–Tasman Sea transition zone on the eastern coast of Australia (Keane and Neira 2008).
Chlorophyll a concentrations at the sites also explained some of the variation in copepod assemblage structure, although its influence was less significant. This is not surprising because any response of copepod assemblages to chlorophyll a concentration would be indirect and subject to lag as the effect worked along the food chain (Legendre 1990). Greater abundances of predominantly herbivorous copepods in assemblages, such as Clausocalanus spp, Temora spp., Temora turbinata and Paracalanus indicus (Kouwenberg 1994), appeared to be associated with an increased chlorophyll a concentration. Previous studies have found that opportunistic herbivorous copepods can achieve rapid growth and production rates in response to a higher chlorophyll a concentration (Peterson and Hutchings 1995; Rosa et al. 2016), and this may also be the case here.
Besides the underlying water masses, the LC may also influence the distribution of zooplankton along the Western Australian coast, particularly in autumn–winter when it is at maximum strength and along-shore transport and onshore intrusion of warm water increases, resulting in entrainment of species that are dispersed poleward or across the shelf (Hutchins and Pearce 1994; Gaughan et al. 2009; Holliday et al. 2012). This conveyance of plankton in the LC plays a role in enhancing the species richness, in particular, the tropical component, of assemblages in temperate Western Australian waters (Maxwell and Cresswell 1981; Gaughan and Fletcher 1997). This study found that, in winter, dissimilarities among the NRS assemblages weakened, the number of copepod species in common across sites increased, and species richness was enhanced, including a stronger presence of offshore species on the shelf and tropical species in the south. This suggests that the seasonal oceanography of the LC enhances connectivity among copepod assemblages across a broad latitudinal range, increasing diversity in temperate waters that are otherwise known for their high level of endemism (Fox and Beckley 2005). Reduced dissimilarities among plankton assemblages in south-eastern Australian waters have, similarly, been attributed to poleward transport of species in a strengthened East Australian Current (Keane and Neira 2008; Kelly et al. 2016).
Populations of copepods with tropical and subtropical affinities also occurred throughout the year at Rottnest and Esperance. It is unclear whether these copepods are representative of a broad trend of range extensions and shifts towards dominance by small-bodied and warm-water copepods in temperate waters, as is evident in Tasmania (Kelly et al. 2016), the Southern Benguela (Huggett et al. 2009), and the large ocean basins (Beaugrand et al. 2002b). Alternatively, the persistence of warm-water copepod species at higher latitudes on the Western Australian coast may be further confirmation of the influence of the LC, which injects warm, subtropical, lower salinity water to the coast. Sustained monitoring is required to investigate this pattern.
This study is the first to relate differences in oceanographic conditions to variation in zooplankton abundance, diversity and distribution among north-western, south-western and southern coastal waters of Western Australia. This study has highlighted the role of the LC in driving variability in zooplankton assemblages at both spatial and temporal scales. Although seawater density and chlorophyll a were identified as significant in explaining copepod assemblage structure, a large amount of variation remained unexplained. Thus, there remains large scope for future research of the zooplankton of this vastly understudied system, using the significant amount of environmental and biological data that IMOS will continue to provide.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This research did not receive any specific funding.
Acknowledgements
The data used in this study were sourced from the Integrated Marine Observing System (IMOS). Australia’s IMOS is enabled by the National Collaborative Research Infrastructure Strategy (NCRIS). It is operated by a consortium of institutions as an unincorporated joint venture, with the University of Tasmania as Lead Agent.
References
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecology 26, 32–46.Anderson, M. J., Gorley, R. N., and Clarke, K. R. (2008). ‘PERMANOVA for PRIMER: Guide to Software and Statistical Methods.’ (PRIMER-E: Plymouth, UK.)
Ayón, P., Criales-Hernandez, M. I., Schwamborn, R., and Hirche, H.-J. (2008). Zooplankton research off Peru: a review. Progress in Oceanography 79, 238–255.
| Zooplankton research off Peru: a review.Crossref | GoogleScholarGoogle Scholar |
Beaugrand, G., Ibanez, F., Lindley, J. A., and Reid, P. C. (2002a). Diversity of calanoid copepods in the North Atlantic and adjacent seas: species associations and biogeography. Marine Ecology Progress Series 232, 179–195.
| Diversity of calanoid copepods in the North Atlantic and adjacent seas: species associations and biogeography.Crossref | GoogleScholarGoogle Scholar |
Beaugrand, G., Reid, P. C., and Ibanez, F. (2002b). Major reorganisation of North Atlantic pelagic ecosystems linked to climate change. GLOBEC International Newsletter 8, 30–33.
Beckley, L. E., Muhling, B. A., and Gaughan, D. J. (2009). Larval fishes off Western Australia: influence of the Leeuwin Current. Journal of the Royal Society of Western Australia 92, 101–109.
Beckley, L. E., Holliday, D., Sutton, A. L., Weller, E., Olivar, M. P., and Thompson, P. A. (2019). Structuring of larval fish assemblages along a coastal-oceanic gradient in the macro-tidal, tropical eastern Indian Ocean. Deep-sea Research Part II: Topical Studies in Oceanography 161, 105–119.
| Structuring of larval fish assemblages along a coastal-oceanic gradient in the macro-tidal, tropical eastern Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Benthuysen, J., Feng, M., and Zhong, L. (2014). Spatial patterns of warming off Western Australia during the 2011 Ningaloo Niño: quantifying impacts of remote and local forcing. Continental Shelf Research 91, 232–246.
| Spatial patterns of warming off Western Australia during the 2011 Ningaloo Niño: quantifying impacts of remote and local forcing.Crossref | GoogleScholarGoogle Scholar |
Brun, P., Stamieszkin, K., Visser, A. W., Licandro, P., Payne, M. R., and Kiørboe, T. (2019). Climate change has altered zooplankton-fuelled carbon export in the North Atlantic. Nature Ecology & Evolution 3, 416–423.
| Climate change has altered zooplankton-fuelled carbon export in the North Atlantic.Crossref | GoogleScholarGoogle Scholar |
Brylinski, J. (1981). Report on the presence of Acartia tonsa Dana (Copepoda) in the Harbor of Dunkirk (France) and its geographical distribution in Europe. Journal of Plankton Research 3, 255–260.
| Report on the presence of Acartia tonsa Dana (Copepoda) in the Harbor of Dunkirk (France) and its geographical distribution in Europe.Crossref | GoogleScholarGoogle Scholar |
Buchanan, P. J., and Beckley, L. E. (2016). Chaetognaths of the Leeuwin Current system: oceanographic conditions drive epi-pelagic zoogeography in the south-east Indian Ocean. Hydrobiologia 763, 81–96.
| Chaetognaths of the Leeuwin Current system: oceanographic conditions drive epi-pelagic zoogeography in the south-east Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Bureau of Meteorology (2012). Record-breaking La Niña events: an analysis of the La Niña life cycle and the impacts and significance of the 2010–11 and 2011–12 La Niña events in Australia. Available at http://www.bom.gov.au/climate/enso/history/ln-2010- 12/?ref=ad [verified 12 September 2016].
Chiba, S., Tadokoro, K., Sugisaki, H., and Saino, T. (2006). Effects of decadal climate change on zooplankton over the last 50 years in the western subarctic North Pacific. Global Change Biology 12, 907–920.
| Effects of decadal climate change on zooplankton over the last 50 years in the western subarctic North Pacific.Crossref | GoogleScholarGoogle Scholar |
Clarke, K. R., and Gorley, R. N. (2006). ‘PRIMER ver. 6: User Manual/ Tutorial.’ (PRIMER-E: Plymouth, UK.)
Commonwealth of Australia (2006). ‘A Guide to the Integrated Marine and Coastal Regionalisation of Australia Version 4.0.’ (Department of Environment and Heritage: Canberra, ACT, Australia.)
Cresswell, G. R. (1991). The Leeuwin Current: observations and recent models. Journal of the Royal Society of Western Australia 74, 1–14.
Cresswell, G. R., and Domingues, C. M. (2009). The Leeuwin Current south of Western Australia. Journal of the Royal Society of Western Australia 92, 83–100.
Cresswell, G. R., and Golding, T. J. (1980). Observations of a southward flowing current in the south-eastern Indian Ocean. Deep-Sea Research 27, 449–466.
| Observations of a southward flowing current in the south-eastern Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Davies, C., and Sommerville, E. (Eds) (2017). National Reference Stations Biogeochemical Operations Manual Version 3.3. Integrated Marine Observing System. Available at https://s3-ap-southeast-2.amazonaws.com/content.aodn.org.au/Documents/IMOS/Facilities/national_mooring/IMOS_NRS_BGCManual_LATEST.pdf [verified 5 May 2019].
Dias, C. O., Araujo, A. V., Vianna, S. C., Loureiro-Fernandes, L. F., Paranhos, R., Suzuki, M. S., and Bonecker, S. L. C. (2015). Spatial and temporal changes in biomass, production and assemblage structure of mesozooplanktonic copepods in the tropical south-west Atlantic Ocean. Journal of the Marine Biological Association of the United Kingdom 95, 483–496.
| Spatial and temporal changes in biomass, production and assemblage structure of mesozooplanktonic copepods in the tropical south-west Atlantic Ocean.Crossref | GoogleScholarGoogle Scholar |
Eriksen, R. S., Davies, C. H., Bonham, P., Coman, F. E., Edgar, S., McEnnulty, F. R., McLeod, D., Miller, M. J., Rochester, W., Slotwinski, A., Tonks, M. L., Uribe-Palomino, J., and Richardson, A. J. (2019). Australia’s long-term plankton observations: the Integrated Marine Observing System National Reference Station Network. Frontiers in Marine Science 6, 161.
| Australia’s long-term plankton observations: the Integrated Marine Observing System National Reference Station Network.Crossref | GoogleScholarGoogle Scholar |
Feng, M., McPhaden, M. J., Xie, S., and Hafner, J. (2013). La Niña forces unprecedented Leeuwin Current warming in 2011. Scientific Reports 3, 1277.
| La Niña forces unprecedented Leeuwin Current warming in 2011.Crossref | GoogleScholarGoogle Scholar | 23429502PubMed |
Fox, N. J., and Beckley, L. E. (2005). Priority areas for conservation of Western Australian coastal fishes: a comparison of hotspot, biogeographical and complementary approaches. Biological Conservation 125, 399–410.
| Priority areas for conservation of Western Australian coastal fishes: a comparison of hotspot, biogeographical and complementary approaches.Crossref | GoogleScholarGoogle Scholar |
Fromentin, J.-M., and Planque, B. (1996). Calanus and environment in the eastern North Atlantic. II. Influence of the North Atlantic oscillation on C. finmarchicus and C. helgolandicus. Marine Ecology Progress Series 134, 111–118.
| Calanus and environment in the eastern North Atlantic. II. Influence of the North Atlantic oscillation on C. finmarchicus and C. helgolandicus.Crossref | GoogleScholarGoogle Scholar |
Frost, B., and Fleminger, A. (1968). A revision of the genus Clausocalanus (Copepoda: Calanoida) with remarks on distributional patterns in diagnostic characters. Bulletin of the Scripps Institution of Oceanography 12, 1–235.
Gaughan, D. J., and Fletcher, W. J. (1997). Effects of the Leeuwin Current on the distribution of carnivorous macrozooplankton in the shelf waters of southern Western Australia. Estuarine, Coastal and Shelf Science 45, 89–97.
| Effects of the Leeuwin Current on the distribution of carnivorous macrozooplankton in the shelf waters of southern Western Australia.Crossref | GoogleScholarGoogle Scholar |
Gaughan, D. J., Pearce, A. F., and Lewis, P. D. (2009). Does the poleward boundary current off Western Australia exert a dominant influence on coastal chaetognaths and siphonophores? Estuarine, Coastal and Shelf Science 83, 443–450.
| Does the poleward boundary current off Western Australia exert a dominant influence on coastal chaetognaths and siphonophores?Crossref | GoogleScholarGoogle Scholar |
Gersbach, G. H., Pattiaratchi, C. B., Ivey, G. N., and Cresswell, G. R. (1999). Upwelling on the south-west coast of Australia: source of the Capes Current? Continental Shelf Research 19, 363–400.
| Upwelling on the south-west coast of Australia: source of the Capes Current?Crossref | GoogleScholarGoogle Scholar |
Godfrey, J. S., and Ridgway, K. R. (1985). The large-scale environment of the poleward-flowing Leeuwin Current, Western Australia: longshore steric height gradients, wind stresses and geostrophic flow. Journal of Physical Oceanography 15, 481–495.
| The large-scale environment of the poleward-flowing Leeuwin Current, Western Australia: longshore steric height gradients, wind stresses and geostrophic flow.Crossref | GoogleScholarGoogle Scholar |
Hanson, C. E., Pattiaratchi, C. B., and Waite, A. M. (2005). Seasonal production regimes off south-western Australia: influence of the Capes and Leeuwin Currents on phytoplankton dynamics. Marine and Freshwater Research 56, 1011–1026.
| Seasonal production regimes off south-western Australia: influence of the Capes and Leeuwin Currents on phytoplankton dynamics.Crossref | GoogleScholarGoogle Scholar |
Hardy, S. A. C. (1967). ‘Great Waters: a Voyage of Natural History to Study Whales, Plankton and the Waters of the Southern Ocean in the old Royal Research Ship Discovery, with the Results Brought up to Date by the Findings of the R.R.S. Discovery II.’ (Collins: London, UK.)
Hays, G. C., Richardson, A. J., and Robinson, C. (2005). Climate change and marine plankton. Trends in Ecology & Evolution 20, 337–344.
| Climate change and marine plankton.Crossref | GoogleScholarGoogle Scholar |
Heron, A. C. (1982). A vertical free fall plankton net with no mouth obstructions. Limnology and Oceanography 27, 380–383.
| A vertical free fall plankton net with no mouth obstructions.Crossref | GoogleScholarGoogle Scholar |
Holliday, D., Beckley, L. E., Weller, E., and Sutton, A. L. (2011). Natural variability of macro-zooplankton and larval fishes off the Kimberley, north-western Australia: preliminary findings. Journal of the Royal Society of Western Australia 94, 181–195.
Holliday, D., Beckley, L. E., Millar, N., Olivar, M. P., Slawinski, D., Feng, M., and Thompson, P. A. (2012). Larval fish assemblages and particle back-tracking define latitudinal and cross-shelf variability in an eastern Indian Ocean boundary current. Marine Ecology Progress Series 460, 127–144.
| Larval fish assemblages and particle back-tracking define latitudinal and cross-shelf variability in an eastern Indian Ocean boundary current.Crossref | GoogleScholarGoogle Scholar |
Hopcroft, R. R., Kosobokova, K. N., and Pinchuk, A. L. (2010). Zooplankton community patterns in the Chukchi Sea during summer 2004. Deep-sea Research Part II: Topical Studies in Oceanography 57, 27–39.
| Zooplankton community patterns in the Chukchi Sea during summer 2004.Crossref | GoogleScholarGoogle Scholar |
Huang, Z., and Feng, M. (2015). Remotely sensed spatial and temporal variability of the Leeuwin Current using MODIS data. Remote Sensing of Environment 166, 214–232.
| Remotely sensed spatial and temporal variability of the Leeuwin Current using MODIS data.Crossref | GoogleScholarGoogle Scholar |
Huggett, J., Verheye, H., Escribano, R., and Fairweather, T. (2009). Copepod biomass, size composition and production in the southern Benguela: spatio-temporal patterns of variation, and comparison with other eastern boundary upwelling systems. Progress in Oceanography 83, 197–207.
| Copepod biomass, size composition and production in the southern Benguela: spatio-temporal patterns of variation, and comparison with other eastern boundary upwelling systems.Crossref | GoogleScholarGoogle Scholar |
Hutchins, J. N., and Pearce, A. F. (1994). Influence of the Leeuwin Current on recruitment of tropical reef fishes at Rottnest Island, Western Australia. Bulletin of Marine Science 54, 245–255.
Jiang, S., Dickey, T. D., Steinberg, D. K., and Madin, L. P. (2007). Temporal variability of zooplankton biomass from ADCP backscatter time series data at the Bermuda testbed mooring site. Deep-sea Research Part I: Oceanographic Research Papers 54, 608–636.
| Temporal variability of zooplankton biomass from ADCP backscatter time series data at the Bermuda testbed mooring site.Crossref | GoogleScholarGoogle Scholar |
Johnson, C. R., Banks, S. C., Barrett, N. S., Cazassus, F., Dunstan, P. K., Edgar, G. J., Frusher, S. D., Gardner, C., Haddon, M., Helidoniotis, F., Hill, K. L., Holbrook, N. J., Hosie, G. W., Last, P. R., Ling, S. D., Melbourne-Thomas, J., Miller, K., Pecl, G. T., Richardson, A. J., Ridgway, K. R., Rintoul, S. R., Ritz, D. A., Ross, D. J., Sanderson, J. C., Shepherd, S. A., Slotwinski, A., Swadling, K. M., and Taw, N. (2011). Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. Journal of Experimental Marine Biology and Ecology 400, 17–32.
| Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania.Crossref | GoogleScholarGoogle Scholar |
Keane, J. P., and Neira, F. J. (2008). Larval fish assemblages along the south-eastern Australian shelf: linking mesoscale non-depth-discriminate structure and water masses. Fisheries Oceanography 17, 263–280.
| Larval fish assemblages along the south-eastern Australian shelf: linking mesoscale non-depth-discriminate structure and water masses.Crossref | GoogleScholarGoogle Scholar |
Kelly, P., Clementson, L., Davies, C., Croney, S., and Swadling, K. (2016). Zooplankton responses to increasing sea surface temperatures in the southeastern Australia global marine hotspot. Estuarine, Coastal and Shelf Science 180, 242–257.
| Zooplankton responses to increasing sea surface temperatures in the southeastern Australia global marine hotspot.Crossref | GoogleScholarGoogle Scholar |
Kimmerer, W. J., McKinnon, A. D., Atkinson, M. J., and Kessell, J. A. (1985). Spatial distributions of plankton in Shark Bay, Western Australia. Marine and Freshwater Research 36, 421–432.
| Spatial distributions of plankton in Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Koslow, J. A., Pesant, S., Feng, M., Pearce, A., Fearns, P., Moore, T., Matear, R., and Waite, A. (2008). The effect of the Leeuwin Current on phytoplankton biomass and production off southwestern Australia. Journal of Geophysical Research 113, C07050.
| The effect of the Leeuwin Current on phytoplankton biomass and production off southwestern Australia.Crossref | GoogleScholarGoogle Scholar |
Kouwenberg, J. H. M. (1994). Copepod distribution in relation to seasonal hydrographics and spatial structure in the north-western Mediterranean (Golfe du Lion). Estuarine, Coastal and Shelf Science 38, 69–90.
| Copepod distribution in relation to seasonal hydrographics and spatial structure in the north-western Mediterranean (Golfe du Lion).Crossref | GoogleScholarGoogle Scholar |
Lara-Lopez, A., Moltmann, T., and Proctor, R. (2016). Australia’s Integrated Marine Observing System (IMOS): data impacts and lessons learned. Marine Technology Society Journal 50, 23–33.
| Australia’s Integrated Marine Observing System (IMOS): data impacts and lessons learned.Crossref | GoogleScholarGoogle Scholar |
Legendre, P. (1990). The significance of microalgal blooms for fisheries and for the export of particulate organic carbon in oceans. Journal of Plankton Research 12, 681–699.
| The significance of microalgal blooms for fisheries and for the export of particulate organic carbon in oceans.Crossref | GoogleScholarGoogle Scholar |
Legendre, P., and Anderson, M. J. (1999). Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments. Ecological Monographs 69, 1–24.
| Distance-based redundancy analysis: testing multispecies responses in multifactorial ecological experiments.Crossref | GoogleScholarGoogle Scholar |
Longhurst, A. R. (1985). The structure and evolution of plankton communities. Progress in Oceanography 15, 1–35.
| The structure and evolution of plankton communities.Crossref | GoogleScholarGoogle Scholar |
Lourey, M. J., Thompson, P. A., McLaughlin, M. J., Bonham, P., and Feng, M. (2013). Primary production and phytoplankton community structure during a winter shelf-scale phytoplankton bloom off Western Australia. Marine Biology 160, 355–369.
| Primary production and phytoplankton community structure during a winter shelf-scale phytoplankton bloom off Western Australia.Crossref | GoogleScholarGoogle Scholar |
Lynch, T. P., Morello, E. B., Evans, K., Richardson, A. J., Rochester, W., Steinberg, C. R., Roughan, M., Thompson, P., Middleton, J. F., Feng, M., Sherrington, R., Brando, V., Tilbrook, B., Ridgway, K., Allen, S., Doherty, P., Hill, K., and Moltmann, T. C. (2014). IMOS National Reference Stations: a continental-wide physical, chemical and biological coastal observing system. PLoS One 9, e113652.
| IMOS National Reference Stations: a continental-wide physical, chemical and biological coastal observing system.Crossref | GoogleScholarGoogle Scholar | 25517905PubMed |
Mackas, D. L., Thomson, R. E., and Galbraith, M. (2001). Changes in the zooplankton community of the British Columbia continental margin, 1985–1999, and their covariation with oceanographic conditions. Canadian Journal of Fisheries and Aquatic Sciences 58, 685–702.
| Changes in the zooplankton community of the British Columbia continental margin, 1985–1999, and their covariation with oceanographic conditions.Crossref | GoogleScholarGoogle Scholar |
Mackas, D. L., Peterson, W. T., Ohman, M. D., and Lavaniegos, B. E. (2006). Zooplankton anomalies in the California Current system before and during the warm ocean conditions of 2005. Geophysical Research Letters 33, L22S07.
| Zooplankton anomalies in the California Current system before and during the warm ocean conditions of 2005.Crossref | GoogleScholarGoogle Scholar |
Mackas, D. L., Pepin, P., and Verheye, H. (2012). Interannual variability of marine zooplankton and their environments: within- and between-region comparisons. Progress in Oceanography 97–100, 1–14.
| Interannual variability of marine zooplankton and their environments: within- and between-region comparisons.Crossref | GoogleScholarGoogle Scholar |
Maxwell, J. G., and Cresswell, G. R. (1981). Dispersal of tropical marine fauna to the Great Australian Bight by the Leeuwin Current. Marine and Freshwater Research 32, 493–500.
| Dispersal of tropical marine fauna to the Great Australian Bight by the Leeuwin Current.Crossref | GoogleScholarGoogle Scholar |
McKinnon, A. D., and Duggan, S. (2014). Community ecology of pelagic copepods in tropical coastal waters. In ‘Copepods: Diversity, Habitat and Behaviour’. (Ed. Laurent Seuront.) pp. 25–49. (Nova Science Publishers, Inc.: United Kingdom.)
McKinnon, A. D., Meekan, M. G., Carleton, J. H., Furnas, M. J., Duggan, S., and Skirving, W. (2003a). Rapid changes in shelf waters and pelagic communities on the southern Northwest Shelf, Australia, following a tropical cyclone. Continental Shelf Research 23, 93–111.
| Rapid changes in shelf waters and pelagic communities on the southern Northwest Shelf, Australia, following a tropical cyclone.Crossref | GoogleScholarGoogle Scholar |
McKinnon, A. D., Duggan, S., Nichols, P. D., Rimmer, M. A., Semmens, G., and Robino, B. (2003b). The potential of tropical paracalanid copepods as live feeds in aquaculture. Aquaculture 223, 89–106.
| The potential of tropical paracalanid copepods as live feeds in aquaculture.Crossref | GoogleScholarGoogle Scholar |
McKinnon, A. D., Duggan, S., Carleton, J. H., and Bottger-Schnack, R. (2008). Summer planktonic copepod communities of Australia’s North West Cape (Indian Ocean) during the 1997–99 El Nino/La Nina. Journal of Plankton Research 30, 839–855.
| Summer planktonic copepod communities of Australia’s North West Cape (Indian Ocean) during the 1997–99 El Nino/La Nina.Crossref | GoogleScholarGoogle Scholar |
McKinnon, A. D., Duggan, S., Holliday, D., and Brinkman, R. (2015). Plankton community structure and connectivity in the Kimberley–Browse region of NW Australia. Estuarine, Coastal and Shelf Science 153, 156–167.
| Plankton community structure and connectivity in the Kimberley–Browse region of NW Australia.Crossref | GoogleScholarGoogle Scholar |
Middleton, J. F., and Bye, J. A. T. (2007). A review of the shelf-slope circulation along Australia’s southern shelves: Cape Leeuwin to Portland. Progress in Oceanography 75, 1–41.
| A review of the shelf-slope circulation along Australia’s southern shelves: Cape Leeuwin to Portland.Crossref | GoogleScholarGoogle Scholar |
Muhling, B. A., and Beckley, L. E. (2007). Seasonal variation in horizontal and vertical structure of larval fish assemblages off south-western Australia, with implications for larval transport. Journal of Plankton Research 29, 967–983.
| Seasonal variation in horizontal and vertical structure of larval fish assemblages off south-western Australia, with implications for larval transport.Crossref | GoogleScholarGoogle Scholar |
Muhling, B. A., Beckley, L. E., Koslow, J. A., and Pearce, A. F. (2008). Larval fish assemblages and water mass structure off the oligotrophic south-western Australian coast. Fisheries Oceanography 17, 16–31.
| Larval fish assemblages and water mass structure off the oligotrophic south-western Australian coast.Crossref | GoogleScholarGoogle Scholar |
O’Hara, T. D., and Poore, G. C. B. (2000). Patterns of distribution for southern Australian marine echinoderms and decapods. Journal of Biogeography 27, 1321–1335.
| Patterns of distribution for southern Australian marine echinoderms and decapods.Crossref | GoogleScholarGoogle Scholar |
Pearce, A. F. (1991). Eastern boundary currents of the southern hemisphere. Journal of the Royal Society of Western Australia 74, 35–45.
Pearce, A. F., and Feng, M. (2013). The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/2011. Journal of Marine Systems 111–112, 139–156.
| The rise and fall of the ‘marine heat wave’ off Western Australia during the summer of 2010/2011.Crossref | GoogleScholarGoogle Scholar |
Pearce, A. F., Lynch, M. J., and Hanson, C. E. (2006). The Hillarys transect (1): seasonal and cross-shelf variability of physical and chemical water properties off Perth, Western Australia, 1996–98. Continental Shelf Research 26, 1689–1729.
| The Hillarys transect (1): seasonal and cross-shelf variability of physical and chemical water properties off Perth, Western Australia, 1996–98.Crossref | GoogleScholarGoogle Scholar |
Peterson, W. T., and Hutchings, L. (1995). Distribution, abundance and production of the copepod Calanus agulhensis on the Agulhas Bank in relation to spatial variations in hydrography and chlorophyll concentration. Journal of Plankton Research 17, 2275–2294.
| Distribution, abundance and production of the copepod Calanus agulhensis on the Agulhas Bank in relation to spatial variations in hydrography and chlorophyll concentration.Crossref | GoogleScholarGoogle Scholar |
Richardson, A. J. (2008). In hot water: zooplankton and climate change. ICES Journal of Marine Science 65, 279–295.
| In hot water: zooplankton and climate change.Crossref | GoogleScholarGoogle Scholar |
Richardson, A. J., and Schoeman, D. S. (2004). Climate impact on plankton ecosystems in the northeast Atlantic. Science 305, 1609–1612.
| Climate impact on plankton ecosystems in the northeast Atlantic.Crossref | GoogleScholarGoogle Scholar | 15361622PubMed |
Richardson, A. J., Eriksen, R. S., and Rochester, W. (2015). Plankton 2015: state of Australia’s oceans. CSIRO report. ISBN 978-1-4863-0565-0. Available at http://imos.org.au/fileadmin/user_upload/shared/Data_Tools/15-00245_OA_Plankton2015_20ppBrochure_WEB_151116.pdf [verified 19 September 2016].
Ridgway, K. R., and Condie, S. A. (2004). The 5500 km long boundary flow off western and southern Australia. Journal of Geophysical Research 109, C04017.
| The 5500 km long boundary flow off western and southern Australia.Crossref | GoogleScholarGoogle Scholar |
Rosa, J. C. L., Monteiro-Ribas, W. M., and Fernandes, L. D. A. (2016). Herbivorous copepods with emphasis on dynamic Paracalanus quasimodo in an upwelling region. Brazilian Journal of Oceanography 64, 67–73.
| Herbivorous copepods with emphasis on dynamic Paracalanus quasimodo in an upwelling region.Crossref | GoogleScholarGoogle Scholar |
Rossi, V., Feng, M., Pattiaratchi, C., Roughan, M., and Waite, A. M. (2013). On the factors influencing the development of sporadic upwelling in the Leeuwin Current system. Journal of Geophysical Research. Oceans 118, 3608–3621.
| On the factors influencing the development of sporadic upwelling in the Leeuwin Current system.Crossref | GoogleScholarGoogle Scholar |
Säwström, C., Beckley, L. E., Saunders, M. I., Thompson, P. A., and Waite, A. M. (2014). The zooplankton prey field for rock lobster phyllosoma larvae in relation to oceanographic features of the south-eastern Indian Ocean. Journal of Plankton Research 36, 1003–1016.
| The zooplankton prey field for rock lobster phyllosoma larvae in relation to oceanographic features of the south-eastern Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Steinberg, D. K., Lomas, M. W., and Cope, J. S. (2012). Long‐term increase in mesozooplankton biomass in the Sargasso Sea: linkage to climate and implications for food web dynamics and biogeochemical cycling. Global Biogeochemical Cycles 26, GB1004.
| Long‐term increase in mesozooplankton biomass in the Sargasso Sea: linkage to climate and implications for food web dynamics and biogeochemical cycling.Crossref | GoogleScholarGoogle Scholar |
Sutton, A. L., and Beckley, L. E. (2016). Influence of the Leeuwin Current on the epipelagic euphausiid assemblages of the south-east Indian Ocean. Hydrobiologia 779, 193–207.
| Influence of the Leeuwin Current on the epipelagic euphausiid assemblages of the south-east Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Thompson, P. A., Baird, M. E., Ingleton, T., and Doblin, M. A. (2009). Long-term changes in temperate Australian coastal waters: implications for phytoplankton. Marine Ecology Progress Series 394, 1–19.
| Long-term changes in temperate Australian coastal waters: implications for phytoplankton.Crossref | GoogleScholarGoogle Scholar |
Thompson, P. A., Wild-Allen, K., Lourey, M., Rousseaux, C., Waite, A. M., Feng, M., and Beckley, L. E. (2011). Nutrients in an oligotrophic boundary current: evidence of a new role for the Leeuwin Current. Progress in Oceanography 91, 345–359.
| Nutrients in an oligotrophic boundary current: evidence of a new role for the Leeuwin Current.Crossref | GoogleScholarGoogle Scholar |
Tranter, D. J., and Kerr, J. D. (1969). Seasonal variations in the Indian Ocean along 110°E. Australian Journal of Marine and Freshwater Research 20, 77–84.
| Seasonal variations in the Indian Ocean along 110°E.Crossref | GoogleScholarGoogle Scholar |
Tranter, D. J., and Kerr, J. D. (1977). Further studies of plankton ecosystems in the eastern Indian Ocean II. Numerical abundance and biomass. Australian Journal of Marine and Freshwater Research 28, 557–583.
| Further studies of plankton ecosystems in the eastern Indian Ocean II. Numerical abundance and biomass.Crossref | GoogleScholarGoogle Scholar |
Valencia, B., Landry, M. R., Décima, M., and Hannides, C. C. S. (2016). Environmental drivers of mesozooplankton biomass variability in the North Pacific Subtropical Gyre. Journal of Geophysical Research. Biogeosciences 121, 3131–3143.
| Environmental drivers of mesozooplankton biomass variability in the North Pacific Subtropical Gyre.Crossref | GoogleScholarGoogle Scholar |
Verheye, H. M., and Richardson, A. J. (1998). Long-term increase in crustacean zooplankton abundance in the southern Benguela upwelling region (1951–1996): bottom-up or top-down control? ICES Journal of Marine Science 55, 803–807.
| Long-term increase in crustacean zooplankton abundance in the southern Benguela upwelling region (1951–1996): bottom-up or top-down control?Crossref | GoogleScholarGoogle Scholar |
Verheye, H. M., Lamont, T., Huggett, J. A., Kreiner, A., and Hampton, I. (2016). Plankton productivity of the Benguela Current Large Marine Ecosystem (BCLME). Environmental Development 17, 75–92.
| Plankton productivity of the Benguela Current Large Marine Ecosystem (BCLME).Crossref | GoogleScholarGoogle Scholar |
Waite, A. M., Thompson, P. A., Pesant, S., Feng, M., Beckley, L. E., Domingues, C. M., Gaughan, D., Hanson, C. E., Holl, C. M., Koslow, T., Meuleners, M., Montoya, J. P., Moore, T., Muhling, B. A., Paterson, H., Rennie, S., Strzelecki, J., and Twomey, L. (2007). The Leeuwin Current and its eddies: an introductory overview. Deep-sea Research Part II: Topical Studies in Oceanography 54, 789–796.
| The Leeuwin Current and its eddies: an introductory overview.Crossref | GoogleScholarGoogle Scholar |
Weller, E., Holliday, D., Feng, M., Beckley, L., and Thompson, P. (2011). A continental shelf scale examination of the Leeuwin Current off Western Australia during the austral autumn–winter. Continental Shelf Research 31, 1858–1868.
| A continental shelf scale examination of the Leeuwin Current off Western Australia during the austral autumn–winter.Crossref | GoogleScholarGoogle Scholar |
Wilson, S. G., Carleton, J. H., and Meekan, M. G. (2003). Spatial and temporal patterns in the distribution and abundance of macrozooplankton on the southern North West Shelf, Western Australia. Estuarine, Coastal and Shelf Science 56, 897–908.
| Spatial and temporal patterns in the distribution and abundance of macrozooplankton on the southern North West Shelf, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Woo, M., and Pattiaratchi, C. (2008). Hydrography and water masses off the western Australian coast. Deep-sea Research Part I: Oceanographic Research Papers 55, 1090–1104.
| Hydrography and water masses off the western Australian coast.Crossref | GoogleScholarGoogle Scholar |
Woo, M., Pattiaratchi, C., and Schroeder, W. (2006). Dynamics of the Ningaloo Current off Point Cloates, Western Australia. Marine and Freshwater Research 57, 291–301.
| Dynamics of the Ningaloo Current off Point Cloates, Western Australia.Crossref | GoogleScholarGoogle Scholar |


