Using optimised otolith sectioning to determine the age, growth and age at sexual maturity of the herbivorous fish Kyphosus bigibbus: with a comparison to using scales
Yoshimi Ogino A , Keisuke Furumitsu A , Takanari Kiriyama B and Atsuko Yamaguchi A CA Laboratory of Marine Zoology, Graduate School of Fisheries and Environmental Sciences, Nagasaki University, 1-14 Bunkyo, Nagasaki, Nagasaki 852-8521, Japan.
B Nagasaki Prefectural Institute of Fisheries, 1551-4 Taira, Nagasaki, Nagasaki 851-2213, Japan.
C Corresponding author. Email: y-atsuko@nagasaki-u.ac.jp
Marine and Freshwater Research 71(7) 855-867 https://doi.org/10.1071/MF19231
Submitted: 1 July 2019 Accepted: 26 August 2019 Published: 18 November 2019
Journal Compilation © CSIRO 2020 Open Access CC BY-NC-ND
Abstract
Determining the population parameters of herbivorous fishes facilitates our understanding of their overall effects on ecosystems. However, this has not been successful with species such as Kyphosus bigibbus that are difficult to age using otoliths. In this study, we estimated the age, growth and age at sexual maturity of K. bigibbus off the west coast of Kyushu, Japan, using fish scales and otoliths. Scales were found unreliable because they caused underestimation of the age of fish older than 5 years, whereas otoliths were reliable when used with our improved otolith-sectioning methodology. The maximum age and fork length were 46 years and 574 mm for females and 32 years and 506 mm for males. According to the von Bertalanffy growth curves, females became slightly larger than males, and both sexes showed nearly asymptotic fork lengths after 10 years. The age at 50% sexual maturity for females and males was 3.2 and 1.9 years respectively, which is extremely early considering their maximum age. The year-class composition of K. bigibbus suggests that its recruitment may have increased rapidly since 1999, following noticeable losses of kelp forests in this region. Our findings will contribute to the understanding of algal forest ecosystems and advancement of fish ageing studies.
Additional keywords: algal deforestation, isoyake, Kyphosidae, life history.
Introduction
Algal forests, particularly kelp forests, are important biogenic habitats in temperate ecosystems (Teagle et al. 2017), providing immense ecological, social and economic benefits (Smale et al. 2013; Bennett et al. 2016; Blamey and Bolton 2018). Although algal forests are declining globally, there is a high degree of geographic variation caused by variations in local stressors, such as harvesting, trampling, habitat modification, eutrophication and overgrazing (Mineur et al. 2015; Krumhansl et al. 2016). Overgrazing of temperate macroalgae is usually caused by sea urchins (Steneck et al. 2002). However, there has recently been an increasing interest in overgrazing by herbivorous fishes (Vergés et al. 2014a). For example, in the Mediterranean Sea, the range expansion of tropical rabbitfishes that first entered the basin via the Suez Canal has caused considerable damage to algal forests (Vergés et al. 2014b). Moreover, Gianni et al. (2017) suggested that a Mediterranean native herbivorous fish (Sarpa salpa) presents a potential threat to algal forests because its abundance may have increased recently. On the west coast of Australia, an extreme marine heat wave in 2011 caused extensive losses of algal forests and a concurrent increase in tropical herbivorous fishes, which are now suppressing the recovery of algal forests (Smale and Wernberg 2013; Wernberg et al. 2013, 2016). Furthermore, on the east coast of Australia, algal forests have gradually declined, whereas there has been a concurrent increase in tropical herbivorous fishes (Basford et al. 2016; Vergés et al. 2016). In these areas of algal deforestation on both sides of Australia, feeding assays have identified Kyphosus bigibbus as a major consumer of kelp (Bennett et al. 2015; Vergés et al. 2016).
There has been increasing concern over the ongoing leaf structure loss of kelp species on the west coast of Kyushu, Japan, caused by overgrazing by herbivorous fishes, which was first detected in the autumn of 1998 (Kiriyama et al. 1999; Kiyomoto et al. 2000). Observations of feeding behaviour and the bite marks of several fish species on algae indicated that Siganus fuscescens, Calotomus japonicus and Kyphosus spp. are responsible for this phenomenon (Kiriyama et al. 2001). Subsequent surveys showed that four Kyphosus species are distributed in this region, with K. bigibbus being the most abundant (Yamaguchi 2010). Yamaguchi (2010) suggested that herbivorous fishes’ greater activity and consumption of newly germinated large brown algae in winter was a response to current global warming-associated increases in winter seawater temperatures, and that it may be changing the balance of algal forest ecosystems.
Areas of algal deforestation (termed ‘isoyake’) exist in most of Japan’s coastal prefectures, with overgrazing by herbivorous fishes representing a potential cause of algal deforestation in southern Japan (Fisheries Agency 2015). Accordingly, a countermeasure currently recommended by the Japanese government to control algal deforestation involves the culling of herbivorous fishes (Fisheries Agency 2015). Within this framework, researchers have attempted to catch schooling K. bigibbus near breakwater structures using gill-nets (Kuwahara 2015). However, the biological and ecological properties of herbivorous fishes are not yet fully understood. Thus, it remains unclear whether culling is an effective strategy to prevent algal deforestation.
K. bigibbus is widely distributed in the subtropical and temperate regions of the Indian, Pacific and Atlantic oceans (Knudsen and Clements 2013, 2016). This species is an obligate herbivore, with a diet consisting predominantly of brown algae (Clements and Choat 1997; Clements and Zemke-White 2008; Yatsuya et al. 2015). In the Ningaloo Reef, Australia, K. bigibbus is one of the main consumers of macroalgae, with feeding rates being highest when individuals of this species form part of monospecific groups (Michael et al. 2013). There are both resident and nomadic individuals of this species (Pillans et al. 2017). The movement patterns of resident individuals demonstrate high fidelity to particular patch reefs and large home ranges, leading to large-scale landscape changes in the form of areas largely devoid of macroalgae (termed ‘halos’) around patch reefs (Downie et al. 2013; Pillans et al. 2017). On the west coast of Kyushu, Japan, K. bigibbus migrates diurnally, and its activity declines markedly when the seawater temperature decreases to ~17°C (Yamaguchi et al. 2006; Yamaguchi 2010). The spawning and maturation patterns of this species have been elucidated only in populations inhabiting this region. Spawning occurs from June to October (with activity peaking in June–July), and the fork length (FL) at 50% sexual maturity is 360 and 284 mm for females and males respectively (Yamaguchi et al. 2011). Inoue et al. (2006) estimated the age and growth of K. bigibbus in this region, reporting a maximum age of 13 years; these authors used fish scales for age determination because otoliths were small, irregularly shaped and their opaque zones were not clearly visible on otolith sections. However, many studies have demonstrated that age estimation using fish scales can result in serious underestimation for some species (e.g. Beamish and Chilton 1982; Gray et al. 2012; Baudouin et al. 2016). Therefore, K. bigibbus age and growth need to be estimated using more accurate age determination methods.
The aim of this study was to compare the use of fish scales and otolith sections (prepared using an improved methodology) to evaluate the efficacy of the methods based on these two structures to determine the age, growth rate and age at sexual maturity of K. bigibbus. The findings obtained here are expected to provide baseline knowledge on the population dynamics of this species. We believe that this will facilitate future studies on how the culling of K. bigibbus will affect algal forests, allowing appropriate measures to be implemented in order to protect algal forest ecosystems and the herbivorous fishes that use them.
Materials and methods
Fish collection and measurements
Between May 2004 and February 2009, 388 specimens of K. bigibbus were collected at depths ranging from ~3 to 25 m off the Nagasaki Peninsula, Japan, using set and gill-nets. Detailed information about the study site has been previously presented (Kume et al. 2010). In addition, five juvenile K. bigibbus specimens found near drifting algae were collected from the same area in August 2007. In the laboratory, the FL (mm) and bodyweight (BW; g) of fish were measured. Individuals were classified as female, male or unsexed based on macroscopic examination of the gonads, which were dissected. Length–weight relationships for females and males (unsexed fish were included in both sexes for the analyses) were analysed by fitting the allometric function:
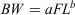
Parameters a and b were estimated using a non-linear least-squares procedure with the ‘Solver’ function in MS Excel (Microsoft, Redmond, WA, USA). To compare the length–weight relationships between females and males, analysis of covariance (ANCOVA) was performed on the log-transformed dataset. This, and all other ANCOVA tests, were performed using BellCurve (Social Survey Research Information, Tokyo, Japan) for MS Excel.
Preparation of scales
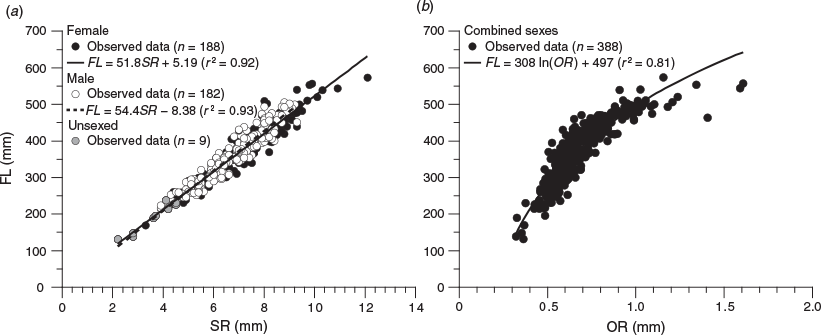
For each fish, the third scale below the scales of the lateral line scale that was the 20th from the posterior end of the lateral line was removed. The scales were washed with 0.5% KOH solution, observed under a light transmission microscope (SMZ1000; Nikon, Tokyo, Japan; magnification 8×) and photographed using a digital camera (DS-Fi1; Nikon) mounted on the microscope. An alternating pattern of translucent and opaque zones in the scales was observed (Fig. 1). If the scale was a regenerated scale, characterised by an opaque focus (Lou 1992), the data were excluded. Scale readings were undertaken using digital images. The scale radius (SR; mm) was measured from the focus to the distal margin of the dorsal side in the anterior field (Fig. 1). The relationship between FL and SR was calculated using MS Excel, and ANCOVA was performed to test the significance of differences between sexes (unsexed fish data were included in the dataset of both sexes).
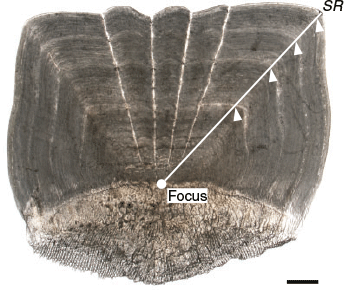
|
Preparation of otolith sections
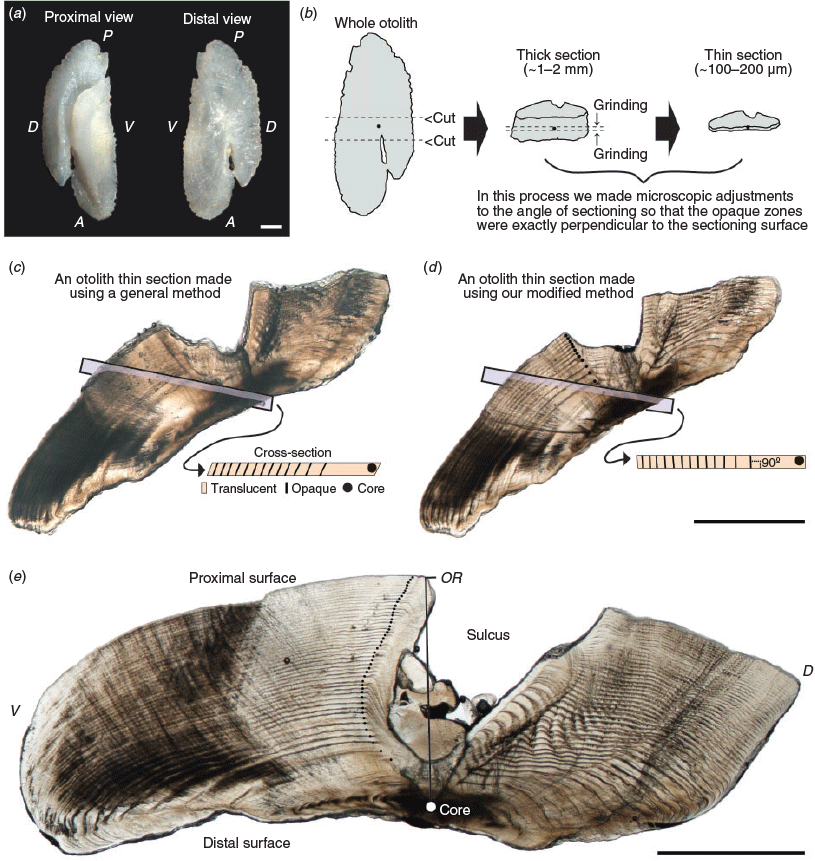
Sagittal otoliths were removed from the fish, cleaned and stored dry (Fig. 2a). A vertical mark was drawn across the core of each otolith with a well-sharpened pencil under the microscope (SMZ1000; Nikon) and each otolith was then embedded in acrylic resin. To obtain otolith sections in which opaque zones were exactly perpendicular to the sectioning surface, we followed a two-step otolith sectioning procedure (Fig. 2b). First, each embedded otolith was cut along the vertical pencil mark with a diamond disc saw to obtain transverse thick sections (~1–2 mm) containing the core. This step was needed because we could not know the slope of opaque zones in the otolith unless we observed the sectioned otoliths under the microscope. Second, both sides of the transverse thick sections were ground using 80- to 4000-grit waterproof sandpaper, making microadjustments to the angle of sectioning with frequent observations under the microscope until the sections were ~100–200 μm thick with an exposed core. This step was very important because the clarity of opaque zones in otolith sections was closely dependent on the perpendicularity of the opaque zones. Comparisons between the left and right otolith sections of the same specimen are shown in Fig. 2c, d. Because the left section (Fig. 2c) was produced using a general method (i.e. through macroscopic adjustments to the angle of sectioning), the opaque zones were not perpendicular to the sectioning surface, and thus are not clearly visible. By contrast, the right section (Fig. 2d), which was produced by using the modified method proposed herein, has clearly visible opaque zones, exactly perpendicular to the sectioning surface. The resulting thin sections were immersed in 100% glycerol, observed under a transmitted light microscope (SMZ1000; Nikon; magnification 20–40×) and photographed using a digital camera (DS-Fi1; Nikon) mounted on the microscope. An alternating pattern of translucent and opaque zones surrounding a central opaque region was observed in otolith sections (Fig. 2d, e). Otolith readings were performed using digital images. The otolith radius (OR; mm) was measured from the core to the edge of the ventral lobe next to the sulcus (Fig. 2e). The relationship between FL and OR was calculated using MS Excel, and ANCOVA was performed on the log-transformed dataset to test the significance of differences between the sexes (unsexed fish data were included in the dataset of both sexes).
Validation of ageing methods
To determine the periodicity of opaque zone formation, edge and marginal increment analyses were performed. Edge analysis was based on the method described by Yamaguchi et al. (2004), and it was performed to examine monthly changes in the percentage occurrence of structures with an opaque margin. Marginal increment analysis, based on that described by Coulson et al. (2009), was used to examine monthly trends in the marginal increment of the structures (i.e. the distance between the outer edge of the outermost opaque zone and the structure periphery). The marginal increment was expressed as the proportion of the distance between the outer edges of the two outermost opaque zones, and differences among months were compared using the Kruskal–Wallis test in BellCurve (Social Survey Research Information).
Precision and bias
The number of opaque zones for each scale and otolith was counted three times by the same person, without knowledge of fish length or previous counts. If the resulting counts were the same on two or more readings, that value was recorded as the number of opaque zones. If the counts differed in all three readings, the specimen was deemed illegible. The average percentage error (APE) and CV were calculated using the three counts, and the precisions of the scale and otolith opaque zone observations were compared (Beamish and Fournier 1981; Chang 1982). Campana (2001) suggested that acceptable levels of APE and CV are less than 5.5 and 7.6% respectively. In addition, an age-bias plot was used to investigate systematic variations in the scale and otolith opaque zone counts (Campana et al. 1995).
Growth curves
Following the method of Fowler and Short (1998), each fish was assigned an age in months based on the number of opaque zones in its age structure and taking into account its date of capture, an ‘average’ birth date (approximate mid-point of the spawning period) of 1 August (Yamaguchi et al. 2011) and a time of year at which the opaque zones were formed. Length-at-age data for females and males were fitted to the von Bertalanffy growth function (VBGF) using a non-linear least-squares procedure with the ‘Solver’ function in MS Excel. The VBGF was calculated as follows:
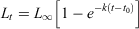
where Lt is the FL at age t, L∞ is the theoretical asymptotic length, k is the growth rate coefficient and t0 is the theoretical time at zero length. To improve the fit of VBGF at young ages, juveniles were added to the length-at-age data as an age-0 group; small, unsexed fish were included in the datasets of both sexes. A likelihood ratio test (Kimura 1980) was used on these datasets to determine whether growth curves differed by sex, following Paul and Horn (2009).
Age at sexual maturity
Considering the individuals (females: n = 53; males: n = 41) collected in the spawning season for which maturity was determined by histological examination of the gonads based on Yamaguchi et al. (2011), the percentage of mature individuals in each age class was fitted to the following logistic model:
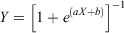
where Y is the proportion of mature individuals at age X, and a and b are empirical parameters estimated using a non-linear least-squares procedure. The age at 50% sexual maturity (A50) for each sex was calculated using the following equation:
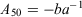
Results
Length–weight relationship
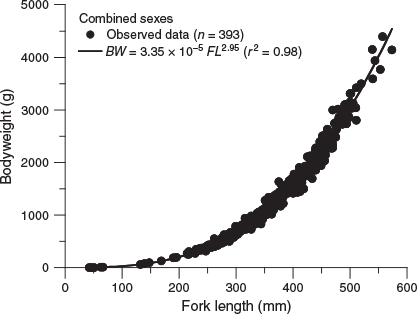
Of the 393 fish that were collected, 190 were female (169–574 mm FL, 131.0–4391.4 g BW), 189 were male (214–506 mm FL, 256.6–3306.4 g BW) and 14 were unsexed (43–238 mm FL, 1.4–333.0 g BW). The relationships between FL and BW for females and males were not significantly different (ANCOVA on log-transformed data: F = 3.46; d.f. = 1, 405; P = 0.064). Therefore, the relationship between FL and BW for combined sexes (Fig. 3) was estimated as follows:
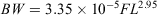
where r2 = 0.98.
|
Growth of scales and otoliths
Nine of the 388 scales collected had regenerated and were therefore excluded from the analyses. The relationship between FL and SR for females and males (Fig. 4a) was significantly different (ANCOVA: F = 4.24; d.f. = 1, 386; P < 0.05) and was expressed using the following linear equations:
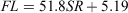
for females, where r2 = 0.92; and
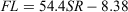
for males, where r2 = 0.93.
|
However, the relationship between FL and OR for females and males (Fig. 4b) was not significantly different (ANCOVA on log-transformed data: F = 0.581; d.f. = 1, 395; P = 0.45) and was better expressed for combined sexes using the following logarithmic (rather than linear) equation:
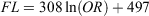
where r2 = 0.81.
Validation of ageing methods
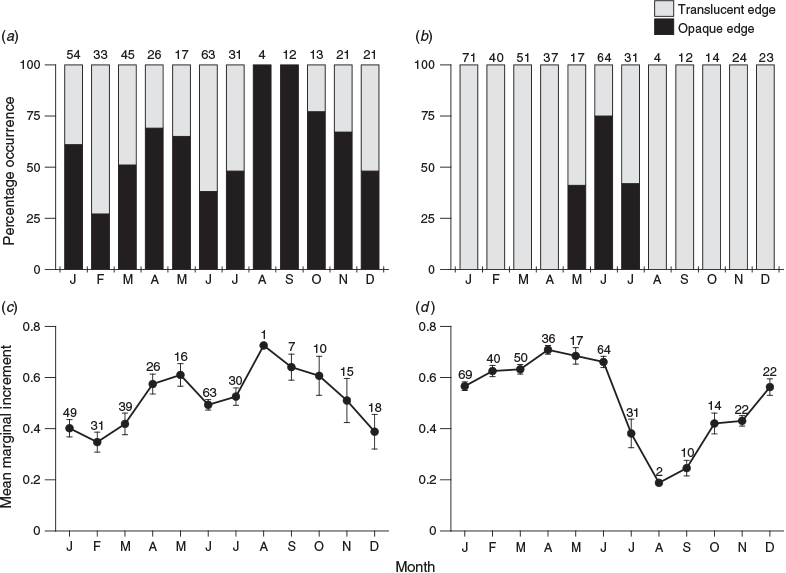
Among the 379 scales examined (excluding the 9 regenerated scales), 340 (89.7%) had legible opaque zones. In comparison, all 388 otoliths examined had legible opaque zones. The scale edge analysis indicated that the monthly proportion of specimens with opaque margins fluctuated throughout the year, regardless of month and season (Fig. 5a). Similarly, although the mean monthly marginal increments on the scales were significantly different (Kruskal–Wallis test: χ2 = 50.7; d.f. = 11; P < 0.001), they fluctuated throughout the year (Fig. 5c). However, the edge analysis of otoliths showed that opaque margins appeared only on the otoliths of specimens caught in May, June and July (Fig. 5b). The mean monthly marginal increments of otoliths were significantly different (Kruskal–Wallis test: χ2 = 120; d.f. = 11; P < 0.001). Specifically, mean monthly increments were high between December and June, and then declined sharply from June until August (Fig. 5d). Thus, even though a clear periodicity of opaque zone formation could not be detected in the scales, a clear periodicity was detected in the otoliths, confirming that the opaque zones become delineated from the edge of the otolith between June and August.
Precision and bias
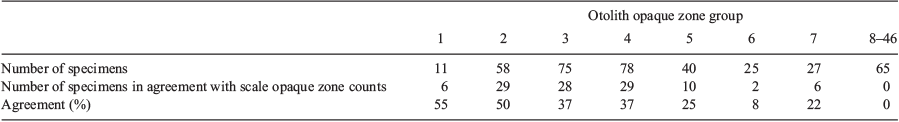
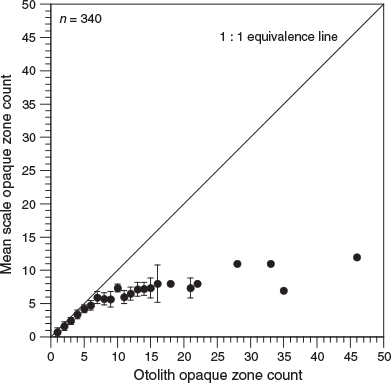
Analysis of opaque zone count precision showed that, for scales, the APE was 9.7% and the CV was 12.8%. However, for otoliths, the APE was 0.2% and the CV was 0.3%, indicating considerably higher reading precision. In the present study, 0–12 and 1–46 zones were recorded in K. bigibbus scales and otoliths respectively. Comparison of opaque zone counts between scales and otoliths of the same fish specimens indicated that the percentage agreement of opaque zone counts between the structures decreased with increasing age, and was 0% for fish older than 8 years (Table 1). The occurrence of systematic variation in counts between scales and otoliths was evaluated using an age-bias plot (Fig. 6). The opaque zone counts for samples with one to five zones generally showed a close correlation, and there was a linear relationship for these samples. Beyond five opaque zones, the difference became larger and the data exhibited a non-linear relationship, with the scales invariably underestimating the age of the specimen. The greatest difference in a fish specimen was 34 zones.
|
|
Growth curves
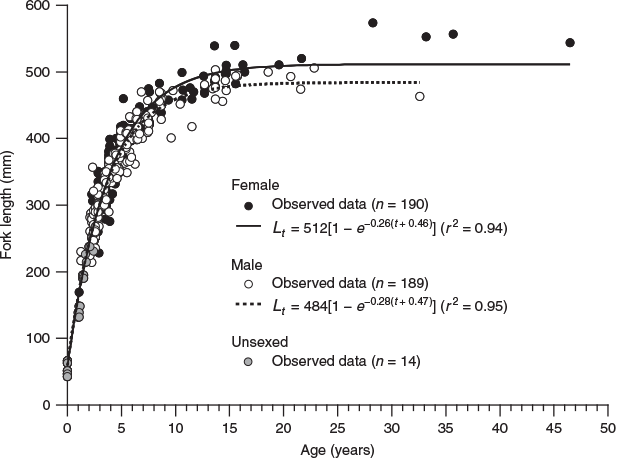
Because the opaque zones in the otoliths were validated as annuli, age estimations were performed based on these otoliths. The length-at-age data fitted the VBGF for each sex (Fig. 7), which was calculated as follows for females:
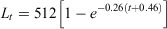
where r2 = 0.94; and for males:
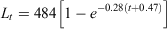
where r2 = 0.95.
|
The likelihood ratio test indicated that the VBGF was significantly different between sexes (χ2 = 22.8; d.f. = 3; P < 0.001). The VBGFs showed that females became slightly larger than males as they became older, and that females and males reached 95% of L∞ at 11 and 10 years of age respectively. The observed maximum age for females and males was 46 years (544 mm FL) and 32 years (463 mm FL) respectively.
Age at sexual maturity
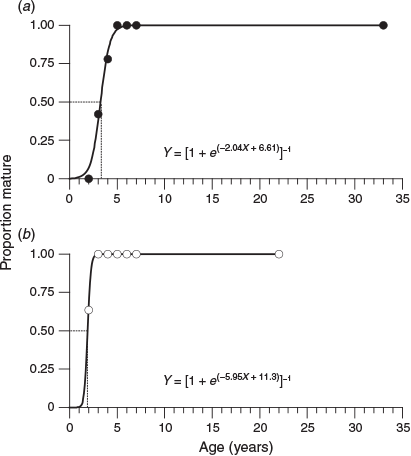
Based on the estimated ages determined from the otoliths, the percentage of mature females in the 2-, 3- and 4-year age classes was 0, 42 and 78% respectively, with all females reaching maturity after 5 years (Fig. 8a). By contrast, 64% of males reached maturity at 2 years of age and all males reached maturity after 3 years (Fig. 8b). The age at 50% sexual maturity was 3.2 years for females and 1.9 years for males.
|
Year-class strength
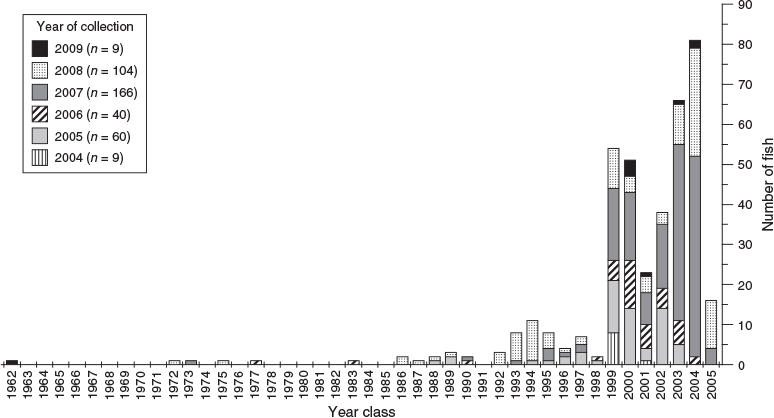
The number of samples differed across the 6-year sampling period. However, the lifespan of K. bigibbus spans multiple decades. Thus, there was virtually no sampling bias for year-class strength. The oldest specimen was born in 1962, with a few individuals being born between 1962 and 1998. However, there was a rapid increase in the number of individuals born from 1999 onwards (Fig. 9).
Discussion
Our comparison of the opaque zones of scales and otoliths of K. bigibbus and their validity as age-determining structures showed that scales should be used with caution. Because the opaque zones were not clearly visible in the scales, there were many errors in reading the number of zones. In particular, the identification of opaque zones in scales was difficult in marginal areas. This may be why a clear periodicity could not be detected in scale opaque zone formation with edge and marginal increment analyses, which depend on the visibility of zones in the marginal areas of structures. Because the opaque zone counts for samples with one to five zones generally showed close correlation between scales and otoliths, the opaque zones in scales may also be formed annually. For samples with more than five zones, the opaque zone counts on scales were lower than those in otoliths, as described by Beamish and McFarlane (1987). Because scale growth is proportional to somatic growth (Fig. 4a), slower somatic growth coincides with a decreased scale growth rate. Therefore, the pattern of newly formed opaque zones in the scales of specimens that exhibit slower growth rates would be extremely dense and virtually unreadable.
In comparison, opaque zones were clearly visible in the otoliths, even in marginal areas. Therefore, a clear periodicity could be detected in otolith opaque zone formation. Moreover, otolith growth continued towards the proximal side after somatic growth began to plateau (Fig. 4b). Thus, sectioned otoliths could be used to determine the age of older specimens, as described by Beamish (1979). In the present study, we initially believed that we would have difficulty observing opaque zones in otolith sections (Fig. 2c). However, by carefully making microscopic adjustments to the section angle, we were able to prepare otolith sections in which the opaque zones could be read clearly in virtually all instances (Fig. 2d, e). To read zones in otolith sections clearly, researchers have used various techniques, such as burning (Christensen 1964; Power 1978; Francis 1981), staining (Albrechtsen 1968; Richter and McDermott 1990; Arneri et al. 2001), thin sectioning (Beamish 1979; Anderson et al. 1992; Wakefield et al. 2017) and image enhancement techniques (Estep et al. 1995; Dwyer et al. 2003; Campana et al. 2016). Moreover, the clarity of zones in otolith sections is affected by various factors, such as section thickness (Chilton and Beamish 1982; Peres and Haimovici 2004), cutting orientation (e.g. perpendicular to the anterior posterior axis or sulcus acusticus; Beamish 1979; Wakefield et al. 2017) and observation methods (e.g. using transmitted or reflected light; Morales-Nin and Panfili 2002; VanderKooy 2009). Differences in shape, size and composition of otoliths among species require species-specific modifications to otolith section preparation (VanderKooy 2009). Here, we found that the clarity of opaque zones in otolith sections of K. bigibbus was closely dependent on the perpendicularity of the opaque zones. VanderKooy (2009) introduced an observation technique for an otolith section that was not cut absolutely perpendicular to the core: by tilting a microscope slide so that the opaque zones were perpendicular to the observation plane, it was possible to correct the double images of the opaque zones. We also used this observation technique to improve opaque zone clarity in K. bigibbus otolith sections. However, this technique alone could not ensure the accurate perpendicularity of opaque zones. Therefore, to ensure that opaque zones were exactly perpendicular to the sectioning surface, we had to adjust the angle of sectioning by careful hand grinding with frequent observation under the microscope while transforming a thick section into a thin section. Although this is a time-consuming procedure, preparing otoliths using the described techniques may also enhance the precision of age determination for other fish species.
Based on otolith sections, the maximum ages of K. bigibbus females and males were determined to be 46 and 32 years respectively. Off South Africa, the maximum age of Neoscorpis lithophilus, also within Kyphosidae (Knudsen and Clements 2016), was 10 years (Mann et al. 2002). Moreover, among the Scorpididae, which is the most closely related family to Kyphosidae (Knudsen and Clements 2016), the maximum age of Scorpis lineolata off eastern Australia was 54 years (Stewart and Hughes 2005) and that of Scorpis aequipinnis off western Australia was 68 years (Coulson et al. 2012). The maximum age of Labracoglossa argenteiventris and Medialuna californiensis, also members of Scorpididae, off central Japan and southern California respectively was 8 years for both (Watari et al. 2005; Bredvik et al. 2011). Although the factors contributing to these lifespan differences are not entirely clear, they illustrate that the lifespan of K. bigibbus is relatively long compared with that of closely related species.
Froese and Binohlan (2000) have developed an empirical relationship between longevity and age at maturity based on the data for over 400 fish species. Coulson et al. (2012) compared the relationships between A50 and maximum age for S. aequipinnis and S. lineolata with that of other fishes using the equation of Froese and Binohlan (2000) and showed that the A50 of 14.1 and 16.1 years for female and male S. aequipinnis respectively was typical for species with similar longevity, whereas the A50 of 2.5 years for S. lineolata would be far more typical of a species with a much shorter life cycle. Based on the equation of Froese and Binohlan (2000), and using the maximum ages obtained for female and male K. bigibbus specimens, we predicted an A50 for female and male K. bigibbus of 14.7 and 10.2 years respectively; these values are higher than the actual A50 estimated in our study (3.2 and 1.9 years for females and males respectively). Therefore, the ages at sexual maturity for K. bigibbus are relatively low compared with those of other species with similar longevity.
Early maturation and considerable longevity are indicative of a potentially long reproductive lifespan and thus long-term population recruitment. Several fishes inhabiting shallow reef habitats have similar life history strategies, with long-term population recruitment (e.g. Stewart and Hughes 2005; Ewing et al. 2007; Andrews et al. 2016). This strategy reduces the risk of a population collapsing during long periods of environmental conditions that are unfavourable for successful recruitment (Leaman and Beamish 1984). Therefore, species with this life history strategy have often been considered to have evolved to compensate for uncertainty in reproductive success in any given year, and often exhibit highly variable year-class strength in their populations (Longhurst 2002; Walsh et al. 2010; Stewart 2011). In addition, Coulson et al. (2010) suggested that, under certain circumstances, far greater mortality of smaller and younger individuals and constraints on the growth of adults may play major roles in selecting for early maturity and a long life.
On the west coast of Kyushu, Japan, three herbivorous fishes (i.e. K. bigibbus, Siganus fuscescens and C. japonicus) are considered to have a critical effect on algal forests (Kiriyama et al. 2001; Yamaguchi 2010). Among these three herbivorous fishes, the maximum age and standard length of 46 years and 506 mm recorded for K. bigibbus in this study greatly exceeded the 13 years and 357 mm for S. fuscescens (Katayama et al. 2009) and the 8 years and 384 mm for C. japonicus (Kume et al. 2010; G. Kume, unpubl. data). Furthermore, K. bigibbus is an obligate herbivore, unlike the other two species (Yamaguchi et al. 2010). Therefore, at the individual level, K. bigibbus could have a more significant effect on algal forests than either S. fuscescens or C. japonicus.
Our results suggest that K. bigibbus recruitment may have increased rapidly since 1999. There has been extensive loss of kelp forests in the study area resulting from overgrazing by herbivorous fishes since the autumn of 1998 (Kiriyama et al. 1999), with recent increases in seawater temperatures being cited as a potential factor contributing to this phenomenon (Kiriyama 2009; Yamaguchi 2010). In the coastal waters of Meshima, Nagasaki Prefecture, which is near the study area, mean annual sea water temperature increased by ~0.9°C from 1955 to 2003, and has remained high since 1998, with a peak record of 22.6°C (Kiriyama 2009). High seawater temperatures may decrease winter mortality of K. bigibbus, leading to an increase in the number of overwintering individuals that are able to spawn the following season (Figueira and Booth 2010). Beck et al. (2017) indicated that temperate macroalgal patches may strongly inhibit the recruitment of many tropical reef fish species, primarily planktivores and herbivores. The noticeable losses of kelp forests in 1998 may have facilitated the rapid increase in K. bigibbus recruitment since 1999. Thus, it is important to elucidate the early life history strategies of this species to address these issues.
In tropical regions, reducing exploitation of herbivores is considered to lead to protection of coral reefs, because herbivores contribute to the maintenance of coral reefs by removing macroalgal species (Bellwood et al. 2004; Hughes et al. 2007; Mumby and Harborne 2010). K. bigibbus also plays a major role in the removal of macroalgae, as reported in Ningaloo Reef, Australia (Michael et al. 2013). The feeding activity of herbivorous fishes could also contribute towards sustaining communities associated with temperate algal forests. For example, their feeding activity causes the detachment and drifting of portions of Sargassum species, which constitute an essential nursery ground for larval or juvenile fishes (Yamaguchi 2008). This process results in some kelp matter sinking to the seabed, providing a food source for abalone and turban shells (Noda 2006). Furthermore, Ruz et al. (2018) suggested that kelp consumption by the herbivorous fish Aplodactylus punctatus may positively affect kelp populations by facilitating the transport of kelp zoospores. Therefore, the loss of herbivorous fishes could adversely affect algal forest ecosystems. In Japan, the government has recommended culling herbivores to preserve algal forests (Fisheries Agency 2015). However, regarding herbivorous fishes as harmful organisms and culling them indiscriminately could lead to ecosystem destruction (Yamaguchi 2006).
We expand on the findings of existing studies on the behavioural and reproductive ecology and feeding habits of K. bigibbus (Yamaguchi et al. 2006, 2011; Yamaguchi 2010) by clarifying several life history traits for this species. However, details of the early life history, population dynamics and population structure of K. bigibbus remain unclear. In conclusion, before implementing any culling strategy as a means to preserve algal forests, we must first establish the biological and ecological characteristics of herbivorous fishes, including K. bigibbus, and determine how they contribute to each ecosystem.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was supported, in part, by Nagasaki Prefecture’s project for countermeasures against rocky shore denudation in coastal areas in Nagasaki Prefecture and JSPS KAKENHI (grant number 18H02268).
Acknowledgements
The authors are grateful to A. Hideshima, J. Fukushima, T. Miura and the commercial fishermen of the Nomozaki Fisheries Cooperative Association for providing assistance with the field survey. The authors thank G. Kume (Kagoshima University, Kagoshima), K. Inoue, K. Hara and many students (Nagasaki University, Nagasaki) for their support and research discussions. The authors also thank two anonymous reviewers for constructive comments on the manuscript. Finally, the authors thank Editage (www.editage.jp) for English language editing.
References
Albrechtsen, K. (1968). A dyeing technique for otolith age reading. Journal du Conseil Permanent International pour l’Exploration de la Mer 32, 278–280.| A dyeing technique for otolith age reading.Crossref | GoogleScholarGoogle Scholar |
Anderson, J. R., Morison, A. K., and Ray, D. J. (1992). Validation of the use of thin-sectioned otoliths for determining the age and growth of golden perch, Macquaria ambigua (Perciformes: Percichthyidae), in the lower Murray–Darling basin, Australia. Marine and Freshwater Research 43, 1103–1128.
| Validation of the use of thin-sectioned otoliths for determining the age and growth of golden perch, Macquaria ambigua (Perciformes: Percichthyidae), in the lower Murray–Darling basin, Australia.Crossref | GoogleScholarGoogle Scholar |
Andrews, A. H., DeMartini, E. E., Eble, J. A., Taylor, B. M., Lou, D. C., and Humphreys, R. L. (2016). Age and growth of bluespine unicornfish (Naso unicornis): a half-century life-span for a keystone browser, with a novel approach to bomb radiocarbon dating in the Hawaiian Islands. Canadian Journal of Fisheries and Aquatic Sciences 73, 1575–1586.
| Age and growth of bluespine unicornfish (Naso unicornis): a half-century life-span for a keystone browser, with a novel approach to bomb radiocarbon dating in the Hawaiian Islands.Crossref | GoogleScholarGoogle Scholar |
Arneri, E., Colella, S., and Giannetti, G. (2001). Age determination and growth of turbot and brill in the Adriatic Sea: reversal of the seasonal pattern of otolith zone formation. Journal of Applied Ichthyology 17, 256–261.
| Age determination and growth of turbot and brill in the Adriatic Sea: reversal of the seasonal pattern of otolith zone formation.Crossref | GoogleScholarGoogle Scholar |
Basford, A. J., Feary, D. A., Truong, G., Steinberg, P. D., Marzinelli, E. M., and Vergés, A. (2016). Feeding habits of range-shifting herbivores: tropical surgeonfishes in a temperate environment. Marine and Freshwater Research 67, 75–83.
| Feeding habits of range-shifting herbivores: tropical surgeonfishes in a temperate environment.Crossref | GoogleScholarGoogle Scholar |
Baudouin, M., Marengo, M., Pere, A., Culioli, J. M., Santoni, M. C., Marchand, B., and Durieux, E. D. (2016). Comparison of otolith and scale readings for age and growth estimation of common dentex Dentex dentex. Journal of Fish Biology 88, 760–766.
| Comparison of otolith and scale readings for age and growth estimation of common dentex Dentex dentex.Crossref | GoogleScholarGoogle Scholar | 26563912PubMed |
Beamish, R. J. (1979). Differences in the age of Pacific hake (Merluccius productus) using whole otoliths and sections of otoliths. Journal of the Fisheries Board of Canada 36, 141–151.
| Differences in the age of Pacific hake (Merluccius productus) using whole otoliths and sections of otoliths.Crossref | GoogleScholarGoogle Scholar |
Beamish, R. J., and Chilton, D. E. (1982). Preliminary evaluation of a method to determine the age of sablefish (Anoplopoma fimbria). Canadian Journal of Fisheries and Aquatic Sciences 39, 277–287.
| Preliminary evaluation of a method to determine the age of sablefish (Anoplopoma fimbria).Crossref | GoogleScholarGoogle Scholar |
Beamish, R. J., and Fournier, D. A. (1981). A method for comparing the precision of a set of age determinations. Canadian Journal of Fisheries and Aquatic Sciences 38, 982–983.
| A method for comparing the precision of a set of age determinations.Crossref | GoogleScholarGoogle Scholar |
Beamish, R. J., and McFarlane, G. A. (1987). Current trends in age determination methodology. In ‘Age and Growth of Fish’. (Eds R. C. Summerfelt and G. E. Hall.) pp. 15–42. (Iowa State University Press: Ames, IA, USA.)
Beck, H. J., Feary, D. A., Nakamura, Y., and Booth, D. J. (2017). Temperate macroalgae impacts tropical fish recruitment at forefronts of range expansion. Coral Reefs 36, 639–651.
| Temperate macroalgae impacts tropical fish recruitment at forefronts of range expansion.Crossref | GoogleScholarGoogle Scholar |
Bellwood, D. R., Hughes, T. P., Folke, C., and Nyström, M. (2004). Confronting the coral reef crisis. Nature 429, 827–833.
| Confronting the coral reef crisis.Crossref | GoogleScholarGoogle Scholar | 15215854PubMed |
Bennett, S., Wernberg, T., Harvey, E. S., Santana-Garcon, J., and Saunders, B. J. (2015). Tropical herbivores provide resilience to a climate-mediated phase shift on temperate reefs. Ecology Letters 18, 714–723.
| Tropical herbivores provide resilience to a climate-mediated phase shift on temperate reefs.Crossref | GoogleScholarGoogle Scholar | 25994785PubMed |
Bennett, S., Wernberg, T., Connell, S. D., Hobday, A. J., Johnson, C. R., and Poloczanska, E. S. (2016). The ‘Great Southern Reef’: social, ecological and economic value of Australia’s neglected kelp forests. Marine and Freshwater Research 67, 47–56.
| The ‘Great Southern Reef’: social, ecological and economic value of Australia’s neglected kelp forests.Crossref | GoogleScholarGoogle Scholar |
Blamey, L. K., and Bolton, J. J. (2018). The economic value of South African kelp forests and temperate reefs: past, present and future. Journal of Marine Systems 188, 172–181.
| The economic value of South African kelp forests and temperate reefs: past, present and future.Crossref | GoogleScholarGoogle Scholar |
Bredvik, J. J., Boerger, C., and Allen, L. G. (2011). Age and growth of two herbivorous, kelp forest fishes, the opaleye (Girella nigricans) and halfmoon (Medialuna californiensis). Bulletin of the Southern California Academy of Sciences 110, 25–34.
| Age and growth of two herbivorous, kelp forest fishes, the opaleye (Girella nigricans) and halfmoon (Medialuna californiensis).Crossref | GoogleScholarGoogle Scholar |
Campana, S. E. (2001). Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. Journal of Fish Biology 59, 197–242.
| Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., Annand, M. C., and McMillan, J. I. (1995). Graphical and statistical methods for determining the consistency of age determinations. Transactions of the American Fisheries Society 124, 131–138.
| Graphical and statistical methods for determining the consistency of age determinations.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., Valentin, A. E., MacLellan, S. E., and Groot, J. B. (2016). Image-enhanced burnt otoliths, bomb radiocarbon and the growth dynamics of redfish (Sebastes mentella and S. fasciatus) off the eastern coast of Canada. Marine and Freshwater Research 67, 925–936.
| Image-enhanced burnt otoliths, bomb radiocarbon and the growth dynamics of redfish (Sebastes mentella and S. fasciatus) off the eastern coast of Canada.Crossref | GoogleScholarGoogle Scholar |
Chang, W. Y. B. (1982). A statistical method for evaluating the reproducibility of age determination. Canadian Journal of Fisheries and Aquatic Sciences 39, 1208–1210.
| A statistical method for evaluating the reproducibility of age determination.Crossref | GoogleScholarGoogle Scholar |
Chilton, D. E., and Beamish, R. J. (1982). Age determination methods for fishes studied by the groundfish program at the Pacific Biological Station. Canadian Special Publication of Fisheries and Aquatic Sciences number 60, Department of Fisheries and Oceans, Ottawa, ON, Canada.
Christensen, J. M. (1964). Burning of otoliths, a technique for age determination of soles and other fish. Journal du Conseil Permanent International pour l’Exploration de la Mer 29, 73–81.
| Burning of otoliths, a technique for age determination of soles and other fish.Crossref | GoogleScholarGoogle Scholar |
Clements, K. D., and Choat, J. H. (1997). Comparison of herbivory in the closely related marine fish genera Girella and Kyphosus. Marine Biology 127, 579–586.
| Comparison of herbivory in the closely related marine fish genera Girella and Kyphosus.Crossref | GoogleScholarGoogle Scholar |
Clements, K. D., and Zemke-White, W. L. (2008). Diet of subtropical herbivorous fishes in northeastern New Zealand. New Zealand Journal of Marine and Freshwater Research 42, 47–55.
| Diet of subtropical herbivorous fishes in northeastern New Zealand.Crossref | GoogleScholarGoogle Scholar |
Coulson, P. G., Hesp, S. A., Hall, N. G., and Potter, I. C. (2009). The western blue groper (Achoerodus gouldii), a protogynous hermaphroditic labrid with exceptional longevity, late maturity, slow growth, and both late maturation and sex change. Fishery Bulletin 107, 57–75.
Coulson, P. G., Hesp, S. A., Potter, I. C., and Hall, N. G. (2010). Life cycle characteristics of the blue morwong Nemadactylus valenciennesi, compared with those of other species of Cheilodactylidae. Marine and Freshwater Research 61, 104–118.
| Life cycle characteristics of the blue morwong Nemadactylus valenciennesi, compared with those of other species of Cheilodactylidae.Crossref | GoogleScholarGoogle Scholar |
Coulson, P. G., Potter, I. C., and Hall, N. G. (2012). The biological characteristics of Scorpis aequipinnis (Kyphosidae), including relevant comparisons with those of other species and particularly of a heavily exploited congener. Fisheries Research 125–126, 272–282.
| The biological characteristics of Scorpis aequipinnis (Kyphosidae), including relevant comparisons with those of other species and particularly of a heavily exploited congener.Crossref | GoogleScholarGoogle Scholar |
Downie, R. A., Babcock, R. C., Thomson, D. P., and Vanderklift, M. A. (2013). Density of herbivorous fish and intensity of herbivory are influenced by proximity to coral reefs. Marine Ecology Progress Series 482, 217–225.
| Density of herbivorous fish and intensity of herbivory are influenced by proximity to coral reefs.Crossref | GoogleScholarGoogle Scholar |
Dwyer, K. S., Walsh, S. J., and Campana, S. E. (2003). Age determination, validation and growth of Grand Bank yellowtail flounder (Limanda ferruginea). ICES Journal of Marine Science 60, 1123–1138.
| Age determination, validation and growth of Grand Bank yellowtail flounder (Limanda ferruginea).Crossref | GoogleScholarGoogle Scholar |
Estep, K. W., Nedreaas, K. H., and Maclntyre, F. (1995). Computer image enhancement and presentation of otoliths. In ‘Recent Developments in Fish Otolith Research’. (Eds D. H. Secor, J. M. Dean, and S. E. Campana.) pp. 303–317. (University of South Carolina Press: Columbia, SC, USA.)
Ewing, G. P., Lyle, J. M., Murphy, R. J., Kalish, J. M., and Ziegler, P. E. (2007). Validation of age and growth in a long-lived temperate reef fish using otolith structure, oxytetracycline and bomb radiocarbon methods. Marine and Freshwater Research 58, 944–955.
| Validation of age and growth in a long-lived temperate reef fish using otolith structure, oxytetracycline and bomb radiocarbon methods.Crossref | GoogleScholarGoogle Scholar |
Figueira, W. F., and Booth, D. J. (2010). Increasing ocean temperatures allow tropical fishes to survive overwinter in temperate waters. Global Change Biology 16, 506–516.
| Increasing ocean temperatures allow tropical fishes to survive overwinter in temperate waters.Crossref | GoogleScholarGoogle Scholar |
Fisheries Agency (2015). ‘Kaitei Isoyake Taisaku Guideline.’ (Fisheries Agency: Tokyo, Japan.) [In Japanese].
Fowler, A. J., and Short, D. A. (1998). Validation of age determination from otoliths of the King George whiting Sillaginodes punctata (Perciformes). Marine Biology 130, 577–587.
| Validation of age determination from otoliths of the King George whiting Sillaginodes punctata (Perciformes).Crossref | GoogleScholarGoogle Scholar |
Francis, M. P. (1981). Age and growth of moki, Latridopsis ciliaris (Teleostei: Latridae). New Zealand Journal of Marine and Freshwater Research 15, 47–49.
| Age and growth of moki, Latridopsis ciliaris (Teleostei: Latridae).Crossref | GoogleScholarGoogle Scholar |
Froese, R., and Binohlan, C. (2000). Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. Journal of Fish Biology 56, 758–773.
| Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data.Crossref | GoogleScholarGoogle Scholar |
Gianni, F., Bartolini, F., Pey, A., Laurent, M., Martins, G. M., Airoldi, L., and Mangialajo, L. (2017). Threats to large brown algal forests in temperate seas: the overlooked role of native herbivorous fish. Scientific Reports 7, 6012.
| Threats to large brown algal forests in temperate seas: the overlooked role of native herbivorous fish.Crossref | GoogleScholarGoogle Scholar | 28729633PubMed |
Gray, C. A., Haddy, J. A., Fearman, J., Barnes, L. M., Macbeth, W. G., and Kendall, B. W. (2012). Reproduction, growth and connectivity among populations of Girella tricuspidata (Pisces: Girellidae). Aquatic Biology 16, 53–68.
| Reproduction, growth and connectivity among populations of Girella tricuspidata (Pisces: Girellidae).Crossref | GoogleScholarGoogle Scholar |
Hughes, T. P., Rodrigues, M. J., Bellwood, D. R., Ceccarelli, D., Hoegh-Guldberg, O., McCook, L., Moltschaniwskyj, N., Pratchett, M. S., Steneck, R. S., and Willis, B. (2007). Phase shifts, herbivory, and the resilience of coral reefs to climate change. Current Biology 17, 360–365.
| Phase shifts, herbivory, and the resilience of coral reefs to climate change.Crossref | GoogleScholarGoogle Scholar | 17291763PubMed |
Inoue, K., Yamaguchi, A., Kiriyama, T., and Yoshimura, T. (2006). Age and growth of the grey sea chub Kyphosus bigibbus off Nomozaki, Nagasaki Prefecture. In ‘Abstracts for the Annual Meeting of the Japanese Society of Fisheries Science’, 30 March 2006, Kochi, Japan. (Ed. Y. Itoh.) p. 48. [Abstract] (The Japanese Society of Fisheries Science: Tokyo, Japan.) [In Japanese].
Katayama, S., Akiyama, S., Naganuma, M., and Shibata, R. (2009). Age and growth of rabbitfish Siganus fuscescens in Tateyama Bay, Japan. Suisan Zoshoku 57, 417–422.
Kimura, D. K. (1980). Likelihood methods for the von Bertalanffy growth curve. Fishery Bulletin 77, 765–776.
Kiriyama, T. (2009). Study on recent decline of large brown alga population in coastal waters around Nagasaki Prefecture. Bulletin of Nagasaki Prefectural Institute of Fisheries 35, 15–78.
Kiriyama, T., Fujii, A., Yoshimura, T., Kiyomoto, S., and Yotsui, T. (1999). Leaf-lost phenomenon observed on three laminariaceous species in coastal waters around Nagasaki prefecture in autumn 1998. Suisan Zoshoku 47, 319–323.
Kiriyama, T., Noda, M., and Fujii, A. (2001). Grazing and bite marks on Ecklonia kurome, caused by several herbivorous fishes. Suisan Zoshoku 49, 431–438.
Kiyomoto, S., Yoshimura, T., Arai, S., Kiriyama, T., Fujii, A., and Yotsui, T. (2000). Recovery of Ecklonia kurome after the occurrence of blade disappearing phenomenon at Nomozaki, Nagasaki Prefecture. Bulletin of Seikai National Fisheries Research Institute 78, 57–65.
Knudsen, S. W., and Clements, K. D. (2013). Revision of the fish family Kyphosidae (Teleostei: Perciformes). Zootaxa 3751, 1–101.
| Revision of the fish family Kyphosidae (Teleostei: Perciformes).Crossref | GoogleScholarGoogle Scholar | 29097648PubMed |
Knudsen, S. W., and Clements, K. D. (2016). World-wide species distributions in the family Kyphosidae (Teleostei: Perciformes). Molecular Phylogenetics and Evolution 101, 252–266.
| World-wide species distributions in the family Kyphosidae (Teleostei: Perciformes).Crossref | GoogleScholarGoogle Scholar | 27143240PubMed |
Krumhansl, K. A., Okamoto, D. K., Rassweiler, A., Novak, M., Bolton, J. J., Cavanaugh, K. C., Connell, S. D., Johnson, C. R., Konar, B., Ling, S. D., Micheli, F., Norderhaug, K. M., Pérez-Matus, A., Sousa-Pinto, I., Reed, D. C., Salomon, A. K., Shears, N. T., Wernberg, T., Anderson, R. J., Barrett, N. S., Buschmann, A. H., Carr, M. H., Caselle, J. E., Derrien-Courtel, S., Edgar, G. J., Edwards, M., Estes, J. A., Goodwin, C., Kenner, M. C., Kushner, D. J., Moy, F. E., Nunn, J., Steneck, R. S., Vásquez, J., Watson, J., Witman, J. D., and Byrnes, J. E. (2016). Global patterns of kelp forest change over the past half-century. Proceedings of the National Academy of Sciences of the United States of America 113, 13785–13790.
| Global patterns of kelp forest change over the past half-century.Crossref | GoogleScholarGoogle Scholar | 27849580PubMed |
Kume, G., Kubo, Y., Yoshimura, T., Kiriyama, T., and Yamaguchi, A. (2010). Life history characteristics of the protogynous parrotfish Calotomus japonicus from northwest Kyushu, Japan. Ichthyological Research 57, 113–120.
| Life history characteristics of the protogynous parrotfish Calotomus japonicus from northwest Kyushu, Japan.Crossref | GoogleScholarGoogle Scholar |
Kuwahara, H. (2015). The evaluation of current status for grazing control techniques of herbivorous fish. Journal of Fisheries Engineering 51, 253–257.
| The evaluation of current status for grazing control techniques of herbivorous fish.Crossref | GoogleScholarGoogle Scholar |
Leaman, B. M., and Beamish, R. J. (1984). Ecological and management implications of longevity in some northeast Pacific groundfishes. International North Pacific Fisheries Commission Bulletin 42, 85–97.
Longhurst, A. (2002). Murphy’s law revisited: longevity as a factor in recruitment to fish populations. Fisheries Research 56, 125–131.
| Murphy’s law revisited: longevity as a factor in recruitment to fish populations.Crossref | GoogleScholarGoogle Scholar |
Lou, D. C. (1992). Validation of annual growth bands in the otolith of tropical parrotfishes (Scarus schlegeli Bleeker). Journal of Fish Biology 41, 775–790.
| Validation of annual growth bands in the otolith of tropical parrotfishes (Scarus schlegeli Bleeker).Crossref | GoogleScholarGoogle Scholar |
Mann, B. Q., Fennessy, S. T., Govender, A., and van der Walt, B. A. (2002). Age and growth and a preliminary stock assessment of stonebream Neoscorpis lithophilus (Pisces: Scorpididae) along the KwaZulu–Natal coast, South Africa. Marine and Freshwater Research 53, 131–138.
| Age and growth and a preliminary stock assessment of stonebream Neoscorpis lithophilus (Pisces: Scorpididae) along the KwaZulu–Natal coast, South Africa.Crossref | GoogleScholarGoogle Scholar |
Michael, P. J., Hyndes, G. A., Vanderklift, M. A., and Vergés, A. (2013). Identity and behaviour of herbivorous fish influence large-scale spatial patterns of macroalgal herbivory in a coral reef. Marine Ecology Progress Series 482, 227–240.
| Identity and behaviour of herbivorous fish influence large-scale spatial patterns of macroalgal herbivory in a coral reef.Crossref | GoogleScholarGoogle Scholar |
Mineur, F., Arenas, F., Assis, J., Davies, A. J., Engelen, A. H., Fernandes, F., Malta, E.-j., Thibaut, T., Van Nguyen, T., Vaz-Pinto, F., Vranken, S., Serrão, E. A., and De Clerck, O. (2015). European seaweeds under pressure: consequences for communities and ecosystem functioning. Journal of Sea Research 98, 91–108.
| European seaweeds under pressure: consequences for communities and ecosystem functioning.Crossref | GoogleScholarGoogle Scholar |
Morales-Nin, B., and Panfili, J. (2002). Preparation and observation techniques D. Observation. In ‘Manual of Fish Sclerochronology’. (Eds J. Panfili, H. de Pontual, H. Troadec, and P. J. Wright.) pp. 358–369. (Institut français de recherche pour l’exploitation de la mer (IFREMER) and Institut de recherche pour le développement (IRD): Brest, France.)
Mumby, P. J., and Harborne, A. R. (2010). Marine reserves enhance the recovery of corals on Caribbean reefs. PLoS One 5, e8657.
| Marine reserves enhance the recovery of corals on Caribbean reefs.Crossref | GoogleScholarGoogle Scholar | 20066158PubMed |
Noda, M. (2006). Characteristics of feeding behavior of Siganus fuscescens. In ‘Marine Herbivorous Fish – Ecology, Fishery and Utilization’. (Eds D. Fujita, M. Noda, and H. Kuwahara.) pp. 114–126. (Seizando-Shoten: Tokyo, Japan.)
Paul, L. J., and Horn, P. L. (2009). Age and growth of sea perch (Helicolenus percoides) from two adjacent areas off the east coast of South Island, New Zealand. Fisheries Research 95, 169–180.
| Age and growth of sea perch (Helicolenus percoides) from two adjacent areas off the east coast of South Island, New Zealand.Crossref | GoogleScholarGoogle Scholar |
Peres, M. B., and Haimovici, M. (2004). Age and growth of southwestern Atlantic wreckfish Polyprion americanus. Fisheries Research 66, 157–169.
| Age and growth of southwestern Atlantic wreckfish Polyprion americanus.Crossref | GoogleScholarGoogle Scholar |
Pillans, R. D., Babcock, R. C., Thomson, D. P., Haywood, M. D. E., Downie, R. A., Vanderklift, M. A., and Rochester, W. A. (2017). Habitat effects on home range and schooling behaviour in a herbivorous fish (Kyphosus bigibbus) revealed by acoustic tracking. Marine and Freshwater Research 68, 1454–1467.
| Habitat effects on home range and schooling behaviour in a herbivorous fish (Kyphosus bigibbus) revealed by acoustic tracking.Crossref | GoogleScholarGoogle Scholar |
Power, G. (1978). Fish population structure in Arctic lakes. Journal of the Fisheries Board of Canada 35, 53–59.
| Fish population structure in Arctic lakes.Crossref | GoogleScholarGoogle Scholar |
Richter, H., and McDermott, J. G. (1990). The staining of fish otoliths for age determination. Journal of Fish Biology 36, 773–779.
| The staining of fish otoliths for age determination.Crossref | GoogleScholarGoogle Scholar |
Ruz, C. S., Muth, A. F., Tala, F., and Pérez-Matus, A. (2018). The herbivorous fish, Aplodactylus punctatus, as a potential facilitator of dispersal of kelp, Lessonia trabeculata, in Chile. Journal of Experimental Marine Biology and Ecology 500, 112–119.
| The herbivorous fish, Aplodactylus punctatus, as a potential facilitator of dispersal of kelp, Lessonia trabeculata, in Chile.Crossref | GoogleScholarGoogle Scholar |
Smale, D. A., and Wernberg, T. (2013). Extreme climatic event drives range contraction of a habitat-forming species. Proceedings of the Royal Society of London – B. Biological Sciences 280, 20122829.
| Extreme climatic event drives range contraction of a habitat-forming species.Crossref | GoogleScholarGoogle Scholar |
Smale, D. A., Burrows, M. T., Moore, P., O’Connor, N., and Hawkins, S. J. (2013). Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective. Ecology and Evolution 3, 4016–4038.
| Threats and knowledge gaps for ecosystem services provided by kelp forests: a northeast Atlantic perspective.Crossref | GoogleScholarGoogle Scholar | 24198956PubMed |
Steneck, R. S., Graham, M. H., Bourque, B. J., Corbett, D., Erlandson, J. M., Estes, J. A., and Tegner, M. J. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation 29, 436–459.
| Kelp forest ecosystems: biodiversity, stability, resilience and future.Crossref | GoogleScholarGoogle Scholar |
Stewart, J. (2011). Evidence of age-class truncation in some exploited marine fish populations in New South Wales, Australia. Fisheries Research 108, 209–213.
| Evidence of age-class truncation in some exploited marine fish populations in New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Stewart, J., and Hughes, J. M. (2005). Longevity, growth, reproduction and a description of the fishery for silver sweep Scorpis lineolatus off New South Wales, Australia. New Zealand Journal of Marine and Freshwater Research 39, 827–838.
| Longevity, growth, reproduction and a description of the fishery for silver sweep Scorpis lineolatus off New South Wales, Australia.Crossref | GoogleScholarGoogle Scholar |
Teagle, H., Hawkins, S. J., Moore, P. J., and Smale, D. A. (2017). The role of kelp species as biogenic habitat formers in coastal marine ecosystems. Journal of Experimental Marine Biology and Ecology 492, 81–98.
| The role of kelp species as biogenic habitat formers in coastal marine ecosystems.Crossref | GoogleScholarGoogle Scholar |
VanderKooy, S. (Ed.) (2009). ‘A Practical Handbook for Determining the Age of Gulf of Mexico Fishes’, 2nd edn. (Gulf States Marine Fisheries Commission: Ocean Springs, MS, USA.)
Vergés, A., Steinberg, P. D., Hay, M. E., Poore, A. G., Campbell, A. H., Ballesteros, E., Heck, K. L., Booth, D. J., Coleman, M. A., Feary, D. A., Figueira, W., Langlois, T., Marzinelli, E. M., Mizerek, T., Mumby, P. J., Nakamura, Y., Roughan, M., van Sebille, E., Gupta, A. S., Smale, D. A., Tomas, F., Wernberg, T., and Wilson, S. K. (2014a). The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proceedings of the Royal Society of London – B. Biological Sciences 281, 20140846.
| The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts.Crossref | GoogleScholarGoogle Scholar |
Vergés, A., Tomas, F., Cebrian, E., Ballesteros, E., Kizilkaya, Z., Dendrinos, P., Karamanlidis, A. A., Spiegel, D., and Sala, E. (2014b). Tropical rabbitfish and the deforestation of a warming temperate sea. Journal of Ecology 102, 1518–1527.
| Tropical rabbitfish and the deforestation of a warming temperate sea.Crossref | GoogleScholarGoogle Scholar |
Vergés, A., Doropoulos, C., Malcolm, H. A., Skye, M., Garcia-Pizá, M., Marzinelli, E. M., Campbell, A. H., Ballesteros, E., Hoey, A. S., Vila-Concejo, A., Bozec, Y.-M., and Steinberg, P. D. (2016). Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proceedings of the National Academy of Sciences of the United States of America 113, 13791–13796.
| Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp.Crossref | GoogleScholarGoogle Scholar | 27849585PubMed |
Wakefield, C. B., O’Malley, J. M., Williams, A. J., Taylor, B. M., Nichols, R. S., Halafihi, T., Humphreys, R. L., Kaltavara, J., Nicol, S. J., and Newman, S. J. (2017). Ageing bias and precision for deep-water snappers: evaluating nascent otolith preparation methods using novel multivariate comparisons among readers and growth parameter estimates. ICES Journal of Marine Science 74, 193–203.
| Ageing bias and precision for deep-water snappers: evaluating nascent otolith preparation methods using novel multivariate comparisons among readers and growth parameter estimates.Crossref | GoogleScholarGoogle Scholar |
Walsh, C. T., Gray, C. A., West, R. J., van der Meulen, D. E., and Williams, L. F. G. (2010). Growth, episodic recruitment and age truncation in populations of a catadromous percichthyid, Macquaria colonorum. Marine and Freshwater Research 61, 397–407.
| Growth, episodic recruitment and age truncation in populations of a catadromous percichthyid, Macquaria colonorum.Crossref | GoogleScholarGoogle Scholar |
Watari, S., Yonezawa, J., Yamada, S., Tanaka, E., and Kitakado, T. (2005). Age and growth of yellowstriped butterfish, Labracoglossa argentiventris, around Izu Oshima Island. Fisheries Science 71, 86–94.
| Age and growth of yellowstriped butterfish, Labracoglossa argentiventris, around Izu Oshima Island.Crossref | GoogleScholarGoogle Scholar |
Wernberg, T., Smale, D. A., Tuya, F., Thomsen, M. S., Langlois, T. J., de Bettignies, T., Bennett, S., and Rousseaux, C. S. (2013). An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nature Climate Change 3, 78–82.
| An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot.Crossref | GoogleScholarGoogle Scholar |
Wernberg, T., Bennett, S., Babcock, R. C., de Bettignies, T., Cure, K., Depczynski, M., Dufois, F., Fromont, J., Fulton, C. J., Hovey, R. K., Harvey, E. S., Holmes, T. H., Kendrick, G. A., Radford, B., Santana-Garcon, J., Saunders, B. J., Smale, D. A., Thomsen, M. S., Tuckett, C. A., Tuya, F., Vanderklift, M. A., and Wilson, S. (2016). Climate-driven regime shift of a temperate marine ecosystem. Science 353, 169–172.
| Climate-driven regime shift of a temperate marine ecosystem.Crossref | GoogleScholarGoogle Scholar | 27387951PubMed |
Yamaguchi, A. (2006). Investigating feeding and behavioral ecology. In ‘Marine Herbivorous Fish – Ecology, Fishery and Utilization’. (Eds D. Fujita, M. Noda, and H. Kuwahara.) pp. 126–137. (Seizando-Shoten: Tokyo, Japan.) [In Japanese, title translated by Y. Ogino].
Yamaguchi, A. (2008). Behavior and migration of herbivorous fish. In ‘Science and Restoration Technology of Marine Deforestation ‘Isoyake’’. (Eds K. Taniguchi, Y. Agatsuma, and N. Saga.) pp. 70–80. (Kouseisha Kouseikaku: Tokyo, Japan.)
Yamaguchi, A. (2010). Biological aspects of herbivorous fishes in the coastal areas of western Japan. Suisan Sougou Kenkyuu Senta Kenkyuu Houkoku 32, 89–94.
Yamaguchi, A., Kume, G., Higuchi, T., and Takita, T. (2004). Geographic variation in the growth of white croaker, Pennahia argentata, off the coast of northwest Kyushu, Japan. Environmental Biology of Fishes 71, 179–188.
| Geographic variation in the growth of white croaker, Pennahia argentata, off the coast of northwest Kyushu, Japan.Crossref | GoogleScholarGoogle Scholar |
Yamaguchi, A., Inoue, K., Furumitsu, K., Kiriyama, T., Yoshimura, T., Koido, T., and Nakata, H. (2006). Behavior and migration of rabbitfish Siganus fuscescens and grey seachub Kyphosus bigibbus off Nomozaki, Kyushu, tracked by biotelemetry method. Nippon Suisan Gakkaishi 72, 1046–1056.
| Behavior and migration of rabbitfish Siganus fuscescens and grey seachub Kyphosus bigibbus off Nomozaki, Kyushu, tracked by biotelemetry method.Crossref | GoogleScholarGoogle Scholar |
Yamaguchi, A., Furumitsu, K., Yagishita, N., and Kume, G. (2010). Biology of herbivorous fish in the coastal areas of Western Japan. In ‘Coastal Environmental and Ecosystem Issues of the East China Sea’. (Eds A. Ishimatsu and H.-J. Lie.) pp. 181–190. (TERRAPUB and Nagasaki University: Tokyo, Japan.)
Yamaguchi, A., Kume, G., Yoshimura, Y., Kiriyama, T., and Yoshimura, T. (2011). Spawning season and size at sexual maturity of Kyphosus bigibbus (Kyphosidae) from northwest Kyushu, Japan. Ichthyological Research 58, 283–287.
| Spawning season and size at sexual maturity of Kyphosus bigibbus (Kyphosidae) from northwest Kyushu, Japan.Crossref | GoogleScholarGoogle Scholar |
Yatsuya, K., Kiyomoto, S., and Yoshimura, T. (2015). Seasonal changes in dietary composition of the herbivorous fish Kyphosus bigibbus in southwestern Japan. Fisheries Science 81, 1025–1033.
| Seasonal changes in dietary composition of the herbivorous fish Kyphosus bigibbus in southwestern Japan.Crossref | GoogleScholarGoogle Scholar |