Isotopic evidence of connectivity between an inshore vegetated lagoon (nursery habitat) and coastal artificial reefs (adult habitats) for the reef fish Lethrinus lentjan on the Terengganu coast, Malaysia
Dung Quang Le A E , Siau Yin Fui A , Rumeaida Mat Piah B , Toyoho Ishimura C , Yuji Sano D , Kentaro Tanaka D and Kotaro Shirai D
A E , Siau Yin Fui A , Rumeaida Mat Piah B , Toyoho Ishimura C , Yuji Sano D , Kentaro Tanaka D and Kotaro Shirai D
A Institute of Oceanography and Environment, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia.
B School of Fisheries and Aquaculture Sciences, Universiti Malaysia Terengganu, 21030 Kuala Nerus, Terengganu, Malaysia.
C Department of Industrial Engineering, National Institute of Technology, Ibaraki College, 866 Nakane, Hitachinaka, Ibaraki 312-8508 Japan.
D Atmosphere and Ocean Research Institute, The University of Tokyo, 5-1-5, Kashiwanoha, Kashiwa-shi, Chiba 277-8564 Japan.
E Corresponding author. Email: lqdungimer@gmail.com; le.dung@umt.edu.my
Marine and Freshwater Research 70(12) 1675-1688 https://doi.org/10.1071/MF18302
Submitted: 1 August 2018 Accepted: 1 May 2019 Published: 6 August 2019
Journal Compilation © CSIRO 2019 Open Access CC BY-NC-ND
Abstract
Stable isotope analyses of muscle tissue (δ13Cmuscle and δ15Nmuscle) and otoliths (δ13Cotolith and δ18Ootolith) were used to retrospectively track habitat uses of Lethrinus lentjan, and to determine any association between Setiu Lagoon (nursery habitat) and coastal artificial reefs (CARs; adult habitats) on the Terengganu coast, Malaysia. Muscle stable isotopes exhibited a spatial change from inshore to offshore habitats associated with growth, possibly related to the reef-ward movement of the fish. Otolith stable isotopes of adult fish from CARs were measured in juvenile (from outside the core to the first opaque zone of otolith) and adult (the edge of otolith) portions and were compared with those of juveniles from Setiu Lagoon, suggesting that the adult fish may not primarily use the lagoon as a nursery before ontogenetically migrating to CARs. The effects of coastal currents between monsoonal seasons could reorientate offshore juvenile migration; hence, adult cohorts in CARs may be replenished from various nursery habitats along the coast. Additionally, similarities in the δ18Ootolith values of juvenile and adult sections suggested that some individuals may not spend their juvenile phases in shallow estuarine habitats. Based on the findings of this study, we recommend that coastal conservation strategies take into account multiple nursery habitats rather than a single one.
Additional keywords: ecological linkage, muscle, otolith, stable isotopes.
Introduction
Coral reefs are an important habitat for numerous commercially important target species, reef fisheries providing an important source of protein and livelihood for coastal human communities (Rioja-Nieto and Alvarez-Filip 2019). However, over- and destructive fishing, coastal development and pollution have threatened coral reefs in many tropical regions, including the east coast of Peninsular Malaysia (ECPM; Burke et al. 2012). The rapid degradation of coral reefs has resulted in a reduction of fisheries products, particularly high-value commercial reef fishes (Cruz-Trinidad et al. 2014). However, an increase in available coastal shelters close to nursery habitats can potentially increase recruitment to a fishery and therefore aid in fisheries recovery (Rilov and Benayahu 2000; Arney et al. 2017). The surrogate shelters provided by coastal artificial reefs (CARs) are considered to provide important habitats for post-settlement fish and other benthic reef macrofauna (Bohnsack 1989; Dupont 2008; Arney et al. 2017). In addition, adult populations on CARs can be replenished from adjacent natural coral reefs as ‘spillover’ effects of protected marine areas (Carr et al. 2017). Accordingly, CARs have been installed off the Terengganu coast as a conservation technique to increase and enhance the abundance of fishes and fish stock, fisheries management, aquaculture, research and recreation (Jensen 2002; Seaman 2007; Ali et al. 2013; Cresson et al. 2014). Some reef fishes, such as snappers, groupers and emperors, are generally recognised as migrating with growth from juvenile nursery areas, such as seagrass meadows and mangroves in estuaries, to adult habitats on reefs (Nagelkerken et al. 2002; Mumby 2006; Luo et al. 2009; Honda et al. 2013; Kimirei et al. 2013a). Nevertheless, limited information is available regarding the fish sources supplying such reefs, or the link between reef fish nursery and CAR habitats, despite the crucial importance of such information for the design of optimal fish management strategies for sustainable use and conservation.
The pink ear emperor fish (Lethrinus lentjan) is a very important commercial species for marine fisheries in subtropical and tropical regions of the Indian and Indo-Pacific oceans, including Malaysian coastal waters (Carpenter et al. 2016). However, this high-value species has been heavily exploited and possibly overfished in and around coral reefs (Carpenter et al. 2016). Being a reef-associated species, L. lentjan occurs in various habitats throughout its lifespan, including mangroves and seagrasses as juveniles before ontogenetically migrating to coral reefs as adults (Kimirei et al. 2013a, 2013b; Carpenter et al. 2016; Le et al. 2018). A previous study reported that juveniles (41–150-mm total length, TL) prefer to inhabit the mangrove–seagrass continua rather than the mangroves without adjacent seagrasses in Setiu Lagoon because mangroves are often exposed during low tide (Le et al. 2018). Furthermore, during our primary surveys conducted in mangrove estuaries, such as Kuala Besut, Sungai Keluang Besar, Sungai Semaerak, Merang Terengganu and Sungai Ibai, along the Terengganu coast in 2015, only a limited number of juveniles was found. Unlike juveniles, high densities of adult cohorts (>160 mm TL) were recorded from coral reefs and CARs off Terengganu coastal waters (Ali et al. 2013). Even though size-distributed populations related to resource utilisation abilities and predation risk support an ontogenetic habitat shift in reef fish species from estuarine and marine areas (Werner and Gilliam 1984; Nakamura et al. 2008), evidence of population connectivity is still lacking. Hence, this raised the possibility that Setiu Lagoon may be one of several important nursery habitats that replenish adult L. lentjan in the CARs along the Terengganu coast.
Resolution of the possible role of Setiu Lagoon was deemed most likely from stable isotope analyses of fish muscle tissue and otoliths due to the difficulty of tracking movements of individual fish between aquatic habitats using conventional methods, such as mark and recapture, biologging and genetic population structure analysis (Amano et al. 2015). Mark and recapture and biologging methods are limited by recapture rates, marking procedures, fish size dependence and technical demands, whereas genetic approaches are limited by the dynamic nature of oceanography and the often subtle genetic structures resulting from large effective population sizes and high potential for larval dispersal (Amano et al. 2015).
Stable isotope analyses of fish muscle tissues have been widely used to determine dietary sources, feeding habits and trophic levels (Post 2002; Vaslet et al. 2011; Reis-Santos et al. 2015; Davis et al. 2015; Abrantes et al. 2015). Whereas stable carbon isotope (δ13C) signatures in fish tissues can reflect corresponding isotope signatures of local vegetation-based food webs in which the fish has grown (photosynthetic pathways of C3 v. C4 plants; Fry and Sherr 1984; Abrantes et al. 2015), the degree of enrichment of stable nitrogen isotopes (δ15N) can be used to estimate the trophic level of fish in the food web (Post 2002). Isotope compositions in fish muscle tissues often reflect time-integrated values of food sources and habitat utilities, the turnover times of the former in tissues varying from weeks to 6 months, allowing the tracking of fish movements or ontogenetic diet changes over short periods during growth (Tieszen et al. 1983; Herzka 2005; Fry 2008; Nakamura et al. 2008). Conversely, during the formation of new otolith material throughout the life of a fish, elements and isotopes in ambient waters are incorporated permanently into the carbonate matrix in a layered manner (Campana 1999; Campana and Thorrold 2001; Elsdon et al. 2010), thereby preserving the timing of deposition, which can be used as a proxy to provide a detailed history of the fish’s environment (Brazner et al. 2004; Kimirei et al. 2013b), ontogenetic migration (Huxham et al. 2007; Reis-Santos et al. 2015), dietary sources (Elsdon et al. 2010) and stock discrimination (Edmonds et al. 1999; Bastow et al. 2002; Gao et al. 2005; Amano et al. 2015). Owing to trophic interactions and ontogenetic niche shifts frequently being size dependent, differences in individual fish sizes are commonly observed within populations (Werner and Gilliam 1984). Gradual changes in the isotope composition of fish tissues associated with size classes (growth) may reflect the mixture of a new diet and habitats because the fish are new arrivals, hence inferring population connectivity, if there is clear isotope discrimination between inhabited environments (Nakamura et al. 2008; Vaslet et al. 2011; Le et al. 2018). Indeed, during ontogenetic offshore migration, reef fishes may move across vegetated nursery estuaries as juveniles (shallow, low saline, warmer water) to adult habitats on coastal reefs (deep, high saline, cooler water). Discrete isotope incorporation in otoliths can reflect environmental changes (Elsdon et al. 2008). In recent years, an increasing number of studies has used otolith δ18O and δ13C as markers to retrospectively identify fish migration, rather than trace elements, because reef fishes usually inhabit environments with uniform water chemistry (Campana 1999; Mateo et al. 2010; Kimirei et al. 2013b; Amano et al. 2015). In fact, stable oxygen isotopes in otoliths (δ18Ootolith) are deposited in, or very near to, isotopic equilibrium with ambient waters (Radtke et al. 1996; Campana 1999; Elsdon and Gillanders 2002; Huxham et al. 2007; Kitagawa et al. 2013). Using δ18Ootolith, Weidman and Millner (2000) found a significant correlation between δ18Ootolith and ambient water temperature at the time of aragonite formation in the otolith. Conversely, Bowen (2010) found spatial variations in oxygen stable isotopes associated with salinity changes in oceanic surface waters, because they both arose from sea surface processes such as evaporation–precipitation, continental run-off, upwelling–advection and melting–freezing (Delaygue et al. 2001; McConnell et al. 2009). Accordingly, Shirai et al. (2018) used δ18Ootolith to reconstruct the salinities experienced by fishes in a mangrove estuary in Urauchi Bay, temperature not being considered because of the narrow variations of water temperature in subtropical and tropical regions. Thus, δ18Ootolith can be used reliably to reconstruct salinity experiences in subtropical and tropical fishes, if the fish move across a spatial salinity gradient (Shirai et al. 2018). Unlike oxygen, stable carbon isotope in otoliths (δ13Cotolith) is more complex, because the carbon sources incorporated may include the diet (Nelson et al. 2011), dissolved inorganic carbon (DIC) in ambient waters taken up via the gills and intestinal interfaces (Kalish 1991; Nelson et al. 2011) and metabolic effects (Kalish 1991; Weidman and Millner 2000; Trueman et al. 2016). Such factors may result in incorrect interpretation of fish migration history. However, in some cases δ13Cotolith may be useful for tracking fish movement if the spatial pattern of isotope signals from the fish environment shows a steep gradient or is strong enough for discrimination between sources of variability (Schwarcz et al. 1998; Mateo et al. 2010; Kimirei et al. 2013b).
The aim of the present study was to retrospectively track the nursery habitat use of adult L. lentjan collected from CARs, using muscle (δ13Cmuscle and δ15Nmuscle) and cross-sectioned otolith (δ13Cotolith and δ18Ootolith) stable isotope analyses. Specifically, the aim of the study was to determine whether fish had developed in the Setiu Lagoon (nursery habitat) before migrating to CARs along the Terengganu coast. Comparisons of stable isotopes in post-larval (juvenile) and margin (adult) otolith portions of adult fish with those in surface otoliths of juvenile fish collected from the lagoon should indicate whether the adult L. lentjan had originated from the Setiu Lagoon nursery. To improve the reliability of different environment determinations, reef (i.e. fully marine) and freshwater fishes were used to establish end-member habitat values as reference points.
Materials and methods
Typical regional monsoonal climates
The ECPM, located in the southern South China Sea, is continuous with the Gulf of Thailand and Vietnam coast to the north and north-east respectively. The region has an annual tropical monsoon climate that controls surface circulation along the coast. The North-east Monsoon (NEM; from October to March) brings greater rainfall compared with the South-west Monsoon (SWM; from April to September), April and October representing transitions between the two monsoons (Akhir 2012; Akhir et al. 2014). Sea circulation, driven by monsoon wind seasons, affects the reef environments along the coast. During the SWM, coastal currents flow northward along the ECPM and advect water masses from the Java Sea (Qu et al. 2005), in association with strong upwelling from Kuantan coast, bringing cool salty water along the coast (Daryabor et al. 2015; Kok et al. 2015). Accordingly, sea surface temperatures and salinity along the Terengganu coast range from 28.6 to 29.0°C and from 32.1 to 32.6.6 practical salinity units (PSU; Akhir et al. 2014). During the NEM, surface current eddies advect water southward from coastal Vietnam, there being an absence of coastal upwelling. Temperature and salinity in the study area ranged from 29.2 to 30.9°C and from 31.5 to 31.8 PSU (Akhir et al. 2014; Daryabor et al. 2015). Unlike the two main monsoon seasons, slow currents are usually observed during the short inter-monsoon periods (Akhir et al. 2014; Daryabor et al. 2015; Kok et al. 2015).
Study sites
The study was conducted to examine ecological connectivity between an inshore area (i.e. the shallow brackish water Setiu Lagoon) and an offshore area (i.e. CARs along the Terengganu coast, ECPM; Fig. 1). The Setiu Lagoon, ~14 km long and 300–800 m wide (Fig. 1), is considered a potential nursery habitat for various commercial fishes, particularly L. lentjan, due to its habitat configuration, hydrology and mosaic vegetation habitat (Le et al. 2018). The mangrove forests are dominated by Rhizophora spp., Avicennia spp. and Nypa spp. Halodule pinifolia, the dominant seagrass species with a mean canopy height of 18 cm, forms meadows or small patches near the Rhizophora mangrove forests or close to the mouths of the lagoon. The lagoon is subjected to semidiurnal tides (tidal range 1.7 m), water depth varying from 0.2 to 2 m above the seagrass beds at low and high tides. Water flow and exchange in the lagoon were driven by tidal regimes through two open inlets during the sampling period. Previous surveys indicated somewhat uniform salinity within the seagrass areas (fluctuating between 25 and 28 PSU) during the SWM (dry season) due to very low precipitation, and stratified salinity during the NEM (wet season), decreasing to 5 PSU in surface water at peak rainfall, but steady at ~18–24 PSU in the bottom layer (Le et al. 2018). The water temperature varied slightly between the seasons, ranging between 28 and 32°C.

|
The CARs (the offshore study area) have been deployed by the Department of Fisheries Malaysia since 1975, being attractive habitats for various reef fish species, including snappers, emperors, groupers and trevally. In addition, the artificial reefs provide hard surfaces where corals, algae and oysters attach (Ali et al. 2013). Owing to adult L. lentjan having been identified as an offshore resident (Lugendo et al. 2005; Carpenter et al. 2016), the CARs are considered to provide adult habitat for the species. The target CARs, constructed from reinforced concrete, included cuboid (size 2.0 × 2.0 × 3.0 m; weight 10 Mg module–1) and cube forms (size 2.5 × 2.5 × 2.5 m, weight 16 Mg module–1), which were deployed at a depth of 15–20 m over an area of ~125 × 50 m (Ali et al. 2013).
Sampling
Pink ear emperor fish L. lentjan were collected in the Setiu Lagoon as juveniles and from offshore CARs along the Terengganu coast as adults (Fig. 1). All juvenile samples from the Setiu Lagoon were collected using gill nets during ebb tides in both 2016 and 2017. The 10 samples from 2016 (size 9–14.5 cm TL, and subject of a previous study; Le et al. 2018), included 5 specimens collected in January (corresponding to NEM) and 5 collected in July (corresponding to SWM; Site 1). In 2017, five juvenile samples were collected in July at a second site (Site 2) in the lagoon to verify spatial and interannual variations in otolith isotope composition, but no juveniles were collected in January (Fig. 1). Following Carpenter et al. (2016), all the juveniles were considered as young-of-the-year or of age 1 year. Based on previous surveys in Le et al. (2018), the juveniles were found only in seagrass beds adjacent to mangroves (salinity 20–28 PSU) and at no time were found in low-salinity areas in the lagoon or freshwater area of the input river basin; hence, the species likely occurs within a certain salinity range (Le et al. 2018).
In all, 23 adult pink emperor fishes of various body size were collected from CARs ~10–12 km off the Terengganu coast. Of the adult samples, 18 were purchased from local fishermen using cage traps at the CARs in April 2016 (corresponding to post-NEM; 11 specimens) and August 2016 (7 specimens), and 5 were speared by divers using SCUBA in August 2016 (corresponding to SWM). All adult samples were confirmed as being caught in the studied CARs by fishermen, although several other CARs, surrounding Bidong and Redang, were ~20 km from the study site. Natural coral reefs also surround islands off the Terengganu coast, including Cipu, Lang Tengah, Perhentian, Bidong and Redang; Perhentian and Redang islands are included in marine protected areas (MPAs) of the Marine Park Malaysia.
To establish end-member habitat values, adult fish from freshwater and marine habitats were collected following Shirai et al. (2018). The adult freshwater fish included Trichogaster pectoralis (two specimens), Barbonymus schwanenfeldii (two specimens) and Channa micropeltes (two specimens), purchased from a local market upstream in the Setiu River, whereas the adult marine fish included Caesio cuning (three specimens), Chlorurus japanesis (three specimens) and Lutjanus vitta (five specimens), collected from a single CAR in August 2016. For an environmental history reference of fishes in the lagoon, samples of the brackish-water resident Apogon hyalosoma (five specimens) were collected for otolith stable isotope analyses.
To determine whether any ontogenetic dietary or habitat shift occurred from inshore to offshore areas, various potential food items were sampled from the lagoon and CAR habitats. According to Carpenter et al. (2016) and Le et al. (2018), L. lentjan is a demersal carnivore, taking primarily crustaceans, molluscs, echinoderms, polychaetes and small fishes. Juvenile L. lentjan prefer crustaceans, polychaetes and molluscs (Le et al. 2018), whereas adults target crustaceans, molluscs and echinoderms (Toor 1964; Carpenter et al. 2016). Lagoon food items were used as described by Le et al. (2018), whereas food items from CARs, including small sea cucumbers (Holothuria spp.; six specimens), molluscs (Pinnidae; four specimens) and annelid worms (two specimens), were collected by divers using SCUBA in August 2016.
All fish samples were packed in labelled polyethylene bags and immediately stored in coolers, transported to the laboratory and frozen at –20°C until further processing.
Ambient water temperature and salinity were measured using a YSI multiparameter meter (ProPlus, Yellow Springs, OH, USA), before water samples were collected for oxygen stable isotope analysis along a salinity gradient from upstream in the Setiu River to the Setiu Lagoon to the offshore CARs. The vertical distributions of salinity and temperature in the water column at the CARs were taken from Akhir et al. (2014), who reported interannual variability from the surface to a depth of 30 m of 28.5–30.9°C in temperature and 31.5–33.3 PSU in salinity.
Water samples for determination of the salinity gradient were taken from the CARs (33 PSU), at three points along the salinity gradient (31, 21.8 and 9 PSU) in the lagoon and in fresh water (0 PSU) upstream in the Setiu river during the ebb tide in August 2017 (Fig. 1) and kept in clean 20-mL glass vials in a cool box. The water samples collected in triplicate from each sampling site were preserved at 5°C and transported to the Atmosphere and Ocean Research Institute, The University of Tokyo, for further analysis.
Sample preparation and stable isotope analysis
The TL (to the nearest millimetre) and bodyweight (to the nearest gram) of fish samples were recorded, and dorsal white muscle tissue was taken for stable isotope analyses. All tissue samples were dried at 60°C for 24 h before grinding into fine powder with a mortar and pestle. Sagittal otoliths were extracted from each fish, and the length and width measured using callipers (to an accuracy of 0.01 mm) before being cleaned with deionized water. The otoliths of adult L. lentjan from CARs were large enough (length 7.3–15.4 mm, width 5.5–13.3 mm) for microdrilling through different life stages. Random juvenile and adult otoliths were selected for analysis, there being no significant differences in stable isotopes between the left and right sides (Campana 1999; Huxham et al. 2007).
Tissue samples
Stable isotope (δ13Cmuscle and δ15Nmuscle) compositions of the tissue samples and potential food items were measured using a stable isotope ratio mass spectrometer (IsoPrime100; IsoPrime, Cheadle, UK) coupled with a combustion device (vario MICRO cube; Elementar, Langenselbold, Germany) at the Atmosphere and Ocean Research Institute, The University of Tokyo, as described by Le et al. (2018). Data for juvenile L. lentjan collected from the lagoon were taken from Le et al. (2018).
Otolith samples
Selected otoliths were ground with a graded series of alumina paste to expose the core along the anterior–posterior axis after being embedded in epoxy resin (Araldite Rapid R30, Nichiban Co., Ltd, Tokyo, Japan) on a glass slide. After polishing, the otoliths were cleaned with Milli-Q water and dried. Based on alternating growth increments and light reflection under a microscope, three otolith zones in large adults (18 specimens; >220 mm TL) were identified as representing larval, post-larval and adult periods (Blacker 1974; Schwarcz et al. 1998; Fig. 2). However, the adult period in otoliths of small adult fish (three specimens; 154–184 mm TL) were either unclear or seen as narrow zones beyond the post-larval period. The larval period was identified as a dark spot in the centre of the otolith (core), and the post-larval period (juvenile portions) was defined as a region of rapid growth apparent as the first opaque zone of the otolith, determined within an interval of ~350–850 µm from the core depending on otolith size and shape. These intervals were considered as representing the first year of life, corresponding to a body size of juvenile fish (6–15 cm TL) collected from the lagoon and noted in an earlier study (Kimirei et al. 2013a). The otolith margin, beyond the post-larval interval region with a regular series of alternating growth bands called translucent and opaque zones, was considered as the adult period (hereafter ‘adult portion’), likely reflecting recent habitats. Otolith margins were analysed for L. lentjan juveniles (hereafter ‘juveniles’) and the end-member fish species. A triangular pyramid drilling bit of a high-precision micromilling system with accurate positioning on a 1/1000-mm scale (GEOMILL326; Izumo-web Ltd., Izumo, Shimane, Japan) was used for post-larval and adult portions in the same individual, two cone-shaped drilling samples (diameter 250 μm, depth 250 μm) being taken from each portion on the same side of the otolith. After milling, the otolith powder was collected onto aluminium foil and transferred to a glass vial for isotope analysis. The otolith was photographed using a digital microscope (VHX-5000; KEYENCE, Itasca, IL, USA) before and after each micromilling exercise to confirm the portions.
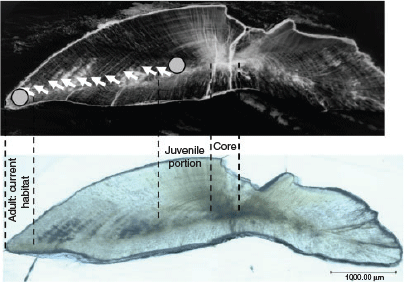
|
Analyses of otolith stable isotopes of carbon (δ13Cotolith) and oxygen (δ18Ootolith) were conducted at the Atmosphere and Ocean Research Institute, The University of Tokyo. The powder sample was measured using an isotope ratio mass spectrometer (Delta V plus; ThermoFisher Scientific, Waltham, MA, USA), equipped with an automated carbonate reaction device (GasBench II; Thermo Fisher Scientific). Otolith powder was introduced into a glass vial, flushed with pure helium and reacted with 100% phosphoric acid at 72°C. Liberated CO2 was transported with the helium gas flow to the mass spectrometer. Detailed analytical conditions have been reported elsewhere (e.g. Kubota et al. 2017; Shirai et al. 2018). All isotope values are reported using delta notation with respect to Pee Dee Belemnite (PDB), based on an NBS-19 (International Atomic Energy Agency, Vienna, Austria) value of –2.20‰ for δ18Ootolith and +1.95‰ for δ13Cotolith. No correction was applied to the acid fractionation factor between calcite and aragonite (phosphoric acid–calcium carbonate reaction temperature 72°C; Kim et al. 2007a). Repeated analysis of the NBS 19 standard yielded an external reproducibility of δ18Ootolith and δ13Cotolith measurements better than 0.14 and 0.13‰ respectively (1σ).
Water samples
Oxygen isotope compositions of the water samples (δ18Owater) were determined using a Picarro L2130-i Analyzer (Picarro, Inc., Santa Clara, CA, USA) at the National Institute of Technology, Ibaraki College. Before introduction to the analyser, samples were filtered using a membrane filter (pore size 0.45 µm; Toyo Roshi Kaisha, Tokyo, Japan) to reduce suspended particles and avoid blocking the sampling line. Samples were analysed and mean values are presented, the deviations being smaller than long-term reproducibility. Data are reported in delta notation against the Vienna Standard Mean Ocean Water (VSMOW) reference standard. Long-term instrument reproducibility was ±0.02‰.
Statistical analysis
Values are expressed as the mean ± s.d. Prior to analysis, all stable isotope data were tested for normality using the Shapiro–Wilk and Levene’s tests. If the data did not fit a normal distribution, non-parametric tests were conducted. Student’s t-test was used to test the significance of differences in the stable isotopic signatures between freshwater and seawater fishes. Mahalanobis distances were used to detect outliers, which were then removed before further tests (Barnett and Lewis 1994). Relationships in this study were examined using linear regressions. To examine a specific nursery and past habitat use of fish in the study areas, multivariate analysis of variance (MANOVA) was conducted to test whether otolith stable isotope signatures of juveniles from the lagoon and the juvenile portions of adult fishes from CARs were distinguishable from each other with regard to different sampling periods (seasons). If adult fish exhibit otolith stable isotope (OSI) areas in juvenile portions similar to those in juveniles, they could be considered to have occupied similar nursery environments. In contrast, different isotope ratio values indicate either different nursery environments experienced during the juvenile phases or that the lagoon is not the only nursery habitat supplying the adult habitat. Mean δ13Cotolith and δ18Ootolith values for juvenile L. lentjan and otolith portions of adults were compared using one-way analysis of variance (ANOVA) or Kruskal–Wallis tests. A one-sided significance level of α < 0.05 was used in all tests. Statistical analyses, including identification of outliers, were performed using SPSS Statistics for Windows (ver. 25, IBM Corp., Armonk, NY, USA). Graphs were drawn using R (ver. 3.2.4, see https://cran.r-project.org/, accessed 11 June 2019) and SigmaPlot for Windows (ver. 11, Systat Software, San Jose, CA, USA).
Results
Fish samples
Fishes used to provide end-member values were identified as marine and freshwater residents, all individuals being adult (Table 1). The target species (L. lentjan) collected from the lagoon ranged in size from 70 to 145 mm TL, four specimens (135–145 mm TL) being subadult. Conversely, L. lentjan collected from CARs varied in size from 154 to 452 mm TL (Table 2), the three smallest samples (154–184 mm TL) being considered subadult. The overall pool of L. lentjan from the lagoon and CARs showed a significant relationship between TL and otolith length (R2 = 0.96, P < 0.001), suggesting that increasing otolith size was consistent with fish growth.

|
Relationship between δ18Owater and salinity regimes
At salinities of 33, 32.5, 21.8, 9.7 and 0 PSU, mean (±s.d. of triplicate measurements) δ18Owater values were –0.42 ± 0.01, –0.47 ± 0.02, –2.00 ± 0.02, –4.11 ± 0.02 and –5.83 ± 0.04‰ (SMOW) respectively (Fig. 3). Seawater and freshwater salinity differed by 33 PSU, corresponding to 4.7‰ SMOW in δ18Owater. A significant positive linear relationship was found between ambient water salinity and δ18Owater from the study areas (R2 = 0.9992; P < 0.0001).
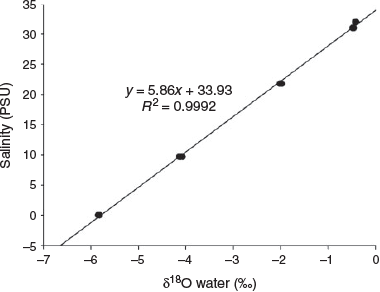
|
Stable isotopes in potential food items compared between the lagoon and CARs
There were different trends in stable isotope values of potential food items between the lagoon and CARs. Although the food items in the lagoon were enriched in 15N and depleted in 13C in both seasons, in CARs the reverse was observed, namely depletion in 15N and enrichment in 13C (Fig. 4). Significant differences were also found in both δ13Cfood and δ15Nfood between the lagoon and CARs (Kruskal–Wallis test, P < 0.001). In addition, a small but non-significant difference was found in the isotope composition of food items in the lagoon between seasons.
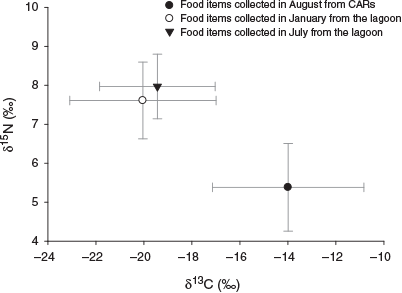
|
Stable isotopes in fish muscle tissue
The δ13Cmuscle values exhibited a gradient from freshwater to marine fishes, the most depleted δ13Cmuscle values being found in freshwater residents, whereas marine residents had the highest δ13Cmuscle values (t-test, P < 0.001). The wide range of δ15Nmuscle values among fish residents likely reflects different feeding modes and trophic levels, as well as different nitrogen sources in the ecosystem (Table 1).
In the lagoon, significant differences in δ13Cmuscle of L. lentjan occurred between seasons and sites (one-way ANOVA, Tukey’s honest significant difference (HSD), P < 0.05), but a significant difference in δ15Nmuscle values was only found between sites (one-way ANOVA, Tukey’s HSD, P < 0.05; Table 2).
Comparisons of stable isotope signatures in L. lentjan between inshore and offshore indicated that the δ13Cmuscle values were more depleted in fish from the lagoon than from CARs (t-tests, P < 0.001), whereas the δ15Nmuscle values were more enriched for lagoon-captured fish than in those from CARs (t-tests, P < 0.001; Table 2).
There was a significant positive relationship between δ13Cmuscle and TL for fish captured in CARs had (R2 = 0.40, P < 0.001; Fig. 5). However, a negative relationship found between δ15Nmuscle and TL that was not significant (R2 = 0.15, P = 0.085; Fig. 5).

|
Otolith analysis of end-member habitat patterns
The δ13Cotolith and δ18Ootolith values discriminated between end-member patterns (Table 1), with values for freshwater residents ranging from –19.9 to –10.7 and from –7.9 to –5.7‰ respectively, compared with more enriched values for marine residents, which ranging from –8.6 to –3.8‰ for δ13Cotolith and from –2.3 to –1.7‰ for δ18Ootolith (Fig. 6). The OSI values for the brackish water resident A. hyalosoma were intermediate between freshwater and marine residents, ranging from –9.3 to –5.2‰ for δ13Cotolith and from –5.0 to –2.8‰ for δ18Ootolith (Fig. 6). The mean ranges of δ13Cotolith and δ18Ootolith within marine and freshwater resident fishes were 9.7 and 4.5‰ respectively, the latter being close to 4.7‰ SMOW determined for δ18Owater.
Otolith analyses of L. lentjan
In the lagoon, the juvenile δ13Cotolith and δ18Ootolith values ranged from –8.1 to –5.9‰ and from –5.1 to –2.8‰ respectively, having a similar range to the brackish-water resident A. hyalosoma. Comparisons of juveniles between seasons and sites revealed that δ13Cotolith values generally had a similar range between seasons, but were significantly different between sites (Table 3), whereas δ18Ootolith values were lowest in January 2016 compared with both July 2016 and July 2017 (Table 3).

|
In the CARs, OSI values for juvenile portions ranged from –7.9 to –3.0‰ (δ13Cotolith) and from –3.4 to –1.9‰ (δ18Ootolith), whereas δ13Cotolith and δ18Ootolith for adult portions ranged from –5.3 to –1.7‰ and from –3.5 to –1.7‰ respectively. There were no significant differences in either isotope value of juvenile portions between August and April, although significant differences in δ13Cotolith and δ18Ootolith of adult portions were found between sampling periods (t-test, P = 0.018 for δ13Cotolith; U-test, P = 0.009 for δ18Ootolith). The juvenile portions showed significantly more depletion of δ13Cotoilth and δ18Ootolith than adult portions in August (t-test, P = 0.001 for both). There was a slight depletion of stable isotope signature in juvenile portions compared with adult portions in April, but the difference did not reach statistical significant (t-test, P = 0.416 for δ13Cotoilth; P = 0.654 for δ18Ootolith). The three small adult fish (<185 mm TL) similarly exhibited the lowest range of δ18Ootolith values in both portions (varying between –3.7 and –2.8‰ for adult portions, and between –3.4 and –2.8‰ for juvenile portions) in the same individuals, but the δ13Cotolith for these fish did not show any overlap with juveniles from Setiu Lagoon. These results suggest that small adult fish may freshly recruit to CARs after their ontogenetic migration from brackish-water habitats. Seven of the samples had δ18Ootolith values ranging between –2.4 and –1.8‰ in both portions (Fig. 7). Interestingly, a higher number of juvenile portions exhibited δ18Ootolith values close to those of juveniles collected in July (both 2016 and 2017; corresponding to SWM), whereas no juvenile portion δ18Ootolith value fell within the δ18Ootolith range of juveniles collected in January 2016 (corresponding to NEM; Fig. 7).
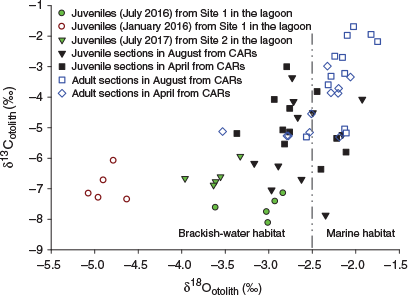
|
MANOVA indicated significant differences in OSI signatures between juveniles from the lagoon and juvenile portions from CARs (Wilks’ λ = 0.083; F = 19.21; P < 0.0001). The results of univariate and multiple comparisons confirmed that differences among sampling periods were significant for both elements (Table 4; see Table S1, available as Supplementary material to this paper).

|
Discussion
In this study both muscle and otolith stable isotopes were used to identify short- and long-term fish movements across habitats. For muscle stable isotope, because of fish muscle-tissue turnover rate, movement patterns covered a few weeks to months (Herzka 2005; Xia et al. 2013), the gradual change in stable isotopes in tissues being related to size-dependent (growth) fish movement recorded across habitats or diet shifts (Vaslet et al. 2011; Le et al. 2018) or seaward migration (Cocheret de la Morinière et al. 2003; Nakamura et al. 2008; Berkström et al. 2012, 2013; Huijbers et al. 2015). Given the size at first maturity (L50) of L. lentjan in seagrass beds, individuals could ontogenetically move to coral reef adult habitats at a size of ~140 mm TL (Kimirei et al. 2013a). Earlier studies have found that there were no adult fish in the lagoon and that juvenile fish ontogenetically shifted diet and habitat in a size range >120 mm TL (Le et al. 2018). Potential food sources also showed differences in stable isotopes between the lagoon and CAR habitats, similar isotopic gradients among habitats having been reported from other tropical coasts (Cocheret de la Morinière et al. 2003; Nakamura et al. 2008). Hence, the depleted 13C and enriched 15N signatures of small adult individuals (<185 mm TL) in CARs suggest that they are recent arrivals from estuarine habitats.
One of the biggest challenges to retrospectively identify fish lifetime movements among habitats is the unknown environment to which the fish has been exposed during different life stages. The δ13Cotolith and δ18Ootolith fingerprints can be used as proxies of temperature or salinity to reconstruct the past environments of migratory fish, because variations in these isotopic signatures have been shown experimentally to be significantly related to temperature or salinities (Elsdon and Gillanders 2002; Kerr et al. 2007; McMahon et al. 2011; Shirai et al. 2018). Because δ13Cotolith values can be affected by several factors, including DIC incorporated into otoliths derived from ambient waters (Weidman and Millner 2000; Solomon et al. 2006; Tohse and Mugiya 2008; Elsdon et al. 2010), species-specific differences in carbon metabolism (Weidman and Millner 2000) and dietary sources (Schwarcz et al. 1998; McMahon et al. 2011), changes in δ13Cotolith provide some ability to differentiate between large trophic- and ecosystem-level shifts, related to inshore–offshore carbon sources or depth gradients of DIC in δ13Cotolith values (Mulcahy et al. 1979; Schwarcz et al. 1998). In the present study, the increasing trend of δ13Cotolith values from small (young) fish to large (old) fish was partly consistent with the hypothesis of movement of fish to deeper offshore waters where the δ13C of DIC is lower compared with inshore wetlands and changes in nutrient sources in new habitats (Bouillon et al. 2011). However, a part of this trend in δ13Cotolith values could also be explained by a reduction in mass-specific field metabolic rates with fish growth during the lifetime (Schwarcz et al. 1998; Jamieson et al. 2004; Trueman et al. 2016). Several studies have successfully used δ18Ootolith as a proxy of salinity or temperature to retrospectively identify habitat use in coastal fishes (Elsdon and Gillanders 2002; Kerr et al. 2007; McMahon et al. 2011; Shirai et al. 2018), because lower δ18Ootolith values indicate high temperatures and low salinity, and vice versa (Campana 1999; Weidman and Millner 2000; Kitagawa et al. 2013; Shirai et al. 2018). However, in the tropical zone, particularly the ECPM, seawater temperatures often vary narrowly (1–3°C) on the surface and in the water column throughout the seasons (Akhir 2012; Akhir et al. 2014; Daryabor et al. 2015). Given that water temperatures in rivers could also be affected by rainfall in the NEM season and upstream water input, annual mean temperature nevertheless varied within 3°C in the study area (Suratman et al. 2015). Furthermore, a change in temperature by 1°C causes isotopic fractionation of ~0.22–0.25‰ during aragonite precipitation (Kim et al. 2007b). It is reasonable to estimate that the effect of temperature change on δ18Ootolith is less than 1.0‰ (corresponding to ~3°C in water), whereas in the present study end-member species showed enriched δ18Ootolith from freshwater to seawater (the difference in δ18Ootolith was ~4.5‰, close to 4.7‰ SMOW in δ18Owater). It is likely that variations in δ18Ootolith between and within species in the present study were affected more by salinity than by temperature. Because the enriched δ18O signatures of pelagic oceanic water vary slightly within ±0.5‰ worldwide, most freshwater bodies can be highly isotopically depleted (Craig 1961; Dansgaard 1964; Schaffner and Swart 1991; Rohling 2013; Shirai et al. 2018). Accordingly, the mixing of run-off water (riverine waters and precipitation) with ocean water (mixed masses of water) in coastal regions may be expected to form a positive gradient in δ18Ootolith values from fishes in fresh water to fishes in marine water habitats. In addition, δ18Ootolith has exhibited quasi-linear relationships with salinity under both experimental conditions (Kalish 1991; Thorrold et al. 1997) and in the field (Weidman and Millner 2000). In addition, δ18Ootolith values may be affected by species-specific fractionation, such variations estimated as ~1‰ by Sakamoto et al. (2017) and Shirai et al. (2018). Hence, salinity is reasonable as the dominant parameter for reconstructing the past environment of L. lentjan.
For adult L. lentjan, no significant difference in OSI values in juvenile portions between sampling periods suggests that these fishes may have been exposed to similar environments as juveniles, whereas the difference in both OSI values in adult portions between sampling periods indicates that the fish may have been exposed to changes in ambient environment or food resources related to current circulation patterns driven by monsoonal seasons. Indeed, water masses off the Terengganu coast have had small changes reported in both salinity and temperature (nearly 1.0 PSU and 1.5°C) between monsoonal seasons (Akhir et al. 2014). Such changes in the ambient environment could be recorded in the otolith chemistry, even if the adult fish remains in the CARs, because the δ13Cotolith would be primarily affected by DIC in different waters or food availability, whereas δ18Ootolith reflects exposure to water temperatures and salinities of different provenance (Ashford and Jones 2007). Furthermore, the ‘spillover’ effect can be considered as another factor replenishing adult populations in the CARs, because some adults may come from adjacent natural coral reefs of MPAs to form mating groups or seeking food sources (Carr et al. 2017), the former being related to the spawning season (April–June; Grandcourt et al. 2011).
Conversely, several juvenile portions showed lower δ18Ootolith values than adult portions from the same individuals in August, suggesting that these fish may have inhabited estuarine waters during their juvenile phase. Furthermore, the otolith of small adult fish had narrow adult zones beyond the post-larval period, the drilled samples taken from otolith edges potentially including a mix of new (seawater) and old (brackish water) material deposited during otolith formation soon after the fish arrived at the CAR. The former included primarily isotope signatures of marine waters (enriched OSI values) in the upper layers, whereas the latter corresponded to estuarine waters (depleted OSI values) in the lower layers. Hence, the small adult fish (<185 mm TL) had the lowest δ18Ootolith signatures with characteristics of fish inhabiting estuarine waters (depleted 18Ootolith), which supports population connectivity between estuarine and marine habitats. Alternatively, juvenile and adult portions in the same fish exhibited δ18Ootolith values similar to marine residents (noted in seven individuals), suggesting that some individual L. lentjan may not spend their juvenile phases in shallow estuarine habitats, instead possibly being self-replenishing in the reef habitats or migrating from surrounding coral reefs. This result is consistent with a previous study that showed that some L. lentjan adults had never passed through brackish-water vegetation habitats as juveniles, instead inhabiting predominantly coral reefs during their entire life (Kimirei et al. 2013b).
For juvenile emperors from the lagoon, the overall more negative δ18Ootolith values in juvenile fish collected in January may reflect the influence of changing water composition during the NEM season, due to the input of fresh water from precipitation and tributaries potentially affecting isotope signatures of water in the lagoon (Cocheret de la Morinière et al. 2003). Furthermore, the effects of unfavourable environmental conditions during peak rainfall in the NEM may force the juvenile reef fish to emigrate or to go deeper in the lagoon because they may have certain salinity tolerances and preferences (Estudillo et al. 2000; Serrano et al. 2010). Unlike δ18Ootolith, a small variation in δ13Cotolith between seasons may not only reflect a change in water composition, because δ13Cotolith values can be affected by several factors (see above), but may also be related to differences in metabolic rates due to seasonal temperature changes (Kalish 1991; Trueman et al. 2016). Therefore, interannual variability in OSI (δ13C and δ18O) values may provide baseline information on the typical nursery habitat of fish. Such information may be valuable for extrapolation to retrospectively track nursery habitats of adult L. lentjan when OSI signatures are compared between juvenile portions of adult fishes from CARs and juvenile fishes from the lagoon. As indicated by the MANOVA, there were offsets in OSI signatures between juvenile portions of adult fish and juvenile fish, suggesting that Setiu Lagoon may not be an important juvenile area for the fish sampled from CARs (Fig. 7). This result may account for the influence of coastal currents when the fish ontogenetically migrate out of the lagoon. The different velocity of coastal currents between monsoonal seasons could profoundly affect the direction of fish migration from inshore to offshore habitats (Carr et al. 2017). Indeed, strong currents that flow southward along the coast during the NEM could have deflected the movement of juveniles to CARs, promoting southward connectivity instead. Accordingly, only a limited number of juvenile fish could successfully recruit to the CARs from the lagoon. This explanation may also be consistent with isotope values in juvenile portions of some adult individuals being outside the isotope range in juveniles collected from the lagoon, whereas a separation in δ13Cotolith among individuals could be attributed to spatial differences in habitat use (Mateo et al. 2010; Pruell et al. 2010; Kimirei et al. 2013b). Thus, the adult fish are considered a mix of cohorts replenished by different nursery habitats. Conversely, low-intensity coastal currents and weak northward currents that flow along the coast during the short inter-monsoon periods and SWM respectively may not significantly obstruct direct offshore migration of juvenile fish to CARs from the lagoon. Although very limited information is available on the effects of ocean circulation on fish migration in the South China Sea, a few studies have reported on the effects of ocean currents on landfall latitudes and migration speed of migratory fish, such as salmon returning to parental spawning grounds (Thomson et al. 1992).
In conclusion, although we could not find clear isotopic evidence of population connectivity between the Setiu Lagoon and CARs by ontogenetic migration of L. lentjan, artificial reef deployments may benefit adult fish populations after ontogenetic migration from other nursery habitats along the coast. Thus, given that Setiu Lagoon may be insufficient as a single nursery habitat for maintaining fish stocks, it is recommended that multiple nursery habitats along the coastal region of the study be included in any conservation strategy. Future multidisciplinary studies should be performed to investigate potential nursery habitats along the coast and to clarify the effects of coastal currents on the contribution of juvenile biomass to adult populations in the CARs and natural coral reefs along ECPM. Such information would be critical for the effective design of a sustainable resources management program for the coastal region, particularly concerning the linkage between inshore nurseries and oceanic habitats.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Declaration of funding
This study was supported by the Ministry of Higher Education Malaysia, under Fundamental Research Grant Scheme (FRGS) Project number FRGS/1/2016/WAB09/UMT/02/5 (Vote number 59425), and partly supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (JSPS; number JP16KT0028, JP16K13912).
Acknowledgements
The authors are grateful to staff of Universiti Malaysia Terengganu for their kind assistance with the fieldwork, and to N. Izumoto for undertaking isotope measurements of otolith samples. The authors also sincerely thank Graham Hardy for the English language review. The authors very much appreciate the constructive comments and suggestions of the reviewers of the manuscript.
References
Abrantes, K. G., Johnston, R., Connolly, R. M., and Sheaves, M. (2015). Importance of mangrove carbon for aquatic food webs in wet–dry tropical estuaries. Estuaries and Coasts 38, 383–399.| Importance of mangrove carbon for aquatic food webs in wet–dry tropical estuaries.Crossref | GoogleScholarGoogle Scholar |
Akhir, M. F. M. (2012). Surface circulation and temperature distribution of southern South China Sea from Global Ocean Model (OCCAM). Sains Malaysiana 41, 701–714.
Akhir, M. F. M., Zakaria, N. Z., and Tangang, F. (2014). Intermonsoon variation of physical characteristics and current circulation along the east coast of Peninsular Malaysia. International Journal of Oceanography 2014, 527587.
| Intermonsoon variation of physical characteristics and current circulation along the east coast of Peninsular Malaysia.Crossref | GoogleScholarGoogle Scholar |
Ali, A., Abdullah, M. P., Hazizi, R., Marzuki, A. H., and Hassan, R. B. R. (2013). Protecting coastal habitats and enhancing fisheries resources using big size artificial reefs in the east coast of Peninsular Malaysia. Malaysian Journal of Science 32, 19–36.
| Protecting coastal habitats and enhancing fisheries resources using big size artificial reefs in the east coast of Peninsular Malaysia.Crossref | GoogleScholarGoogle Scholar |
Amano, Y., Shiao, J., Ishimura, T., Yokouchi, K., and Shirai, K. (2015). Otolith geochemical analysis for stock discrimination and migratory ecology of tunas. In ‘Biology and Ecology of Bluefin Tuna’. (Eds T. Kitagawa, and S. Kimura.) Chapt. 10, pp. 225–257. (CRC Press: Boca Raton, FL, USA.)
Arney, R. N., Froehlich, C. Y. M., and Kline, R. J. (2017). Recruitment patterns of juvenile fish at an artificial reef area in the Gulf of Mexico. Marine and Coastal Fisheries 9, 79–92.
| Recruitment patterns of juvenile fish at an artificial reef area in the Gulf of Mexico.Crossref | GoogleScholarGoogle Scholar |
Ashford, J., and Jones, C. (2007). Oxygen and carbon stable isotopes in otoliths record spatial isolation of Patagonian toothfish (Dissostichus eleginoides). Geochimica et Cosmochimica Acta 71, 87–94.
| Oxygen and carbon stable isotopes in otoliths record spatial isolation of Patagonian toothfish (Dissostichus eleginoides).Crossref | GoogleScholarGoogle Scholar |
Barnett, V., and Lewis, T. (1994). ‘Outliers in Statistical Data’, 3rd edn. (Wiley: New York, NY, USA.)
Bastow, T. P., Jackson, G., and Edmonds, J. S. (2002). Elevated salinity and isotopic composition of fish otolith carbonate: stock delineation of pink snapper, Pagrusauratus, in Shark Bay, Western Australia. Marine Biology 141, 801–806.
| Elevated salinity and isotopic composition of fish otolith carbonate: stock delineation of pink snapper, Pagrusauratus, in Shark Bay, Western Australia.Crossref | GoogleScholarGoogle Scholar |
Berkström, C., Gullstrom, M., Lindborg, G., Mwandya, A. W., Yahya, S. A., Kautsky, N., and Nystrom, M. (2012). Exploring ‘knowns’ and ‘unknowns’ in tropical seascape connectivity with insights from East African coral reefs. Estuarine, Coastal and Shelf Science 107, 1–21.
| Exploring ‘knowns’ and ‘unknowns’ in tropical seascape connectivity with insights from East African coral reefs.Crossref | GoogleScholarGoogle Scholar |
Berkström, C., Jörgensen Lund, T., and Hellström, M. (2013). Ecological connectivity and niche differentiation between two closely related fish species in the mangroves-seagrass-coral reef continuum. Marine Ecology Progress Series 477, 201–215.
| Ecological connectivity and niche differentiation between two closely related fish species in the mangroves-seagrass-coral reef continuum.Crossref | GoogleScholarGoogle Scholar |
Blacker, R. W. (1974). Recent advances in otolith studies. In ‘Sea Fisheries Research’. (Ed. F. R. H. Jones.) pp. 67–90. (Wiley: New York, NY, USA.)
Bohnsack, J. A. (1989). Are high densities of fishes at artificial reefs the result of habitat limitation or behavioral preference? Bulletin of Marine Science 44, 631–645.
Bouillon, S., Connolly, R. M., and Gillikin, D. P. (2011). Use of stable isotopes to understand food webs and ecosystem functioning in estuaries. In ‘Treatise on Estuarine and Coastal Science’. (Eds E. Wolanski and D. S. McLusky.) Chapt. 7, pp. 143–173. (Academic Press: Waltham, MA, USA.)
Bowen, G. J. (2010). Isoscapes: spatial pattern in isotopic biogeochemistry. Annual Review of Earth and Planetary Sciences 38, 161–187.
| Isoscapes: spatial pattern in isotopic biogeochemistry.Crossref | GoogleScholarGoogle Scholar |
Brazner, J. C., Campana, S. E., Tanner, D. K., and Schram, S. T. (2004). Reconstructing habitat use and wetland nursery origin of yellow perch from Lake Superior using otolith elemental analysis. Journal of Great Lakes Research 30, 492–507.
| Reconstructing habitat use and wetland nursery origin of yellow perch from Lake Superior using otolith elemental analysis.Crossref | GoogleScholarGoogle Scholar |
Burke, L., Reytar, K., Spalding, M., and Perry, A. (2012). Reefs at risk revisited in the coral triangle. (World Resources Institute: Washington, DC, USA.) Available at http://www.wri.org/our-work/project/reefs-risk/reefs-risk-revisited-coral-triangle-2012 [Verified 6 June 2019].
Campana, S. E. (1999). Chemistry and composition of fish otolith; pathways, mechanisms and applications. Marine Ecology Progress Series 188, 263–297.
| Chemistry and composition of fish otolith; pathways, mechanisms and applications.Crossref | GoogleScholarGoogle Scholar |
Campana, S. E., and Thorrold, S. (2001). Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations? Canadian Journal of Fisheries and Aquatic Sciences 58, 30–38.
| Otoliths, increments, and elements: keys to a comprehensive understanding of fish populations?Crossref | GoogleScholarGoogle Scholar |
Carpenter, K. E., Lawrence, A., and Myers, R. (2016). Pinkear emperor Lethrinus lentjan. In ‘The IUCN Red List of Threatened Species 2016’. e.T16720036A16722340. (International Union for Conservation of Nature and Natural Resources.) https://www.iucnredlist.org/species/16720036/16722340 [Verified 6 June 2019].
Carr, M. H., Robinson, S. P., Wahle, C., Davis, G., Kroll, S., Murray, S., Schumacker, E. J., and Williams, M. (2017). The central importance of ecological spatial connectivity to effective coastal marine protected areas and to meeting the challenges of climate change in the marine environment. Aquatic Conservation 27, 6–29.
| The central importance of ecological spatial connectivity to effective coastal marine protected areas and to meeting the challenges of climate change in the marine environment.Crossref | GoogleScholarGoogle Scholar |
Cocheret de la Morinière, E., Pollux, B. J. A., Nagelkerken, I., Hemminga, M. A., Huiskes, A. H. L., and van der Velde, G. (2003). Ontogenetic dietary changes of coral reef fishes in the mangrove–seagrass–reef continuum: stable isotopes and gut-content analysis. Marine Ecology Progress Series 246, 279–289.
| Ontogenetic dietary changes of coral reef fishes in the mangrove–seagrass–reef continuum: stable isotopes and gut-content analysis.Crossref | GoogleScholarGoogle Scholar |
Craig, H. (1961). Isotopic variations in meteoric waters. Science 133, 1702–1703.
| Isotopic variations in meteoric waters.Crossref | GoogleScholarGoogle Scholar | 17814749PubMed |
Cresson, P., Ruitton, S., and Harmelin-Vivien, M. (2014). Artificial reefs do increase secondary biomass production: mechanisms evidenced by stable isotopes. Marine Ecology Progress Series 509, 15–26.
| Artificial reefs do increase secondary biomass production: mechanisms evidenced by stable isotopes.Crossref | GoogleScholarGoogle Scholar |
Cruz-Trinidad, A., Aliño, P. M., Geronimo, R. C., and Cabral, R. B. (2014). Linking food security with coral reefs and fisheries in the coral triangle Coastal Management 42, 160–182.
| Linking food security with coral reefs and fisheries in the coral triangleCrossref | GoogleScholarGoogle Scholar |
Dansgaard, W. (1964). Stable isotopes in precipitation. Tellus 16, 436–468.
| Stable isotopes in precipitation.Crossref | GoogleScholarGoogle Scholar |
Daryabor, F., Samah, A. A., and Ooi, S. H. (2015). Dynamical structure of the sea off the east coast of Peninsular Malaysia. Ocean Dynamics 65, 93–106.
| Dynamical structure of the sea off the east coast of Peninsular Malaysia.Crossref | GoogleScholarGoogle Scholar |
Davis, J. P., Pitt, K. A., Fry, B., and Connoly, R. M. (2015). Stable isotopes as tracers of residency for fish on inshore coral reefs. Estuarine, Coastal and Shelf Science 167, 368–376.
| Stable isotopes as tracers of residency for fish on inshore coral reefs.Crossref | GoogleScholarGoogle Scholar |
Delaygue, G., Bard, E., Rollion, C., Jouzel, J., Stievenard, M., Duplessy, J. C., and Ganssen, G. (2001). Oxygen isotope/salinity relationship in the northern Indian Ocean. Journal of Geophysical Research 106, 4565–4574.
| Oxygen isotope/salinity relationship in the northern Indian Ocean.Crossref | GoogleScholarGoogle Scholar |
Dupont, J. M. (2008). Artificial reefs as restoration tools: a case study on the West Florida Shelf. Coastal Management 36, 495–507.
| Artificial reefs as restoration tools: a case study on the West Florida Shelf.Crossref | GoogleScholarGoogle Scholar |
Edmonds, J. S., Steckis, R. A., Moran, M. J., Caputi, N., and Morita, M. (1999). Stock delineation of pink snapper and tailor from Western Australia by analysis of stable isotope and strontium/calcium ratios in otolith carbonate. Journal of Fish Biology 55, 243–259.
| Stock delineation of pink snapper and tailor from Western Australia by analysis of stable isotope and strontium/calcium ratios in otolith carbonate.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., and Gillanders, B. M. (2002). Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish. Canadian Journal of Fisheries and Aquatic Sciences 59, 1796–1808.
| Interactive effects of temperature and salinity on otolith chemistry: challenges for determining environmental histories of fish.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., Wells, B. K., Campana, S. E., Gillandres, B. M., Jones, C. M., Limburg, K. E., Secor, D. H., Thorrold, S. R., and Walther, B. D. (2008). Otolith chemistry to describe movements and life history parameters of fishes; hypotheses, assumptions, limitations and inferences. Oceanography and Marine Biology – an Annual Review 46, 297–330.
| Otolith chemistry to describe movements and life history parameters of fishes; hypotheses, assumptions, limitations and inferences.Crossref | GoogleScholarGoogle Scholar |
Elsdon, T. S., Ayvazian, S., McMahon, K. W., and Thorrold, S. R. (2010). Experimental evaluation of stable isotope fractionation in fish muscle and otoliths. Marine Ecology Progress Series 408, 195–205.
| Experimental evaluation of stable isotope fractionation in fish muscle and otoliths.Crossref | GoogleScholarGoogle Scholar |
Estudillo, C. B., Duray, M. N., Marasigan, E. T., and Emata, A. C. (2000). Salinity tolerance of larvae of the mangrove red snapper (Lutjanus argentimaculatus) during ontogeny. Aquaculture 190, 155–167.
| Salinity tolerance of larvae of the mangrove red snapper (Lutjanus argentimaculatus) during ontogeny.Crossref | GoogleScholarGoogle Scholar |
Fry, B. (2008). ‘Stable Isotope Ecology’, 3rd edn. (Springer Science+Business Media, LLC: New York, NY, USA.)
Fry, B., and Sherr, E. B. (1984). δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems Contributions in Marine Science 27, 13–47.
Gao, Y., Bargmann, G. G., Brand, U., and Noakes, D. L. G. (2005). Stable isotopic and trace elemental compositions of otoliths and the stock structure of Pacific cod, Gadus macrocephalus. Environmental Biology of Fishes 74, 335–348.
| Stable isotopic and trace elemental compositions of otoliths and the stock structure of Pacific cod, Gadus macrocephalus.Crossref | GoogleScholarGoogle Scholar |
Grandcourt, E., Al Abdessalaam, T. Z., Francis, F., and Al Shamsi, A. (2011). Demographic parameters and status assessments of Lutjanus ehrenbergii, Lethrinus lentjan, Plectorhinchus sordidus and Rhabdosargus sarba in the southern Arabian Gulf. Journal of Applied Ichthyology 27, 1203–1211.
| Demographic parameters and status assessments of Lutjanus ehrenbergii, Lethrinus lentjan, Plectorhinchus sordidus and Rhabdosargus sarba in the southern Arabian Gulf.Crossref | GoogleScholarGoogle Scholar |
Herzka, S. Z. (2005). Assessing connectivity of estuarine fishes based on stable isotope ratio analysis. Estuarine, Coastal and Shelf Science 64, 58–69.
| Assessing connectivity of estuarine fishes based on stable isotope ratio analysis.Crossref | GoogleScholarGoogle Scholar |
Honda, K., Nakamura, Y., Nakaoka, M., Uy, W. H., and Fortes, M. D. (2013). Habitat use by fishes in coral reefs, seagrass beds and mangrove habitats in the Philippines. PLoS One 8, e65735.
| Habitat use by fishes in coral reefs, seagrass beds and mangrove habitats in the Philippines.Crossref | GoogleScholarGoogle Scholar | 24312621PubMed |
Huijbers, C. M., Nagelkerken, I., and Layman, C. A. (2015). Fish movement from nursery bays to coral reefs: a matter of size? Hydrobiologia 750, 89–101.
| Fish movement from nursery bays to coral reefs: a matter of size?Crossref | GoogleScholarGoogle Scholar |
Huxham, M., Kimuni, E., Newton, J., and Augley, J. (2007). Stable isotope records from otoliths as tracers of fish migration in a mangrove system. Journal of Fish Biology 70, 1554–1567.
| Stable isotope records from otoliths as tracers of fish migration in a mangrove system.Crossref | GoogleScholarGoogle Scholar |
Jamieson, R. E., Schwarcz, H. P., and Brattey, J. (2004). Carbon isotopic records from the otoliths of Atlantic cod (Gadus morhua) from eastern Newfoundland, Canada. Fisheries Research 68, 83–97.
| Carbon isotopic records from the otoliths of Atlantic cod (Gadus morhua) from eastern Newfoundland, Canada.Crossref | GoogleScholarGoogle Scholar |
Jensen, A. (2002). Artificial reefs of Europe: perspective and future. ICES Journal of Marine Science 59, S3–S13.
| Artificial reefs of Europe: perspective and future.Crossref | GoogleScholarGoogle Scholar |
Kalish, J. M. (1991). 13C and 18O isotopic disequilibria in fish otoliths: metabolic and kinetic effects. Marine Ecology Progress Series 75, 191–203.
| 13C and 18O isotopic disequilibria in fish otoliths: metabolic and kinetic effects.Crossref | GoogleScholarGoogle Scholar |
Kerr, L. A., Secor, D. H., and Kraus, R. T. (2007). Stable isotope (δ13C and δ18O) and Sr/Ca composition of otoliths as proxies for environmental salinity experienced by an estuarine fish. Marine Ecology Progress Series 349, 245–253.
| Stable isotope (δ13C and δ18O) and Sr/Ca composition of otoliths as proxies for environmental salinity experienced by an estuarine fish.Crossref | GoogleScholarGoogle Scholar |
Kim, S. T., Mucci, A., and Taylor, B. E. (2007a). Phosphoric acid fractionation factors for calcite and aragonite between 25 and 75°C: revisited. Chemical Geology 246, 135–146.
| Phosphoric acid fractionation factors for calcite and aragonite between 25 and 75°C: revisited.Crossref | GoogleScholarGoogle Scholar |
Kim, S. T., O’Neil, J. R., Hillaire-Marcel, C., and Mucci, A. (2007b). Oxygen isotope fractionation between synthetic aragonite and water: influence of temperature and Mg2+ concentration. Geochimica et Cosmochimica Acta 71, 4704–4715.
| Oxygen isotope fractionation between synthetic aragonite and water: influence of temperature and Mg2+ concentration.Crossref | GoogleScholarGoogle Scholar |
Kimirei, I. A., Nagelkerken, I., Trommelen, M., Blankers, P., van Hoytema, N., Hoeijmakers, D., Huijbers, C. M., Mgaya, Y. D., and Rypel, A. L. (2013a). What drives ontogenetic niche shifts of fishes in coral reef ecosystems? Ecosystems 16, 783–796.
| What drives ontogenetic niche shifts of fishes in coral reef ecosystems?Crossref | GoogleScholarGoogle Scholar |
Kimirei, I. A., Nagelkerken, I., Mgaya, Y. D., and Huijbers, C. M. (2013b). The mangrove nursery paradigm revisited: otolith stable isotopes support nursery-to-reef movements by Indo-Pacific fishes. PLoS One 8, e66320.
| The mangrove nursery paradigm revisited: otolith stable isotopes support nursery-to-reef movements by Indo-Pacific fishes.Crossref | GoogleScholarGoogle Scholar | 23776658PubMed |
Kitagawa, T., Ishimura, T., Uozato, R., Shirai, K., Amano, Y., Shinoda, A., Otake, T., Tsunogai, U., and Kimura, S. (2013). Otolith δ18O of Pacific bluefin tuna Thunnus orientalis as an indicator of ambient water temperature. Marine Ecology Progress Series 481, 199–209.
| Otolith δ18O of Pacific bluefin tuna Thunnus orientalis as an indicator of ambient water temperature.Crossref | GoogleScholarGoogle Scholar |
Kok, P. H., Akhir, M. F., and Tangang, F. T. (2015). Thermal frontal zone along the east coast of Peninsular Malaysia. Continental Shelf Research 110, 1–15.
| Thermal frontal zone along the east coast of Peninsular Malaysia.Crossref | GoogleScholarGoogle Scholar |
Kubota, K., Shirai, K., Murakami-Sugihara, N., Seike, K., Hori, M., and Tanabe, K. (2017). Annual shell growth pattern of the Stimpson’s hard clam Mercenaria stimpsoni as revealed by sclerochronological and oxygen stable isotope measurements. Palaeogeography, Palaeoclimatology, Palaeoecology 465, 307–315.
| Annual shell growth pattern of the Stimpson’s hard clam Mercenaria stimpsoni as revealed by sclerochronological and oxygen stable isotope measurements.Crossref | GoogleScholarGoogle Scholar |
Le, D. Q., Tanaka, K., Hii, Y. S., Sano, Y., Nanjo, K., and Shirai, K. (2018). Importance of seagrass–mangrove continuum as feeding grounds for juvenile pink ear emperor Lethrinus lentjan in Setiu Lagoon, Malaysia: stable isotope approach. Journal of Sea Research 135, 1–10.
| Importance of seagrass–mangrove continuum as feeding grounds for juvenile pink ear emperor Lethrinus lentjan in Setiu Lagoon, Malaysia: stable isotope approach.Crossref | GoogleScholarGoogle Scholar |
Lugendo, B. R., Pronker, A., Cornelissen, I., de Groene, A., Nagelkerken, I., Dorenbosch, M., van der Velde, G., and Yunus, D. M. (2005). Habitat utilisation by juveniles of commercially important fish species in a marine embayment in Zanzibar, Tanzania. Aquatic Living Resources 18, 149–158.
| Habitat utilisation by juveniles of commercially important fish species in a marine embayment in Zanzibar, Tanzania.Crossref | GoogleScholarGoogle Scholar |
Luo, J., Serafy, J. E., Sponaugle, S., Teare, P. B., and Kieckbusch, D. (2009). Diel and seasonal movement of gray snapper (Lutjanus griseus) among subtropical seagrass, mangrove and coral reef habitats. Marine Ecology Progress Series 380, 255–269.
| Diel and seasonal movement of gray snapper (Lutjanus griseus) among subtropical seagrass, mangrove and coral reef habitats.Crossref | GoogleScholarGoogle Scholar |
Mateo, I., Durbin, E. G., Appeldoorn, R. S., Adams, A. J., Juanes, F., Kingsley, R., Swart, P., and Durant, D. (2010). Role of mangroves as nurseries for French grunt Haemulon flavolinatum and schoolmaster Lutjanus apodus assessed by otolith elemental fingerprints. Marine Ecology Progress Series 402, 197–212.
| Role of mangroves as nurseries for French grunt Haemulon flavolinatum and schoolmaster Lutjanus apodus assessed by otolith elemental fingerprints.Crossref | GoogleScholarGoogle Scholar |
McConnell, M. C., Thunell, R. C., Lorenzoni, L., Astor, Y., Wright, J. D., and Fairbanks, R. (2009). Seasonal variability in the salinity and oxygen isotopic composition of seawater from the Cariaco Basin, Venezuela: implications for paleosalinity reconstructions. Geochemistry Geophysics Geosystems 10, Q06019.
| Seasonal variability in the salinity and oxygen isotopic composition of seawater from the Cariaco Basin, Venezuela: implications for paleosalinity reconstructions.Crossref | GoogleScholarGoogle Scholar |
McMahon, K. W., Fogel, M. L., Johnson, B. J., Houghton, L. A., and Thorrold, S. R. (2011). A new method to reconstruct fish diet and movement patterns from δ13C values in otolith amino acids. Canadian Journal of Fisheries and Aquatic Sciences 68, 1330–1340.
| A new method to reconstruct fish diet and movement patterns from δ13C values in otolith amino acids.Crossref | GoogleScholarGoogle Scholar |
Mulcahy, S. A., Killingley, J. S., Phleger, C. F., and Berger, W. H. (1979). Isotopic composition of otoliths from a benthopelagic fish, Coryphaenoides acrolepis, Macrouridae: Gadiformes. Oceanologica Acta 2, 423–427.
Mumby, P. J. (2006). Connectivity of reef fish between mangroves and coral reefs: algorithms for the design of marine reserves at seascape scales. Biological Conservation 128, 215–222.
| Connectivity of reef fish between mangroves and coral reefs: algorithms for the design of marine reserves at seascape scales.Crossref | GoogleScholarGoogle Scholar |
Nagelkerken, I., Roberts, C. M., van der Velde, G., Dorenbosch, M., van Riel, M. C., de la Morinere, E. C., and Nienhuis, P. H. (2002). How important are mangroves and seagrass beds for coral-reef fish? The nursery hypothesis tested on an island scale. Marine Ecology Progress Series 244, 299–305.
| How important are mangroves and seagrass beds for coral-reef fish? The nursery hypothesis tested on an island scale.Crossref | GoogleScholarGoogle Scholar |
Nakamura, Y., Horinouchi, M., Shibuno, T., Tanaka, Y., Miyajima, T., Koike, I., Kurokura, H., and Sano, M. (2008). Evidence of ontogenetic migration from mangroves to coral reefs by black-tail snapper Lutjanus fulvus: stable isotope approach. Marine Ecology Progress Series 355, 257–266.
| Evidence of ontogenetic migration from mangroves to coral reefs by black-tail snapper Lutjanus fulvus: stable isotope approach.Crossref | GoogleScholarGoogle Scholar |
Nelson, J., Hanson, C. W., Koenig, C., and Chanton, J. (2011). Influence of diet on stable carbon isotope composition in otoliths of juvenile red drum Sciaenops ocellatus. Aquatic Biology 13, 89–95.
| Influence of diet on stable carbon isotope composition in otoliths of juvenile red drum Sciaenops ocellatus.Crossref | GoogleScholarGoogle Scholar |
Post, D. M. (2002). Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83, 703–718.
| Using stable isotopes to estimate trophic position: models, methods, and assumptions.Crossref | GoogleScholarGoogle Scholar |
Pruell, R. J., Taplin, B. K., and Karr, J. D. (2010). Stable carbon and oxygen isotope ratios of otoliths differentiate juvenile winter flounder (Pseudopleuronectes americanus) habitats. Marine and Freshwater Research 61, 34–41.
| Stable carbon and oxygen isotope ratios of otoliths differentiate juvenile winter flounder (Pseudopleuronectes americanus) habitats.Crossref | GoogleScholarGoogle Scholar |
Qu, T., Du, Y., Strachan, J., Meyers, G., and Slingo, J. (2005). Sea surface temperature and its variability in the Indonesian region. Journal of Oceanography 18, 50–61.
| Sea surface temperature and its variability in the Indonesian region.Crossref | GoogleScholarGoogle Scholar |
Radtke, R. L., Showers, W., Moksness, E., and Lenz, P. (1996). Environmental information stored in otoliths: insights from stable isotopes. Marine Biology 127, 161–170.
| Environmental information stored in otoliths: insights from stable isotopes.Crossref | GoogleScholarGoogle Scholar |
Reis-Santos, P., Tanner, S. E., Franca, S., Vasconcelos, R. P., Gillanders, B. M., and Cabral, H. N. (2015). Connectivity within estuaries: an otolith chemistry and muscle stable isotope approach. Ocean and Coastal Management 118, 51–59.
| Connectivity within estuaries: an otolith chemistry and muscle stable isotope approach.Crossref | GoogleScholarGoogle Scholar |
Rilov, G., and Benayahu, Y. (2000). Fish assemblage on natural versus vertical artificial reefs: the rehabilitation perspective. Marine Biology 136, 931–942.
| Fish assemblage on natural versus vertical artificial reefs: the rehabilitation perspective.Crossref | GoogleScholarGoogle Scholar |
Rioja-Nieto, R., and Alvarez-Filip, L. (2019). Coral reef systems of the Mexican Caribbean: status, recent trends and conservation. Marine Pollution Bulletin 140, 616–625.
| Coral reef systems of the Mexican Caribbean: status, recent trends and conservation.Crossref | GoogleScholarGoogle Scholar | 30005908PubMed |
Rohling, E. J. (2013). Oxygen isotope composition of seawater. In ‘The Encyclopedia of Quaternary Science, Vol. 2’. (Ed. S. A. Elias.) pp. 915–922. (Elsevier: Amsterdam, Netherlands.)
Sakamoto, T., Komatsu, K., Yoneda, M., Ishimura, T., Higuchi, T., Shirai, K., Kamimura, Y., Watanabe, C., and Kawabata, A. (2017). Temperature dependence of δ18O in otolith of juvenile Japanese sardine: laboratory rearing experiment with micro-scale analysis. Fisheries Research 194, 55–59.
| Temperature dependence of δ18O in otolith of juvenile Japanese sardine: laboratory rearing experiment with micro-scale analysis.Crossref | GoogleScholarGoogle Scholar |
Schaffner, F. C., and Swart, P. K. (1991). Influence of diet and environmental water on the carbon and oxygen stable isotopic signatures of seabird eggshell carbonate. Bulletin of Marine Science 48, 23–38.
Schwarcz, H. P., Gao, Y., Campana, S., Browne, D., Knyf, M., and Brand, U. (1998). Stable carbon isotope variations in otoliths of Atlantic cod (Gadus morhua). Canadian Journal of Fisheries and Aquatic Sciences 55, 1798–1806.
| Stable carbon isotope variations in otoliths of Atlantic cod (Gadus morhua).Crossref | GoogleScholarGoogle Scholar |
Seaman, W. (2007). Artificial habitats and the restoration of degraded marine ecosystems and fisheries. Hydrobiology 580, 143–155.
| Artificial habitats and the restoration of degraded marine ecosystems and fisheries.Crossref | GoogleScholarGoogle Scholar |
Serrano, X., Grosell, M., and Serafy, J. E. (2010). Salinity selection and preference of the grey snapper Lutjanus griseus: field and laboratory observations. Journal of Fish Biology 76, 1592–1608.
| Salinity selection and preference of the grey snapper Lutjanus griseus: field and laboratory observations.Crossref | GoogleScholarGoogle Scholar | 20557618PubMed |
Shirai, K., Koyama, F., Murakami-Sugihara, N., Nanjo, K., Higuchi, T., Kohno, H., Watanabe, Y., Okamoto, K., and Sano, M. (2018). Reconstruction of the salinity history associated with movements of mangrove fishes using otolith oxygen isotopic analysis. Marine Ecology Progress Series 593, 127–139.
| Reconstruction of the salinity history associated with movements of mangrove fishes using otolith oxygen isotopic analysis.Crossref | GoogleScholarGoogle Scholar |
Solomon, C. T., Weber, P. K., Cech, J. J., Ingram, B. L., Conrad, M. E., Machavaram, M. V., Pogodina, A. R., and Franklin, R. L. (2006). Experimental determination of the sources of otolith carbon and associated isotopic fractionation. Canadian Journal of Fisheries and Aquatic Sciences 63, 79–89.
| Experimental determination of the sources of otolith carbon and associated isotopic fractionation.Crossref | GoogleScholarGoogle Scholar |
Suratman, S., Mohd Sailan, M. I., Hee, Y. Y., Bedurus, E. A., and Latif, M. T. (2015). A preliminary study of water quality index in Terengganu River Basin, Malaysia. Sains Malaysiana 44, 67–73.
| A preliminary study of water quality index in Terengganu River Basin, Malaysia.Crossref | GoogleScholarGoogle Scholar |
Thomson, K. A., Ingraham, W. J., Healey, M. C., Leblond, P. H., Groot, C., and Healey, C. G. (1992). The influence of ocean currents on latitude of landfall and migration speed of sockeye salmon returning to the Fraser River. Fisheries Oceanography 1, 163–179.
| The influence of ocean currents on latitude of landfall and migration speed of sockeye salmon returning to the Fraser River.Crossref | GoogleScholarGoogle Scholar |
Thorrold, S. R., Campana, S. E., Jones, C. M., and Swart, P. K. (1997). Factors determining 13C and 18O fractionation in aragonitic otoliths of marine fish. Geochimica et Cosmochimica Acta 61, 2909–2919.
| Factors determining 13C and 18O fractionation in aragonitic otoliths of marine fish.Crossref | GoogleScholarGoogle Scholar |
Tieszen, L. L., Boutton, T. W., Tesdahl, K. G., and Slade, N. A. (1983). Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia 57, 32–37.
| Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet.Crossref | GoogleScholarGoogle Scholar | 28310153PubMed |
Tohse, H., and Mugiya, Y. (2008). Sources of otolith carbonate: experimental determination of carbon incorporation rates from water and metabolic CO2, and their diel variations. Aquatic Biology 1, 259–268.
| Sources of otolith carbonate: experimental determination of carbon incorporation rates from water and metabolic CO2, and their diel variations.Crossref | GoogleScholarGoogle Scholar |
Toor, H. S. (1964). Biology and fishery of the pig-face bream, Lethrinus lentjan Lacepede from Indian waters. III. Age and growth Indian Journal of Fisheries 11A, 597–620.
Trueman, C. N., Chung, M.-T., and Shores, D. (2016). Ecogeochemistry potential in deep time biodiversity illustrated using a modern deep-water case study. Philosophical Transactions of the Royal Society of London – B. Biological Sciences 371, 20150223.
| Ecogeochemistry potential in deep time biodiversity illustrated using a modern deep-water case study.Crossref | GoogleScholarGoogle Scholar | 26977063PubMed |
Vaslet, A., France, C., Phillips, D. L., Feller, I. C., and Baldwin, C. C. (2011). Stable-isotope analyses reveal the importance of seagrass beds as feeding areas for juveniles of the speckled worm eel Myrophis punctatus (Teleostei: Ophichthidae) in Florida. Journal of Fish Biology 79, 692–706.
| Stable-isotope analyses reveal the importance of seagrass beds as feeding areas for juveniles of the speckled worm eel Myrophis punctatus (Teleostei: Ophichthidae) in Florida.Crossref | GoogleScholarGoogle Scholar | 21884107PubMed |
Weidman, C., and Millner, R. (2000). High-resolution stable isotope records from North Atlantic cod. Fisheries Research 46, 327–342.
| High-resolution stable isotope records from North Atlantic cod.Crossref | GoogleScholarGoogle Scholar |
Werner, E. E., and Gilliam, J. F. (1984). The ontogenetic niche and species interactions in size-structured populations. Annual Review of Ecology and Systematics 15, 393–425.
| The ontogenetic niche and species interactions in size-structured populations.Crossref | GoogleScholarGoogle Scholar |
Xia, B., Gao, Q. F., Dong, S. L., and Wang, F. (2013). Carbon stable isotope turnover and fractionation in grass carp Ctenopharyngodon idella tissues. Aquatic Biology 19, 207–216.
| Carbon stable isotope turnover and fractionation in grass carp Ctenopharyngodon idella tissues.Crossref | GoogleScholarGoogle Scholar |




