Does wood type influence the colonisation of this habitat by macroinvertebrates in large lowland rivers?
Jarod P. Lyon A C , Simon J. Nicol A B , Jason A. Lieschke A and David S. L. Ramsey AA Department of Sustainability and Environment, Arthur Rylah Institute for Environmental Research, 123 Brown St, Heidelberg, Vic. 3084, Australia.
B Oceanic Fisheries Program, Secretariat of the Pacific Community, BP D5, 98848 Noumea CEDEX, New Caledonia.
C Corresponding author. Email: jarod.lyon@dse.vic.gov.au
Marine and Freshwater Research 60(5) 384-393 https://doi.org/10.1071/MF07233
Submitted: 3 December 2007 Accepted: 24 October 2008 Published: 25 May 2009
Abstract
Submerged woody habitat provides the major structure around which ecological processes operate in many lowland rivers. Colonisation by macroinvertebrates was measured in a south-eastern Australian river over a 32-day period in an experiment testing the hypothesis that wood type influences the invertebrate assemblage structure. The wood types were green wood, dry wood, and dry but previously waterlogged wood. All wood used was river red gum (Eucalyptus camaldulensis). Macroinvertebrates colonised previously waterlogged wood more rapidly than green or dry wood. The assemblage structure varied significantly over the sampling period, with copepods and cladocerans numerically dominating the assemblage during the first few days after the introduction of the wood. The assemblage became more diverse through time and was numerically dominated by dipterans, ephemeropterans and trichopterans. The results indicate that there was little difference in the time taken for macroinvertebrate colonisation after wood introduction when using either green or dry wood. This has implications for large-scale restoration projects, where green wood is likely to be a more readily available option for reintroduction than dry wood.
Additional keywords: Australia, habitat complexity, large woody debris, Murray River, restoration.
Introduction
In freshwater ecosystems, submerged woody habitat collectively describes the habitat provided by tree branches, tree trunks, root balls or entire trees (Harmon et al. 1986). Strong associations between submerged woody habitats and freshwater biota have been observed globally (see reviews by Benke and Wallace 2003; Dolloff and Warren 2003; Zalewski et al. 2003). Not surprisingly, this information has led to an increased interest for restoration of this habitat in rivers where it was once removed (Gerhard and Reich 2000; Roni and Quinn 2001; Brooks et al. 2004; Lester et al. 2007). Conversely, a number of studies have also failed to detect an association between submerged woody habitats and species diversity, richness and the abundance of macroinvertebrates and fish (see reviews by Wondzell and Bisson 2003; Spänhoff et al. 2006a). These observations introduce some uncertainty in determining what is expected of submerged woody habitat restoration and suggest that more information is required to explain the variable results.
The restoration of submerged woody habitats has only recently been applied in Australia (e.g. Erskine and Webb 2003; Brooks et al. 2004; Bond and Lake 2005; Lester et al. 2007; Scealy et al. 2007). Between 1870 and 1970, millions of large woody habitats in the rivers of the Murray-Darling Basin in eastern Australia were removed (Phillips 1972). The removal was carried out to aid in the navigation of riverboats, which, at the time, were the main form of goods transport in inland Australia. During the later stages of this time period, water authorities removed submerged wood in the belief that this would improve the water delivery capability of rivers and, therefore, make rivers operate more efficiently for irrigation (Gippel et al. 1996). Removal continued in the Murray River until as recently as the early 1990s. In areas where such removal had occurred, re-introduction of submerged woody habitats is now considered a major component in the reinstatement of natural ecological processes (Barrett 2004).
The ecological effects of submerged wood removal are potentially substantial because these items can serve as habitat for native biota, including fish, birds, invertebrates, plants and algae (Harmon et al. 1986). For invertebrates, submerged wood in relatively high-gradient mountainous streams can retain particulate organic matter, serve as stable substrate for attachment, and provide a food resource for wood-feeding individuals (Bilby and Likens 1980; Bilby 1981; O’Connor 1992; Collier and Halliday 2000; Lemly and Hilderbrand 2000). In larger, low-gradient streams, submerged wood is often a major provider of stable substrata and consequently can be a ‘hot spot’ of invertebrate production (Wallace et al. 1995; Growns et al. 1999; Brown and May 2000). Such areas are likely to provide important refuge areas during floods and can be the source of new colonists to the floodplain (Boulton and Lloyd 1991).
Invertebrate responses to submerged woody habitat reintroduction have been examined across a range of stream types from upland and foothill zones (O’Connor 1991; Hax and Golladay 1993; Wallace et al. 1995; McKie and Cranston 2001; Pretty and Dobson 2004) to lowland rivers (Mathooko and Otieno 2002; Johnson et al. 2003; Bond et al. 2006). However, large lowland rivers have generally been overlooked, although they are often likely to have been affected by submerged woody habitat removal.
The importance of wood surface complexity has been consistently reported in many studies documenting the response of invertebrates to wood reintroduction (O’Connor 1991; Magoulick 1998; Collier et al. 2004), with greater diversity and richness associated with higher heterogeneity owing to the provision of more microhabitat diversity (O’Connor 1991). Wood hardness has also been reported as an important influence on invertebrate community composition, with softer decaying substrates providing habitat for wood shredders and burrowers as well as substrate for collectors, predators and scrapers (Phillips and Kilambi 1994; Magoulick 1998; Collier and Halliday 2000).
The restoration of submerged woody habitat can pursue either long-term or short-term objectives, being either passive (i.e. revegetation of riparian zones and wood recruitment into the river through natural erosion and tree fall processes) or active where trees are sourced externally and mechanically placed into the river channel (Wondzell and Bisson 2003). For invertebrates, the restored wood would have ideally also experienced some level of decay to maximise surface heterogeneity and wood softness to capitalise on the increased taxa richness often associated with these types of substrates (O’Connor 1991). The submerged wood observed in Australian lowland streams is typically derived from large trees (Erskine and Webb 2003; Koehn et al. 2004). Obtaining trees of this size and decay for active restoration, however, may be problematic as fallen trees in riparian zones also serve as important habitat for terrestrial organisms (Mac Nally et al. 2001, 2002; Boyd et al. 2005); consequently, the removal of this wood may have wider negative effects. Transporting harvested wood from an external source and using this for mechanical restoration is also a possibility. However, such wood is likely to have lower surface heterogeneity and higher hardness than dead trees. Consequently, those undertaking submerged wood restoration could expect that the easily obtainable green wood for restoration would support a less diverse and abundant community assemblage than decayed wood, which has higher surface heterogeneity. The present study examines whether the type of wood introduced into lowland rivers (green timber v. two types of timber that had been dead for a long period) influences the early colonisation (as measured by the change in numerical abundance and in assemblage composition) of this habitat by macroinvertebrates.
Materials and methods
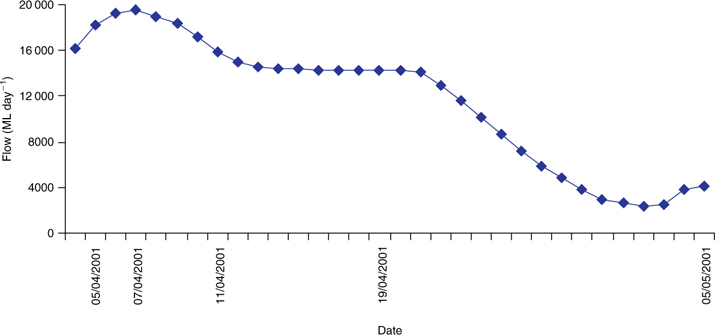
The study was undertaken in a 4-km stretch of the Murray River ~10 km upstream from Corowa (146°27′48″E, 35°58′23″S) between 3 April 2001 (Day 0) and 5 May 2001 (Day 32). The Murray River at this site is a large lowland river, ~80 m in width and up to 5 m deep, and its flow is highly regulated owing to Lake Hume, 40 km upstream. The average daily flow in this section of the Murray River varies between 1400 and 27 000 ML day–1, depending on downstream irrigation needs. The discharge at the study site decreased markedly over the sampling period (Fig. 1), with flows varying from 19 518 ML day–1 on 7 April 2001 to 2339 ML day–1 on 2 May 2001.
|
During the 1960s and 1970s, over 25 000 submerged woody habitats were removed from this section of the river to allow for more efficient water passage to downstream Lake Mulwala (Murray-Darling Basin Ministerial Council 1987). Nevertheless, some areas remain where submerged woody habitats are abundant, and as such, this provided a potential source of colonists for newly introduced submerged wood.
River red gum, Eucalyptus camaldulensis, was used as a substrate in the present study because this tree dominates the riparian vegetation and submerged woody habitats in much of the southern Murray-Darling Basin. Green wood (i.e. wood from a live tree that had fallen less than 1 month previously) was compared with two types of dry wood. The first type comprised wood from a fallen tree that had been dead for >24 months, but had never been waterlogged. The second type was wood that had previously been waterlogged, but had been removed from the river and dried out for several years, eliminating the possibility of aquatic macroinvertebrates being present. Each piece of wood was cut to ~300 mm long × 125 mm diameter.
Thirty sets were used, each consisting of one piece of each type of wood tied individually beneath a float. The order of each set beneath a float was randomised so that the wood types were in a different position for each set. Two sets were attached to a floating 6-m length of 90-mm diameter stormwater pipe (float), with the pieces separated by 1 m; there was a gap of 2 m between sets. The pieces of wood were weighed down to a depth of 400 mm below the surface using a 1-kg weight attached to the bottom of each piece of wood with a piece of twine. This allowed the samples to remain at a constant distance from the water surface, and compensated for fluctuations in river height at the time of sampling. The distance from the bottom changed according to the discharge from Lake Hume, but was generally between 0.5 and 1.5 m. Pre-existing submerged woody habitat occurred in the study reach and to account for any effect that proximity to this pre-existing wood might have on the results, half of the replicates were placed within five areas considered to be ‘snag rich’ (i.e. >10 submerged fallen trees per 100 m2) and the other half were placed in five areas considered to be ‘snag poor’ (i.e. <2 submerged fallen trees per 100 m2). Rich and poor areas were interspersed (with a minimum of 600–700 m between each area) to avoid spatial confounding, and within these areas all floats were kept a minimum of 6 m apart.
The duration of the present study was based on a study of colonisation in a nearby small lowland Australian stream (O’Connor 1991), which found that the bulk of macroinvertebrate colonisation was completed in less than 2 weeks, with species composition stabilising after 4 weeks. Sampling was conducted on days 2, 4, 8, 16 and 32 after the wood was placed in the river. Two sets (i.e. one float) from a ‘snag poor’ area were not sampled on Day 32 owing to a decrease in the river level causing grounding of that particular float. Three replicates were chosen randomly from ‘rich’ areas and three from ‘poor’ areas. A boat was driven into the current and up behind the last log in the set (keeping at least 500 mm away from the float at all times) to avoid disturbing the organisms situated on the experimental pieces of wood. A 50-μm mesh net was used to scoop up the wood. The twine attaching the wood to the pipe was cut, and the wood was placed in a sorting tray in the boat. All material collected in the net was washed into the tray. The wood was then scrubbed thoroughly into the tray with a coarse brush. The contents of the tray were then tipped through a 180-μm mesh sieve. Invertebrates collected in the sieve were stored in 70% ethanol and later counted and identified to order level, and for some taxa to family level. Most individuals were not mature enough to display the characters required for their identification to genus or species level. We assumed that the scrubbing process was sufficient to adequately collect animals that may have dug into the wood, such as elmids and boring chironomids (Growns et al. 1999).
Data analysis
To assess changes in the assemblage structure (the numbers of taxa and the numbers of individuals at the identification level of order) over the study period and to evaluate differences between groups (wood type and presence/absence of an existing submerged woody habitat load), we used non-metric multi-dimensional scaling (NMDS) in combination with permutational multivariate ANOVA (Anderson 2001). The data were square-root transformed and standardised using the Wisconsin double standardisation procedure. A matrix of pair-wise dissimilarities between the samples was constructed using a Bray–Curtis dissimilarity metric. The R add-in package ‘vegan’ (http://cran.au.r-project.org/; Oksanen et al. 2007) was used to ordinate the order level data set. Following NMDS, linear vectors were fitted to the ordination space by finding directions within the ordination space that had maximal correlation with each of the original taxonomic orders using the ‘vegan’ function ‘envfit’ based on 1000 permutations (Oksanen et al. 2007). Multivariate dispersion assumptions for the permutational multivariate ANOVA were tested using permutational analysis of multivariate dispersions (program ‘PERMDISP’; Anderson 2004). The Day 32 samples were excluded from the permutational multivariate ANOVA and the permutational analysis of multivariate dispersions tests to satisfy the assumption of a balanced design required for these tests.
Generalised linear mixed-effect models were fitted to the natural logarithm of the count data assuming Poisson errors. Float was fitted as a random effect with day and wood type (type) fitted as fixed effects. Over-dispersion was evident in some of the models so quasi-likelihood methods (Wedderburn 1974) were used, which allow for the dispersion parameter to be estimated and used in the calculation of the standard errors.
For each invertebrate order, four models were fitted as follows:




where Y is the natural log of the counts, day and type are the fixed effects of day and wood type, respectively, and float is the random effect. The relative support for each of these models was assessed by calculating Akaike’s Information Criterion (AIC), corrected for small sample size and over-dispersion (QAICc) (Burnham and Anderson 1998). The over-dispersion parameter was initially calculated for Model 1 and the resulting estimate was used to calculate QAICc for the remaining three models. The QAICc values for each model were rescaled as differences from the model with the lowest QAICc value (ΔQAICc). The likelihood of each model, given the data, was then calculated as:

where P(Mk | y) is the likelihood of model Mk given data y (Hoeting et al. 1999).
Results
A total of 29 651 individuals from 15 orders and 31 families were collected. The most abundant taxa collected were Diptera, Ephemeroptera and small Trichoptera. Ten families were recorded on only one occasion. Within Diptera, the most abundant taxa found were juvenile chironomids.
The macroinvertebrate assemblage structure differed over the 32-day time period of the study (Fig. 2a; Table 1). At the completion of the study on Day 32, the community structure, although not stable, was more diverse and dominated by insects in the Ephemeroptera, Plecoptera, Diptera and Trichoptera in comparison to Day 2 and Day 4 when the community was dominated by crustaceans in the order Copepoda and Cladocera (Figs 2b and 3).

|

|

|
A difference in the assemblage structure was not detected between areas where the pre-existing submerged woody habitat load was >10 fallen trees per 100 m2 and those areas where it was <2 fallen trees per 100 m2 (Fig. 2c; Table 1). Wood that had been previously waterlogged had a significantly different assemblage structure to green wood, but a difference was not detected between previously waterlogged and dry or dry and green wood (Fig. 2d; Table 1: pairwise comparisons, waterlogged v. dry, t23 = 1.3892, P = 0.0731; waterlogged v. green, t23 = 1.5210, P = 0.0335; dry v. green, t23 = 0.8854, P = 0.5519). A significant interaction between day and wood type was detected for assemblage structure (Table 1). The permutational analysis of the multivariate dispersions test indicated that the variance assumptions were met for the test of mean day and mean type (Table 2). Unequal variances between groups, however, could not be ruled out as an explanation for the significant day × type interaction term (Table 2).

|
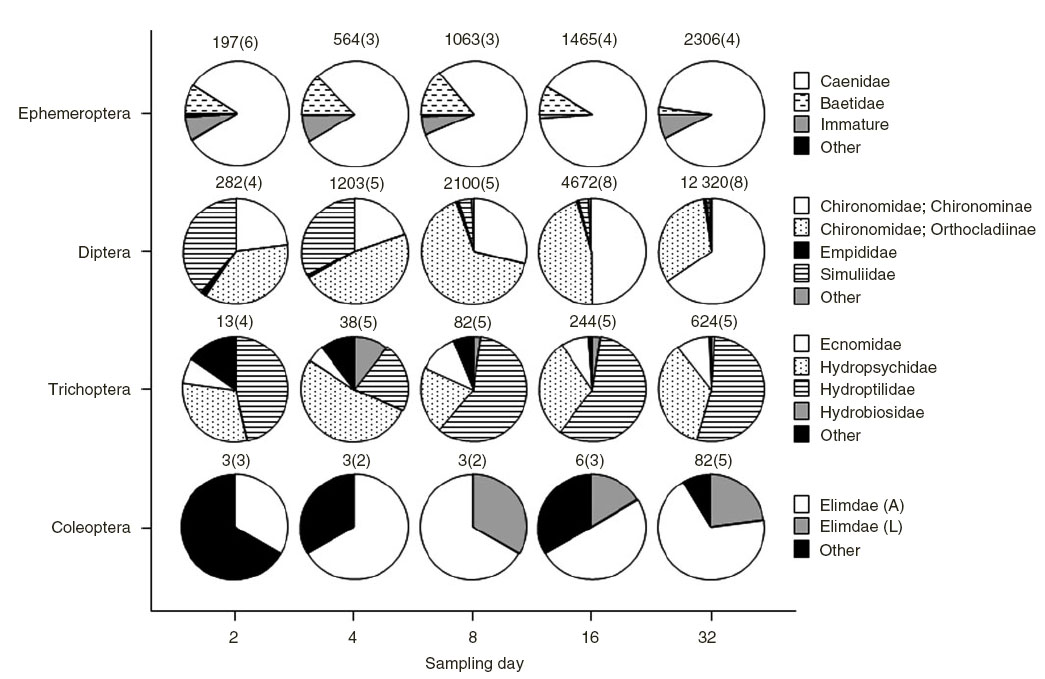
Results of the model selection procedure for the four generalised linear mixed-models fitted to the counts of each dominant order indicated that there was significant support for models where the counts varied by type (wood type) and day, either additively (Trichoptera and Coleoptera) or interactively (Ephemeroptera, Plecoptera, Diptera, Copepoda, Oligochaeta and Hydracarina) (Table 3). The exception to this was counts for the order Cladocera, which had more support for model 3, indicating an effect of wood type, but not day, and for the order Odonata where there was equal support for Model 2 and Model 3 (Table 3). The number of crustaceans in the orders Copepoda and Cladocera decreased over the 32-day period, whereas insects in the orders Diptera, Trichoptera, Plecoptera, Coleoptera and Ephemeroptera increased as did oligochaete worms and water mites (Hydracarina) (Fig. 3). Previously waterlogged wood generally had higher average counts than the other wood types (Fig. 3). The response of particular families varied within the taxonomic orders where organisms could be identified to family level (Fig. 4). Within Ephemeroptera, the family composition remained relatively constant over the 32 days, with Caenidae the dominant family (Fig. 4). The composition of Diptera varied over the study period; Simuliidae was dominant early with Chironominae and Orthocladiinae; however, the abundance of Simuliidae was reduced by Day 32 (Fig. 4). The order Trichoptera was dominated by Hydropsychidae and Hydroptilidae over the entire 32 days (Fig. 4). Elmid adults and larvae dominated Coleoptera over the 32 days (Fig. 4).

|
Discussion
Our observations concur with the general colonisation patterns reported for submerged woody habitats (O’Connor 1991; McKie and Cranston 1998; Scealy et al. 2007). The initial colonisation we observed increased on each consecutive sampling occasion and was higher on previously waterlogged wood in comparison to dry and green wood. As samples that came into contact with the river bed were excluded from the analysis, there was little opportunity for colonisation by non-drifting organisms that may contribute to the assemblage structure on resnagged wood. Many factors can influence the length of time for colonisation of woody habitats by macroinvertebrates, including season, position of the wood within the stream, flow rate and biofilm development, and although this study was undertaken over a 32-day period, it is important to note that the assemblage structure had not stabilised by Day 32.
The similarities between samples placed in ‘snag rich’ and ‘snag poor’ areas suggest that the small quantities of naturally occurring wood in the ‘snag rich’ areas did not contribute greatly to the pool of colonists available. During the study period, the flow rate in the area was particularly high. Flow has been identified as an important determinant of macroinvertebrate assemblage composition (Way et al. 1995; Johnson et al. 2003; Spänhoff et al. 2006b). Borchardt (1993) identified the effects of differing amounts of woody debris and differing flow rates on the drift loss (caused by the effects of water scour) of two species of benthic macroinvertebrates (Gammarus pulex L. and Ephemerella ignita Poda) and observed an increased drift loss of up to 44% with an increase in flow rate. Borchardt (1993) also found that drift loss could be mitigated to a certain degree by increasing the amount of woody debris as available habitat. It is plausible that, owing to the lack of snags in the area, the highly abrasive effects of the fast-flowing water from Lake Hume had removed most of the macroinvertebrates from the remaining snags, thereby limiting the potential for drift movements from the remaining snags beyond any ‘background’ drift.
The dominance of the macroinvertebrate community by taxa in the orders Diptera, Ephemeroptera and Trichoptera was also consistent with other studies (Magoulick 1998; Collier and Halliday 2000; Mathooko and Otieno 2002; Johnson et al. 2003; Bond et al. 2006). Dipterans are dominant consumers of biofilms that form on the surface of both submerged and semi-submerged wood (Bond et al. 2006), and consequently it is not surprising that they dominated the macrofauna. Chironominae was the dominant taxon by Day 32, and Simuliidae had correspondingly decreased. Simuliids colonise new clean surfaces and their abundance decreases once a thick biofilm develops, whereas this biofilm provides preferred habitat for chironomids (Downes and Lake 1991). Caenids are the dominant mayfly family in southern Australian lowland rivers (Marchant et al. 2000) and consequently it is not unexpected that they were the dominant taxon within Ephemeroptera in the present study. Hydropsychidae and hydroptilids were the dominant taxa in Trichoptera. Hydropsychidae are filter feeders and the surface of submerged wood provides suitable habitat for this taxon (Lake and Doeg 1985). Similarly, hydroptilids typically consume algal communities that are associated with logs (Gooderham and Tsyrlin 2002).
Invertebrates can change the surface complexity on submerged wood. Xylophagous elmid beetles (Coleoptera : Elmidae) are responsible for gouging grooves in wood in south-eastern Australian streams, thereby allowing colonisation by other macroinvertebrates and microorganisms, such as algae and fungi (McKie and Cranston 1998). The hardness of wood has also been reported to be an important determinant of community structure, with densities of wood-eating and burrowing invertebrates higher on softer woods compared with harder wood (Magoulick 1998), although other authors (Spänhoff et al. 2000; Collier et al. 2004) have shown that surface complexity is more important than wood genus in determining macroinvertebrate colonisation. This has implications for our findings because often the timber used in restoration projects is obtained opportunistically, and could be one of several Australian hardwood species. The Elmidae found in the present study were primarily on the previously waterlogged and dry wood, indicating that, at least over the first 32 days, these wood types provided better points of attachment for this taxon.
The rapid colonisation and then numerical decline of copepods and cladocerans suggest that colonisation was probably by drift and that these animals were seeking shelter from the river currents, an observation that has been observed in other experiments (Tank and Winterbourn 1995; Magoulick 1998; Jenkins et al. 2005). As the occupation of the logs by other taxa, including predators, increased with time, the quantity of available shelter for copepods and cladocerans is likely to have declined, resulting in the reduced abundance of both taxa. The dominance of oligochaetes on green wood may be related to their feeding habitats. Oligochaetes are typically detritivores (Gooderham and Tsyrlin 2002) and their numerical dominance on green wood indicates that the taxa observed in the present study were probably seeking food sources that live on the dissolved organic matter and nutrients coming from the this wood type.
Green wood is generally more readily available for restoration activities than other wood types through felling for land clearance. Although our study did not observe a stable assemblage structure within its 32-day duration, we were able to observe increasing similarity in the macroinvertebrate assemblages between green wood and the two other types of decayed wood through time. The results presented here, in addition to findings from similar studies (i.e. Johnson et al. 2003; Scealy et al. 2007), show that the complexity of the wood surface and not the species of tree or the time since falling/felling is the more important factor in terms of macroinvertebrate colonisation on newly introduced timber. As such, river restoration practitioners should have confidence that implementing habitat restoration with green wood may provide adequate physical habitat for colonisation by macroinvertebrate communities in a large lowland river in the absence of older wood with a higher surface complexity.
Acknowledgements
The present study was undertaken with the support of the Murray-Darling Basin Commission and the Department of Agriculture, Forestry and Fisheries Australia. We would like to thank Shanaugh McKay and Mike Nicol (ARI) and Russell Shiel for their identification of macroinvertebrates. We thank Sabine Schreiber for assistance with data analysis and Nick Bond and Alison King for comments on the colonisation patterns observed. We also thank Sam Lake and three anonymous referees for their comprehensive reviews, which greatly improved the manuscript.
Anderson, M. J. (2001). A new method for non-parametric multivariate analysis of variance. Austral Ecology 26, 32–46.
| Crossref | GoogleScholarGoogle Scholar |
Barrett, J. (2004). Introducing the Murray-Darling Basin Native Fish Strategy and initial steps towards demonstration reaches. Ecological Management & Restoration 5, 15–23.
| Crossref | GoogleScholarGoogle Scholar |
Bilby, R. E. (1981). Role of organic debris dams in regulating the export of dissolved and particulate matter from a forested watershed. Ecology 62, 1234–1243.
| Crossref | GoogleScholarGoogle Scholar | CAS |
Bond, N. R. , and Lake, P. S. (2005). Ecological restoration and large-scale ecological disturbance: the effect of drought on the response by fish to a habitat restoration experiment. Restoration Ecology 13, 39–48.
| Crossref | GoogleScholarGoogle Scholar |
Collier, K. J. , and Halliday, J. N. (2000). Macroinvertebrate–wood associations during decay of plantation pine in New Zealand pumice-bed streams: stable habitat or trophic subsidy? Journal of the North American Benthological Society 19, 94–111.
| Crossref | GoogleScholarGoogle Scholar |
Downes, B. J. , and Lake, P. S. (1991). Different colonization patterns of two closely related stream insects (Austrosimulium spp.) following disturbance. Freshwater Biology 26, 295–306.
| Crossref | GoogleScholarGoogle Scholar |
Growns, J. E. , King, A. J. , and Betts, F. M. (1999). The snag bag: a new method for sampling macroinvertebrate communities on large woody debris. Hydrobiologia 405, 67–77.
| Crossref | GoogleScholarGoogle Scholar |
O’Connor, N. A. (1991). The effects of habitat complexity on the macroinvertebrates colonising wood substrates in a lowland stream. Oecologia 85, 504–512.
| Crossref | GoogleScholarGoogle Scholar |
Phillips, E. C. , and Kilambi, R. V. (1994). Use of coarse woody debris by Diptera in Ozark streams, Arkansas. Journal of the North American Benthological Society 13, 151–159.
| Crossref | GoogleScholarGoogle Scholar |