Feasibility of a physiotherapist-supervised walking program with telephone coaching to increase physical activity following acquired brain injury
Caitlyn Payne A , Janelle Gesch A B , Esther Smits C , Charlotte Brakenridge
C , Charlotte Brakenridge  C D , Venerina Johnston
C D , Venerina Johnston  E F , Paul A. Gardiner
E F , Paul A. Gardiner  G , Tracy Comans
G , Tracy Comans  H , Ryan Bell B and Elise Gane
H , Ryan Bell B and Elise Gane  A E I *
A E I *
A
B
C
D
E
F
G
H
I
Abstract
Physical activity has health benefits for adults with acquired brain injury, but it is a challenge to increase physical activity during inpatient rehabilitation. The objectives of this pilot study were to determine whether a physiotherapy-supervised inpatient walking program was feasible and able to improve physical activity and sedentary behaviour in the short and medium term.
Adults with acquired brain injury receiving inpatient rehabilitation undertook twice-weekly supervised walks plus behavioural therapy for 4 weeks. Feasibility was measured via recruitment, participation and drop out rates, adverse events and intervention delivery costs. Physical activity and sedentary behaviour were measured with an activPAL. Assessments were conducted at baseline, post-intervention and 3–6 months post-intervention.
The program was safe to deliver (no adverse events), recruitment rate was 55% (16/29) and the participation rate for eligible individuals was high (14/19, 74%). However, the program had a high drop out rate (7/16, 44%) and physical activity and sedentary behaviour did not significantly change during the 4-week intervention. Costs were AU$427.71/participant. Physical activity and sedentary behaviour did improve 3–6 months after the intervention (vs baseline, on average: +3913 steps per day, 95% CI: 671, 7156).
This pilot study demonstrated a supervised physiotherapy walking program is safe and feasible to recruit in an inpatient setting. However, drop out during the study was high and behaviour change did not occur. More work is required to boost physical activity during sub-acute rehabilitation for acquired brain injury.
Keywords: behavioural therapy, brain injury, hospital rehabilitation, physical activity, physiotherapy, sedentary behaviour, self-management, walking.
Introduction
Acquired brain injury (ABI) is defined as a brain injury that occurs after birth, caused by trauma (traumatic brain injury (TBI)), stroke, tumours, infection, hypoxia or substance misuse (Teasell et al. 2007; Turner‐Stokes et al. 2015). This is a condition that affects many Australians. In 2020, more than 445,087 Australians were dealing with the consequences of a stroke (Deloitte Access Economics 2020). Across 2015–2020, 16,350 Australians were hospitalised with a moderate to severe TBI (O’Reilly et al. 2023). The consequences of ABI are complex and often result in significant restrictions on an individual’s ability to participate fully in daily tasks, employment and physical activity. Their rehabilitation needs may encompass physical, communicative, behavioural, psychosocial and environmental concerns (Turner‐Stokes et al. 2015).
Physical activity guidelines recommend that all adults, including those living with a disability, should participate in 150–300 min of moderate intensity or 75–150 min of vigorous intensity aerobic physical activity per week (or a combination of both); muscle strengthening exercises on 2 or more days per week; reduce their time spent sitting and break up long periods of sitting (World Health Organization 2020; Department of Health 2021). In adults with ABI, however, this is often difficult to achieve, with a multitude of physical deficits (Basford et al. 2003), cognitive deficits (Slovarp et al. 2012), psychosocial changes (Hart et al. 2011) and fatigue (Chaudhuri and Behan 2004; Guggisberg et al. 2020) negatively impacting participation in physical activity (Hamilton et al. 2016; Fini et al. 2017; Ramsey et al. 2018) and resulting in high levels of sedentary time (sitting or lying with low energy expenditure, ≤1.5 metabolic equivalents (METS)) (Tremblay et al. 2017). Being physically inactive predisposes adults with ABI to experiencing poorer long-term health outcomes and prolonged disability, along with an increased risk of developing subsequent health issues including stroke (Hartmann et al. 2001; Burke et al. 2013), depression (Zgaljardic et al. 2015; Shi et al. 2017) and dementia (Li et al. 2016; Kuzma et al. 2018). As participation in regular physical activity is an important factor in maintaining health and wellbeing and reducing the risks of these secondary complications (Hoffman et al. 2010; Moore et al. 2015; D’Isabella et al. 2017), there is a need to improve and maintain physical activity levels in adults with ABI and reduce their sedentary time.
Previous studies have explored engaging adults with ABI in physical activity programs, using a variety of supportive tools and in different settings. Driver and colleagues (2023) implemented a 12-month educational diabetes prevention program, supported by a wrist-worn activity tracker to promote physical activity, for community-dwelling adults with TBI in the United States. Participants in the intervention group lost more weight than the control group who received a general health education group program. Bellon and colleagues (2015) progressively increased the step count goal for adults with TBI using a pedometer over 12 weeks and found their walking program to significantly reduce depression and stress when compared to education about nutrition (control group). Quality of life improved for sub-acute stroke survivors after a 9-month group physical activity program conducted within a seniors community centre (Lund et al. 2012). These programs employed physical activity as a component of their interventions, but did not measure physical activity as an outcome. One such program that did measure physical activity delivered a group education program (1 h, twice per week) to adults with a brain injury undertaking outpatient rehabilitation (Driver and Woolsey 2016). This education was designed to teach social and behavioural strategies, so that participants could adopt and maintain physical activity within a healthy lifestyle (Driver et al. 2012b). Participants did improve their (self-reported) physical activity levels as a result of the program. This evidence is promising, but also serves to highlight that translating the effect of physical activity programs into increased free-living physical activity is still a challenge.
Initiating and maintaining a physically active lifestyle is a significant challenge for healthy adults (Kelly et al. 2016; Australian Institute of Health and Welfare 2018); adults with ABI may face even greater challenges due to the nature of their injury (Driver et al. 2012a). An important aspect of maintaining an active lifestyle is having positive thoughts and beliefs about physical activity. Previous research suggests that behavioural therapy (changing thoughts and beliefs, and thus behaviours) can improve self-confidence to exercise in adults with ABI (Bell et al. 2005; Driver et al. 2012b). Behavioural therapy can be delivered in person or via telephone to adults with ABI to treat conditions such as depression (Fann et al. 2015; Kirkness et al. 2017). Behaviour change programs delivered via telephone have been shown to increase physical activity levels in adult patients of an ambulatory care clinic (Barrett et al. 2018). Thus, promoting self-confidence to exercise in adults with ABI could be achieved via telephone coaching, and might contribute to increasing their levels of physical activity.
It is common for adults with ABI who are hospitalised to receive an initial period of intensive rehabilitation within a hospital inpatient setting, with ongoing community-based follow up in specialised outpatient clinics after discharge (Turner‐Stokes et al. 2015; Stroke Foundation 2023). During inpatient rehabilitation, many patients achieve a level of functional mobility, meaning that they can engage in walking as a form of physical activity. Walking as a regular form of physical activity has many known health benefits in free living adult populations and certain neurological populations (Halabchi et al. 2017; Jones et al. 2021). Prior studies have implemented physical activity programs with adults in the sub-acute and chronic phase post-ABI (Lund et al. 2012; Bellon et al. 2015; Driver et al. 2023). There is a need to explore the feasibility of such programs for the inpatient rehabilitation setting, as patients in these settings are physically inactive (Kunkel et al. 2015). Therefore, we designed a 4-week, hospital-based, supervised walking program complimented with behavioural therapy including telephone coaching in order to increase physical activity and decrease sedentary behaviour of patients with brain injury.
Pilot studies are a sub-set of feasibility studies, in that researchers are seeking to determine whether something can be done (a feasibility study) as well conducting some part of a future study on a smaller scale (a pilot study) (Eldridge et al. 2016). According to Thabane and colleagues (2010), there are four concepts to assess within a pilot study, related to a larger future study: the process, resources, management and treatment response. We had three objectives for this pilot study that addressed the process, resources and treatment response concepts. The first objective was to determine the recruitment rate, participation rate, drop out rate (process), adverse events (treatment response) and associated cost (resources) of the intervention. The second objective was to explore if the intervention had an effect (treatment response) on physical activity and sedentary behaviour primarily, and health-related quality of life, exercise self-efficacy and fatigue secondarily, in the short term (immediately after the 4-week intervention) and in the medium term (3 months after the intervention). The third objective was to explore feedback from participants with ABI, their caregivers and physiotherapy staff involved in the walking program concerning the acceptability of and satisfaction with the assessment and intervention components of the program (process).
Materials and methods
Study design and setting
This pilot study with one group of participants was conducted at Princess Alexandra Hospital in 2019–2020; specifically, the Brain Injury Rehabilitation Unit, which is the 26-bed specialist multidisciplinary inpatient rehabilitation unit for people with brain injury in Queensland and northern New South Wales, Australia. This study was approved by Metro South Health (HREC/2019/QMS/48750) and The University of Queensland (2019/HE001243) Ethics Committees. Written informed consent was obtained from each participant. A corresponding hypothesis was not set for objective 2 (treatment response) as a formal power calculation was not done and any results from hypothesis testing in pilot studies are recommended to be treated with caution (Lancaster et al. 2004).
Participants
Eligible participants were adults (18 years and older) with a history of ABI; current inpatients of the Brain Injury Rehabilitation Unit; able to safely mobilise outdoors continuously for at least 10 min (±walking aid, ±stand by assistance only) at a minimum step rate of 80 steps per minute; able to follow three stage commands and provide informed consent. People were excluded from the study if they had other medical conditions that limited walking or made walking unsafe; had ABI-associated behavioural concerns or a mental health condition that limited constructive group engagement; had a cognitive impairment or insufficient English that impaired provision of informed consent; or were likely to discharge from the unit within 2 weeks of admission. The participants’ treating physiotherapists, and medical team if required, were consulted to establish eligibility.
Caregivers or family members of participants with ABI and physiotherapy staff delivering the intervention were also invited to formally consent as research participants in order to provide feedback about the program. Caregivers were eligible to participate if they were within the social support network of a participant with ABI, as nominated by that participant. Physiotherapy staff were eligible to participate if they worked within the brain injury clinical team at Princess Alexandra Hospital and had actively supervised participants in at least one walking session.
Sample size
A sample size of 20 individuals with ABI was considered practical for this study, allowing for potential participant drop out (Julious 2005; Sim and Lewis 2012) as well as being feasible to achieve with predicted patient flow through the Brain Injury Rehabilitation Unit.
Procedures
Treating physiotherapists within the Brain Injury Rehabilitation Unit referred patients who they identified would benefit from the program and broadly met eligibility criteria to the first author (physiotherapy clinician) or the senior author (physiotherapy researcher) for formal screening and recruitment. Baseline data collection included 7 days of continuous accelerometer wear and completion of clinical and patient-reported outcome measures (Supplementary material S1). The 4-week intervention period commenced after the 7-day activity monitoring. The post-intervention assessment occurred as shortly after the intervention completion as possible (i.e. at the next hospital visit). The medium-term follow up assessment was planned to occur at 3 months after intervention completion, at the next most convenient hospital outpatient appointment. Caregivers of participants were identified in the last 1–2 weeks of the intervention period for their associated participant and were consented to provide feedback via written questionnaire about the program, not to actively be involved in the walking sessions. Staff involved with the study provided feedback via anonymous online questionnaire at the end of the study period.
Intervention
All participants received the intervention, which had two components: a supervised walking program twice a week and a weekly behavioural therapy program delivered by a trained physiotherapist. This was in addition to usual care provided within the rehabilitation unit.
The participants undertook two supervised walking sessions per week for 4 weeks in small groups of up to six. Each session was supervised by one physiotherapist and either a physiotherapy assistant or final year physiotherapy student. The walking session consisted of a 5-min warm up, 30-min walk on a pre-specified walking route around the hospital campus, and a 5-min cool down. Music with a tempo of 80 beats per minute was played to keep participants walking at a pace considered to be of moderate intensity for other neurological populations (Manns and Baldwin 2009; Billinger et al. 2014) to achieve a physiological effect.
The behavioural therapy component was designed by following the steps to design a behaviour change intervention advocated by Michie et al. (2011, 2014): understand the behaviour, identify intervention options and identify content and implementation options. Behaviour occurs as a result of capability, opportunity and motivation. We identified intervention functions (e.g. education, training, enablement) and their corresponding behaviour change techniques (e.g. information, demonstration of the behaviour, goal setting) to influence capability, opportunity or motivation. An in-person one-on-one initial education session of 20–30 min duration was delivered to the participant (and their caregivers, if available) by a physiotherapist trained in goal setting (Prescott et al. 2015) and motivational interviewing principles (Medley and Powell 2010). The purpose of this session was to facilitate discussion of the participant’s beliefs, expectations and concerns around physical activity, and commence goal setting toward physical activity targets. An A4-sized paper copy of a presentation was used as a tool in the education session, meaning the education session was semi-scripted. Either individual face-to-face or telephone coaching (depending on inpatient vs outpatient status) was then delivered once per week. During these coaching sessions, participants were facilitated to set goals that increased their total physical activity each week, working towards the adult physical activity recommendations of 30 min of moderate intensity physical activity per day for 5 days per week (Department of Health 2021) by Week 4. However, as the intervention was tailored to each participant, participants were not forced to achieve this recommended amount by Week 4. If a participant was discharged before completing the program, they were facilitated via these coaching telephone calls to continue twice-weekly community walking sessions equivalent to the hospital-based sessions. The goal setting diary and weekly coaching phone call were used to support engagement, and a family member or friend was encouraged to supervise the sessions if available, although was not mandated.
At the start of the intervention, participants also received a generic physical activity brochure; an activity diary to plan, record and reflect on physical activity levels; a pedometer to use as a goal setting tool; and a hat, water bottle and sunscreen.
Outcome measures
Feasibility data included recording the number of eligible patients screened and recruited, along with participant drop out. The recruitment rate was defined as the consented participants as a percentage of participants screened. The participation rate was defined as the number of participants who commenced the intervention as a percentage of those eligible. Participants who were uncontactable after discharge as well as participants who formally withdrew were considered to have dropped out.
To contribute to feasibility data, staff conducting the supervised walking sessions were instructed to ask all participants how they felt after the previous walking session (e.g. to identify any musculoskeletal injuries associated with the walking session), and to make a record in the medical chart and in the research record of any adverse events (e.g. mechanical fall) that may have occurred during the supervised walking sessions. The treating medical team was also to be notified of any adverse events arising from the walking sessions. Costs of implementing the program were determined by the recording of staff time spent screening, recruiting, collecting data and delivering the walking program; and by recording the use of equipment and consumables.
Physical activity and sedentary behaviour were captured by the activPAL4 accelerometer (PAL Technologies Ltd, Glasgow, UK). This device has been found to be a valid and reliable measure of physical activity and sedentary behaviour in healthy adults and neurological populations (Ryan et al. 2006; Lamont 2013; Mahendran et al. 2016). For each monitoring period, participants were asked to wear the device for 7 continuous days, affixed to the right thigh via a hypoallergenic dressing. Time points for waking, sleeping and device removal >15 min were recorded by the participants in a paper-based diary. Days were regarded as invalid if >4 h of non-wear occurred. All participants with ≥1 day of valid data were included in analyses. Outcomes derived from this device included step count, stepping time, upright time, standing time, sitting time, primary lying and secondary lying, metabolic equivalents per hour (MET/h), and number and duration of sitting bouts. Primary lying is the longest bout of lying of ≥60 min (i.e. night time sleep) and secondary lying is any other time spent lying for ≥60 min (e.g. daytime nap, lying on the couch) (PAL Technologies 2021). Physical activity was then further classified by intensity as either light or moderate-vigorous (Lyden et al. 2017). This range of variables was used in order to fully describe the concepts of physical activity and sedentary behaviour.
Health-related quality of life was measured with the EuroQol-5 Dimensions (EQ-5D) questionnaire. A utility score was calculated, anchored at 0 for poor health and 1 for perfect health (Herdman et al. 2011). The visual analogue scale (VAS) component of the EQ-5D was also used, where respondents reported their perceived health status with a grade ranging from 0 (worst possible) to 100 (best possible) (Herdman et al. 2011). In people undergoing rehabilitation after stroke, the minimal clinically importance difference (MCID) has been reported as 0.10 for the EQ-5D Utility score, and 8.61 for the EQ-VAS (Chen et al. 2016).
In addition to the EQ-5D, the Short Form-36 (SF-36) was also used to measure health-related quality of life (Ware and Sherbourne 1992). The SF-36 has eight sub-scales, each of which is scored on a 0–100 scale with a higher score denoting a more favourable health state (Ware and Sherbourne 1992). An MCID of eight points has been reported across healthy and neurological populations (Norman et al. 2003).
The Self-efficacy for Exercise Scale (Resnick and Jenkins 2000) was also utilised to measure the individual’s beliefs in their ability to continue exercising across nine different situations covering environmental, physical and psychosocial barriers to exercise. The total score is a sum of the question scores, ranging between 0 and 90, with a higher score indicating greater self-efficacy for exercise (Resnick and Jenkins 2000). The MCID value has not been established for this outcome measure.
The Modified Fatigue Impact Scale (MFIS) was used to measure fatigue and the impact it has on daily function (Schiehser et al. 2015). In this scale, items are aggregated into three subscales: physical (0–36), cognitive (0–40) and psychosocial (0–8), which can also be represented as a total score (0–84). Higher scores indicating a greater impact of fatigue (Schiehser et al. 2015). Studies in people with multiple sclerosis have reported an MCID of four points (Rooney et al. 2019).
Acceptability and satisfaction with aspects of the walking program were captured via two 5-point Likert scales anchored with 0 being ‘not at all acceptable/satisfied’ up to 5 being ‘very acceptable/satisfied’. The questions were individualised to participants with ABI, caregivers and staff.
In order to characterise the cohort, data concerning sociodemographics (age, gender, country of birth, language spoken at home, education, marital status, residence) were collected from participants at baseline via a paper-based questionnaire. Health data (time since injury, initial Glasgow Coma Scale, type of brain injury) were extracted from the medical chart by the first author. All participants had their height and weight measured at baseline by the first author or senior author, in order to calculate body mass index. Level-ground gait performance was measured at baseline as an additional descriptor of the cohort (not outcome). Gait was evaluated by completing a 12-m walk over an instrumented GAITRite walkway (GAITRite® CIR Systems Inc., USA). GAITRite® instrumentation has been shown to have high reliability capturing spatio-temporal gait characteristics (Batey et al. 2003). The variables velocity, step count, step time and step length were processed by the GAITRite® software. Participants completed three walks along the walkway, and the results of the three walks were averaged.
Statistical analyses
Statistical analysis was performed using SPSS (ver. 25, IBM Corp, Armonk, NY, USA) and STATA (vSE 17.0, StataCorp LLC, College Station, TX, USA) and statistical significance was set at α = 0.05. Summary statistics (mean, standard deviation, frequency, percentage) were produced to describe the participant cohort. A comparison between characteristics of those who remained in the study versus those who withdrew was conducted using the Independent t-test (parametric) and Mann Whitney U test (non-parametric) for continuous data, and Pearson’s Chi-squared or Fisher’s exact test for categorical variables. For the first objective, feasibility data were presented descriptively. The total cost of the pilot intervention and the cost per participant were calculated using the known hourly rates for physiotherapists (level HP3.4) and physiotherapy assistants (level OO3.4) within Queensland Health applicable to 2020, plus facility usage fees and on-costs. For the second objective, data obtained from the activPAL4 activity monitors were batch processed and analysed using the activPAL software PALanalysis (v8.10.3.8). These data were also processed and analysed using MATLAB software (ver. R2017b) to provide intensity of physical activity (light, moderate-vigorous intensity). To explore the second objective, the Shapiro-Wilk test was used to test for assumption of normality, with paired T-Test or Wilcoxon Signed Rank tests being used to calculate mean differences (with 95% confidence intervals) between (i) baseline to 4-weeks, and (ii) baseline to long-term follow up. Partial eta-squared values (partial η2) were used as a measure of effect size and calculated using repeated measures analysis of variance. These effect sizes were interpreted as a small (0.01), medium (0.06) or large (0.14) effect (Richardson 2011). This analysis for the second objective applied to the variables of physical activity and sedentary behaviour, as well as SF-36, EQ. 05D, Self-Efficacy for Exercise Scale and MFIS. Missing data were not replaced. For the third objective, acceptability and satisfaction results were tabulated and presented as a frequency count.
Results
Interruptions to study procedures
Recruitment commenced in April 2019. Research activity was paused in September 2019 due to unforeseen staff redeployment out of the Brain Injury Rehabilitation Unit and did not resume until July 2020 due to the health system’s response to the COVID-19 pandemic in the first half of 2020 (group treatment sessions were suspended). Research activity and participant recruitment continued from July 2020 until November 2020. Another aspect of the hospital’s response to COVID-19 during 2020 was to shift from in-person outpatient clinical appointments to telehealth clinical appointments. Consequently, some of our participants did not return to the hospital for in-person testing post-intervention (if they had been discharged during their 4-week walking program) or at the 3-month medium term follow up assessment timepoint. The general unease in the community due to COVID also made it harder to reach existing participants and arrange even remote data collection. As a result, some of the 3-month follow up appointments were conducted up to 6 months after the end of the intervention. To reflect this change, this medium term follow up time point will be referred to as 3–6 months post-intervention through the remainder of the present study. Accelerometer data and patient-reported outcomes were measured remotely under these circumstances if participants agreed.
Feasibility – recruitment rate and drop out rate
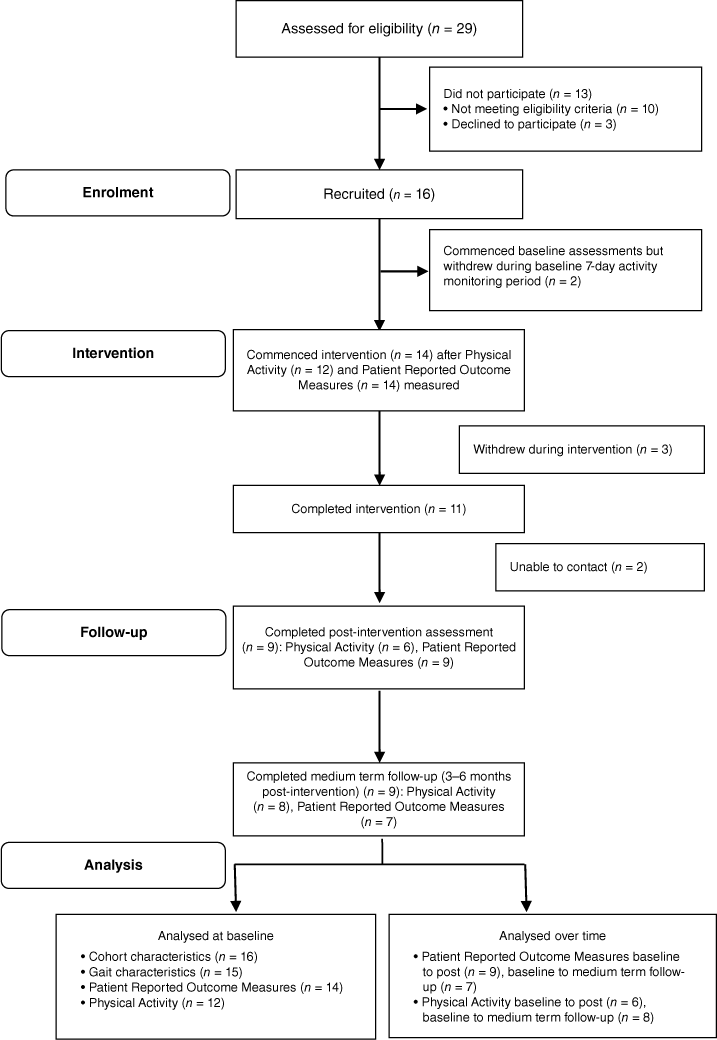
During the accumulative 11 months of active research activity, 29 inpatients were identified by clinical staff as potentially eligible. When formal eligibility screening was conducted, 19/29 (66%) were eligible, and 16 patients went on to consent to participate. This resulted in a recruitment rate of 16/29 (55%). Table 1 presents the reasons for ineligibility for n = 10 patients. The main reason for ineligibility (n = 4) was too short a duration in inpatient treatment, followed by having post-traumatic amnesia (n = 3). Fig. 1 presents the flow of participants through the study. The 16 participants who consented to the project were reduced to 14 by the start of the intervention because two participants withdrew during the baseline activPAL testing period of 7 days (n = 1 change of mind, n = 1 unexpected early discharge). This resulted in a participation rate of 14/19 (74%). A further three participants dropped out during the intervention period because of: a change to their exercise ability due to surgical complications (n = 1); failure to respond to telephone coaching calls (n = 1); and withdrawing from the research project at the same time as discharging against medical advice (n = 1). This resulted in 11 participants completing the intervention. Two participants were unable to be contacted for post-intervention assessment. Therefore, the drop out rate was 7/16 (44%). The same nine participants were assessed post-intervention and at medium-term follow up (3–6 months post-intervention). There were incomplete data at each time point, mostly activPAL data, missing due to researcher error (see Fig. 1).
| Recruitment | n (%) | |
|---|---|---|
| Participants referred for screening | 29 (100%) | |
| Participants eligible for recruitment | 19 (65%) | |
| Consented to recruitment (recruitment rate) | 16 (55%) | |
| Declined to participate | 3 (10%) | |
| Commenced intervention (participation rate) | 14 of 19 (74%) | |
| Participants interested in participating but excluded due to not meeting eligibility criteria | 10 (35%) | |
| Failure to emerge from post-traumatic amnesia, thus being unable to provide informed consent | 3 | |
| Discharge date from rehabilitation unit unexpectedly brought forward, shortening length of stay to <2 weeks | 4 | |
| Onset of new medical condition making it unsafe to perform intervention | 2 | |
| Deterioration in acquired brain injury associated behaviour, making it unsafe to participate in group setting | 1 |
Of the 14 who commenced the intervention, six discharged home from hospital prior to completing the last 2 weeks of the intervention. These participants continued their walking in the community and received their weekly coaching and behavioural therapy via telephone. Additionally, the rolling nature of the program meant that on rare occasions, the walking sessions at the hospital were conducted with only one participant.
Participant characteristics
The characteristics of participants who consented (n = 16) are presented in Table 2. Participants were predominantly male (n = 11, 69%), with a mean (s.d.) age of 36 (12) years and a mean (s.d.) BMI in the overweight range (26.0 (5.5) kg/m2). The age range was 18–56 years. The most frequent cause of brain injury was trauma (n = 5, 31%) or cerebral haemorrhage of non-traumatic origin (n = 5, 31%). These participants had a mean (s.d.) first documented Glasgow Coma Scale of 9 (5) indicating a moderately severe brain injury (Teasdale and Jennett 1974), with mean (s.d.) length of time of 38 (21) days between date of injury and study recruitment.
| Characteristics | Recruited cohort (n = 16) | Retained cohort (n = 9) | Dropped out (n = 7) | |
|---|---|---|---|---|
| Mean (s.d.) or n (%) | Mean (s.d.) or n (%) | Mean (s.d.) or n (%) | ||
| Age (years) | 36.3 (12.0) | 42.1 (12.3) | 28.7 (6.5) | |
| Gender (male) | 11 (68.8%) | 5 (56%) | 6 (86%) | |
| Height (m) | 1.74 (0.1) | 1.70 (0.1) | 1.78 (0.1) | |
| Weight (kg) | 78.4 (19.2) | 75.6 (14.6) | 82.1 (24.7) | |
| Body mass index (kg/m2) | 26.0 (5.5) | 26.0 (4.6) | 26.1 (6.9) | |
| Country of birth | ||||
| Australia | 12 (75%) | 7 (78%) | 5 (71%) | |
| China | 1 (6%) | 0 (0%) | 1 (14%) | |
| England | 1 (6%) | 1 (11%) | 0 (0%) | |
| New Zealand | 1 (6%) | 1 (11%) | 0 (0%) | |
| South Africa | 1 (6%) | 0 (0%) | 1 (14%) | |
| Language spoken at home | 2 (22%) | 2 (29%) | ||
| English | 15 (94%) | |||
| Chinese | 1 (6%) | 9 (100%) | 6 (86%) | |
| Highest educational level achieved | ||||
| Certificate I–IV | 2 (13%) | 1 (11%) | 1 (14%) | |
| Secondary education | 13 (81%) | 7 (78%) | 6 (86%) | |
| Primary education | 1 (6%) | 1 (11%) | 0 (0%) | |
| Marital status | ||||
| Married or de facto | 6 (38%) | 4 (44%) | 2 (29%) | |
| Never married | 6 (38%) | 3 (33%) | 3 (43%) | |
| Divorced | 3 (19%) | 2 (22%) | 1 (14%) | |
| Widowed | 1 (6%) | 0 (0%) | 1 (14%) | |
| Residential address within greater Brisbane (yes) | 9 (56%) | 3 (33%) | 6 (86%) | |
| Time since injury (days between injury and consent) | 38 (21) | 36 (22) | 41 (22) | |
| Initial Glasgow Coma Scale | 9 (5) | 9 (5) | 10 (5) | |
| Category of brain injury | ||||
| Traumatic | 5 (31%) | 4 (44%) | 1 (14%) | |
| Haemorrhage | 5 (31%) | 2 (22%) | 3 (43%) | |
| Hypoxic | 3 (19%) | 2 (22%) | 1 (14%) | |
| Surgical resection of tumour | 2 (13%) | 1 (11%) | 1 (14%) | |
| Abscess | 1 (6%) | 0 | 1 (14%) | |
| Gait characteristics A | ||||
| Velocity (cm/s) | 112.0 (12.0) | 114.2 (13.7) | 108.8 (9.2) | |
| Step count (n) | 8.6 (1.1) | 8.5 (1.0) | 8.8 (1.3) | |
| Cadence (steps/min) | 108.7 (8) | 110.3 (7.9) | 106.3 (8.4) | |
| Step time – left (s) | 0.55 (0.04) | 0.54 (0.04) | 0.56 (0.04) | |
| Step time – right (s) | 0.56 (0.04) | 0.55 (0.04) | 0.57 (0.04) | |
| Step length – left (cm) | 61.3 (5.5) | 62.0 (5.9) | 60.2 (5.2) | |
| Step length – right (cm) | 62.2 (5.5) | 61.9 (6.4) | 62.7 (5.7) | |
Due to the considerable drop out rate (44%), characteristics were then compared between the participants who were retained in the study (n = 9, 56%) and those who withdrew (n = 7, 44%). Those who withdrew were significantly younger (mean (s.d.) 28.7 (6.5) vs 42.1 (12.3); P = 0.02), and were significantly more likely to reside within the greater Brisbane area (86% vs 33%) (see Table 2).
Feasibility – safety and cost
When asked, no adverse events were reported by participants to the physiotherapists, and no adverse events occurred during the supervised walking sessions. The cost per participant was AU$427.71. Intervention costs and research costs are outlined in Supplementary material S2.
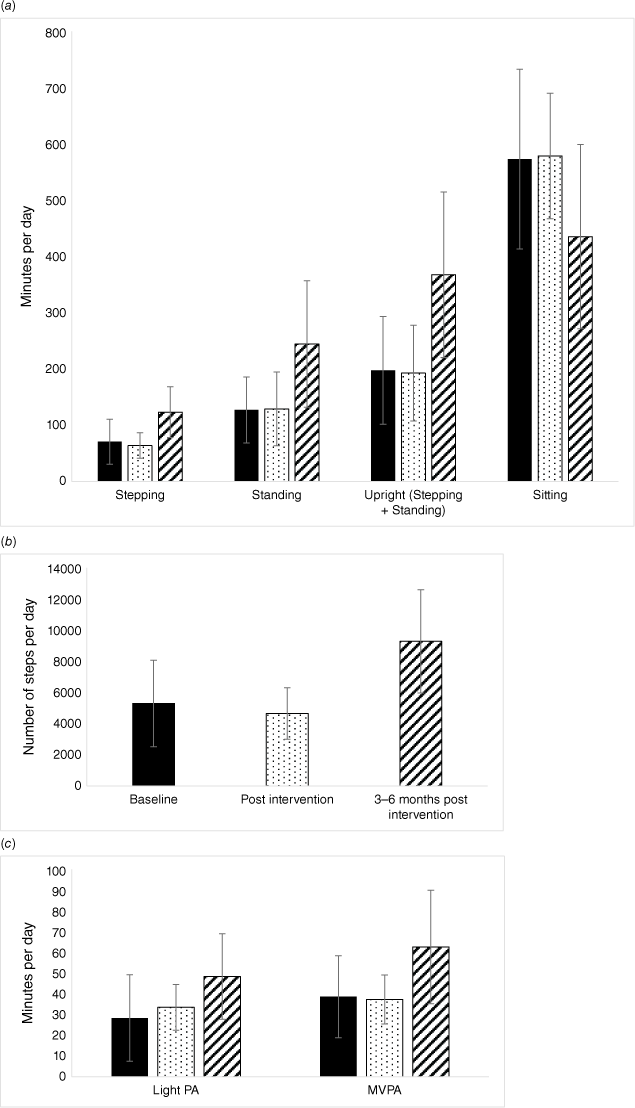
Physical activity and sedentary behaviour outcomes
Summary data for measures of physical activity and sedentary behaviour are presented in Table 3 and Fig. 2a–c. The available data demonstrated no change to physical activity or sedentary behaviour across the intervention period (n = 6, post-intervention vs baseline). The number of available data sets for analysis was small, and results should be interpreted with caution. In comparing medium-term follow up (3–6 months post-intervention) with baseline, there were a total of n = 8 datasets available for analysis – still a small number to be interpreted with caution. When comparing medium-term follow up (3–6 months post-intervention) with baseline, on average, participants took an extra 3913 steps per day (95% CI 671, 7156; P = 0.03) and spent an extra 52 min stepping (95% CI 9, 96; P = 0.03). On average, participants sat for a total of 213 min less each day (95% CI −341, −85; P < 0.01). Time spent in light and moderate-vigorous physical activity or in primary lying and secondary lying were not significantly different at either follow up. Large effect sizes (partial η2 > 0.14) were identified for all of the physical activity and sedentary behaviour outcomes, with the exception of primary and secondary lying time.
| Outcome measures | Baseline (n = 12) | Post-intervention (n = 6) | 3–6 months post-intervention (n = 9) | Post-intervention vs baseline (n = 6) | 3–6 months post-intervention vs baseline (n = 8) | Partial η2 (effect size) for time | |
|---|---|---|---|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | Mean difference (95% CI) | Mean difference (95% CI) | |||
| Total number of steps/day | 5331 (2793) | 4682 (1664) | 9355 (3338) | −551 (−5187, 4084) | 3913 (670, 7156) | 0.36 | |
| Total stepping time (min/day) | 71 (40) | 64 (23) | 124 (45) | −5 (−70, 60) | 52 (9, 96) | 0.36 | |
| Upright time (min/day) | 199 (96) | 194 (86) | 369 (148) | 3 (−169, 174) | 163 (31, 294) | 0.42 | |
| Standing time (min/day) | 128 (59) | 130 (66) | 246 (113) | 8 (−103, 118) | 111 (15, 206) | 0.26 | |
| Sitting time (min/day) | 576 (160) | 582 (112) | 437 (164) | −66 (−187, 56) | −213 (−341, −85) | 0.40 | |
| Primary lying time (min/day) | 619 (187) | 620 (174) | 561 (162) | 93 (−102, 288) | 29 (−81, 138) | 0.10 | |
| Secondary lying time (min/day) | 46 (50) | 44 (67) | 64 (85) | −30 (−110, 50) | 13 (−51, 76) | 0.09 | |
| Activity score (MET.h/day) | 32 (1) | 32 (1) | 34 (1) | 0 (−2, 2) | 2 (0, 3) | 0.42 | |
| Number of sitting bouts >30 min/day | 4.3 (2.5) | 3.8 (1.7) | 2.4 (1.8) | −1.8 (−4.3, 0.6) | −2.5 (−5.0, −0.0) | 0.36 | |
| Number of sitting bouts >60 min/day | 0.8 (0.6) | 0.8 (1.7) | 0.1 (0.3) | −0.2 (−1.0, 0.6) | −0.8 (−1.3, −0.2) | 0.48 | |
| Time spent in sitting bouts >30 min (min/day | 256 (126) | 222 (96) | 131 (87) | −97 (−204, 10) | −149 (−261, −37) | 0.48 | |
| Time spent in sitting bouts >60 min (min/day) | 105 (58) | 85 (47) | 39 (24) | −47 (−101, 7) | −74 (−128, −20) | 0.52 | |
| Time spent in light physical activity (min/day) | 29 (21) | 34 (11) | 49 (21) B | n/a A | n/a A | 0.37 | |
| Time spent in moderate-vigorous physical activity (min/day) | 39 (20) | 38 (12) | 63 (28) B | 2 (−26, 30) | 15 (−33, 3) | 0.34 |
Bold data indicates a significant (P < 0.05) mean difference. n/a, not applicable.
Physical activity and sedentary behaviour outcomes at baseline (solid), post-intervention (dots) and medium-term follow up (3–6 months post-intervention) (stripes). Error bars represent standard deviation. (a) Average time spent stepping, standing, upright (combination of stepping and standing) and sitting, minutes per day. (b) Average steps per day. (c) Average time spent in light vs moderate-vigorous intensity physical activity (MVPA), minutes per day.
Participants who withdrew after baseline spent significantly less time sitting (mean, s.d.) (427, 150 min) than the retained cohort (650, 109 min; P = 0.01), however spent significantly more time in primary lying (784, 250 min) compared to the retained cohort (536, 71 min; P = 0.01). Further comparisons of physical activity measures between the retained and withdrawn cohorts are depicted in Supplementary material S3.
Self-reported quality of life, exercise self-efficacy and fatigue
Summary data for patient-reported outcome measures are presented in Table 4 and Supplementary material S4. At the post-intervention assessment, a statistically significant improvement of 14.4 points was observed for the energy/fatigue (vitality) subscale of the SF-36 (95% CI 5.0, 23.7, P = 0.008). Clinically significant improvements were observed across multiple domains of the SF-36 (+≥8 points) and MFIS (−17 points) scales during the intervention period, and multiple sub-sections of the EQ-5D, SF36 and MFIS at long-term follow up, however none of these changes were statistically significant. Exercise self-efficacy scores were not statistically significantly different across the study at either timepoint. Large effect sizes (partial η2 > 0.14) were identified for five of the eight sub-scales of the SF-36, the EQ-5D VAS and the MFIS total score.
| Self-reported outcome measures | Baseline (n = 14) | Post-intervention (n = 9) | 3–6 months pest-intervention (n = 7) | Post-intervention vs baseline (n = 9) | 3–6 months post-intervention vs baseline (n= 7) | Partial η2 (effect size) for time | |
|---|---|---|---|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | Mean difference (95% CI) | Mean difference (95% CI) | |||
| EQ-5D | |||||||
| Utility | 0.88 (0.08) | 0.89 (0.11) | 0.91 (0.13) | n/a A | n/a A | 0.09 | |
| VAS | 74.3 (15.3) | 78.9 (22.0) | 86.0 (11.4) | 6.7 (−6.2, 19.5) | 11.7 (−7.6, 31.0) | 0.19 | |
| SF-36 | |||||||
| Physical functioning | 67.5 (23.1) | 76.9 (20.6) | 87.2 (16.7) | 16.3 (−4.4, 37.0) | 25.8 (−7.0, 58.6) | 0.35 | |
| Role limitations due to physical health | 22.0 (32.5) | 40.6 (40.0) | 50.0 (47.9) | 33.3 (−2.2, 68.8) | 41.7 (−11.2, 94.5) | 0.26 | |
| Role limitations due to emotional problems | 42.9 (42.2) | 58.3 (38.8) | 61.9 (48.8) | 12.5 (−34.5, 59.5) | 23.8 (−44.5, 92.1) | 0.08 | |
| Energy/fatigue (vitality) | 48.6 (20.0) | 61.3 (19.2) | 56.4 (20.8) | 14.4 (5.0, 23.7) | 12.9 (−5.2, 30.9) | 0.30 | |
| Emotional wellbeing | 66.6 (14.9) | 70.0 (17.0) | 66.3 (17.9) | 8.0 (−8.5, 24.5) | 4.0 (−16.3, 24.3) | 0.10 | |
| Social functioning | 54.5 (23.8) | 67.19 (26.67) | 60.71 (24.40) | 18.75 (−9.2, 46.7) | 14.29 (−24.3, 52.9) | 0.18 | |
| Pain | 58.8 (20.3) | 66.88 (22.35) | 75 (23.40) | 14.38 (−8.8, 37.6) | 19.64 (−8.6, 47.9) | 0.28 | |
| General health | 63.6 (14.5) | 63.75 (20.13) | 70.71 (12.72) | −0.63 (−12.3, 11.1) | 5.00 (−9.6, 19.6) | 0.03 | |
| MFIS | |||||||
| Physical subscale | 5.3 (3.0) | 4.4 (2.5) | 4.1 (2.9) | −1.1 (−3.9, 1.7) | −1.6 (−4.3) | ||
| Cognitive subscale | 10.6 (6.0) | 8.8 (4.9) | 8.3 (5.8) | −2.3 (−7.9, 3.4) | −3.1 (−8.7, 2.4) | ||
| Psychosocial subscale | 15.9 (9.0) | 13.1 (7.3) | 12.4 (8.7) | −3.4 (−11.8, 5.0) | −4.7 (−13.0, 3.6) | ||
| Total score (/84) | 66.9 (28.9) | 52.4 (11.6) | 52.3 (25.3) | −17.0 (−36.4, 2.4) | −20.1 (−45.5, 5.2) | 0.29 | |
| Self-efficacy for exercise | |||||||
| Total score (/90) | 55.4 (24.8) | 56.8 (22.5) | 65.0 (19.0) | 1.3 (−29.5, 32.0) | 13.0 (−21.4, 47.4) | 0.10 | |
Acceptability and satisfaction
Seven participants completed the feedback forms, and the results are presented in Supplementary material S5. In summary, all participants found the walking program, coaching and the combination of the two either mostly or very acceptable, and mostly or very satisfactory. Four staff members (two physiotherapists and two physiotherapy assistants) completed the feedback forms (see Supplementary material S6). All staff found the walking program and telephone coaching to be very acceptable (100%) and were very satisfied (100%). Of the two caregivers who were approached to provide feedback, neither consented to do so.
Discussion
This physical activity program with behavioural coaching for adults with ABI, delivered in an inpatient care setting, was found to be safe and feasible to recruit to (55% recruitment rate; 74% of those eligible commenced the intervention). However, physical activity and sedentary behaviour did not change during the intervention period and the cost per participant was high at AU$427.71 per participant given the lack of behaviour change. Encouragingly, participants were found to be more physically active and less sedentary at the medium-term follow up; however, it is unclear how much this can be attributed to the intervention given the lack of control group. In contrast, there were clinically significant improvements in measures of quality of life and fatigue at both post-intervention and medium term. Very high levels of acceptability and satisfaction were reported by both staff and participants; however, the carers’ perspectives were unable to be ascertained. In summary, this combined walking program with concurrent behavioural therapy was found to be feasible to recruit, safe and acceptable. However, the potential impact on physical activity remains unclear.
Study feasibility was greatly influenced by the flow of patients through the unit. Length of stay within the Brain Injury Rehabilitation Unit varies greatly between patients because admission is guided by the patient’s goals, rate of progress and supports available on discharge, rather than being a standardised fixed length of time. Admission duration for higher functioning patients is often much shorter than for those with more severe impairments and restricted function. Given this environment, recruiting 16 of 29 eligible, specifically highly mobile, patients to this program was considered a positive outcome by our research team. The nature of the intervention in the present study meant that our target participants were already highly mobile and had intact basic cognition. As such, these individuals tended to progress quickly through their rehabilitation, and many transitioned home from hospital sooner than originally expected when they were first admitted to the unit. This affected recruitment rates in the study, as we excluded patients who were planned to have short admissions, and our retention of participants – one participant had an unexpected early discharge between consenting to the study and baseline testing. Participants who dropped out were more likely to be younger than those who remained in the study. It is possible that younger patients (in their 20s and 30s) are more likely to move quickly through inpatient rehabilitation than older patients, perhaps due to fewer comorbidities or more robust social support networks in the community (e.g. parents). A fully telehealth program or a program delivered as a part of their outreach outpatient services may be better suited to addressing physical activity and sedentary behaviour with such younger patients.
The cost analysis provides useful information for implementing a walking program within a rehabilitation program. Costs were high at AU$427.71per participant for no demonstrable return on investment in physical activity or sedentary behaviour; however, formal comparison of costs and benefits was not possible in this study design. Previous economic analyses have identified that programs with exercise as a core intervention are cost effective for improving mobility outcomes and decreasing falls in elderly adults and neurological populations such as Parkinson’s disease (Davis et al. 2010; Farag et al. 2015, 2016). However, in this context, a larger study with a comparison group would be required to explore the cost-effectiveness of this particular intervention.
The combined walking and behaviour change program was developed by clinicians and clinician-scientists in response to a perceived need to better foster physical activity levels and exercise self-efficacy in adults with ABI, particularly during inpatient rehabilitation. However, there were no changes in physical activity and sedentary behaviour during this intervention period. It is possible that the supervised sessions were unable to overcome the environmental influence on sedentary behaviour – the Brain Injury Rehabilitation Unit is a locked ward that patients cannot freely come and go from. It is also possible that patients were not yet cognitively ready for such a program. Driver and colleagues used this observation as justification for piloting their health promotion program with adults in an outpatient, transitional rehabilitation program for adults with TBI (Driver et al. 2013).
Our study found a significant increase in levels of physical activity at medium-term follow up (3–6 months post-intervention). This may be explained in part by the fact that community-dwelling adults have more potential opportunities for participating in physical activity than those residing in hospital rehabilitation settings (Ramsey et al. 2021). This is consistent with research in people with stroke who were observed to spend less time sitting and more time standing and walking in their homes following discharge compared with the last week of their hospital stay (Simpson et al. 2018). Another potential explanation is the task-specific practice of ambulating within real-life physical environments that our participants received in the walking sessions. This element of the program is in line with task-specific rehabilitation principles (Shumway-Cook and Woollacott 2017) and may have had a flow on effect to post-discharge community-based physical activity. Another explanation, and an acknowledged limitation of the study, is the data loss across the study, meaning participants included in analyses at post-intervention may not have been the participants included in analyses at follow up.
Strengths and limitations
This study has some strengths but several significant weaknesses that need to be acknowledged. As a feasibility study, several outcomes relevant to future larger studies and to clinical implementation were monitored, including recruitment rate, patient and staff satisfaction, adverse events, and costs. The use of device-based measures of physical activity and sedentary behaviour is a strength, as the reliability of self-reported measures of physical activity could be impacted by cognitive changes in adults with ABI. Using device-based measures in this study is part of the growing trend for such monitoring in hospital patients (Fazio et al. 2020).
The study setting was a specialised neurorehabilitation unit with experienced clinical staff associated with intervention delivery; however, this may limit the generalisability of findings to other more generalised hospital wards. There is risk of bias present in the recording of adverse events by intervention staff, who may have an interest in the intervention being considered safe. The timeframe in which the study was carried out did not occur as originally planned due to the COVID-19 pandemic. Inpatient flow through the Brain Injury Rehabilitation Unit was reduced compared with pre-COVID expectations and follow up appointments with the treating clinical team were rescheduled to telehealth, leading to slower than expected recruitment rates and a small sample size. The slow pace of recruitment also meant that on some occasions, only one participant was walking with two staff (or one staff and one student). The absence of peers may have affected how participants felt about the study and their ongoing participation. The gender, age and body mass index of participants were consistent with the usual patient population in our unit; however, the proportion of participants with TBI (vs non-TBI) in the study was lower than what is normally encountered in the unit. Findings may not be generalisable to all forms of ABI. For those who were able to commence the intervention but were discharged home prior to completing it, they continued to engage in walking and coaching via telephone. As these walking sessions were not supervised, true compliance rates are unknown, and this may have influenced study findings.
Critically, data loss and drop out rate mean the results must be interpreted with caution as there is significant potential for bias, particularly of the physical activity and sedentary behaviour outcomes. A power calculation was not performed in the context of this pilot study. Finally, there was no control group, meaning that these findings must be interpreted with care. Future randomised controlled trials may be planned on the output from this feasibility study to better understand the potential effectiveness of the intervention over standard care.
Conclusions
Participants within a specialist inpatient rehabilitation unit for adults with ABI engaged in a 4-week program of twice-weekly supervised walking sessions with behaviour change counselling. This pilot study demonstrated that while the program was safe, the drop out rate was high and behaviour change did not occur. More work is required to boost physical activity during sub-acute rehabilitation for ABI.
Data availability
The data that support this study cannot be publicly shared due to ethical or privacy reasons and may be shared upon reasonable request to the corresponding author if appropriate.
Ethics standard
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
References
Barrett S, Begg S, O’Halloran P, Kingsley M (2018) Integrated motivational interviewing and cognitive behaviour therapy can increase physical activity and improve health of adult ambulatory care patients in a regional hospital: the Healthy4U randomised controlled trial. BMC Public Health 18(1), 1166.
| Crossref | Google Scholar | PubMed |
Basford JR, Chou LS, Kaufman KR, Brey RH, Walker A, Malec JF, Moessner AM, Brown AW (2003) An assessment of gait and balance deficits after traumatic brain injury. Archives of Physical Medicine and Rehabilitation 84(3), 343-349.
| Crossref | Google Scholar | PubMed |
Batey P, Rome K, Finn P, Hanchard N (2003) Assessing Reliability of Measurement of Gait Velocity. Physiotherapy 89(5), 313-317.
| Crossref | Google Scholar |
Bell KR, Temkin NR, Esselman PC, Doctor JN, Bombardier CH, Fraser RT, Hoffman JM, Powell JM, Dikmen S (2005) The effect of a scheduled telephone intervention on outcome after moderate to severe traumatic brain injury: a randomized trial. Archives of Physical Medicine and Rehabilitation 86(5), 851-856.
| Crossref | Google Scholar | PubMed |
Bellon K, Kolakowsky-Hayner S, Wright J, Huie H, Toda K, Bushnik T, Englander J (2015) A home-based walking study to ameliorate perceived stress and depressive symptoms in people with a traumatic brain injury. Brain Injury 29(3), 313-319.
| Crossref | Google Scholar | PubMed |
Billinger SA, Arena R, Bernhardt J, Eng JJ, Franklin BA, Johnson CM, MacKay-Lyons M, Macko RF, Mead GE, Roth EJ, Shaughnessy M, Tang A (2014) Physical activity and exercise recommendations for stroke survivors: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 45(8), 2532-2553.
| Crossref | Google Scholar | PubMed |
Burke JF, Stulc JL, Skolarus LE, Sears ED, Zahuranec DB, Morgenstern LB (2013) Traumatic brain injury may be an independent risk factor for stroke. Neurology 81(1), 33-39.
| Crossref | Google Scholar | PubMed |
Chaudhuri A, Behan PO (2004) Fatigue in neurological disorders. The Lancet 363(9413), 978-988.
| Crossref | Google Scholar | PubMed |
Chen P, Lin KC, Liing RJ, Wu CY, Chen CL, Chang KC (2016) Validity, responsiveness, and minimal clinically important difference of EQ-5D-5L in stroke patients undergoing rehabilitation. Quality of Life Research 25(6), 1585-1596.
| Crossref | Google Scholar | PubMed |
Davis JC, Robertson MC, Ashe MC, Liu-Ambrose T, Khan KM, Marra CA (2010) Does a home-based strength and balance programme in people aged ≥80 years provide the best value for money to prevent falls? A systematic review of economic evaluations of falls prevention interventions. British Journal of Sports Medicine 44(2), 80-89.
| Crossref | Google Scholar | PubMed |
Department of Health (2021) ‘Australia’s Physical Activity and Sedentary Behaviour Guidelines for Adults Aged 18-64.’ (Department of Health and Aged Care: Canberra, ACT, Australia) Available at https://www.health.gov.au/topics/physical-activity-and-exercise/physical-activity-and-exercise-guidelines-for-all-australians/for-adults-18-to-64-years
D’Isabella NT, Shkredova DA, Richardson JA, Tang A (2017) Effects of exercise on cardiovascular risk factors following stroke or transient ischemic attack: a systematic review and meta-analysis. Clinical Rehabilitation 31(12), 1561-1572.
| Crossref | Google Scholar | PubMed |
Driver S, Woolsey A (2016) Evaluation of a Physical Activity Behavior Change Program for Individuals With a Brain Injury. Archives of Physical Medicine and Rehabilitation 97(9, Supplement), S194-S200.
| Crossref | Google Scholar | PubMed |
Driver S, Ede A, Dodd Z, Stevens L, Warren AM (2012a) What barriers to physical activity do individuals with a recent brain injury face? Disability and Health Journal 5(2), 117-125.
| Crossref | Google Scholar | PubMed |
Driver S, Irwin K, Woolsey A, Pawlowski J (2012b) Creating an effective physical activity-based health promotion programme for adults with a brain injury. Brain Injury 26(12), 1482-1492.
| Crossref | Google Scholar | PubMed |
Driver S, Irwin K, Woolsey A, Warren AM (2013) Piloting a physical activity centred education programme for adults with a brain injury. Brain Injury 27(10), 1173-1180.
| Crossref | Google Scholar | PubMed |
Driver S, McShan E, Swank C, Calhoun S, Bennett M, Callender L, Holden A, Juengst S, Bell K, Douglas M, Kramer K, Dubiel R (2023) Efficacy of the Diabetes Prevention Program Group Lifestyle Balance Program Modified for Individuals with TBI (GLB-TBI): results from a 12-month Randomized Controlled Trial. Annals of Behavioral Medicine 57(2), 131-145.
| Crossref | Google Scholar | PubMed |
Eldridge SM, Lancaster GA, Campbell MJ, Thabane L, Hopewell S, Coleman CL, Bond CM (2016) Defining Feasibility and Pilot Studies in Preparation for Randomised Controlled Trials: Development of a Conceptual Framework. PLoS One 11(3), e0150205.
| Crossref | Google Scholar | PubMed |
Fann JR, Bombardier CH, Vannoy S, Dyer J, Ludman E, Dikmen S, Marshall K, Barber J, Temkin N (2015) Telephone and in-person cognitive behavioral therapy for major depression after traumatic brain injury: a randomized controlled trial. Journal of Neurotrauma 32(1), 45-57.
| Crossref | Google Scholar | PubMed |
Farag I, Howard K, Hayes AJ, Ferreira ML, Lord SR, Close JT, Vogler C, Dean CM, Cumming RG, Sherrington C (2015) Cost-effectiveness of a Home-Exercise Program Among Older People After Hospitalization. Journal of the American Medical Directors Association 16(6), 490-496.
| Crossref | Google Scholar | PubMed |
Farag I, Sherrington C, Hayes A, Canning CG, Lord SR, Close JC, Fung VS, Howard K (2016) Economic evaluation of a falls prevention exercise program among people with Parkinson’s disease. Movement Disorders 31(1), 53-61.
| Crossref | Google Scholar | PubMed |
Fazio S, Stocking J, Kuhn B, Doroy A, Blackmon E, Young HM, Adams JY (2020) How much do hospitalized adults move? A systematic review and meta-analysis. Applied Nursing Research 51, 151189.
| Crossref | Google Scholar | PubMed |
Fini NA, Holland AE, Keating J, Simek J, Bernhardt J (2017) How physically active are people following stroke? Systematic review and quantitative synthesis. Physical Therapy 97, 707-717.
| Crossref | Google Scholar |
Guggisberg AG, Chauvigné L, Pignat JM (2020) [Fatigue after acquired brain injury and its impact on socio-professional reintegration]. Revue Medicale Suisse 16(692), 901-903.
| Google Scholar | PubMed |
Halabchi F, Alizadeh Z, Sahraian MA, Abolhasani M (2017) Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurology 17(1), 185.
| Crossref | Google Scholar | PubMed |
Hamilton M, Khan M, Clark R, Williams G, Bryant A (2016) Predictors of physical activity levels of individuals following traumatic brain injury remain unclear: a systematic review. Brain Injury 30(7), 819-828.
| Crossref | Google Scholar | PubMed |
Hart T, Brenner L, Clark AN, BOgner JA, Novack TA, Chervoneva I, Nakase-Richardson R, Arango-Lasprilla JC (2011) Major and Minor Depression After Traumatic Brain Injury. Archives of Physical Medicine and Rehabilitation 92(8), 1211-1219.
| Crossref | Google Scholar | PubMed |
Hartmann A, Rundek T, Mast H, Paik MS, Boden-Albala B, Mohr JP, Sacco RL (2001) Mortality and causes of death after first ischemic stroke. Neurology 57, 2000-2005.
| Crossref | Google Scholar | PubMed |
Herdman M, Gudex C, Lloyd A, Janssen MF, Kind P, Parkin D, Bonsel G, Badia X (2011) Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research 20(10), 1727-1736.
| Crossref | Google Scholar | PubMed |
Hoffman JM, Bell KR, Powell JM, Behr J, Dunn EC, Dikmen S, Bombardier CH (2010) A randomized controlled trial of exercise to improve mood after traumatic brain injury. Physical Medicine and Rehabilitation 2, 911-919.
| Crossref | Google Scholar | PubMed |
Jones K, Hawke F, Newman J, Miller JA, Burns J, Jakovljevic DG, Gorman G, Turnbull DM, Ramdharry G (2021) Interventions for promoting physical activity in people with neuromuscular disease. Cochrane Database of Systematic Reviews 5(5), CD013544.
| Crossref | Google Scholar | PubMed |
Julious SA (2005) Sample size of 12 per group rule of thumb for a pilot study. Pharmaceutical Statistics 4(4), 287-291.
| Crossref | Google Scholar |
Kelly S, Martin S, Kuhn I, Cowan A, Brayne C, Lafortune L (2016) Barriers and Facilitators to the Uptake and Maintenance of Healthy Behaviours by People at Mid-Life: A Rapid Systematic Review. PLoS One 11(1), e0145074.
| Crossref | Google Scholar |
Kirkness CJ, Cain KC, Becker KJ, Tirschwell DL, Buzaitis AM, Weisman PL, McKenzie S, Teri L, Kohen R, Veith RC, Mitchell PH (2017) Randomized trial of telephone versus in-person delivery of a brief psychosocial intervention in post-stroke depression. BMC Research Notes 10(1), 500.
| Crossref | Google Scholar | PubMed |
Kunkel D, Fitton C, Burnett M, Ashburn A (2015) Physical inactivity post-stroke: a 3-year longitudinal study. Disability and Rehabilitation 37(4), 304-310.
| Crossref | Google Scholar | PubMed |
Kuzma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ (2018) Stroke and dementia risk: a systematic review and meta-analysis. Alzheimer’s & Dementia 14, 1416-1426.
| Crossref | Google Scholar | PubMed |
Lancaster GA, Dodd S, Williamson PR (2004) Design and analysis of pilot studies: recommendations for good practice. Journal of Evaluation in Clinical Practice 10(2), 307-312.
| Crossref | Google Scholar | PubMed |
Li W, Risacher SL, McAllister TW, Saykin AJ (2016) Traumatic brain injury and age at onset of cognitive impairment in older adults. Journal of Neurology 263(7), 1280-1285.
| Crossref | Google Scholar | PubMed |
PAL Technologies (2021) CREA. Available at https://kb.palt.com/articles/crea
Lund A, Michelet M, Sandvik L, Wyller T, Sveen U (2012) A lifestyle intervention as supplement to a physical activity programme in rehabilitation after stroke: a randomized controlled trial. Clinical Rehabilitation 26(6), 502-512.
| Crossref | Google Scholar | PubMed |
Lyden K, Keadle SK, Staudenmayer J, Freedson PS (2017) The activPALTM Accurately Classifies Activity Intensity Categories in Healthy Adults. Medicine and Science in Sports and Exercise 49(5), 1022-1028.
| Crossref | Google Scholar | PubMed |
Mahendran N, Kuys SS, Downie E, Ng P, Brauer SG (2016) Are Accelerometers and GPS Devices Valid, Reliable and Feasible Tools for Measurement of Community Ambulation after Stroke? Brain Impairment 17(2), 151-161.
| Crossref | Google Scholar |
Manns PJ, Baldwin E (2009) Ambulatory activity of stroke survivors: measurement options for dose, intensity, and variability of activity. Stroke 40(3), 864-867.
| Crossref | Google Scholar | PubMed |
Medley AR, Powell T (2010) Motivational Interviewing to promote self-awareness and engagement in rehabilitation following acquired brain injury: a conceptual review. Neuropsychological Rehabilitation 20(4), 481-508.
| Crossref | Google Scholar | PubMed |
Michie S, van Stralen MM, West R (2011) The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implementation Science 6(1), 42.
| Crossref | Google Scholar |
Moore SA, Hallsworth K, Jakovljevic DG, Blamire AM, He J, Ford GA, Rochester L, Trenell MI (2015) Effects of Community Exercise Therapy on Metabolic, Brain, Physical, and Cognitive Function Following Stroke: A Randomized Controlled Pilot Trial. Neurorehabilitation and Neural Repair 29(7), 623-635.
| Crossref | Google Scholar | PubMed |
Norman GR, Sloan JA, Wyrwich KW (2003) Interpretation of changes in health related quality of life: the remarkable universality of half a standard deviation. Medical Care 41(5), 582-592.
| Crossref | Google Scholar | PubMed |
O’Reilly GM, Curtis K, Mitra B, Kim Y, Afroz A, Hunter K, Ryder C, Hendrie DV, Rushworth N, Tee J, D’Angelo S, Solly E, Bhattacharya O, Fitzgerald MC (2023) Hospitalisations and in-hospital deaths following moderate to severe traumatic brain injury in Australia, 2015–20: a registry data analysis for the Australian Traumatic Brain Injury National Data (ATBIND) project. Medical Journal of Australia 219(7), 316-324.
| Crossref | Google Scholar | PubMed |
Prescott S, Fleming J, Doig E (2015) Goal setting approaches and principles used in rehabilitation for people with acquired brain injury: a systematic scoping review. Brain Injury 29(13–14), 1515-1529.
| Crossref | Google Scholar | PubMed |
Ramsey J, Driver S, Swank C, Bennett M, Dubiel R (2018) Physical activity intensity of patient’s with traumatic brain injury during inpatient rehabilitation. Brain Injury 32(12), 1518-1524.
| Crossref | Google Scholar | PubMed |
Ramsey KA, Loveland P, Rojer AGM, Denehy L, Goonan R, Marston C, Kay JE, Brenan J, Trappenburg MC, Lim WK, Reijnierse EM, Meskers CGM, Maier AB (2021) Geriatric Rehabilitation Inpatients Roam at Home! A Matched Cohort Study of Objectively Measured Physical Activity and Sedentary Behavior in Home-Based and Hospital-Based Settings. Journal of the American Medical Directors Association 22(12), 2432-2439.e1.
| Crossref | Google Scholar | PubMed |
Resnick B, Jenkins LS (2000) Testing the reliability and validity of the Self-Efficacy for Exercise scale. Nursing Research 49(3), 154-159.
| Crossref | Google Scholar | PubMed |
Richardson JTE (2011) Eta squared and partial eta squared as measures of effect size in educational research. Educational Research Review 6(2), 135-147.
| Crossref | Google Scholar |
Rooney S, McFadyen A, Wood L, Moffat F, Paul L (2019) Minimally important difference of the fatigue severity scale and modified fatigue impact scale in people with multiple sclerosis. Multiple Sclerosis and Related Disorders 35, 158-163.
| Crossref | Google Scholar | PubMed |
Ryan CG, Grant PM, Tigbe WW, Granat MH (2006) The validity and reliability of a novel activity monitor as a measure of walking. British Journal of Sports Medicine 40(9), 779-784.
| Crossref | Google Scholar | PubMed |
Schiehser DM, Delano-Wood L, Jak AJ, Matthews SC, Simmons AN, Jacobson MW, Filoteo JV, Bondi MW, Orff HJ, Liu L (2015) Validation of the Modified Fatigue Impact Scale in mild to moderate traumatic brain injury. Journal of Head Trauma Rehabilitation 30(2), 116-121.
| Crossref | Google Scholar | PubMed |
Shi Y, Yang D, Zeng Y, Wu W (2017) Risk Factors for Post-stroke Depression: A Meta-analysis. Frontiers in Aging Neuroscience 9, 218.
| Crossref | Google Scholar | PubMed |
Sim J, Lewis M (2012) The size of a pilot study for a clinical trial should be calculated in relation to considerations of precision and efficiency. Journal of Clinical Epidemiology 65(3), 301-308.
| Crossref | Google Scholar | PubMed |
Simpson DB, Breslin M, Cumming T, de Zoete S, Gall SL, Schmidt M, English C, Callisaya ML (2018) Go Home, Sit Less: The Impact of Home Versus Hospital Rehabilitation Environment on Activity Levels of Stroke Survivors. Archives of Physical Medicine and Rehabilitation 99(11), 2216-2221.e1.
| Crossref | Google Scholar | PubMed |
Slovarp L, Azuma T, Lapointe L (2012) The effect of traumatic brain injury on sustained attention and working memory. Brain Injury 26(1), 48-57.
| Crossref | Google Scholar | PubMed |
Stroke Foundation (2023) Clinical Guidelines for Stroke Management: Chapter 5 - Rehabilitation. Available at https://informme.org.au/en/Guidelines/Clinical-Guidelines-for-Stroke-Management [accessed 1 March 2024]
Teasdale G, Jennett B (1974) Assessment of coma and impaired consciousness: a practical scale. The Lancet 304(7872), 81-84.
| Crossref | Google Scholar | PubMed |
Teasell R, Bayona N, Marshall S, Cullen N, Bayley M, Chundamala J, Villamere J, Mackie D, Rees L, Hartridge C, Lippert C, Hilditch M, Welch-West P, Weiser M, Ferri C, McCabe P, McCormick A, Aubut JA, Comper P, Salter K, Van Reekum R, Collins D, Foley N, Nowak J, Jutai J, Speechley M, Hellings C, Tu L (2007) A systematic review of the rehabilitation of moderate to severe acquired brain injuries. Brain Injury 21(2), 107-112.
| Crossref | Google Scholar | PubMed |
Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, Robson R, Thabane M, Giangregorio L, Goldsmith CH (2010) A tutorial on pilot studies: the what, why and how. BMC Medical Research Methodology 10, 1.
| Crossref | Google Scholar | PubMed |
Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, Chastin SFM, Altenburg TM, Chinapaw MJM, On behalf of SBRN Terminology Consensus Project Participants (2017) Sedentary Behavior Research Network (SBRN) – Terminology Consensus Project process and outcome. International Journal of Behavioral Nutrition and Physical Activity 14(1), 75.
| Crossref | Google Scholar | PubMed |
Turner‐Stokes L, Pick A, Nair A, Disler PB, Wade DT (2015) Multi‐disciplinary rehabilitation for acquired brain injury in adults of working age. Cochrane Database of Systematic Reviews 2015(12), CD004170.
| Crossref | Google Scholar | PubMed |
Ware JE Jr, Sherbourne CD (1992) The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care 30, 473-483.
| Google Scholar | PubMed |
World Health Organization (2020) ‘WHO guidelines on physical activity and sedentary behaviour.’ (WHO: Geneva, Switzerland) Available at https://iris.who.int/bitstream/handle/10665/336656/9789240015128-eng.pdf
Zgaljardic DJ, Seale GS, Schaefer LA, Temple RO, Foreman J, Elliott TR (2015) Psychiatric Disease and Post-Acute Traumatic Brain Injury. Journal of Neurotrauma 32(23), 1911-1925.
| Crossref | Google Scholar | PubMed |