Antibiotic resistance and prescribing in Australia: current attitudes and practice of GPs
Rachel Hardy-Holbrook A B , Svetlana Aristidi A , Vandana Chandnani A , Daisy DeWindt A and Kathryn Dinh AA NPS MedicineWise, PO Box 1147, Strawberry Hills, NSW 2012, Australia.
B Corresponding author. Email: Rholbrook@nps.org.au
Healthcare Infection 18(4) 147-151 https://doi.org/10.1071/HI13019
Submitted: 3 June 2013 Accepted: 22 July 2013 Published: 3 September 2013
Journal Compilation © Australian Infection Control Association 2013
Abstract
Background: Antimicrobial resistance is a growing public health issue influenced by inappropriate prescribing and use. In Australia the prevalence of antibiotic-resistant bacteria in hospital, nursing home and community settings is on the rise. To address this issue, a 5-year program focuses on reducing the prescribing and inappropriate use of antibiotics. In order to inform development of the program, a cross-sectional survey was conducted.
Methods: The survey was sent to a random sample of 1570 Australian general practitioners (GPs), and data was collected on GP knowledge, attitudes, awareness and self-reported behaviour in relation to antibiotic resistance, medical imaging referrals and antibiotic prescribing.
Results: 730 GPs participated in the survey (46.5% response rate). While GPs perform very well in many areas, especially in recommending symptomatic management rather than prescribing an antibiotic, there is some possible confusion amongst GPs about the factors that increase antibiotic resistance. The results showed that patient expectation also plays a role in the decision to prescribe antibiotics, with almost 40% of respondents admitting that they would prescribe antibiotics to meet a patient’s expectations. Antibiotic resistance is generally not discussed with patients (only half [50%] of respondents would always or often discuss the issue of antibiotic resistance).
Conclusion: Programs to address the prescribing of antibiotics must be informed by existing knowledge, attitudes, awareness and practice of GPs. There is room for improvement in GPs’ knowledge of prescribing behaviours that decrease antibiotic resistance. GPs should be encouraged to discuss the issue of antibiotic resistance with patients and to not provide an antibiotic prescription to be dispensed at a later date or to meet patient expectation.
Implications
-
Programs to address antibiotic resistance must be informed by current prescribing behaviours and practice and specifically address these behaviours.
-
Further support for GPs in relation to dealing with patient expectation in regard to antibiotics should be provided.
Introduction
Antimicrobial resistance is an increasing public health issue influenced by inappropriate prescribing and use. The strong association between antibiotic-prescribing practices in primary care and the rate of antibiotic resistance1 suggests that GPs have an important role in maintaining the efficacy of antibiotics. A study in 21 European countries2 evaluated population-adjusted use of antimicrobial agents in ambulatory care and the resistance trends of Streptococcus pneumoniae and Escherichia coli over 6 years (2000 to 2005). Prescribed drugs were grouped by the active substance as the number of defined daily doses (DDD) per 1000 inhabitants (DID). Total outpatient antimicrobial drug use differed significantly between countries: for example, in 2004, Greece’s consumption (33.4 DID) was much higher than the Netherlands (9.7 DID). Similarly, resistance proportions, in 2005, differed for penicillin non-susceptible S. pneumoniae isolates: France (36%) was much higher than the Czech Republic (2%); and for fluoroquinolone resistance in E. coli, Portugal (29%) was much higher than Iceland (3%) – showing resistance correlating with the use of those agents.1
In Australia, the prevalence of antibiotic-resistant bacteria in hospital, nursing home and community settings is on the rise. An increase in the prevalence of antibiotic resistance in common pathogens causing respiratory tract infections (RTIs) over the past 20 years has been demonstrated in the Australian Group on Antimicrobial Resistance (AGAR) surveys.3 The rate of resistance to macrolides (erythromycin, roxithromycin, clarithromycin, azithromycin) in S. pneumoniae increased from 8.7% in 1994 to 20.4% in 2007 and continues to increase.4 Multi-resistant strains (resistant to two or more classes of antibiotics) were identified in 12.7% of non-invasive S. pneumoniae isolates, which further reduces treatment options.4 By contrast, fluoroquinolone resistance remains uncommon in Australia because of restrictions placed on their use.
Over 19 million prescriptions for 11 selected antibiotics (amoxicillin, cefalexin, amoxicillin with clavulanic acid, roxiithromycin, doxycycline, cefaclor, erythromycin, clarithromycin, phenoxymethylpenicillin, ciprofloxacin and cefuroxime) were dispensed in 2009–10. Use of antibiotics (DDD per 1000 population per day) has increased by almost 10% over the last 10 years and reached 19.8 in 2009–10. This has increased to 24 over the past few years and respiratory system illness has become the most frequently managed problem in general practice in Australia with more than half of antibiotic ordering in primary care for respiratory tract infections.6 In 2009–10, the two most common problems for which antibiotics were prescribed or supplied in Australian primary care were generalised upper respiratory tract infection (URTI), mostly the common cold (14.0%), and acute bronchitis or bronchiolitis (14.0%).
In order to address the issue of antibiotic resistance in Australia, a 5-year program was launched in February 2012 and directed at health professionals and consumers. The program focuses on reducing antimicrobial resistance through the appropriate prescribing and use of antibiotics. The overall program target is to reduce antibiotic usage by 25% over 5 years, from 24 to 19 DDD per day based on 5% per annum reductions to achieve concordance with international best practice and the Australian Commission on Safety and Quality in Health Care (ACSQHC) recommended benchmarks. It is assumed empirically that a corresponding reduction in the incidence of new antibiotic resistance will ensue.
A cross-sectional survey of general practitioners (GPs) was conducted before the program launch and will be repeated at the end of the 5-year program. This paper presents the results of the GP survey.
Methods
The aim of the cross-sectional survey is to identify any short- to medium-term improvements in GP knowledge, attitudes, awareness and self-reported behaviour in relation to antibiotic resistance and prescribing, as well as medical imaging referrals.
The survey is a self-completed, paper-based questionnaire of a random sample of Australian GPs who have participated in any National Prescribing Service (NPS) intervention in the last 5 years (n = 20 120). The number of registered Australian GPs as of June 2011 was 24 720.5
The survey questions relate, where possible, to the objectives of the 5-year program. However, the questions needed to be specific and therefore the topic of upper respiratory tract infections (URTI) was used for specific case scenarios and questions. This topic was selected as the focus of activities for the first year of the program.
The paper-based survey was pre-tested with six GPs and then mailed to 1570 GPs around Australia (number based on sample size calculation) at the end of November 2011. The survey was in field for 10 weeks, and two reminder letters were posted. On completion of the 5-year program, the same questionnaire will be mailed to a random sample of Australian GPs. For each year of the program several interventions will be conducted and the GPs who participate in interventions will be surveyed via a retrospective pre-survey with a control group to assess changes based on individual interventions. These results will be reported at a later date.
Results
Survey response
The survey achieved a response rate of 46.5% (n = 730). The majority of GP respondents were male, working in a multiple GP practice and had been practicing as a GP for an average of 20 years. The first reminder letter was successful in doubling the initial response rate from 18.2% to 36.4%, with a further 10.1% increase achieved after the second reminder. The proportion of respondents by state corresponded to the proportions of GPs practicing in each state. Over half of respondents (56%) indicated that a significant proportion of patients in their practice were concession card holders.
Antibiotic resistance
When asked about antibiotic resistance, 55% (n = 361) of respondents agreed or strongly agreed that antibiotic resistance was a problem in the community serviced by their practice, with 29% neither agreeing nor disagreeing. Forty-six percent (n = 302) believed antibiotic resistance was more of a problem in hospitals, against evidence suggesting antibiotic resistance to be an issue in both the community and the hospital setting.7 Forty-three percent (n = 279) of respondents took a neutral stance on whether antibiotic resistance may last up to 12 months in an individual after a single use, despite evidence showing this to be the case.7
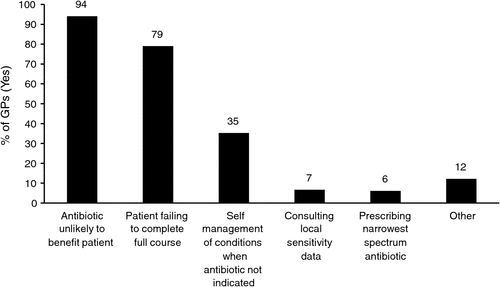
Respondents were asked about behaviours believed to increase antibiotic resistance (see Fig. 1). Respondents were asked to select all the behaviours they believed increased antibiotic resistance. When asked about behaviours believed to increase resistance, 35% of respondents believed that self-management of conditions by the patient when an antibiotic is not indicated did increase resistance. This statement could have been interpreted as patients using old or leftover antibiotics from previously unfinished prescriptions. This could contribute to increasing resistance and is therefore a correct response. This specific question requires further exploration before a post-survey is conducted.
|
The 12% of GPs (n = 80) who selected ‘other’ behaviours believed to increase antibiotic resistance reported use of broad-spectrum antibiotics (n = 24), agricultural use of antibiotics (n = 19), frequent use or overuse (n = 16), hospital use of antibiotics (n = 8), patient request (n = 5), and patient self-management with antibiotics and/or complementary medications (n = 4). All of these can contribute to antibiotic resistance.
Treatment and treatment choices: case scenarios
Respondents were given two case scenarios to explore management of patients with an acute upper respiratory tract infection (URTI). They were asked how they would most likely respond, and which factors helped them make their decision. Case one detailed a 27-year-old male with no significant past medical history who attends with a 3-day history of dry cough and sore throat. He has stayed home for 3 days and requires a medical certificate for any further time off work. On examination, his temperature (tympanic) is 37.8°C, his throat appears slightly red and there is no exudate or cervical lymphadenopathy. His chest is clear.
While less than 1% of respondents would prescribe amoxicillin, 19% of GP respondents would provide a prescription for an antibiotic to be dispensed if symptoms do not improve. This was deemed inappropriate for the purposes of this study although the context of healthcare in Australia must be considered. With many rural practices it may be appropriate, and indeed the only option, for a prescription to be provided to be dispensed at a later date. At a patient’s return visit, respondents were much more likely to prescribe an antibiotic, which is appropriate according to current Australian guidelines.8 The likelihood of ordering a sinus CT scan also increased at a return visit. The majority of respondents (93%) stated they would recommend over-the-counter medicines and/or self-management of symptoms rather than prescribe an antibiotic. This was the desired response.
This question also sought to identify factors which contributed to a respondent’s decision about treatment. Most respondents (78%) selected multiple factors. Overall, respondents were almost equally likely to consider clinical presentation (80%) and their own experience in managing similar problems (81%) when making a decision about treatment. Patient request (12%) and knowledge of local sensitivity data about antibiotic-resistant bacteria (9%) were less likely to contribute towards a respondent’s decision.
Case two detailed a 20-year-old female non-smoker who presents with a 3-day history of nasal congestion and headaches. She has been off work today and hasn’t taken any medicines to manage her symptoms. Examination was normal.
A majority of respondents would recommend over-the-counter medicines and self-management of symptoms (83%). Less than 1% would order a sinus CT scan at this initial visit and only 1% would prescribe a course of amoxicillin. A sinus CT scan or prescription for antibiotics is inappropriate.
At a return visit with worsening symptoms, the proportion of respondents who would prescribe a course of amoxicillin increased to 89%, in line with current Australian guidelines.8 29% of respondents would prescribe intranasal corticosteroids. The percentage of respondents ordering a CT scan increased to 7% which is not appropriate for the purposes of this study.
Similarly to case study one, clinical presentation (87%) and clinical experience managing similar problems (84%) were the main factors considered when making a decision about treatment. However, respondents were more likely to take into account local sensitivity data in case two (14% vs 9%).
Diagnostic tests
When asked about diagnostic tests (in particular X-rays and CT scans) the majority of respondents (89%) did not believe that diagnostic tests should support preliminary diagnosis of a URTI. This is appropriate. However, respondents were equally likely to agree (38%) and disagree (38%) that they use diagnostic tests to confirm empirical management.
Patient communication
In relation to patient communication and URTIs an overwhelming majority of GP respondents (98%) would always or often discuss the benefits of symptomatic management with their patients. Respondents hold discussions with patients about antibiotic expectations (80%), the lack of benefit or potential harms of antibiotic use (85%), adherence to the antibiotic regime prescribed (88%) and vaccination when appropriate (71%). However, only half would always or often discuss the issue of antibiotic resistance (50%).
The usefulness of imaging in confirming a diagnosis was rarely or never discussed by 73% of respondents; however, 22% of respondents responded that they would sometimes speak with a patient about the usefulness of imaging in confirming a diagnosis. Thirty-three percent would often, and 32% would sometimes, discuss the associated risks of imaging. It is desirable for the risks of imaging to be discussed with a patient when imaging is being considered.
Frequency of patient management behaviour
The overwhelming majority of respondents would rarely or never order imaging to confirm a preliminary diagnosis (92%). This is the appropriate response. The majority of respondents (74%) stated they would rarely or never seek advice from specialists to assist when making a decision about requesting diagnostic imaging. This may be because imaging is never or rarely ordered by the majority of GPs. It is however appropriate to seek advice when unsure if a test is necessary.
The majority of respondents would also rarely or never use a chest X-ray to help in making a diagnosis (77%) or order imaging to alleviate a patient’s fears (77%). Again these are the desired responses.
However, 22% responded that they would sometimes order imaging to alleviate patient fear which is inappropriate, especially considering that the majority of respondents reported that the results of an imaging test rarely or never changed management decisions (59%).
An overwhelming majority of respondents (90%) would always or often recommend symptomatic management. This is the desired response. Although most respondents (61%) would rarely or never prescribe antibiotics to meet their patient’s expectations, 37% would sometimes and 2% always or often did, which is inappropriate for the purposes of this study. Most respondents would often (51%) or always (19%) prescribe a narrow-spectrum antibiotic when required (this is appropriate and in line with current Australian guidelines8), and most respondents would always or often consider the issue of antibiotic resistance when prescribing (82%), which is the desired response.
Discussion
The results of this GP survey provide useful information that can assist in developing appropriate programs to address GP knowledge, attitudes and awareness of antibiotic resistance and current practice in prescribing antibiotics. Australian GPs perform very well in many areas, especially in the area of recommending symptomatic management rather than prescribing an antibiotic. However, there is some possible confusion amongst GPs about the factors that increase antibiotic resistance. There is some room for improvement in GP knowledge of the factors that increase resistance, including that resistance is an issue in the community as much as in hospitals (for example, methicillin-resistant Staphylococcus aureus (MRSA) has become more of a community-derived problem in most jurisdictions of Australia), and that antibiotic resistance may last up to 12 months in an individual after a single use of antibiotics. There is also a need to encourage GPs not to provide a prescription for antibiotics to be dispensed at a later date if symptoms do not improve, where this is possible. There is also room for further discussion and knowledge in the health professional community around the completion of a course of antibiotics (Therapeutic Guidelines promote early cessation in some cases8).
Factors that influence a respondent’s decision can help focus interventions or programs to better assist in decision-making. One important reference is current national guidelines8 and another is local sensitivity data (if available) when selecting and prescribing antibiotics. It is important to note that many GPs may already be aware of the current guidelines, and that local sensitivity data may not always be available.
One important area relating to antibiotic prescribing is patient expectation. While patient education is vital, it is also important to help GPs strengthen their existing skills in discussing antibiotic resistance with patients and not to prescribe antibiotics because of patient expectation or pressure. The same consideration is needed for medical imaging referrals, especially in the case of URTIs.
The findings and recommendations from this survey have been integrated into program activities such as academic detailing, clinical e-Audit, webinars, online modules, and facilitated group discussions. The program also includes a consumer campaign that calls on the general public to be ‘Antibiotic Resistance Fighters’.
Prescribing outcomes from the program interventions will be evaluated utilising data from the Pharmaceutical Benefits Scheme (PBS), Drug Utilisation Sub-Committee (DUSC) Pharmacy Guild Survey, Australian Institute of Health and Welfare (AIHW), and the Organisation for Economic Cooperation and Development (OECD). Changes in the dispensing of 11 specified antibiotics, changes in prescription volume and associated costs of specified antibiotics as well as changes in daily defined dose for antibiotics will be analysed. The same survey will be conducted at the end of the 5-year program to determine the general effects of the program and assess changes in GP knowledge, attitudes and awareness of antibiotic resistance and self-reported behaviours in relation to antibiotic prescribing.
Conclusions
Programs to address the prescribing of antibiotics must be informed by existing knowledge, attitudes, awareness and practice of GPs. Results of this survey of Australian GPs indicate that there is some room for improvement in GP awareness of the sources of antibiotic resistance and prescribing-related factors that increase antibiotic resistance. GPs should be encouraged to discuss the issue of antibiotic resistance with patients. GPs should be encouraged to not prescribe antibiotics to meet patient expectation or at the first presentation, to be dispensed at a later date unless required due to circumstance.
Recommendations based on survey results were used to inform the 5-year program.
Conflicts of interest
None reported by any of the authors.
Funding
NPS MedicineWise is funded by the Australian Government Department of Health and Ageing (DoHA).
References
[1] Goossens H, Ferech M, Stichele RV, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005; 365 579–87.| 15708101PubMed |
[2] Goossens H, Ferech M, Coenen S, Stephens P, European Surveillance of Antimicrobial Consumption Project Group. Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries. Clin Infect Dis 2007; 44 1091–5.
| Comparison of outpatient systemic antibacterial use in 2004 in the United States and 27 European countries.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2sXltlCksrw%3D&md5=28928c289132ddea7fea799aa17a8509CAS | 17366456PubMed |
[3] Australian Group on Antimicrobial Resistance (AGAR). AGAR Surveys: schedule and overview. Available from: http://www.antimicrobial-resistance.com/ [verified August 2013].
[4] Australian Group on Antimicrobial Resistance (AGAR) .Streptococcus pneumoniae Survey Antimicrobial Susceptibility Report. Perth: AGAR; 2007.
[5] Australian Bureau of Statistics. General Practice Workforce Statistics. Australian Demographic Statistics. cat. no. 3101.0. Canberra: ABS; 2011.
[6] Britt H, Miller GC, Charles J, Henderson J, Bayram C, Pan Y, et al. General practice activity in Australia 2009–10. General practice series no. 27. cat. no. GEP 27. Sydney: AIHW; 2010.
[7] Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ 2010; 340 c2096
| Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis.Crossref | GoogleScholarGoogle Scholar |
[8] Antibiotic Expert group. Therapeutic guidelines: antibiotic. Version 14. Melbourne: Therapeutic Guidelines Limited; 2010.

