Long-term survival outcome following Staphylococcus aureus bacteraemia
Chong W. Ong A D , Jan L. Roberts B and Peter J. Collignon CA Department of Infectious Diseases and Microbiology, Royal Hobart Hospital, Hobart, Tas. 7000, Australia.
B Infection Prevention and Control Unit, ACT Health Directorate, Canberra, ACT 2605, Australia.
C Department of Infectious Diseases and Microbiology, The Canberra Hospital, Canberra, ACT 2605, Australia.
D Corresponding author. Email: chong.ong.work@gmail.com
Healthcare Infection 18(3) 102-109 https://doi.org/10.1071/HI12062
Submitted: 23 December 2012 Accepted: 21 February 2013 Published: 30 April 2013
Journal Compilation © Australasian College for Infection Prevention and Control 2013
Abstract
Aims: To describe long-term survival (beyond 200 days) following Staphylococcus aureus bacteraemia (SAB) and to determine if certain patient subgroups had poorer long-term survival outcomes.
Methods: A single-centre, retrospective, cohort study of all SAB cases at The Canberra Hospital, a tertiary referral centre, from January 1998 to December 2007 was performed. Clinical and demographic data were obtained from a pre-existing prospectively collected database. Patients were followed-up for a minimum of 9 months. Subsets within the cohort were analysed for differences in long-term survival. The main outcome measure was death from any cause.
Results: During the 3889-day study observation period, 439 patients had SAB and were followed for a total of 546 360 person-days. The overall median survival was 3169 days. The mortality rates were 9.6%, 17%, 24%, 29% and 32% at 7, 30, 90, 180 and 365 days respectively. A total of 188 (43%) patients died. Of the 188 deaths, 22%, 40%, 55%, 67% and 75% occurred within the first 7, 30, 90, 180 and 365 days respectively after their SAB episode. Initial analysis showed poorer long-term survival in those patients with older age, MRSA, with an unknown focus of infection, and who were not admitted under the Infectious Diseases team. However, on multivariate analysis, the only independent risk factors for poorer survival were older age, unknown focus of infection and not being admitted under the Infectious Diseases team. MRSA, sex, surgical vs non-surgical admitting team and an association with an intravascular device were not associated with poorer long-term survival.
Conclusions: High rates of death continue for many months after patients have an episode of SAB. Short-term follow-up studies (30 days or less) may miss large numbers of SAB-associated deaths. If accurate data on SAB-associated mortality is needed, then follow-up of these patients will be needed for at least 90 days, ideally performed prospectively with a matched control group consisting of hospitalised patients without SAB.
Additional keywords: bacteraemia, Staphylococcus aureus, survival.
Implications
-
High rates of death continue for many months after patients have an episode of SAB.
-
Short-term follow-up studies (30 days or less) will miss large numbers of SAB-associated deaths.
-
More medium- to long-term follow-up studies, ideally performed prospectively with a suitable control group, are required to fully understand the impact of SAB on mortality.
Introduction
Staphylococcus aureus bacteraemia (SAB) is common and associated with high morbidity and mortality.1–8 Prognostic factors have been identified but studies are usually of short-term survival and/or in-hospital mortality.1–6 Serious complications from SAB (e.g. endocarditis) are common and may lead to death, weeks or months later. These deaths will be missed if there is inadequate follow-up. Few groups have studied long-term outcome7–9 and even 90 day follow up is unusual. The present study was conducted to provide data on short-, medium- and long-term survival (200 days and beyond). It also determined if certain subgroups had poorer long-term survival outcomes and examined independent risk factors for mortality.
Methods
Study design
A retrospective cohort study at The Canberra Hospital – a 500-bed, tertiary-care academic hospital in Canberra, Australian Capital Territory (ACT), Australia – was performed. All patients known to be resident within ACT who were admitted with SAB during the period 1 January 1998 to 31 December 2007 were included in this study. Follow up was until either death or the end of the study period (25 August 2008, i.e. a minimum of 238 days). Patients resident within other states were excluded.
Data were obtained from prospectively-collected information in the Blood Stream Infection Surveillance Database (part of a quality improvement project involving all hospitalised patients with bacteraemia), as described elsewhere.10 Briefly, trained infection control practitioners visited the microbiology laboratory daily to directly obtain information on all new positive blood cultures. All cases were then reviewed while they were inpatients, with standardised definitions applied.11 Classification consensus was obtained at a weekly team meeting with a specialist infectious diseases physician, who reviewed all cases before data entry. All patients were followed-up for 7 days or to discharge, if earlier. Early clinical outcome was assessed at 7 days (or discharge, if earlier). For this study, early outcome was categorised into one of three groups: Patients with expected ‘full recovery in up to 3 weeks’, those with evidence of ‘ongoing sepsis or new morbidity’ and those who had died at or before 7 days.
The only study outcome was death from any cause. All deaths occurring during this period were included. For patients who did not have a date of death obtainable from the database, the date was obtained from the hospital medical records system. The ACT government Register of Deaths was also searched to ascertain if any other death had been missed.
Ethics approval was obtained from the ACT Health Human Research Ethics Committee.
Definitions
SAB was defined as a patient having at least one blood culture positive with Staphylococcus aureus – either Methicillin-sensitive Staphylococcus aureus (MSSA) or Methicillin-resistant Staphylococcus aureus (MRSA). Further positive blood cultures obtained within the first 14 days of the initial positive culture were regarded as the same episode of bacteraemia.
Residency within ACT was defined as a patient having a home address and postal code within the ACT.
Acquisition location was categorised as ‘community-associated’ if the positive blood culture was obtained within the first 48 h of admission and there was no recent healthcare contact, ‘inpatient healthcare-associated’ if the positive blood culture was obtained after the patient had been in a hospital for at least 48 h, and ‘non-inpatient healthcare-associated’ if the positive blood culture had been obtained within the first 48 h of admission but SAB was a complication of a pre-existing indwelling medical device, a surgical site infection (within 30 days of surgery), an invasive procedure or associated with neutropenia from cytotoxic therapy.
Foci of infection were classified based on the primary body system involved (e.g. cardiovascular, genitourinary, gastrointestinal or as unknown). Foci were also categorised as ‘eradicable’ and ‘non-eradicable’ similarly to Kim and colleagues.12 Eradicable foci included surgically-removable infections or drainable abscesses and indwelling foreign bodies, such as peripheral and central venous catheters. Non-eradicable foci included unknown primary site, pneumonia, endocarditis, osteomyelitis and arthritis.
Data analysis
Survival was defined as the number of days from the index positive blood culture until the date of death. Survival was censored at the end of the study observation period (25 August 2008). All patients not known to be dead at the end of the study observation period were assumed to be alive. Patients who had more than one new episode of SAB during the study period were included only once, based on their first episode of SAB. The Mann-Whitney U test was used to compare continuous variables and Fisher’s exact test for categorical variables. A time-to-event method was used to estimate the effect of SAB on survival for both the entire cohort and on defined subsets of the cohort. Kaplan-Meier analysis was used to evaluate the unadjusted relationship between SAB and death. The log-rank test was applied for survival curve comparisons. Unadjusted hazard ratios (HR) and corresponding confidence intervals (CI) were calculated using the Mantel-Haenszel method. All tests were two-tailed and P ≤ 0.05 was considered significant, except in the case of comparing three survival curves by three paired comparisons, where the Bonferroni-corrected threshold of P < 0.02 was considered significant. Statistical analyses were performed using GraphPad Prism 5 for Windows version 5.01 (GraphPad Software, Inc., San Diego, California, USA). In order to identify independent risk factors for mortality, multiple Cox regression analysis was performed, using MedCalc for Windows, version 12.4.0.0 (MedCalc Software, Mariakerke, Belgium).
Results
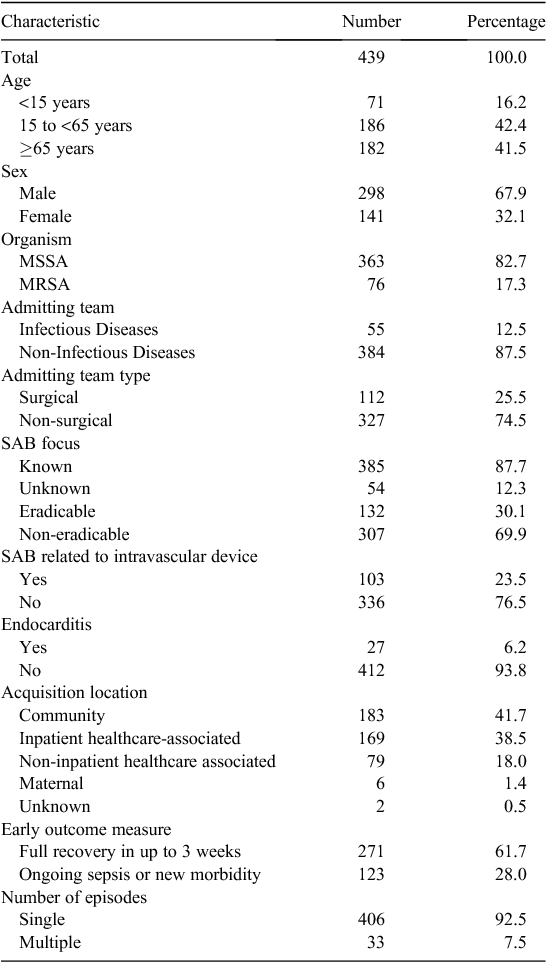
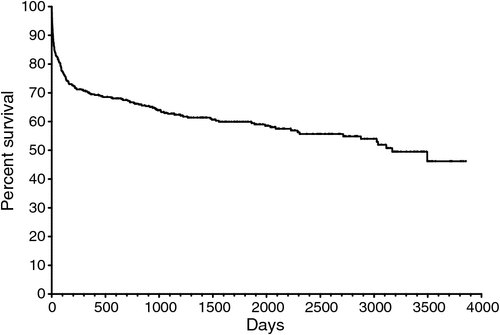
During the 10 year study period, there were 188 deaths in 439 SAB patients (43%). Descriptive characteristics of the patients are shown in Table 1. The observation period was 3889 days and they were followed for 546 360 person-days. Their mean age was 51.3 years (range 0 to 98.6). Median survival was 3169 days.
|
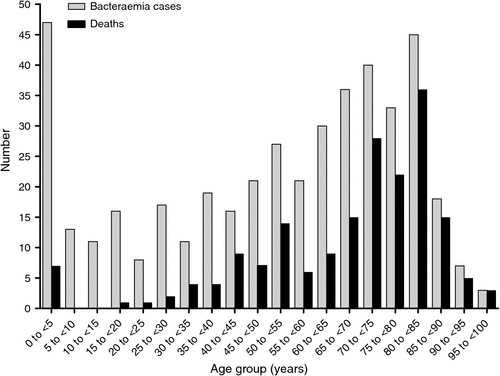
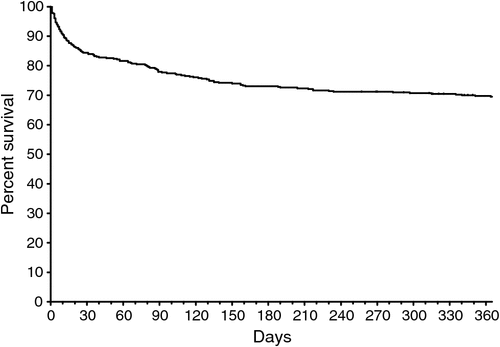
Most SAB episodes occurred in older patients, but with another peak in those <5 years (Fig. 1). Of the 188 patients who died, the mean age was 66.4 (range 0 to 98.6) years, which is significantly older than the mean age of 39.9 (range 0 to 92.7) years for the 251 (57%) surviving patients (P < 0.0001). The mortality rates were 9.6%, 17%, 24%, 29% and 32% at 7, 30, 90, 180 and 365 days respectively (Figs 2 and 3).
|
|
|
Of the 188 deaths, 42 (22%), 75 (40%), 104 (55%), 125 (67%) and 140 (75%) occurred within the first 7, 30, 90, 180 and 365 days respectively.
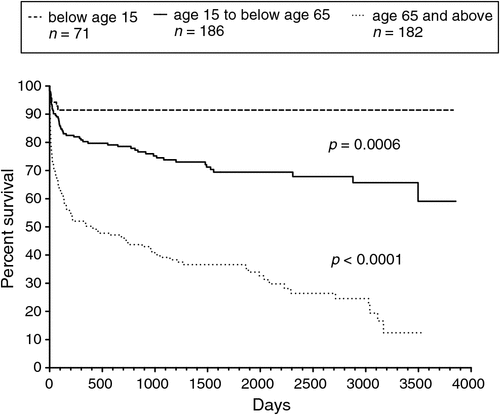
Different age groups differed markedly in long-term survival (Fig. 4). The percentage of patients dead at the end of the study was 9.9%, 31% and 68% for those aged <15 years, aged 15 to <65 years and aged ≥ 65 years, respectively. The median survival was 398 days for those aged ≥ 65 years. Long-term survival was significantly better for those aged <15 years compared with those aged 15 to <65 years (log-rank P = 0.0006; HR 0.38, 95% CI 0.22 to 0.66) and to those aged ≥ 65 years (log-rank P < 0.0001; HR 0.22, 95% CI 0.15 to 0.32). Long-term survival was also significantly better for those aged 15 to <65 years compared with those aged ≥ 65 years (log-rank P < 0.0001; HR 0.30, 95% CI 0.22 to 0.40). For the subset of patients with MSSA bacteraemia, similar significant differences in survival trends were also observed across the three age groups. There was no statistically significant difference in survival curves for males and females.
|
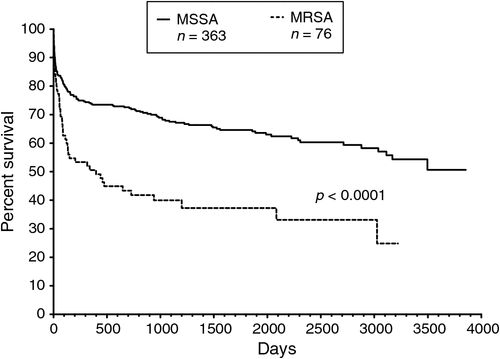
Sixty-three percent of patients with MRSA bacteraemia were dead by the end of the study, compared with 39% of those with MSSA bacteraemia. Those with MRSA bacteraemia had a median survival of 398 days and were more likely to die than those with MSSA bacteraemia (log-rank P < 0.0001; HR 2.87, 95% CI 1.87 to 4.39) (Fig. 5). However, there were major differences in age. Of the 76 patients with MRSA bacteraemia, the median age was 69.5 (range 8.6 to 98.6) years, which was significantly older than the median age of 53.7 (range 0 to 97.3) years for the 363 patients with MSSA bacteraemia (P < 0.0001).
|
With regard to admitting team, 26% of patients with SAB admitted under Infectious Diseases had died by the end of the study, compared with 45% for those admitted under other teams (log-rank P = 0.02; HR 0.60, 95% CI 0.39 to 0.93). There was no significant difference in survival following SAB whether a patient was admitted under a surgical or non-surgical team (median 3025 and 3495 days, respectively).
For patients with SAB who had a known focus of infection, 40% had died by the end of the study, compared with 63% for those who had an unknown focus of infection. The median survival times were 3495 and 440 days for those with known and unknown infection foci, respectively (log-rank P = 0.001; HR 0.44, 95% CI 0.27 to 0.73).
There was no significant difference in survival between those with eradicable and non-eradicable foci of infection (median 3037 and 3169 days, respectively). There was also no significant difference in survival between those with SAB related to an intravascular device and those with SAB not related to such a device (median 3037 and 3169 days, respectively). Patients with endocarditis as a primary focus did not have a difference in survival compared with those without endocarditis (median 3113 and 3169 days, respectively).
With regard to acquisition location, the percentage of patients dead at the end of the study were 34%, 51% and 48% for those with community-associated, inpatient healthcare-associated and non-inpatient healthcare-associated SAB, respectively. The median survival was 3495, 1987 and 2712 days, respectively. Long-term survival was significantly better for those with community-associated SAB compared with those with inpatient healthcare-associated SAB (log-rank P = 0.004; HR 0.61, 95% CI 0.44 to 0.85) but not statistically significantly different compared with those with non-inpatient healthcare-associated SAB. For the subset with MSSA bacteraemia, the survival curves for all three groups of patients with different acquisition location were not significantly different.
Poorer early clinical outcome was associated with lower long-term survival. A total of 394 patients who were not already dead at 7 days were analysed. Of those designated ‘full recovery in up to 3 weeks’, 34% were dead by the end of the study observation period, compared with 43% for those designated ‘ongoing sepsis or new morbidity’ (log-rank P = 0.03; HR 0.66, 95% CI 0.46 to 0.95).
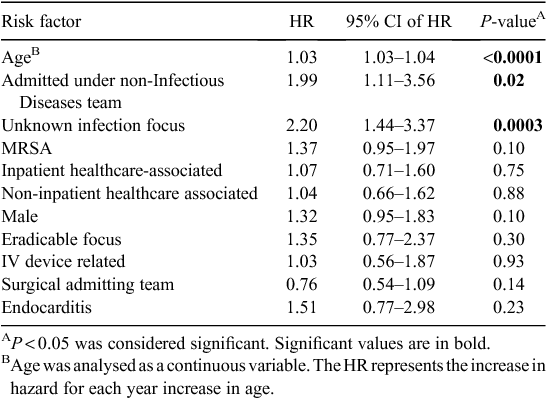
A multiple Cox regression model incorporated age (as a continuous variable), MRSA vs MSSA, Infectious Diseases vs non-Infectious Diseases admitting team, known vs unknown infection focus, community-associated vs inpatient healthcare-associated, community vs non-inpatient healthcare associated, sex, eradicability of focus, involvement of IV device, surgical vs non-surgical admitting team and presence or absence of endocarditis as covariates. Only older age, admission under a non-Infectious Diseases team and unknown infection focus were identified as independent risk factors for poorer long-term survival, as shown in Table 2.
|
Discussion
To our knowledge, this is the largest group of patients with SAB for which survival data is available over such a long observation time following bacteraemia. Our 30-day mortality of 17% is similar to the 19%, 19.7% and 23.2% reported by Hill and colleagues,13 Fätkenheuer and colleagues7 and Mylotte and colleagues,2 respectively. Our 24% mortality at 90 days is the same as reported by Ringberg and colleagues at 12 weeks.14 We have shown, however, that ongoing deaths continue beyond the periods of those studies and high rates are still present 6 to 12 months after the episode of sepsis. In their 1-year follow-up study, Hanses and colleagues have also demonstrated considerable additional mortality beyond 90 days following SAB, reporting crude-mortality at 30 days, 90 days and 1 year of 28.8%, 37.5% and 47.5%, respectively.15 Not all of these deaths are necessarily attributable to SAB, and the differences between all-cause and attributable mortality from SAB varies between studies. For example, Hill and colleagues13 reported an all-cause mortality of 22.4% and an attributable mortality of 18.9% whereas the corresponding figures reported by Sharma and colleagues16 were 22.8% and 14.1%. However, deaths after SAB are not due to underlying disease in patients and thus their hospitalisation. Berger and colleagues17 (at 30 days following SAB), de Kraker and colleagues18 (at 30 days following SAB), Wolkewitz and colleagues19 (at 90 days following admission) and Primo and colleagues,20 all showed that survival was significantly worse in patients hospitalised with SAB compared with hospitalised controls without SAB.
Our data showed long-term survival was poorer with older age. Others have also shown this at 30 days,2,13 3 months21 and 1 year.7 This is likely related to co-morbidities. MRSA was more common in elderly patients but this was not likely the major factor causing an increased mortality. We found that increased age was associated with poorer long-term survival even in the subset with MSSA bacteraemia. Chia and colleagues22 found that an age over 65 years was an independent predictor of mortality in a study of MSSA bacteraemia. Others have shown that, in the short-term, SAB mortality is lower in children than adults.23 Although we know of no published long-term studies in children, our data suggests that mortality risk stabilises after an initial 3-month period (Fig. 4). This is probably because multiple other co-morbidities are relatively rare, compared with adults. We saw 5 (6.8%) deaths in 74 children with SAB within the first 30 days, which is similar to the 4 (3.2%) deaths in 125 children reported by Hill and colleagues.24
In our study, 50.9% of patients with MRSA bacteraemia had died within one year, which is comparable to the 46.2% overall all-cause mortality following MRSA bacteremia noted in a recent Australian study, which was a 10-year retrospective review of all cases at a single institution.25 Patients with MRSA bacteraemia had poorer survival than those with MSSA bacteraemia. This was reported in other studies at 30 days5,8 even with adjustments for disease severity.6 In a multicentre, prospective, matched-cohort study carried out in 13 European countries, de Kraker and colleagues clearly demonstrated that 30-day mortality was higher following MRSA than MSSA bacteraemia.18 MRSA bacteraemia is also associated with higher mortality at 6 and 12 months.8 A large meta-analysis of 31 cohort studies has clearly demonstrated that mortality rates from MRSA were higher than those from MSSA.26 The reason for this is not entirely clear. While this difference has been attributed to a delay in initiation of appropriate antibiotic therapy for MRSA,27 there have also been findings to the contrary.28,29 One recent study did not support the hypothesis that earlier empirical glycopeptide use reduces mortality in hospital-acquired MRSA bacteraemia.9 Whether MRSA directly increases the likelihood of death or merely serves as a marker for a population with greater co-morbidity who are at risk of death from other causes needs to be further addressed by other studies. It should be noted, however, that in our study, after multivariate analysis, MRSA was not identified as an independent risk factor for poorer outcome. It is interesting to note that the Australia New Zealand Cooperative on Outcomes in Staphylococcal Sepsis (ANZCOSS) data for 1865 SAB patients followed up to 30 days also had age and MRSA as predictors of increased mortality on their univariate analysis but, on multivariate analysis, MRSA infection was not an independent predictor of mortality although age still was.30 The ANZCOSS investigators concluded that this could be explained, at least in part, by the inferior efficacy of treatment (i.e. vancomycin), since treatment with a glycopeptide was identified as an independent predictor of mortality. Unfortunately, our study did not include treatment information to enable similar analysis.
Patients admitted under our hospital’s Infectious Diseases team had better long-term survival. Jenkins and colleagues31 reported that routine consultation with an Infectious Diseases Service for all cases of SAB resulted in more investigations (e.g. echocardiography) and adherence to all 4 standards of care in SAB management – removal of intravascular foci of infection, obtaining follow-up blood cultures, use of parenteral β-lactams for MSSA where possible and administration of at least 28 days of therapy for complicated infections. Fowler and colleagues32 demonstrated that where Infectious Diseases specialist recommendations were followed by the attending physician of a SAB patient, cure was more likely and relapse less likely over a 12 week follow-up period. Robinson and colleagues have reported that Infectious Diseases consultation was associated with decreased mortality at 7 days, 30 days and 1 year, because patients were more likely to have received effective initial antibiotics.33
Our study shows that long-term survival is poorer if the focus of infection is unknown. This may be due to an undiscovered metastatic focus or deep infection which increases the risk of inadequate antibiotic treatment dose or duration and/or, failure to eradicate infective foci. Another explanation is that the patients die too early for the focus to be located and therefore represent a subset whose ‘unknown’ focus is a marker of the severity of illness rather than the direct cause of mortality. The association between unknown infection focus and increased mortality has been observed in some studies – at 30 days2,13 and 1 year7 follow-up – but not in others21 (at 3 month follow-up).
Our study indicates that an early clinical assessment of outcome (at Day 7) can provide some longer-term prognostic information. Patients assessed as ‘full recovery in up to 3 weeks’ had better survival than those with ‘ongoing sepsis or new morbidity’. However, this prospective assessment was subjective, based on the judgement of the clinician collecting the data at the time, with reference to the patient’s clinical and laboratory data.
Although long-term survival appeared to be better in the group with community-associated SAB compared with the group with inpatient healthcare-associated SAB on direct comparison, this difference was not significant in the multivariate model. Furthermore, when only MSSA cases were considered, there was no significant difference. In the study by Chia and colleagues22 of community-onset MSSA bacteraemia (as in our study) there was no significant difference in 12 week mortality between community-acquired and healthcare-associated MSSA SAB. Jensen and colleagues21 showed that community or hospital acquisition had no significant impact on 3 month mortality following SAB.
One limitation of our study is that there was no control group comprising hospitalised patients who did not have SAB. While other studies have clearly demonstrated that early deaths following SAB can be largely attributed to SAB, this may not necessarily be the case in deaths occurring in the longer term. We looked at all-cause mortality, not attributable mortality, in this study and, therefore, it is unclear when exactly (if ever) during the long follow-up period did the effect on mortality of having had a SAB become relatively negligible compared with the effects of advanced age or co-morbidity or the fact of hospitalisation itself. Another limitation of our study is the retrospective nature of data collection, which did not allow specific variables of interest to be prospectively defined and data collected such as co-morbidity. Co-morbidity contributes to death in patients with SAB, as shown in a prospective study with at least 3 months follow-up.34 However, some of the limitations of a retrospective design will have been offset because all of the Blood Stream Infection Surveillance Database information had been prospectively collected. Another issue is whether any deaths were missed in ACT residents because they died at a later stage outside of our hospital and out of the ACT (although we have no reason to suppose that such cases constitute large numbers). Of the 188 deaths in the study, we obtained dates of death for 180 patients from the Blood Stream Infection Surveillance Database and the hospital medical records system. The search of the ACT Register of Deaths yielded only 8 additional deaths. Our method does, however, mean that the estimates of rates of death events are conservative and therefore the actual mortality rates may be higher than what we have indicated.
In summary, high rates of death appear to continue for many months after patients have an episode of SAB. Short-term follow-up studies (30 days or less) will miss large numbers of SAB-associated deaths. On this basis, we support the call of others for longer-term data (beyond 90 days) to be taken into account in outcome studies.15 If accurate data on SAB-associated mortality is needed, then follow-up of these patients will need to be for at least 90 days, ideally performed prospectively with a matched control group consisting of hospitalised patients without SAB.
Conflicts of interest
The authors have no conflicts of interest to declare.
Funding
No funding has been received.
References
[1] Collignon P, Nimmo GR, Gottlieb T, Gosbell IB, Australian Group on Antimicrobial Resistance Staphylococcus aureus bacteremia, Australia. Emerg Infect Dis 2005; 11 554–61.| Staphylococcus aureus bacteremia, Australia.Crossref | GoogleScholarGoogle Scholar | 15829193PubMed |
[2] Mylotte JM, Tayara A. Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort. Clin Infect Dis 2000; 31 1170–4.
| Staphylococcus aureus bacteremia: predictors of 30-day mortality in a large cohort.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FotVSmtw%3D%3D&md5=4a3e3fdcfec880e1a20f948f4f98f842CAS | 11073748PubMed |
[3] Gottlieb GS, Fowler VG, Kong LK, McClelland RS, Gopal AK, Marr KA, et al Staphylococcus aureus bacteremia in the surgical patient: a prospective analysis of 73 postoperative patients who developed Staphylococcus aureus bacteremia at a tertiary care facility. J Am Coll Surg 2000; 190 50–7.
| Staphylococcus aureus bacteremia in the surgical patient: a prospective analysis of 73 postoperative patients who developed Staphylococcus aureus bacteremia at a tertiary care facility.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c%2FovVGksg%3D%3D&md5=ac9b7bd951f0d79bbb4a544f0be19307CAS | 10625232PubMed |
[4] Gopal AK, Fowler VG, Shah M, Gesty-Palmer D, Marr KA, McClelland RS, et al Prospective analysis of Staphylococcus aureus bacteremia in nonneutropenic adults with malignancy. J Clin Oncol 2000; 18 1110–5.
| 1:STN:280:DC%2BD3c7mtVOmug%3D%3D&md5=ba489992f6a381c160bd2d851f7f3e88CAS | 10694564PubMed |
[5] Wang FD, Chen YY, Chen TL, Liu CY. Risk factors and mortality in patients with nosocomial Staphylococcus aureus bacteremia. Am J Infect Control 2008; 36 118–22.
| Risk factors and mortality in patients with nosocomial Staphylococcus aureus bacteremia.Crossref | GoogleScholarGoogle Scholar | 18313513PubMed |
[6] Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med 2002; 162 2229–35.
| Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus.Crossref | GoogleScholarGoogle Scholar | 12390067PubMed |
[7] Fätkenheuer G, Preuss M, Salzberger B, Schmeißer N, Cornely OA, Wisplinghoff H, et al Long-term outcome and quality of care of patients with Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis 2004; 23 157–62.
| Long-term outcome and quality of care of patients with Staphylococcus aureus bacteremia.Crossref | GoogleScholarGoogle Scholar | 14986158PubMed |
[8] Haessler S, Mackenzie T, Kirkland KB. Long-term outcomes following infection with meticillin-resistant or meticillin-susceptible Staphylococcus aureus. J Hosp Infect 2008; 69 39–45.
| Long-term outcomes following infection with meticillin-resistant or meticillin-susceptible Staphylococcus aureus.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1c3ns1ersg%3D%3D&md5=4ab532bd2edcddf867a65e3ba3428dc3CAS | 18353493PubMed |
[9] Fang CT, Shau WY, Hsueh PR, Chen YC, Wang JT, Hung CC, et al Early empirical glycopeptide therapy for patients with methicillin-resistant Staphylococcus aureus bacteraemia: impact on the outcome. J Antimicrob Chemother 2006; 57 511–9.
| Early empirical glycopeptide therapy for patients with methicillin-resistant Staphylococcus aureus bacteraemia: impact on the outcome.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD28XhtlOgurw%3D&md5=cfa26cdb1fd690125669cd200c8e5bcaCAS | 16443700PubMed |
[10] Collignon PJ, Dreimanis DE, Beckingham WD, Roberts JL, Gardner A. Intravascular catheter bloodstream infections: an effective and sustained hospital-wide prevention program over 8 years. Med J Aust 2007; 187 551–4.
| 18021041PubMed |
[11] Australian Infection Control Association Expert Working Group. Blood stream infection (BSI) definition. Health-Care Associated Infection Advisory Committee. Canberra: Australian Council for Safety and Quality in Health Care; 2004.
[12] Kim SH, Park WB, Lee KD, Kang CI, Kim HB, Oh MD, et al Outcome of Staphylococcus aureus bacteremia in patients with eradicable foci versus noneradicable foci. Clin Infect Dis 2003; 37 794–9.
| Outcome of Staphylococcus aureus bacteremia in patients with eradicable foci versus noneradicable foci.Crossref | GoogleScholarGoogle Scholar | 12955640PubMed |
[13] Hill PC, Birch M, Chambers S, Drinkovic D, Ellis-Pegler RB, Everts R, et al Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality. Intern Med J 2001; 31 97–103.
| Prospective study of 424 cases of Staphylococcus aureus bacteraemia: determination of factors affecting incidence and mortality.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2FisVarsQ%3D%3D&md5=ca120177589388e8d0e7b88c5258fbd1CAS | 11480485PubMed |
[14] Ringberg H, Thorén A, Lilja B. Metastatic complications of Staphylococcus aureus septicemia. To seek is to find. Infection 2000; 28 132–6.
| Metastatic complications of Staphylococcus aureus septicemia. To seek is to find.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FhsFektQ%3D%3D&md5=6030c54aaf29e81cea68038f1daba5a9CAS | 10879635PubMed |
[15] Hanses F, Spaeth C, Ehrenstein BP, Linde HJ, Schölmerich J, Salzberger B. Risk factors associated with long-term prognosis of patients with Staphylococcus aureus bacteremia. Infection 2010; 38 465–70.
| Risk factors associated with long-term prognosis of patients with Staphylococcus aureus bacteremia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3M%2FjtVGitw%3D%3D&md5=19cb83ca6952c96d6e068c0d31d0f4d4CAS | 20878456PubMed |
[16] Sharma M, Szpunar S, Khatib R. Validating severity of illness scoring systems in the prediction of outcomes in Staphylococcus aureus bacteremia. Am J Med Sci 2013; [Epub ahead of print].
| Validating severity of illness scoring systems in the prediction of outcomes in Staphylococcus aureus bacteremia.Crossref | GoogleScholarGoogle Scholar |
[17] Berger J, Diab-Elschahawi M, Blacky A, Pernicka E, Spertini V, Assadian O, et al A matched prospective cohort study on Staphylococcus aureus and Escherichia coli bloodstream infections: extended perspectives beyond resistance. Am J Infect Control 2010; 38 839–45.
| A matched prospective cohort study on Staphylococcus aureus and Escherichia coli bloodstream infections: extended perspectives beyond resistance.Crossref | GoogleScholarGoogle Scholar | 20650546PubMed |
[18] de Kraker ME, Wolkewitz M, Davey PG, Grundmann H, BURDEN Study Group Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections. Antimicrob Agents Chemother 2011; 55 1598–605.
| Clinical impact of antimicrobial resistance in European hospitals: excess mortality and length of hospital stay related to methicillin-resistant Staphylococcus aureus bloodstream infections.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC38XhsFymsrs%3D&md5=fcffc82390fb9dbb0a6716a9936213baCAS | 21220533PubMed |
[19] Wolkewitz M, Frank U, Philips G, Schumaker M, Davey P, BURDEN Study Group Mortality associated with in-hospital bacteraemia caused by Staphylococcus aureus: a multistate analysis with follow-up beyond hospital discharge. J Antimicrob Chemother 2011; 66 381–6.
| Mortality associated with in-hospital bacteraemia caused by Staphylococcus aureus: a multistate analysis with follow-up beyond hospital discharge.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BC3MXnsFaitA%3D%3D&md5=aa27c96586e96834037d4e1b6fee84f7CAS | 21098543PubMed |
[20] Primo MG, Guilarde AO, Martelli CM, Batista LJ, Turchi MD. Healthcare-associated Staphylococcus aureus bloodstream infection: length of stay, attributable mortality, and additional direct costs. Braz J Infect Dis 2012; 16 503–9.
| Healthcare-associated Staphylococcus aureus bloodstream infection: length of stay, attributable mortality, and additional direct costs.Crossref | GoogleScholarGoogle Scholar | 23158266PubMed |
[21] Jensen AG, Wachmann CH, Espersen F, Scheibel J, Skinhøj P, Frimodt-Møller N. Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases. Arch Intern Med 2002; 162 25–32.
| Treatment and outcome of Staphylococcus aureus bacteremia: a prospective study of 278 cases.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD38XhtFWqurY%3D&md5=5075ce8b559d7997e4b7f4ccd9ee4a9fCAS | 11784216PubMed |
[22] Chia JW, Hsu LY, Chai LY, Tambyah PA. Epidemiology and outcomes of community-onset methicillin-susceptible Staphylococcus aureus bacteraemia in a university hospital in Singapore. BMC Infect Dis 2008; 8 14
| Epidemiology and outcomes of community-onset methicillin-susceptible Staphylococcus aureus bacteraemia in a university hospital in Singapore.Crossref | GoogleScholarGoogle Scholar | 18254979PubMed |
[23] Suryati BA, Watson M. Staphylococcus aureus bacteraemia in children: a 5-year retrospective review. J Paediatr Child Health 2002; 38 290–4.
| Staphylococcus aureus bacteraemia in children: a 5-year retrospective review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38zotlCltQ%3D%3D&md5=ea11a03530eeb5e230a453eecc20060aCAS | 12047699PubMed |
[24] Hill PC, Wong CG, Voss LM, Taylor SL, Pottumarthy S, Drinkovic D, et al Prospective study of 125 cases of Staphylococcus aureus bacteremia in children in New Zealand. Pediatr Infect Dis J 2001; 20 868–73.
| Prospective study of 125 cases of Staphylococcus aureus bacteremia in children in New Zealand.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD38%2FivFGmug%3D%3D&md5=e295f6a205458249a39a851fa3b63873CAS | 11734766PubMed |
[25] Robinson JO, Pearson JC, Christiansen KJ, Coombs GW, Murray RJ. Community-associated versus healthcare-associated methicillin-resistant Staphylococcus aureus bacteraemia: a 10-year retrospective review. Eur J Clin Microbiol Infect Dis 2009; 28 353–61.
| Community-associated versus healthcare-associated methicillin-resistant Staphylococcus aureus bacteraemia: a 10-year retrospective review.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD1M3ksF2rtA%3D%3D&md5=b698401ac4ca72e84d28a7fb6cc25593CAS | 18850122PubMed |
[26] Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 2003; 36 53–9.
| Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 12491202PubMed |
[27] Lodise TP, McKinnon PS, Swiderski L, Rybak MJ. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis 2003; 36 1418–23.
| Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia.Crossref | GoogleScholarGoogle Scholar | 12766837PubMed |
[28] Roghmann MC. Predicting methicillin resistance and the effect of inadequate empiric therapy on survival in patients with Staphylococcus aureus bacteremia. Arch Intern Med 2000; 160 1001–4.
| Predicting methicillin resistance and the effect of inadequate empiric therapy on survival in patients with Staphylococcus aureus bacteremia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3c3it1KjsQ%3D%3D&md5=8e3b08789b1fcb3cfcb86a58f229cc96CAS | 10761966PubMed |
[29] Kim SH, Park WB, Lee KD, Kang CI, Bang JW, Kim HB, et al Outcome of inappropriate initial antimicrobial treatment in patients with methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 2004; 54 489–97.
| Outcome of inappropriate initial antimicrobial treatment in patients with methicillin-resistant Staphylococcus aureus bacteraemia.Crossref | GoogleScholarGoogle Scholar | 1:CAS:528:DC%2BD2cXnt1Witrw%3D&md5=f926cd404db581c04b9469aa6cd9599bCAS | 15254028PubMed |
[30] Turnidge JD, Kotsanas D, Munckhof W, Roberts S, Bennett CM, Nimmo GR, et al Staphylococcus aureus bacteraemia: a major cause of mortality in Australia and New Zealand. Med J Aust 2009; 191 368–73.
| 19807625PubMed |
[31] Jenkins TC, Price CS, Sabel AL, Mehler PS, Burman WJ. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46 1000–8.
| Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia.Crossref | GoogleScholarGoogle Scholar | 18444816PubMed |
[32] Fowler VG, Sanders LL, Sexton DJ, Kong L, Marr KA, Gopal AK, et al Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27 478–86.
| Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients.Crossref | GoogleScholarGoogle Scholar | 9770144PubMed |
[33] Robinson JO, Pozzi-Langhi S, Phillips M, Pearson JC, Christiansen KJ, Coombs GW, et al Formal infectious diseases consultation is associated with decreased mortality in Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 2012; 31 2421–8.
| Formal infectious diseases consultation is associated with decreased mortality in Staphylococcus aureus bacteraemia.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC38vltVKjtg%3D%3D&md5=a41905f3db255bddc2a767ea9c68a24eCAS | 22382823PubMed |
[34] Lesens O, Methlin C, Hansmann Y, Remy V, Martinot M, Bergin C, et al Role of comorbidity in mortality related to Staphylococcus aureus bacteremia: a prospective study using the Charlson weighted index of comorbidity. Infect Control Hosp Epidemiol 2003; 24 890–6.
| Role of comorbidity in mortality related to Staphylococcus aureus bacteremia: a prospective study using the Charlson weighted index of comorbidity.Crossref | GoogleScholarGoogle Scholar | 14700403PubMed |

