A unified approach to infection control: hand hygiene as the entrance door
Didier Pittet A B C and Benedetta Allegranzi BA Infection Control Program, University of Geneva Hospitals and Faculty of Medicine, 1211 Geneva 14, Switzerland.
B World Health Organization World Alliance for Patient Safety, WHO Headquarters, 1211 Geneva 27, Switzerland.
C Corresponding author. Email: didier.pittet@hcuge.ch
Healthcare Infection 13(2) 25-28 https://doi.org/10.1071/HI08014
Published: 18 June 2008
In modern healthcare settings in developed countries, healthcare-associated infections (HAI) are clearly identified as one of the major threats to patient safety, and their epidemiologic profile and impact have been extensively described in terms of morbidity, mortality and costs.1,2 Successful implementation of infection prevention measures has therefore become one of the key indicators of quality of care, reflecting an important evolution of the concept of infection control.3 The true magnitude of the problem worldwide remains unknown, but considering both developed and developing countries, it is likely to be more devastating than other major diseases that afflict humanity.4 In countries with limited resources, the reality of unsafe care is a hidden problem because of the lack of data available and results from malfunctioning health systems with deficiencies at all levels: from defective knowledge dissemination, to lack of infrastructure and equipment, to understaffing and overcrowding.5 To ensure minimum standards for infection control in these settings, deficits in care must be tackled at their roots and actions undertaken at the most basic level. In contrast, in developed countries, targets for infection control have been pitched at a substantially higher level, not only to keep pace with a more sophisticated and complex medicine, but also because of the need to move forward from well-established achievements.6
The minimum requirements for infection control consist of the optimal implementation of standard measures during routine care, followed by the addition of specific precautions according to the transmission route of the potential or identified infectious agent.7,8 Nevertheless, beyond recommended precautions, modern infection control has become a much broader concept that needs to be firmly integrated into an institutional safety culture and visibly supported by senior management and a solid infrastructure.9
Prevention must be guided by the measurement of indicators that identify gaps and highlight the most appropriate solutions. This is known as the ‘recognize–explain–intervene’ concept, which originated in the very early days of Ignaz Semmelweis, one of the 19th century pioneers of infection control, and was validated for the first time on a large scale by the results of the Study on Efficacy of Nosocomial Infection Control (SENIC) project conducted in hospitals in the USA in the 1980s.10 The study identified the cornerstones of a successful infection control program (key infection prevention measures, infection control professionals, HAI surveillance), which were then adopted by most hospitals across the developed world. Out of these basic requirements, a remarkable and fascinating evolution of the concept of infection control has occurred over the past two decades.6,11 The composition of the infection control team has changed, with a recommended increase in the number of infection control nurses and physicians required, and an expansion to a multidisciplinary approach, including different professionals such as clinical microbiologists, hospital engineers, data managers, epidemiologists and administrative staff. The building of such a team in an institution necessarily requires strong support from senior managers who should not only provide adequate resources, but also demonstrate a visible commitment to integrate infection control as part of an inherent, institutional safety culture, and actively promote the improvement of individual healthcare worker behaviour, work environment and infrastructure. As a result, infection control committees should no longer function as a static entity but rather act as a catalyst to promote the integration of the daily work of the infection control team within the institutional plans and management of other in-house key players, especially with regard to staff education and policy development. Antimicrobial stewardship is a good example of an innovative strategy that involves the institution as a whole, being based on the integration of different competencies and requiring strong management support.
New issues also challenge surveillance activities such as the need for the standardisation of diagnostic methods and adjustment for population case mix.12,13 For these reasons, in many cases, measurement of success has switched from outcome indicators, such as HAI rates, to other indicators such as structure (e.g. alcohol-based hand rub available at the point of care) and process (e.g. hand hygiene compliance), which are easier to monitor and are a valid alternative to guide prevention approaches. For example, audits using checklists to assess if correct procedures and equipment are in place have become a very useful monitoring tool contributing to limit the occurrence of HAI.14
An essential pragmatic element of a modern infection control approach is the development of effective strategies to translate scientific, evidence-based guidelines or protocols and expert consensus opinion into practice. One of the most common actions initiated by infection control committees at hospital and national levels, as well as by international societies and institutions, is the preparation of scientific guidelines. Nevertheless, parallel efforts to develop a related implementation strategy to support guideline usage are extremely rare. Even leading organisations such as the World Health Organization (WHO) have been criticised for neglecting this aspect when issuing guidelines.15 However, successful examples of multimodal implementation strategies (sometimes called ‘bundles’) have been recently published, thus bringing a novel approach to guideline dissemination. For instance, in a research project carried out in the intensive care unit at the University of Geneva Hospitals in Switzerland, the incidence of catheter exit site infection and catheter-related bloodstream infection decreased by more than 60% following a multimodal intervention based on education, hand hygiene improvement, locally tailored guidelines including evidence-based recommendations, catheter duration minimisation and prompt removal when sepsis is suspected.16 A follow-up study revealed a sustained 90% reduction after 5 years.17 A similar approach, broader in scope, was applied in a multicentre study involving 103 intensive care units in Michigan, USA, and led to a significant reduction of overall catheter-related bloodstream infection rates from 7.7 to 1.4 episodes per 1000 catheter-days up to 18 months of follow-up.18 In addition to the previously cited successful components, the peculiarities of this model were the building of a strong patient safety climate, commitment by team leaders, creation of a central line chart and the use of a checklist for infection control essential measures, with interruption of the procedure in case of defective compliance. Checklists for process indicator monitoring are now considered not only an audit tool but also an effective instrument to foster better compliance with preventive measures and standard operating protocols.
The University of Geneva Hospitals were also a fertile environment to demonstrate the success of a multifaceted strategy that has changed the approach to hand hygiene promotion in healthcare worldwide, now known as the ‘Geneva model’. Since the 1990s, this model has demonstrated its capacity to produce a significant and sustained reduction of HAI rates, associated with behavioural change and containment of costs.19–21 As the single most important measure to prevent HAIs, WHO has considered hand hygiene as the entrance door for better infection control in healthcare settings.22 Hand hygiene is indeed a transversal measure, at the basis of both standard and transmission-based precautions, and is essential to limit the spread of very diverse pathogens. Moreover, the concentration of efforts catalysed around the implementation of a hand hygiene campaign in a healthcare setting can be the starting point for further improvement and the subsequent engagement in other infection control initiatives. Nevertheless, as highlighted by Bastian et al. in this issue of the Journal,23 an innovative and captivating approach must be proposed to improve the practice of hand hygiene, an infection control measure known since antiquity but still inadequately carried out.
Hand hygiene is the essential component of the First Global Patient Safety Challenge ‘Clean Care is Safer Care’, one of the flagship programs of the WHO World Alliance for Patient Safety, aimed at reducing the burden of HAI worldwide.1,24,25 Endeavouring to reach beyond hand hygiene, the program has also focussed attention on the promotion of clean care practices in their broadest sense, in particular those related to already established WHO programs around injection and immunisation safety (clean equipment), emergency and surgical procedure safety (clean procedures), blood transfusion safety (clean products), and safe water and sanitation (clean environment).1 As evidenced from other papers in this issue of the Journal, these are other essential areas of work in the field of infection control.
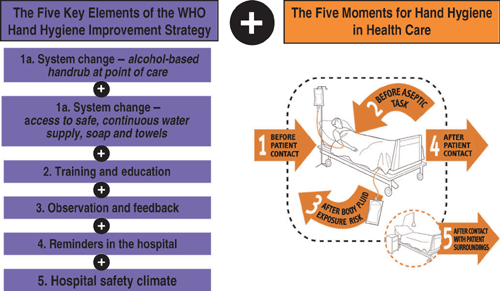
To provide healthcare workers with the best available evidence for its efficacy and to foster hand hygiene improvement on a global scale, the WHO recently issued the Guidelines for Hand Hygiene in Health Care.26 In the wake of the ‘Geneva model’, which has been further validated on a larger scale in Switzerland as well as by single institutions worldwide27 and other national campaigns, a stepwise implementation strategy and a practical toolkit have been developed by WHO to assist regions, countries and facilities in the development of hand hygiene improvement initiatives based on the WHO guidelines. The central element of the strategy is the promotion of alcohol-based handrubs at the point of care as an international standard.26,28 This implies the achievement of a system change that should include the continuous access to products and equipment to make optimal hand hygiene possible (Figure 1). In addition, the strategy consists of four other evidence-based components: (i) staff education; (ii) observation of hand hygiene practices and performance feedback; (iii) reminders (posters and other visual material) at the work place; and (iv) the creation of an institutional safety climate (Figure 1). The educational approach is strengthened by a novel concept of the hand hygiene indications, the newly developed ‘Five Moments for Hand Hygiene’(Figure 1), incorporating social marketing, human factors and the science behind hand hygiene compliance.29 It proposes a unified vision for trainers, observers and healthcare workers to facilitate the understanding of why and when hand hygiene is needed within the healthcare flow. The final aims are to minimise inter-individual variation and lead to a global increase of adherence to correct hand hygiene practices. Although many studies have demonstrated that success relies on the integration and additive effect of these different elements, further research is needed to determine the relative effectiveness of each component of a successful hand hygiene promotion strategy and to identify those most clearly associated with a sustained effect. Additional elements are currently undergoing testing, in particular patient participation.
|
Through the First Global Patient Safety Challenge, it has been possible to mobilise countries, organisations and individuals to help achieve the cleanest and safest possible healthcare. Technical efforts to produce scientifically sound recommendations and propose practical solutions would have no value if not accompanied by actions to raise high-level political commitment and secure the involvement of leading stakeholders in the field of patient safety and infection control. So far, 83 ministries of health have signed a statement of commitment/pledge outlining an intention to take action to address the problem of HAI within their respective countries. Among various other initiatives, this has led to the launch of more than 20 national/subnational hand hygiene campaigns worldwide, including Australia.
Leadership at different political levels, implementation of basic infection control measures and easy and continuous access to preventive equipment and supplies by healthcare workers are the essential elements for improvement identified by key opinion leaders in the field of infection control in Australia.30 Indeed, Collignon and coauthors have urged their colleagues to follow existing guidelines rather than inventing new recommendations and have warned them to never settle for mediocre hand hygiene compliance rates, even as high as 50%.30
Lessons learned from Victoria are indeed promising: system and culture changes, especially related to hand hygiene practices, led to significant reductions in hospital-wide rates of total clinical methicillin-resistant Staphylococcus aureus (MRSA) isolates (40% reduction) and of episodes of MRSA bacteraemia (57% reduction).27 These results, first achieved at the Austin Health in Melbourne,27 have motivated the expansion of the strategy to the entire state of Victoria and have led the authors to advocate a massive infection control campaign at the national level, with hand hygiene as the cornerstone. A perfect example of how hand hygiene can become the entrance door for better infection control, as suggested by the WHO World Alliance for Patient Safety!
[1]
[2] Wenzel RP. Health care-associated infections: major issues in the early years of the 21st century. Clin Infect Dis 2007; 45 S85–8.
| Crossref | GoogleScholarGoogle Scholar | PubMed | [accessed April 2008].
[9] Allegranzi B, Storr J, Dziekan G, Leotsakos A, Donaldson L, Pittet D. The first global patient safety challenge “clean care is safer care”: from launch to current progress and achievements. J Hosp Infect 2007; 65 115–23.
| Crossref | GoogleScholarGoogle Scholar | [verified May 2008].
[15] Oxman AD, Lavis JN, Fretheim A. Use of evidence in WHO recommendations. Lancet 2007; 369 1883–9.
| Crossref | GoogleScholarGoogle Scholar | [verified May 2008].
[27] Johnson PD, Martin R, Burrell LJ, Grabsch EA, Kirsa SW, O’Keeffe J, et al. Efficacy of an alcohol/chlorhexidine hand hygiene program in a hospital with high rates of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection. Med J Aust 2005; 183 509–14.

[28] Pittet D, Boyce JM. Hand hygiene and patient care: pursuing the Semmelweis legacy. Lancet Infect Dis 2001; April 9–20.

[29] Sax H, Allegranzi B, Uçkay I, Larson E, Boyce J, Pittet D. “My five moments for hand hygiene” – a user-centred design approach to understand, train, monitor and report hand hygiene. J Hosp Infect 2007; 67 9–21.
| Crossref | GoogleScholarGoogle Scholar |

[30] Collignon PJ, Grayson ML, Johnson PD. Methicillin-resistant Staphylococcus aureus in hospitals: time for a culture change. Med J Aust 2007; 187 4–5.


