Self-reported participation and beliefs about bowel cancer screening in New South Wales, Australia
Megan Varlow A , Ingrid Stacey A , Sally Dunlop A B C , Jane Young A B , James Kite A , Anita Dessaix A and Claire McAulay BA Cancer Institute NSW, PO Box 41, Alexandria, NSW 1435, Australia.
B Sydney School of Public Health, The University of Sydney, Edward Ford Building (A27), NSW 2006, Australia.
C Corresponding author. Email: sally.dunlop@sydney.edu.au
Health Promotion Journal of Australia 25(2) 97-103 https://doi.org/10.1071/HE13102
Submitted: 14 November 2013 Accepted: 11 April 2014 Published: 14 July 2014
Abstract
Issue addressed: To describe self-reported bowel cancer screening participation, beliefs and attitudes in a sample of New South Wales (NSW) adults, and to identify beliefs and demographic factors associated with self-reported bowel cancer screening participation.
Methods: This study used data from the International Cancer Benchmarking Partnership Module 2, a representative population-based telephone survey. Self-reported participation in and beliefs about bowel cancer screening were measured using the Awareness and Beliefs about Cancer survey of people aged 50 years and over living in NSW, Australia (n = 2001). Logistic regression modelling was used to identify explanatory variables associated with bowel cancer screening participation.
Results: Half of all women (54.1%, 95% CI: 50.8–57.4%) and two-thirds of men (65.7%, 95% CI: 61.5–69.9%) reported screening for bowel cancer within the previous 5 years. Believing that screening was only necessary when experiencing symptoms was more likely to be endorsed by people aged 65 years and over (25.5%, 95% CI: 22.2–28.7%) rather than younger (50–64 years; 16.7%, 95% CI: 13.8–19.7%), non-English-speaking migrants (35.4%, 95% CI: 26.7–44.1%) versus others (18.6%, 95% CI: 16.4–20.7%), and people in metropolitan (23.3%, 95% CI: 20.4–26.1%) versus non-metropolitan areas (16.4%, 95% CI: 12.8–20%). People who disagreed that screening was only necessary when experiencing symptoms were four times more likely to report screening participation (OR 3.96, 95% CI: 3.11–5.03).
Conclusions: Community education about bowel cancer screening is needed to correct misperceptions regarding screening in the absence of symptoms. Tailored strategies for older, migrant and urban communities may be beneficial.
So what?: Education strategies that promote the need for screening in the absence of symptoms and correct misconceptions about bowel cancer screening amongst subgroups of the NSW population may improve screening rates and decrease the burden of bowel cancer in NSW.
Key words: bowel cancer screening, colorectal cancer, health beliefs, International Cancer Benchmarking Partnership.
Introduction
Bowel (or colorectal) cancer presents a significant burden on health and health care in the Australian state of New South Wales (NSW). It is one of the most common cancers diagnosed in NSW, ranking second in both incidence and mortality.1 It has been predicted that the burden will increase over time, with recent forecasts that 1 in 10 males and 1 in 15 females in NSW will develop bowel cancer by 85 years of age.1
When detected early, the majority of bowel cancers can be successfully treated,2 and randomised trials demonstrate that screening with faecal occult blood tests (FOBT) reduces mortality from bowel cancer.3–6 Implementation of population screening in Australia using FOBT commenced in 2006 (with a pilot program 2002–04) via the National Bowel Cancer Screening Program (NBCSP). The NBCSP offers screening with FOBT to individuals turning 50, 55 and 65 years of age in any given year.7 However, it is also possible for individuals to be screened outside of the NBCSP by purchasing a FOBT kit and completing the test independently, or to be screened by direct referral for a colonoscopy or sigmoidoscopy. Before population screening was introduced, an analysis of bowel cancer cases diagnosed in South Australia found that 15% of cancers were detected at an early and treatable stage.8 Subsequent analysis of bowel cancer cases diagnosed between 2006 and 2008 (after the introduction of population screening) found that nearly triple the number of early-stage cancers were detected by screening (40%) compared with those diagnosed through symptoms (14%).9
The success of cancer screening depends on high participation rates; however, participation is often less than optimal.10 The Hunter Community Study reports that 63% of NSW adults aged 55–85 years had participated in some form of bowel cancer screening, with 43% having completed a FOBT, 30% having had a colonoscopy and 7% having had a sigmoidoscopy.11 Internationally, barriers to screening that have been identified include poor cancer awareness12,13 and negative beliefs and attitudes about bowel cancer and screening procedures.14,15 Cultural factors,16,17 lower education levels, and rurality or low socio-economic status13,17,18 have also been associated with lower rates of screening participation.
There are few studies that have examined the demographics, beliefs and attitudes specifically associated with bowel cancer screening participation in Australia. A Victorian study found that being in an early stage of readiness to screen by FOBT or colonoscopy (i.e. being less likely to participate) is associated with being female, of younger age and speaking a language other than English at home.19 The 45 and Up Study (NSW) has recently reported that lower levels of education are associated with lower levels of bowel cancer screening and has suggested targeting negative attitudes towards screening to improve participation; however, this was within the context of multiple screening participation across different cancer types.20 A more recent analysis of data from this study showed that lower education, lower income, not speaking English at home, and a variety of unhealthy lifestyle behaviours are associated with decreased likelihood of screening.21 Two studies from South Australia measuring beliefs about bowel cancer screening include a qualitative study that found that barriers and beliefs varied across five culturally distinct groups22 and a factor analysis that found that perceived barriers to and benefits of screening predict participation in FOBT screening.15 A study from Queensland has identified that intention to screen is associated with beliefs that screening is necessary for people without symptoms and belief that early detection of bowel cancer results in increased survival.23 To date, there has been no population-level study investigating the association between self-reported bowel cancer screening and beliefs specifically relating to bowel cancer screening in a representative sample of Australian adults.
This study had two aims: (1) to describe self-reported bowel cancer screening participation and beliefs and attitudes about bowel cancer screening in a sample of NSW adults aged 50 years and over, and (2) to identify beliefs and demographic factors associated with self-reported bowel cancer screening participation. Specifically, we drew on elements of the Health Belief Model24,25 to identify beliefs associated with participation in bowel cancer screening. The Health Belief Model is a psychological theory of behaviour change which attributes changes in health-promoting behaviour to beliefs about the health issue, perceived barriers to action, perceived benefits of action, and self-efficacy.
Methods
This study was approved by the NSW Population and Health Services Research Ethics Committee.
Overview
This study used data from the International Cancer Benchmarking Partnership (ICBP) Module 2 Awareness and Beliefs about Cancer (ABC) measure administered by telephone interview. ICBP Module 2 aimed to provide an international comparison of the attitudes and beliefs about cancer held by the general public in Australia (Victoria and NSW), Canada, Denmark, Sweden, Norway and the UK. The development, validation and structure of the ABC survey has been described previously.26 In NSW, additional questions about bowel cancer screening beliefs and self-reported participation were included in the ABC survey; these methods are described here in detail because they formed the basis of the present study.
Measures
The NSW version of the questionnaire included four questions relevant to bowel cancer screening. Self-reported screening participation was measured with the question: ‘Have you had a test to detect bowel cancer in the past 5 years?’ A description of the FOBT bowel cancer screening method was only read to the respondent if requested (no other test was described to participants). Respondents were asked to answer either ‘yes’ or ‘no’, with ‘don’t know’ and refusal responses recorded where necessary.
Beliefs about bowel cancer screening were assessed by asking respondents to rate their level of agreement (1 = ‘strongly disagree’ to 4 = ‘strongly agree’) with the following statements: ‘I would be so worried about what might be found from bowel cancer screening that I would prefer not to do it’ (perceived barrier),‘Bowel cancer screening could reduce my chances of dying from bowel cancer’ (perceived benefit) and ‘Bowel cancer screening is only necessary if I have symptoms’ (understanding of screening). Responses to these variables were highly skewed and so were collapsed into binary variables indicating agreement (‘strongly agree’ or ‘tend to agree’) with each statement.
Demographic information was also collected, including age, sex, language spoken at home, country of birth, relationship status, smoking status, own experience of cancer, self-rated health status and level of education. Residential postcodes were used to determine metropolitan or non-metropolitan locality and were matched to an index of relative socio-economic disadvantage and then collapsed into two categories for analysis (quintiles 4–5 = disadvantaged, quintiles 1–3 = advantaged).27 A culturally and linguistically diverse indicator variable was derived from the language spoken at home and country of birth information, where people born overseas who were also non-English speaking were considered to be ‘culturally and linguistically diverse’, whereas Australian-born and/or English-speaking people were not.
Data collection
The telephone survey was administered between May and September 2011 to adults living in NSW aged 50 years and older (n = 2001). Telephone numbers were obtained from a commercially available electronic listing of landline telephone numbers in NSW and a number-replacement procedure was used to bring unlisted numbers into the sampling frame. The Rizzo method was used to randomly select a respondent when there was more than one eligible adult in a household.28 The American Association for Public Opinion Research response rate (formula 3) was 48%.29
Analysis
Where indicated, responses were weighted using design weights and population weights; comparison of proportions was done using Chi-square tests. Population characteristics for which weights were applied included age, sex, tertiary education status, metropolitan (the areas in and around Sydney, Newcastle and Wollongong) versus non-metropolitan residence and country of birth. Details of the weighting method have been provided elsewhere.30
Logistic regression analysis using backward stepwise elimination was used to model self-reported bowel cancer screening with beliefs about screening and demographic factors as explanatory variables. A cut-off of P < 0.05 was used for the step-wise elimination procedure, and a Hosmer–Lemeshow goodness-of-fit test was used to assess the final model fit. Analyses were conducted using SAS ver. 9.2 (weighted frequencies using the PROC SURVEYFREQ procedure) and Stata ver. 11 (logit command).
Results
Demographic characteristics of the sample are shown in Table 1.
Overall, 1187 out of 2001 respondents reported having had a bowel cancer screening test in the previous 5 years. After weighting for sampling and response distributions, the estimate for the NSW population was 60% (95% CI: 57–62%). Males, people aged 65 years and older, and people who had previously been diagnosed with cancer themselves were significantly more likely to report having been screened in the last 5 years (Table 1).
There was substantial variation among respondents in beliefs about bowel cancer screening (Table 1). People who had not completed tertiary education or who were from the most socioeconomically disadvantaged areas of NSW were significantly more likely to agree that they would be so worried about the results of bowel cancer screening that they would prefer not to do it. People who were aged 65 years and older, those who were born overseas and did not speak English as their main language at home, and people living in the metropolitan area were more likely to agree that bowel cancer screening was only necessary if they had symptoms. People aged 50–64 years and those born in Australia and/or who spoke English as their main language at home were significantly more likely to agree that bowel cancer screening could reduce their chances of dying from bowel cancer.
Table 2 shows the results from the logistic regression model predicting participation in screening based on the three bowel cancer screening beliefs, controlling for demographic covariates. Each of the beliefs about bowel cancer screening was found to be significant and independent factors associated with self-reported screening participation (Table 2).
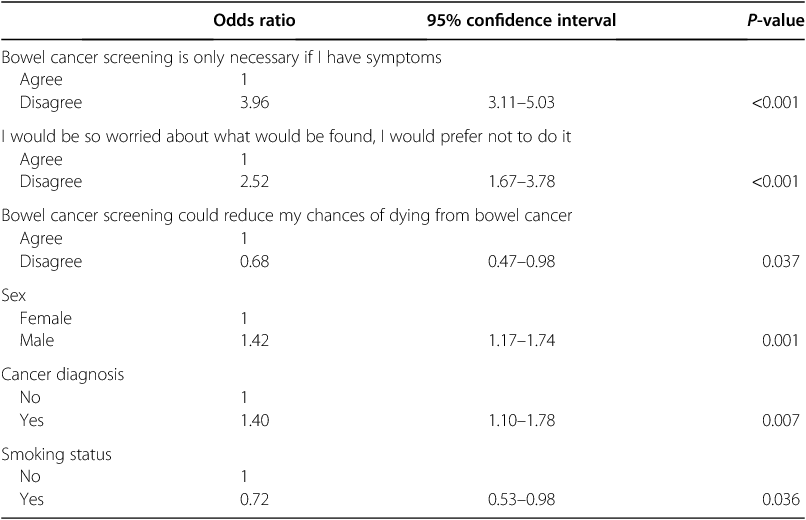
|
Discussion
This study found variations in bowel cancer screening beliefs and behaviours within the NSW population. Males, those who had a previous cancer diagnosis, and non-smokers were more likely to report participation in bowel cancer screening. In general, older people, the disadvantaged and migrants were more likely to endorse negative or incorrect beliefs, and these beliefs were found to be negatively associated with self-reported participation in screening.
The self-reported bowel cancer screening participation rates for NSW estimated by this study (53.7% for females and 65.6% for males) were higher than those reported in the NBCSP (41.2% for females and 36.0% for males).12 However, our estimates were for a 5-year period (compared with the NBCSP, which was for a 1-year period following a single invitation to screen) and possibly includes non-FOBT screening, such as colonoscopy and sigmoidoscopy.11 Additionally, a recent South Australian study examining intentions to maintain adherence to FOBT rescreening reported that 70% of participants are ‘re-screeners’31, which would contribute to the larger estimate of screening prevalence in the current study. Men reported higher levels of screening participation than women in the present study, which is opposite to the actual screening participation reported by the NBCSP7 but consistent with other self-reported participation studies.11
Knowledge gaps were evident in respondents who were born overseas and spoke a language other than English at home. These respondents were nearly twice as likely to agree that screening was only necessary when symptoms are noticed and were significantly less likely to agree that screening could decrease their chances of dying from bowel cancer. This observation is supported by similar findings in other Australian states.15,22,23 These results may go some way to explaining the lower screening uptake in migrant groups, and support the growing body of Australian literature calling for targeted interventions to these groups to help to alleviate these disparities.13,21 While participation in screening was not predicted in our study by membership of a culturally or linguistically diverse group alone, there have been several studies asserting that inequalities do exist and that bowel cancer screening is disproportionately adopted by Caucasian or English-speaking (Anglo-Saxon) Australians.18
Our findings are generally consistent with and build upon previous findings related to bowel cancer screening participation. A study that investigated the Transtheoretical Model of Behaviour Change found that people in the earliest stages of readiness to participate in bowel cancer screening are generally female, younger and speak a language other than English,19 which is supported by the prevalence of beliefs associated with actual participation measured in our study. Results from the 45 and Up study found that low levels of education are specifically associated with low levels of bowel cancer screening.20 Similarly, we found completion of tertiary education to be associated with endorsement of a belief strongly associated with participation. The same study reported that people in regional or remote areas are more likely to be screened for bowel cancer, which is supported by the prevalence of beliefs associated with participation measured in our study.
In the current study, the three beliefs investigated were all associated with participation in bowel cancer screening, and provide some guidance as to potential education and communication opportunities for improving population involvement in screening. In accordance with the Health Belief Model,24 an individual’s perceived benefit of bowel cancer screening was associated with participation. Conversely, a lack of understanding about screening (believing that screening is only necessary if you have symptoms) and cancer worry were identified as barriers. These findings add support to the growing evidence regarding barriers to colorectal cancer screening32–35 and suggest that reducing these barriers might go some way to improving participation rates. Evidence indicates that these barriers might be reduced by using physician-led communications to raise awareness about the importance and efficacy of bowel screening, as well as educating the public about the processes involved in screening.36,37 The role of public communication in educating the public and reducing cancer worry with regard to bowel cancer screening has not yet received much attention in the literature, though evidence from cervical cancer screening promotion indicates that public communication campaigns can be effective in encouraging uptake of screening services.38
The results of the current study need to be considered in relation to the limitations. Given the cross-sectional nature of the study design, we cannot make any conclusions about cause and effect. It is possible that the people in our survey who had participated in screening could have developed more positive or accurate beliefs about screening as a result of the screening experience. However, health promotion theories such as the Health Belief Model39 posit that knowledge and beliefs are precursors for behaviour, making investigation of associations between beliefs and behaviour useful nonetheless. The administration of this survey in English means that culturally and linguistically diverse groups were likely to have been undersampled, resulting in a bias towards underestimation of the effect of this factor on bowel screening participation. The use of landline phone numbers only may also have resulted in selection bias, but given the target age group for the survey, this effect is likely to have been minimal given that the majority of individuals living in mobile-phone-only households are younger. This study was limited by measuring self-reported bowel cancer screening only, which relies on the participant understanding what bowel cancer screening is, or asking for further information if they are unsure. It is possible that tests such as digital rectal examination or other physical examinations may have been mistakenly recalled as bowel cancer screening, resulting in over-reporting of screening participation rates. We do not believe this to be an issue for this study however, since meta-analysis has demonstrated that self-report has high specificity and sensitivity for bowel cancer screening participation recall.40
Together these findings suggest that health promotion programs that successfully address beliefs and misconceptions about the efficacy and processes involved in bowel cancer screening have the potential to improve screening behaviour. To date, NSW has not conducted a population-level campaign regarding bowel cancer awareness or bowel cancer screening. A state-wide campaign was implemented in 2009, using mass media to target NSW residents aged 50 years and older, but this campaign has not been followed up with any additional interventions, and the NBCSP is not supported by a public education campaign or similar activities. The present study highlights that there is scope for further research to determine whether public education or other forms of public health interventions might be effective in changing unhelpful beliefs and attitudes about bowel cancer screening.
Conclusion
Our results suggest there is a need to correct misconceptions amongst subgroups of the NSW population regarding bowel cancer screening. These findings provide a theme for future education strategies to tailor or target messages to these specific groups in order to encourage screening participation.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
The authors would like to acknowledge the Module 2 central team in the UK, who led the design of the ABC measure and the survey: Professor Jane Wardle (University College London), Professor Amanda Ramirez and Dr Lindsay Forbes (King’s College London), and Dr Alice Simon (City University London). Ingrid Stacey was supported by the NSW Biostatistical Officer Training Program conducted by the NSW Ministry of Health.
References
[1] Tracey E, Kerr T, Dobrovic A, Currow D. Cancer in NSW: incidence and mortality report 2008. Sydney: Cancer Institute NSW; 2010.[2] Smith RA, von Eschenbach AC, Wender R, Levin B, Byers T, Rothenberger D, et al (2001) American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001 – testing for early lung cancer detection. CA Cancer J Clin 51, 38–75.
| American Cancer Society guidelines for the early detection of cancer: update of early detection guidelines for prostate, colorectal, and endometrial cancers. Also: update 2001 – testing for early lung cancer detection.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3MrjtFGisw%3D%3D&md5=cc7b5024b46bbd7abd5d38a8cc7c471bCAS | 11577479PubMed |
[3] Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F (1993) Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 328, 1365–71.
| Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK3s3jtlOhtQ%3D%3D&md5=b0e68f635cbdb24b61e8f5ba90c553f4CAS | 8474513PubMed |
[4] Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM (1996) Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 348, 1472–7.
| Randomised controlled trial of faecal-occult-blood screening for colorectal cancer.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FpvVagtg%3D%3D&md5=de8cf465ac710e768a5b3f39bd614c5aCAS | 8942775PubMed |
[5] Kronborg O, Fenger C, Olsen J, Jorgensen OD, Sondergaard O (1996) Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 348, 1467–71.
| Randomised study of screening for colorectal cancer with faecal-occult-blood test.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DyaK2s%2FpvVagsQ%3D%3D&md5=804f959ab21359051f04e9a5826f0553CAS | 8942774PubMed |
[6] Hewitson P, Glasziou P, Irwig L, Towler B, Watson E (2007) Screening for colorectal cancer using the faecal occult blood test, Hemoccult. Cochrane Database Syst Rev CD001216
[7] AIHW. National Bowel Cancer Screening Program monitoring report: phase 2, July 2008–June 2011. Cancer series no. 65. Cat. no. CAN 61. Canberra: AIHW; 2012. Available from http://www.aihw.gov.au/publication-detail/?id=10737421408 [Verified 12 June 2014]
[8] Australian Health Technology Advisory Committee, AGPS. Colorectal cancer screening: a report of the Australian Health Technology Advisory Committee (pp. 1–147). Canberra: Publications Production Unit, Australian Commonwealth Department of Health and Family Services; 1997.
[9] Ananda SS, McLaughlin SJ, Chen F, Hayes IP, Hunter AA, Skinner IJ, et al (2009) Initial impact of Australia’s National Bowel Cancer Screening Program. Med J Aust 191, 378–81.
[10] Subramanian S, Klosterman M, Amonkar MM, Hunt TL (2004) Adherence with colorectal cancer screening guidelines: a review. Prev Med 38, 536–50.
| Adherence with colorectal cancer screening guidelines: a review.Crossref | GoogleScholarGoogle Scholar | 15066356PubMed |
[11] Courtney RJ, Paul CL, Sanson-Fisher RW, Macrae FA, Carey ML, Attia JR, McEvoy MA (2012) Colorectal cancer screening in Australia: a community-level perspective. Med J Aust 196, 516–20.
| Colorectal cancer screening in Australia: a community-level perspective.Crossref | GoogleScholarGoogle Scholar | 22571309PubMed |
[12] Ait Ouakrim D, Boussioutas A, Lockett T, Winship I, Giles GG, Flander LB, Keogh L, Hopper JL, Jenkins MA (2012) Screening practices of unaffected people at familial risk of colorectal cancer. Cancer Prev Res (Phila) 5, 240–7.
| Screening practices of unaffected people at familial risk of colorectal cancer.Crossref | GoogleScholarGoogle Scholar | 22030089PubMed |
[13] Ward PR, Javanparast S, Wilson C (2011) Equity of colorectal cancer screening: which groups have inequitable participation and what can we do about it? Aust J Prim Health 17, 334–46.
| Equity of colorectal cancer screening: which groups have inequitable participation and what can we do about it?Crossref | GoogleScholarGoogle Scholar | 22112702PubMed |
[14] Cole SR, Zajac I, Gregory T, Mehaffey S, Roosa N, Turnbull D, Esterman A, Young GP (2011) Psychosocial variables associated with colorectal cancer screening in South Australia. Int J Behav Med 18, 302–9.
| Psychosocial variables associated with colorectal cancer screening in South Australia.Crossref | GoogleScholarGoogle Scholar | 20496170PubMed |
[15] Gregory TA, Wilson C, Duncan A, Turnbull D, Cole SR, Young G (2011) Demographic, social cognitive and social ecological predictors of intention and participation in screening for colorectal cancer. BMC Public Health 11, 38
| Demographic, social cognitive and social ecological predictors of intention and participation in screening for colorectal cancer.Crossref | GoogleScholarGoogle Scholar | 21232156PubMed |
[16] Christou A, Katzenellenbogen JM, Thompson SC (2010) Australia’s national bowel cancer screening program: does it work for indigenous Australians? BMC Public Health 10, 373
| Australia’s national bowel cancer screening program: does it work for indigenous Australians?Crossref | GoogleScholarGoogle Scholar | 20579344PubMed |
[17] Ward PR, Javanparast S, Matt MA, Martini A, Tsourtos G, Cole S, et al (2011) Equity of colorectal cancer screening: cross-sectional analysis of National Bowel Cancer Screening Program data for South Australia. Aust N Z J Public Health 35, 61–5.
| Equity of colorectal cancer screening: cross-sectional analysis of National Bowel Cancer Screening Program data for South Australia.Crossref | GoogleScholarGoogle Scholar | 21299702PubMed |
[18] Martini A, Javanparast S, Ward PR, Baratiny G, Gill T, Cole S, et al (2011) Colorectal cancer screening in rural and remote areas: analysis of the National Bowel Cancer Screening Program data for South Australia. Rural Remote Health 11, 1648
[19] Duncan A, Wilson C, Cole SR, Mikocka-Walus A, Turnbull D, Young GP (2009) Demographic associations with stage of readiness to screen for colorectal cancer. Health Promot J Austr 20, 7–1.
[20] Weber MF, Cunich M, Smith DP, Salkeld G, Sitas F, O’Connell D (2013) Sociodemographic and health-related predictors of self-reported mammogram, faecal occult blood test and prostate specific antigen test use in a large Australian study. BMC Public Health 13, 429
| Sociodemographic and health-related predictors of self-reported mammogram, faecal occult blood test and prostate specific antigen test use in a large Australian study.Crossref | GoogleScholarGoogle Scholar | 23641775PubMed |
[21] Weber MF, Banks E, Ward R, Sitas F (2008) Population characteristics related to colorectal cancer testing in New South Wales, Australia: results from the 45 and Up Study cohort. J Med Screen 15, 137–42.
| Population characteristics related to colorectal cancer testing in New South Wales, Australia: results from the 45 and Up Study cohort.Crossref | GoogleScholarGoogle Scholar | 18927096PubMed |
[22] Javanparast S, Ward PR, Carter SM, Wilson CJ (2012) Barriers to and facilitators of colorectal cancer screening in different population subgroups in Adelaide, South Australia. Med J Aust 196, 521–3.
| Barriers to and facilitators of colorectal cancer screening in different population subgroups in Adelaide, South Australia.Crossref | GoogleScholarGoogle Scholar | 22571311PubMed |
[23] Tong S, Hughes K, Oldenburg BB, Mar CD (2006) Colorectal cancer screening with faecal occult blood testing: community intention, knowledge, beliefs and behaviour. Asia Pac J Public Health 18, 16–23.
| Colorectal cancer screening with faecal occult blood testing: community intention, knowledge, beliefs and behaviour.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD283it1Sgtg%3D%3D&md5=132d3c062ad4eeec21b048d2fdcfa9fdCAS | 16629434PubMed |
[24] Becker M (1974) The health belief model and personal health behavior. Health Educ Monogr 2, 324–473.
[25] Rosenstock IM. The health belief model: explaining health behavior through expectancies. In Glanz K, Lewis FM, Rimer BK, editors. Health behavior and health education: theory, research, and practice (pp. 39–62). San Francisco: Jossey-Bass; 1990.
[26] Simon AE, Forbes LJ, Boniface D, Warburton F, Brain KE, Dessaix A, et al (2012) An international measure of awareness and beliefs about cancer: development and testing of the ABC. BMJ Open 2, e001758
| An international measure of awareness and beliefs about cancer: development and testing of the ABC.Crossref | GoogleScholarGoogle Scholar | 23253874PubMed |
[27] Australian Bureau of Statistics. 2006 census of population and housing: socio-economic indexes for areas (SEIFA). 2008. Available from: http://www.abs.gov.au/AUSSTATS/abs@.nsf/allprimarymainfeatures/356A4186CCDDC4D1CA257B3B001AC22C?opendocument [Verified 12 June 2014]
[28] Rizzo L, Brick JM, Park I (2004) A minimally intrusive method for sampling persons in random digit dial surveys. Public Opin Q 68, 267–74.
| A minimally intrusive method for sampling persons in random digit dial surveys.Crossref | GoogleScholarGoogle Scholar |
[29] American Association for Public Opinion Research. Standard definitions: final dispositions of case codes and outcome rates for surveys. Lenexa, Kansas: AAPOR; 2008.
[30] Forbes LJ, Simon AE, Warburton F, Boniface D, Brain KE, Dessaix A, et al (2013) Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): do they contribute to differences in cancer survival? Br J Cancer 108, 292–300.
| Differences in cancer awareness and beliefs between Australia, Canada, Denmark, Norway, Sweden and the UK (the International Cancer Benchmarking Partnership): do they contribute to differences in cancer survival?Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BC3szksVeksg%3D%3D&md5=2106ee30ccd1aa40e5c94b287eb2ce32CAS | 23370208PubMed |
[31] Duncan A, Turnbull D, Gregory T, Cole SR, Young GP, Flight I, Wilson C (2012) Using the Transtheoretical Model of Behaviour Change to describe readiness to rescreen for colorectal cancer with faecal occult blood testing. Health Promot J Austr 23, 122–8.
[32] Paddison JS, Yip MJ (2010) Exploratory study examining barriers to participation in colorectal cancer screening. Aust J Rural Health 18, 11–5.
| Exploratory study examining barriers to participation in colorectal cancer screening.Crossref | GoogleScholarGoogle Scholar | 20136809PubMed |
[33] Wardle J, Sutton S, Williamson S, Taylor T, McCaffery K, Cuzick J, Hart A, Atkin W (2000) Psychosocial influences on older adults’ interest in participating in bowel cancer screening. Prev Med 31, 323–34.
| Psychosocial influences on older adults’ interest in participating in bowel cancer screening.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3M%2FhtlSjug%3D%3D&md5=6411309b17def50f44cea6fc67683a59CAS | 11006057PubMed |
[34] Power E, Miles A, von Wagner C, Robb K, Wardle J (2009) Uptake of colorectal cancer screening: system, provider and individual factors and strategies to improve participation. Future Oncol 5, 1371–88.
| Uptake of colorectal cancer screening: system, provider and individual factors and strategies to improve participation.Crossref | GoogleScholarGoogle Scholar | 19903066PubMed |
[35] Hay JL, Buckley TR, Ostroff JS (2005) The role of cancer worry in cancer screening: a theoretical and empirical review of the literature. Psychooncology 14, 517–34.
| The role of cancer worry in cancer screening: a theoretical and empirical review of the literature.Crossref | GoogleScholarGoogle Scholar | 15490428PubMed |
[36] Cole SR, Young GP, Byrne D, Guy JR, Morcom J (2002) Participation in screening for colorectal cancer based on a faecal occult blood test is improved by endorsement by the primary care practitioner. J Med Screen 9, 147–52.
| Participation in screening for colorectal cancer based on a faecal occult blood test is improved by endorsement by the primary care practitioner.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD3s%2FktlCnsg%3D%3D&md5=b9481127e0f6283681db6c474037c098CAS | 12518003PubMed |
[37] Cole SR, Smith A, Wilson C, Turnbull D, Esterman A, Young GP (2007) An advance notification letter increases participation in colorectal cancer screening. J Med Screen 14, 73–5.
| An advance notification letter increases participation in colorectal cancer screening.Crossref | GoogleScholarGoogle Scholar | 1:STN:280:DC%2BD2svgvVyguw%3D%3D&md5=6adea1d590447e52c661045b135ead42CAS | 17626705PubMed |
[38] Morrell S, Perez DA, Hardy M, Cotter T, Bishop JF (2010) Outcomes from a mass media campaign to promote cervical screening in NSW, Australia. J Epidemiol Community Health 64, 777–83.
| Outcomes from a mass media campaign to promote cervical screening in NSW, Australia.Crossref | GoogleScholarGoogle Scholar | 19822553PubMed |
[39] Rosenstock IM (1966) Why people use health services. Milbank Mem Fund Q 44, 94–127.
[40] Rauscher GH, Johnson TP, Cho YI, Walk JA (2008) Accuracy of self-reported cancer-screening histories: a meta-analysis. Cancer Epidemiol Biomarkers Prev 17, 748–57.
| Accuracy of self-reported cancer-screening histories: a meta-analysis.Crossref | GoogleScholarGoogle Scholar | 18381468PubMed |


