Flooding and low oxygen responses in plants
Ole Pedersen A D , Pierdomenico Perata B and Laurentius A. C. J. Voesenek CA Department of Biology, The University of Copenhagen, Universitetsparken 4, 3rd floor, 2100 Copenhagen, Denmark.
B PlantLab, Institute of Life Sciences, Scuola Superiore Sant’Anna, Via Mariscoglio 34, Pisa 56124, Italy.
C Institute of Environmental Biology, Utrecht University, Padualaan 8, 3584 CH Utrecht, The Netherlands.
D Corresponding author. Email: opedersen@bio.ku.dk
Functional Plant Biology 44(9) iii-vi https://doi.org/10.1071/FPv44n9_FO
Published: 11 August 2017
Abstract
The world is currently experiencing dramatic increases in flood events impacting on natural vegetation and crops. Flooding often results in low O2 status in root tissues during waterlogging, but sometimes also in shoot tissues when plants become completely submerged. Plants possess a suite of traits enabling tissue aeration and/or adjusted metabolism during hypoxia or even in the absence of O2. This special issue of Functional Plant Biology presents key papers for plant scientists on the quest to further address and improve flood tolerance of terrestrial plants. The papers address low O2 responses in roots, shoots or whole plants in controlled laboratory conditions or in the field situation using natural wetland plants as models as well as economically important crops, such as rice, wheat and barley. The studies advance our understanding of low O2 responses in plant tissues as caused by O2 shortage during flooding. However, in most instances, submergence not only leads to hypoxic or anoxic tissues, but inundation in water also results in accumulation of CO2 and the important plant hormone ethylene. Thus, carefully designed laboratory studies are often needed to unravel the mechanistic relationships between a combined decline in O2 followed by increases in CO2 and ethylene at tissue as well as on the cellular level.
Additional keywords: anaerobiosis, anoxia, climate change, flood tolerance, flooding tolerance, hypoxia, underwater photosynthesis.
Global background
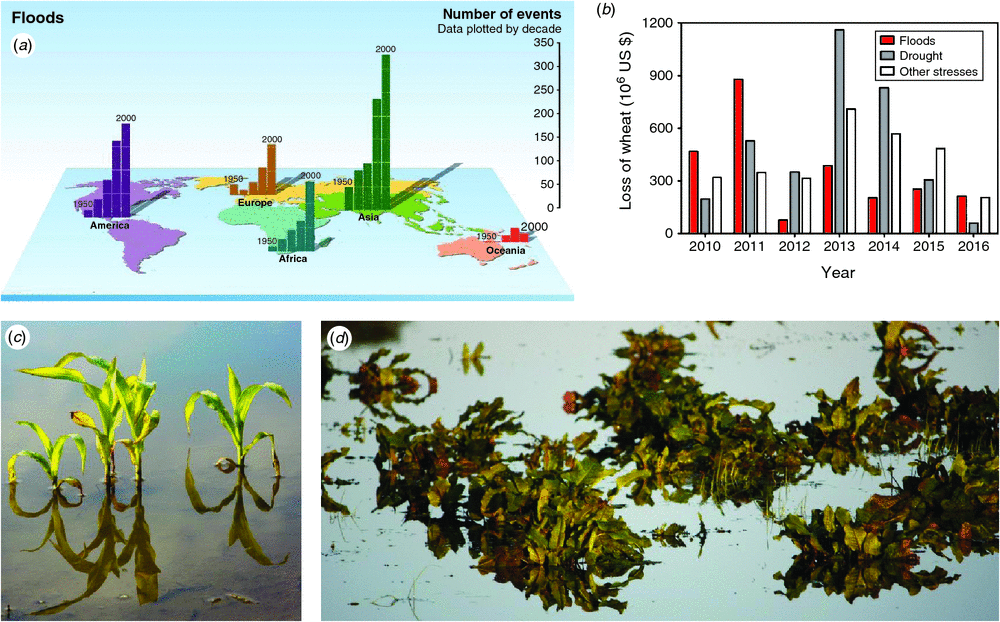
Since 1950, the world has experienced a dramatic increase in flood events that has led to tragic loss of human life, but also to flood disasters impacting on natural vegetation and crops (Fig. 1a–d). Based on insurance enumerations from the past 7 years in the USA, mean annual losses of wheat production due to floods amount US$360M. In 2010, 2011 and 2016 losses caused by floods even exceeded those caused by drought or other natural disasters (Fig. 1b). The calamities inflicted by floods upon plants call for action, and since the challenges span several disciplines the road map to solutions should be cross-disciplinary as well. The International Society for Plant Anaerobiosis (ISPA) hosts plant scientists representing areas from genetics to macro-ecology and thus ISPA serves as an excellent umbrella to address flood-related issues in crops and natural vegetation. The 12th International ISPA Conference held in Elsinore, Denmark, in 2016 gathered plant scientists from around the world to share ideas and recent discoveries related to low O2 stress, a common result of floods causing waterlogging or submergence of terrestrial plants. In this special issue of Functional Plant Biology, key papers present a great resource for plant scientists on the quest to further address and improve flood tolerance of terrestrial plants.
Flooding-induced low O2 conditions re-orchestrate plant metabolism to manage energy production and consumption. In general, plants elevate starch and sucrose catabolism, glycolysis and ethanolic fermentation to increase substrate-level production of ATP (Bailey-Serres and Voesenek 2008), and energy production can be further stimulated through alanine metabolism to succinate (Bailey-Serres and Voesenek 2010). Moreover, many plants are also able to reduce energy consumption and growth upon low O2 conditions (Bailey-Serres and Voesenek 2010).
Responses of roots to low O2
Excess water in terrestrial systems initially results in waterlogging, a situation where all gas spaces in a well-ventilated soil are replaced by water. After some time, waterlogging leads to soil anoxia since the slow diffusion of O2 in water is unable to balance the quick O2 consumption by soil microorganisms and plant roots (Ponnamperuma 1972, 1984). This is nicely shown in a study by Pellegrini et al. (2017) from a saltmarsh where natural tides result in recurrent soil flooding or even complete plant submergence. Soils that are permanently waterlogged remain anoxic whereas soils that are drained at low tide experience times of re-aeration (Pellegrini et al. 2017). The vegetation inhabiting the saltmarsh tackles flooding in different ways and high porosity tissues enabling fast gas phase diffusion of O2 (Armstrong 1979; Colmer 2003) is an important trait for one of the studied halophytes, whereas unidentified bottlenecks in the porous pathway lead to severe tissue hypoxia during submergence in another species (Pellegrini et al. 2017). The authors propose that natural wetland plants may serve as important inspiration for plant biologists that work towards improving flood tolerance of crops.
Waterlogging and partial submergence in many cases spark a rich growth of adventitious roots stimulated by the death of the primary root system (Sauter 2013). For example, in Solanum dulcamara, numerous adventitious roots are formed during partial submergence whereas few adventitious roots are formed when completely submerged (Zhang et al. 2015). Interestingly, high external O2 concentrations in the floodwater could not compensate for the lack of shoot contact to the atmosphere (Zhang et al. 2017). Moreover, carbohydrate depletion before flooding also reduced adventitious root formation unless high light conditions were provided (Zhang et al. 2017).
Adventitious roots of many wetland plants form a barrier to radial O2 loss (ROL) in order to enable supply of O2 to the growing root tip and to maintain a longer root length (Armstrong 1979; Colmer 2003). The barrier is formed by cell wall depositions of lignin and/or suberin, but in the case of Hordeum marinum, only components of suberin differed significantly between waterlogging tolerant accessions (with a strong barrier to ROL and up to 38% root porosity) and intolerant (with only a weak barrier to ROL and up to 26% root porosity) (Kotula et al. 2017).
Responses of shoots to low O2
Recent studies show that some terrestrial plants possess superhydrophobic leaf cuticles, which retain a thin gas film when submerged. The trait confers flood tolerance because these films enhance gas exchange with the floodwater and thus stimulate O2 uptake in the darkness and CO2 uptake – and thus carbohydrate and O2 production – in the light (Colmer and Pedersen 2008; Pedersen et al. 2009). Also the dryland crop, wheat, possesses superhydrophobic leaf cuticles (Raskin and Kende 1983) and initially a gas film is retained when submerged, but the superhydrophobic properties are lost in time and the gas film subsequently vanishes (Winkel et al. 2014, 2016). Wheat loses the superhydrophobic properties after only 2–3 days of submergence, whereas a natural wetland grass (Glyceria fluitans) used as a trait benchmark retained the gas film for more than 4 days of submergence (Konnerup et al. 2017). Despite the fast loss of the gas film in wheat the gas films result in 3 days of extended survival as compared with wheat where the leaf gas films had been experimentally removed before submergence (Winkel et al. 2017).
Complete submergence not only results in reduced O2 levels in soils and roots, but also in densely packed shoot tissues, such as meristems, especially when the flood water is turbid and hampers light penetration (van Dongen and Licausi 2015). However, the impact of hypoxia on shoot tissues is rarely studied. Abadie et al. (2017) studied metabolic shifts upon low O2 in illuminated leaves and found that low O2 had a negative effect on photosynthesis and, furthermore, it clearly showed signs of the typical hypoxia responses.
Among crops, rice is well known to be exceptionally tolerant to submergence. Rice can germinate even in complete absence of O2 and rice varieties possessing the Sub1A or Snorkel genes can adopt survival strategies also at the adult stage (Bailey-Serres and Voesenek 2008). At the germination stage, rice differs from other cereals in its ability to degrade starch, thus providing the germinating embryo with soluble carbohydrates that are channelled through glycolysis to the fermentative pathway (Perata et al. 1993). One specific α-amylase gene, RAMY3D, plays a special role in rice germination under submergence. Different from other α-amylase genes, RAMY3D is not regulated by gibberellins, whose synthesis is hampered under submergence, but rather on sugar availability (Umemura et al. 1998). The rapid consumption of soluble carbohydrates during the early phases of anaerobic germination in rice seeds triggers sugar starvation that activates RAMY3D. Ho et al. (2017) describes a Calcineurin B-like protein, namely CBL4, that interacts with CIPK15, a key player in the mechanism by which sugar starvation and low O2 converge in the activation of RAMY3D (Lee et al. 2009). CBL proteins directly sense Ca2+ and consequently interact with CIPK15, which transduces the signal, thus linking calcium sensing to the sugar-dependent regulation of RAMY3D. Together with the recent identification of trehalose-6-phosphate phosphatase (OsTPP7) as a key player for rice germination under submergence (Kretzschmar et al. 2015), the work by Ho et al. (2017) contributes to drawing a more complete picture of the events enabling rice to germinate under hypoxia.
Shoot elongation is a common flood response of many wetland plants and crops, and the elongation serves to restore contact with the atmosphere so that internal aeration is sustained (Colmer and Voesenek 2009). Similarly to Rumex palustris (Chen et al. 2011), the tropical forage grass, Chloris gayana, benefits from this escape strategy only during prolonged flooding (Striker et al. 2017). When exposed to 2 weeks of continuous complete submergence, restoration of aerial contact by shoot elongation resulted in 2.9-fold higher dry mass accumulation compared with repeated submergence of shorter duration (1 week) but still with 2 weeks of total submergence (Striker et al. 2017).
Flooding may also occur in combination with other stressors such as salinity (Colmer and Flowers 2008). It is well described that flooding in combination with salinity can result in a severe impact on plant performance since dysfunctional roots caused by waterlogging result in dramatic increases first in roots (Kotula et al. 2015) and later in the shoot Na+ and Cl– concentration when compared with exposure to salinity stress only (Colmer and Flowers 2008). Falakboland et al. (2017) exposed a range barley (Hordeum vulgare) cultivars to waterlogging, salt stress or the combination of both stressors. The combined stress had more severe impact on these cultivars than individual stressors. Interestingly, a much stronger correlation was found between the combined stress and waterlogging than with salinity, indicating that waterlogging tolerance had a large contribution to the tolerance of combined stress. Most likely this is related to severe energy limitation induced by waterlogging (Falakboland et al. 2017).
In rice grown in the field, application of K+ alleviated the stress caused by complete submergence so that survival was enhanced and chlorophyll retained at a higher level when compared with plots with no additional K+ added (Dwivedi et al. 2017).
The studies briefly summarised above advance our understanding of low O2 responses in plant tissues as caused by O2 shortage during flooding. However, in most instances, submergence not only leads to hypoxic or anoxic tissues, but inundation in water also results in accumulation of CO2 and ethylene. Thus, carefully designed laboratory studies are often needed to unravel the mechanistic relationships between a combined decline in O2 followed by increases in CO2 and ethylene at tissue as well as on the cellular level.
References
Abadie C, Blanchet S, Carroll A, Tcherkez G (2017) Metabolomics analysis of postphotosynthetic effects of gaseous O2 on primary metabolism in illuminated leaves. Functional Plant Biology 44, 929–940.| Metabolomics analysis of postphotosynthetic effects of gaseous O2 on primary metabolism in illuminated leaves.Crossref | GoogleScholarGoogle Scholar |
Armstrong W (1979) Aeration in higher plants. Advances in Botanical Research 7, 225–332.
| Aeration in higher plants.Crossref | GoogleScholarGoogle Scholar |
Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annual Review of Plant Biology 59, 313–339.
| Flooding stress: acclimations and genetic diversity.Crossref | GoogleScholarGoogle Scholar |
Bailey-Serres J, Voesenek LACJ (2010) Life in the balance: a signaling network controlling survival of flooding. Current Opinion in Plant Biology 13, 489–494.
| Life in the balance: a signaling network controlling survival of flooding.Crossref | GoogleScholarGoogle Scholar |
Chen X, Visser EJW, de Kroon H, Pierik R, Voesenek LACJ, Huber H (2011) Fitness consequences of natural variation in flooding-induced shoot elongation in Rumex palustris. New Phytologist 190, 409–420.
| Fitness consequences of natural variation in flooding-induced shoot elongation in Rumex palustris.Crossref | GoogleScholarGoogle Scholar |
Colmer TD (2003) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26, 17–36.
| Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots.Crossref | GoogleScholarGoogle Scholar |
Colmer TD, Flowers TJ (2008) Flooding tolerance in halophytes. New Phytologist 179, 964–974.
| Flooding tolerance in halophytes.Crossref | GoogleScholarGoogle Scholar |
Colmer TD, Pedersen O (2008) Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist 177, 918–926.
| Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange.Crossref | GoogleScholarGoogle Scholar |
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology 36, 665–681.
| Flooding tolerance: suites of plant traits in variable environments.Crossref | GoogleScholarGoogle Scholar |
Dwivedi S, Kumar S, Bhakta N, Singh S, Rao K, Mishra J, Singh A (2017) Improvement of submergence tolerance in rice through efficient application of potassium under submergence-prone rainfed ecology of Indo-Gangetic Plains. Functional Plant Biology 44, 907–916.
| Improvement of submergence tolerance in rice through efficient application of potassium under submergence-prone rainfed ecology of Indo-Gangetic Plains.Crossref | GoogleScholarGoogle Scholar |
Falakboland Z, Zhou M, Zeng F, Kiani-Pouya A, Shabala L, Shabala S (2017) Plant ionic relation and whole-plant physiological responses to waterlogging, salinity and their combination in barley. Functional Plant Biology 44, 941–953.
| Plant ionic relation and whole-plant physiological responses to waterlogging, salinity and their combination in barley.Crossref | GoogleScholarGoogle Scholar |
Ho VT, Tran AN, Cardarelli F, Perata P, Pucciariello C (2017) A calcineurin B-like protein participates in low oxygen signalling in rice. Functional Plant Biology 44, 917–928.
| A calcineurin B-like protein participates in low oxygen signalling in rice.Crossref | GoogleScholarGoogle Scholar |
Konnerup D, Winkel A, Herzog M, Pedersen O (2017) Leaf gas film retentions during submergence of 14 cultivars of wheat (Triticum aestivum). Functional Plant Biology 44, 877–887.
| Leaf gas film retentions during submergence of 14 cultivars of wheat (Triticum aestivum).Crossref | GoogleScholarGoogle Scholar |
Kotula L, Clode PL, Striker GG, Pedersen O, Läuchli A, Shabala S, Colmer TD (2015) Oxygen deficiency and salinity affect cell-specific ion concentrations in adventitious roots of barley (Hordeum vulgare). New Phytologist 208, 1114–1125.
| Oxygen deficiency and salinity affect cell-specific ion concentrations in adventitious roots of barley (Hordeum vulgare).Crossref | GoogleScholarGoogle Scholar |
Kotula L, Schreiber L, Colmer TD, Nakazono M (2017) Anatomical and biochemical characterisation of a barrier to radial O2 loss in adventitious roots of two contrasting Hordeum marinum accessions. Functional Plant Biology 44, 845–857.
| Anatomical and biochemical characterisation of a barrier to radial O2 loss in adventitious roots of two contrasting Hordeum marinum accessions.Crossref | GoogleScholarGoogle Scholar |
Kretzschmar T, Pelayo MAF, Trijatmiko KR, Gabunada LFM, Alam R, Jimenez R, Mendioro MS, Slamet-Loedin IH, Sreenivasulu N, Bailey-Serres J (2015) A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nature Plants 1, 15124
| A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice.Crossref | GoogleScholarGoogle Scholar |
Lee K-W, Chen P-W, Lu C-A, Chen S, Ho T-HD, Yu S-M (2009) Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Science Signaling 2, ra61
| Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding.Crossref | GoogleScholarGoogle Scholar |
Pedersen O, Rich SM, Colmer TD (2009) Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. The Plant Journal 58, 147–156.
| Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice.Crossref | GoogleScholarGoogle Scholar |
Pellegrini E, Konnerup D, Winkel A, Casolo V, Pedersen O (2017) Internal tissue aeration in two halophytes of the Mediterranean region, during partial and complete submergence. Functional Plant Biology 44, 867–876.
| Internal tissue aeration in two halophytes of the Mediterranean region, during partial and complete submergence.Crossref | GoogleScholarGoogle Scholar |
Perata P, Geshi N, Yamaguchi J, Akazawa T (1993) Effect of anoxia on the induction of α-amylase in cereal seeds. Planta 191, 402–408.
| Effect of anoxia on the induction of α-amylase in cereal seeds.Crossref | GoogleScholarGoogle Scholar |
Ponnamperuma FN (1972) The chemistry of submerged soils. Advances in Agronomy 24, 29–96.
| The chemistry of submerged soils.Crossref | GoogleScholarGoogle Scholar |
Ponnamperuma FN (1984) Effects of flooding on soils. In ‘Flooding and plant growth’. (Ed. T Kozlowski.) pp. 9–45. (Academic Press: New York)
Raskin I, Kende H (1983) How does deep water rice solve its aeration problem? Plant Physiology 72, 447–454.
| How does deep water rice solve its aeration problem?Crossref | GoogleScholarGoogle Scholar |
Sauter M (2013) Root responses to flooding. Current Opinion in Plant Biology 16, 282–286.
| Root responses to flooding.Crossref | GoogleScholarGoogle Scholar |
Striker GG, Casas C, Kuang X, Grimoldi AA (2017) No escape? Costs and benefits of leaf de-submergence in the pasture grass Chloris gayana under different flooding regimes. Functional Plant Biology 44, 899–906.
| No escape? Costs and benefits of leaf de-submergence in the pasture grass Chloris gayana under different flooding regimes.Crossref | GoogleScholarGoogle Scholar |
Umemura T-a, Perata P, Futsuhara Y, Yamaguchi J (1998) Sugar sensing and α-amylase gene repression in rice embryos. Planta 204, 420–428.
| Sugar sensing and α-amylase gene repression in rice embryos.Crossref | GoogleScholarGoogle Scholar |
van Dongen JT, Licausi F (2015) Oxygen sensing and signaling. Annual Review of Plant Biology 66, 345–367.
| Oxygen sensing and signaling.Crossref | GoogleScholarGoogle Scholar |
Winkel A, Pedersen O, Ella ES, Ismail AM, Colmer TD (2014) Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes. Journal of Experimental Botany 65, 3225–3233.
| Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes.Crossref | GoogleScholarGoogle Scholar |
Winkel A, Visser EJW, Colmer TD, Brodersen KP, Voesenek LACJ, Sand-Jensen K, Pedersen O (2016) Leaf gas films, underwater photosynthesis and plant species distributions in a flood gradient. Plant, Cell & Environment 39, 1537–1548.
| Leaf gas films, underwater photosynthesis and plant species distributions in a flood gradient.Crossref | GoogleScholarGoogle Scholar |
Winkel A, Herzog M, Konnerup D, Floytrup AH, Pedersen O (2017) Flood tolerance of wheat – the importance of leaf gas films during complete submergence. Functional Plant Biology 44, 888–898.
| Flood tolerance of wheat – the importance of leaf gas films during complete submergence.Crossref | GoogleScholarGoogle Scholar |
Zhang Q, Visser EJ, de Kroon H, Huber H (2015) Life cycle stage and water depth affect flooding-induced adventitious root formation in the terrestrial species Solanum dulcamara. Annals of Botany 116, 279–290.
| Life cycle stage and water depth affect flooding-induced adventitious root formation in the terrestrial species Solanum dulcamara.Crossref | GoogleScholarGoogle Scholar |
Zhang Q, Huber H, Boerakker JW, Bosch D, de Kroon H, Visser EJ (2017) Environmental factors constraining adventitious root formation during flooding of Solanum dulcamara. Functional Plant Biology 44, 858–866.
| Environmental factors constraining adventitious root formation during flooding of Solanum dulcamara.Crossref | GoogleScholarGoogle Scholar |