Flooding-adaptive root and shoot traits in rice
Chen Lin A B * , Zhao Zhang A , Xuwen Shen A , Dan Liu
A B * , Zhao Zhang A , Xuwen Shen A , Dan Liu  B C and Ole Pedersen
B C and Ole Pedersen  C D *
C D *
A
B
C
D
Abstract
Wetland plants, including rice (Oryza spp.), have developed multiple functional adaptive traits to survive soil flooding, partial submergence or even complete submergence. In waterlogged soils and under water, diffusion of O2 and CO2 is extremely slow with severe impacts on photosynthesis and respiration. As a response to shallow floods or rising floodwater, several rice varieties, including deepwater rice, elongate their stems to keep their leaves above the water surface so that photosynthesis can occur unhindered during partial submergence. In stark contrast, some other varieties hardly elongate even if they become completely submerged. Instead, their metabolism is reduced to an absolute minimum so that carbohydrates are conserved enabling fast regrowth once the floodwater recedes. This review focuses on the fascinating functional adaptive traits conferring tolerance to soil flooding, partial or complete submergence. We provide a general analysis of these traits focusing on molecular, anatomical and morphological, physiological and ecological levels. Some of these key traits have already been introgressed into modern high-yielding genotypes improving flood tolerance of several cultivars used by millions of farmers in Asia. However, with the ongoing changes in climate, we propose that even more emphasis should be placed on improving flood tolerance of rice by breeding for rice that can tolerate longer periods of complete submergence or stagnant flooding. Such tolerance could be achieved via additional tissues; i.e. aquatic adventitious roots relevant during partial submergence, and leaves with higher underwater photosynthesis caused by a longer gas film retention time.
Keywords: aerenchyma formation, flooding tolerance, gas film, Oryza sativa, ROL barrier, root traits, stem elongation, submergence.
Introduction
Flooding is one of the most severe natural disasters threatening the food supply worldwide, and both frequency and severity have increased in the past decades (Pedersen et al. 2017). Flooding can cause severe damage to both the root and shoot systems of plants, and the soil root system is particularly prone to flooding stress due to the reduced gas diffusion rate in waterlogged soils (Armstrong 1979). Waterlogged soils prevent plants from obtaining sufficient O2 for root respiration from the rhizosphere since the O2 is consumed by microorganisms, and slow diffusion restricts the replenishment of O2 from the atmosphere above (Ponnamperuma 1972). Consequently, root tissues become severely hypoxic, or even anoxic, with consequences for water and nutrient uptake (Pedersen et al. 2021; Tong et al. 2023). Deeper floods inundating the shoot result in additional stress caused by the slow gas exchange under water (CO2 for photosynthesis and O2 for respiration (Colmer and Pedersen 2008)) as well as the reduction in light (resulting from reflection and absorption (Kirk 1994)). The resulting decline in carbohydrate production further accelerates tissue death (Voesenek and Bailey-Serres 2009; Kurokawa et al. 2018).
Wetland plants, including rice (Oryza spp.), have developed multiple functional adaptive traits to survive soil flooding, partial submergence, or even complete submergence. In shallow floods, several rice varieties elongate their stems to keep their leaves above the water surface (known as snorkelling) so that photosynthesis can occur unhindered (Hattori et al. 2009). Aerenchyma is another important trait present in the roots, stems and leaves (Yamauchi et al. 2016), where it serves as a low-resistance pathway for O2 diffusion to the submerged parts of the plant. However, if O2 becomes insufficient to fuel aerobic respiration, rice can switch to anaerobic respiration allowing the plant to produce energy in the absence of molecular O2 (Lee et al. 2014). During partial submergence, a brand new root system develops in some rice types, and these roots emerge from the stem in the water and are referred to as aquatic adventitious roots (Lorbiecke and Sauter 1999; Lin et al. 2021). In addition to nutrient and water uptake, aquatic adventitious roots also help anchoring the plant as the original soil roots are perishing due to lack of O2. Therefore, rice is used as a model plant to study the molecular mechanisms of plant tolerance to flooding since responses to flooding vary among the different rice varieties.
This review focuses on the fascinating functional adaptive traits conferring tolerance to soil flooding as well as partial or complete submergence. We provide a general analysis of these traits at the molecular, anatomical and morphological, physiological and ecological levels. Finally, we identify a number of traits to focus on in the quest of breeding for more flood-resilient rice cultivars in order to tackle the ongoing climate changes.
Escape response to flooding
Growing along river or lake banks with fluctuating water levels, deepwater rice has a striking capacity for stem and leaf elongation to keep track with rising floodwaters. This capacity allows deepwater rice to remain in contact with atmospheric O2 and CO2 so that respiration and photosynthesis are maintained even during severe floods (Fig. 1) (Raskin and Kende 1984a; Bleecker et al. 1986; Catling 1992; Lorbiecke and Sauter 1999; Nagai et al. 2020; Lin et al. 2023). This kind of ‘escape response’ to rising floodwater is classified as low-O2 escape syndrome (LOES) (Voesenek and Bailey-Serres 2015). Stem elongation mainly occurs at the intercalary meristem of the second and third internode (Kende et al. 1998; Nagai and Ashikari 2023), and the involvement of gibberellin acids (GA) in regulating stem elongation has been extensively studied (Raskin and Kende 1984b; van der Knaap et al. 2000; Hattori et al. 2009; Nagai et al. 2020). Compared to paddy rice (cv. T65), deepwater rice (cv. C9285) accumulated much more GA1 and GA4 after being exposed to partial submergence (Hattori et al. 2009; Nagai et al. 2020). Three major quantitative trait loci (QTLs) are involved in stem elongation with QTL12 having the largest effect (Hattori et al. 2009). Two ERFVII transcription factors, i.e. SNORKEL1 and SNORKEL2, play a significant role in controlling stem elongation, but ethylene rather than GA induces the expression of SNORKEL1 and SNORKEL2, revealing a function of SNORKEL1 and SNORKEL2 in the interplay between ethylene and GA signalling (Hattori et al. 2009).
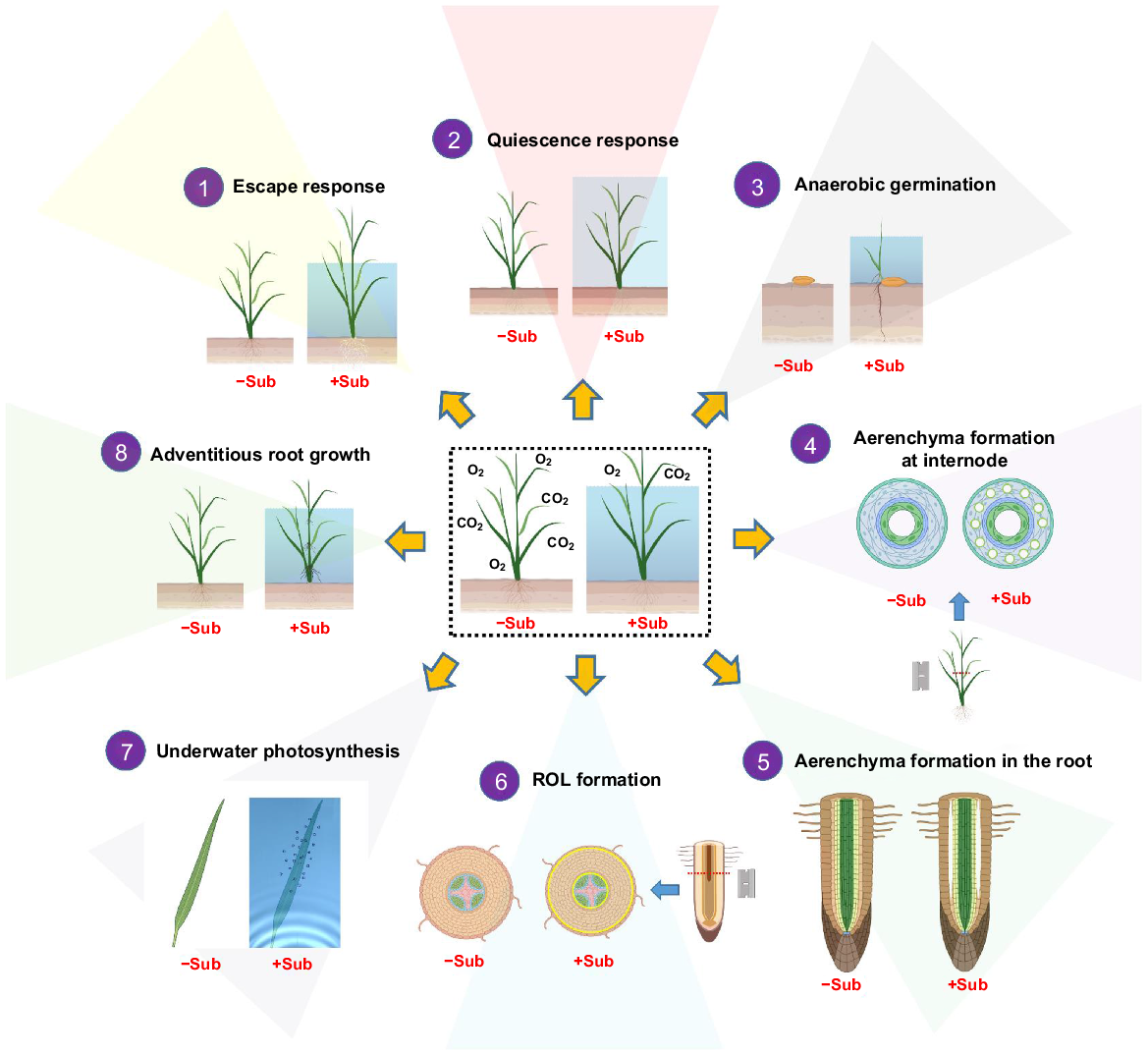
Adaptive traits conferring flood tolerance in rice. (1) Escape response: stem elongation occurs when the intercalary meristem is activated and cell division takes place in response to ethylene and GA during submergence. (2) Quiescence response: SUB1A boosts GA signalling repressors Slender Rice-1 (SLR1) and SLR1 Like-1 (SLRL1), and reduces the expression of the GA-induced genes. In addition, the growth rate and metabolism are reduced. (3) Anaerobic germination: anaerobic germination helps rice to geminate under water and promotes elongation of the coleoptile. (4) Aerenchyma formation at the internode and (5) in the roots facilitates rapid O2 diffusion to submerged tissues. (6) The root barrier to radial O2 loss: formation of a root barrier to radial O2 loss prevents O2 loss to the rhizosphere and enhances the O2 status in roots. (7) Underwater photosynthesis: superhydrophobic leaf cuticles retain a gas film enabling gas exchange with the floodwater, which enhances underwater photosynthesis and respiration. (8) Aquatic adventitious root growth: aquatic adventitious roots emerge and grow in the water in response to partial submergence and take over the function of the soil root system. Several components are created with BioRender.com. ROL, radial O2 loss.
GAs activate the intercalary meristem of the rice stem and regulate the stem cell elongation during submergence. The GA biosynthesis gene SEMIDWARF1 (SD1) controls the stem elongation in deepwater rice (Kuroha et al. 2018), and it has been shown that the domestication of paddy rice resulted in the loss of function of this allele (Sasaki et al. 2002). The ethylene-responsive transcription factor OsEIL1a activates the SD1 haploid gene in deepwater rice cv. C9285 upon submergence, and SD1 further stimulates the biosynthesis of GA4, enabling the internode elongation (Kuroha et al. 2018). Evolutionary analysis revealed that the characteristic SD1 haploid gene of deepwater rice was originally evolved from Oryza rufipogon (Kuroha et al. 2018). Excitingly, two genes controlling internode elongation in response to GA acting in an antagonistic manner have recently been discovered (Nagai et al. 2020). In the presence of GA, the expression of ACCELERATOR OF INTERNODE ELONGATION 1 (ACE1) gene was highly induced, and cell division in the region of intercalary meristem was further activated, eventually leading to internode elongation. By contrast, DECELERATOR OF INTERNODE ELONGATION 1 (DEC1), encoding a zinc-finger transcription factor, suppressed internode elongation. Based on the analysis of genetic diversity, ACE1 and DEC1 were both frequently engaged in domesticating shorter plants to improve lodging resistance in rice populations as well as taller rice plants that were suitable to grow in deepwater habitats (Nagai et al. 2020). In addition, QTLs governing internode elongation during the early vegetative stage with relative minor effect were discovered on chromosomes 2 and 4 in deepwater rice, despite that the associated genes have not been cloned within the QTLs (Nagai et al. 2012).
Quiescence response to flooding
Quiescence enables plants to survive flooding via carbohydrate conservation. In other words, in deep but short floods, it might be more fruitful to wait out the submergence event rather than investing carbohydrates in elongation (see above) and then run out of reserves before surfacing. FR13A is a flood-tolerant Indian landrace of rice (Xu et al. 2006; Bailey-Serres et al. 2010), and via map-based positional cloning, SUBMERGENCE1A (SUB1A) was identified to play a major role in its flood tolerance via repression of shoot elongation (Xu et al. 2006). This strategy is referred to as low-O2 quiescence syndrome (LOQS), and it reduces metabolism and preserves energy reserves during submergence (Fig. 1) (Voesenek and Bailey-Serres 2009, 2015). It has also been shown that the action of SUB1A reduces the rate of starch hydrolysis and accumulation of ethanol, lactate, and alanine as well as metabolic by-products of amino acids (Fukao et al. 2006; Barding et al. 2012). FR13A and the related nearly isogenic lines are all able to survive complete submergence for more than 2 weeks (Das et al. 2005; Fukao et al. 2006). Once the floodwater subsides, plants recover to grow due to the better carbohydrate status, and since they have only elongated little during the submergence event, they are also much less prone to lodging (Ismail et al. 2013).
The ability of paddy rice to tolerate submergence is consequently greatly enhanced by the SUB1A gene. SUB1A, a member of the ERFVII transcription factor family, has been extensively studied to understand the physiological and molecular mechanisms of submergence tolerance in rice, and it has been found that SUB1A restricts ethylene biosynthesis, and reduces metabolism and energy consumption early in the submergence event (Tamang and Fukao 2015; Fukao et al. 2019). Furthermore, SUB1A suppresses shoot elongation and prevents excessive energy consumption by reducing the response to GA and by activating brassinosteroid biosynthesis (Fukao and Bailey-Serres 2008; Schmitz et al. 2013). Moreover, SUB1A stimulates the onset of mitosis and increases the expression of phosphorylated protein kinase 3 (MPK3), and it has therefore been proposed that SUB1A also promotes flooding tolerance by controlling MPK3 activity in rice (Singh and Sinha 2016). During flooding, SUB1A also enhances leaf gas film thickness and retention time (see section on underwater photosynthesis) (Chakraborty et al. 2021).
Following de-submergence, rice also suffers from oxidative stress followed by rapid increase in cell dehydration (Fukao and Xiong 2013). Interestingly, SUB1A increases plant survival through detoxification of reactive O2 species (ROS) during the recovery phase after a submergence event. However, the decrease in ABA caused by submergence is not due to SUB1A (Fukao et al. 2011). SUB1A likewise promotes photosynthesis and metabolic recovery from flooding damage during de-submergence (Alpuerto et al. 2016). It has been shown that SUB1A contributes to the rapid restoration of PSII performance, such as Fv/Fm, ØPSII and metabolism of protein and amino acids including arginine, phenylalanine, proline, threonine and valine. The ERFVII family proteins often serve as substrates of the N-degron pathway in an O2-dependent manner (Bailey-Serres et al. 2012), but the SUB1A protein is not a substrate for the N-degron pathway (Gibbs et al. 2011). Recent research has demonstrated that the direct interaction of N-terminus and C-terminus of SUB1A helps stabilising the protein and prevents degradation via the N-degron pathway (Lin et al. 2019). Transcriptomic analysis also supports the role of SUB1A in rapid recovery during submergence and de-submergence (Jung et al. 2010; Locke et al. 2018).
Ectopic expression of SUB1A results in GA-insensitive phenotype plants. With submergence, SUB1A boosted the abundance of the GA signalling repressors Slender Rice-1 (SLR1) and SLR1 Like-1 (SLRL1), and remarkably reduced the expression of the GA-induced genes (Fukao and Bailey-Serres 2008). Ethylene, which promotes the expression of SUB1A, further stimulates the increased abundance of GA signalling inhibitory factors and decreases GA responsiveness (Fukao and Bailey-Serres 2008). By contrast, GA responsiveness and stem elongation was enhanced by ethylene in waterlogging-intolerant rice varieties (Fukao and Bailey-Serres 2008). The above shows that SUB1A enhances the abundance of the GA signal repressors SLR1 and SLRL1, which in turn decreases GA responsiveness by ethylene during flooding (Fukao and Bailey-Serres 2008).
In conclusion, the quiescence response coded by SUB1 has been found to be effective at all growth stages from early seedling stage to about a week before flowering, and therefore new Sub1 varieties have been spreading fast and are now grown by millions of farmers in Asia (Ismail et al. 2013).
Anaerobic germination and anaerobic seedling development
Anaerobic germination and anaerobic seedling development are extremely important for wetland plants, given that these traits enable seedlings to grow rapidly above the water and take in O2 from the atmosphere (Fig. 1). Anaerobic germination and anaerobic seedling development are both linked with rapid seed germination and coleoptile elongation, with the activities of alcoholic fermentation enzymes taking over energy production from aerobic respiration (Miro and Ismail 2013; Miro et al. 2017; Yu et al. 2021). The majority of crops are unable to germinate underwater due to the limited ATP produced by glycolysis and ethanolic fermentation (Yu et al. 2021), and it is particularly the root development and growth, which are hampered by low O2 availability (Sauter 2013; Pedersen et al. 2021). Therefore, the ability of rice to germinate underwater is unique, and the majority of rice varieties can germinate under severe hypoxia, or even anoxia, and they also extend their coleoptile to a certain degree, but they fail to produce proper leaves and roots (Alpi and Beevers 1983; Miro and Ismail 2013; Yu et al. 2021). However, the coleoptile of some wild rice relatives elongates prior to the development of a root system when it sprouts in wet soils (Fig. 1), allowing the coleoptile, (which is of high tissue porosity; see section on aerenchyma below) to reach the surface of the water where it can obtain O2 from the air; the O2 subsequently diffuses downwards to support root development (Lee and Lin 1995; Ella and Setter 1999; Magneschi et al. 2009). Anaerobic germination and anaerobic seedling development are highly beneficial to agricultural production, since direct-seeded rice reduces labour costs and water requirements for rice transplanting (Ismail et al. 2012; Tuong et al. 2015).
Anaerobic Germination 1 (AG1) derived from a Japanese landrace Khao Hlan On (KHO) is so far the most important QTL related with tolerance to anaerobic germination and anaerobic seedling development (Angaji et al. 2010; Kretzschmar et al. 2015). Several other QTLs with major and minor impacts on anaerobic germination have been identified through a variety of genetic linkage mapping analyses (Ling et al. 2004; Angaji et al. 2010; Baltazar et al. 2019). Trehalose-6-phosphate phosphatase 7 (TPP7), which was found on chromosome 9 in KHO, has ultimately been identified as Anaerobic Germination 1 (AG1) (Kretzschmar et al. 2015). Trehalose-6-phosphate (T6P) helps monitoring the amount of sucrose, the coordination of carbon catabolism and the transfer of carbon from source to sink tissues (Yadav et al. 2014; Figueroa and Lunn 2016). Interestingly, the energy sensor sucrose non-fermenting 1-related protein kinase 1 (SnRK1A) is inhibited by increased T6P levels (Zhang et al. 2009). Under water, the activation of the α-Amylase activities in the early coleoptile improves the trehalose content in the seed coleoptile and it also enhances anaerobic germination and elongation (Kretzschmar et al. 2015). Consequently, chromosomal deletion, which include the loss of the TPP7 gene, results in an impaired anaerobic germination tolerance of the high-yield cv. IR64 (Kretzschmar et al. 2015).
Anaerobic germination involves multiple processes, and rapid starch breakdown is the most important among them to maintain the metabolism of carbohydrates and supply energy for germination (Ella and Setter 1999; Ismail et al. 2009; Magneschi et al. 2009). It has been shown that enzyme activity of α-Amylase was increased to promote starch breakdown (Bailey-Serres and Chang 2005; Ismail et al. 2012). Moreover, alcohol dehydrogenase (ADH), a key enzyme in the anaerobic pathway of carbohydrate metabolism, was highly activated during anaerobic germination (Waters et al. 1991; Gibbs et al. 2000; Ismail et al. 2012). In addition, the enzyme activity for cellular expansion and cell wall loosening was enhanced (Choi et al. 2003; Ismail et al. 2009), and the level of GA was increased (Gu et al. 2010).
At the molecular level, the calcineurin b-like interacting protein kinase 15 (CIPK15) is seen as a central hub in response to low-O2 conditions and sugar starvation. The expression of CIPK15 is highly induced in embryonic tissues of the rice seeds (Lee et al. 2009; Yim et al. 2012). CIPK15 further promotes the activation of the energy sensor SnRK1A, which amplifies transcription of specific amylase genes in the starchy endosperm enabling the nutrient transport from the endosperm to the embryo (Lee et al. 2009; Yu et al. 2015, 2021). Both hypoxia and sucrose starvation promote the expression of SnRK1A (Ramon et al. 2019). SnRK1A phosphorylates the transcription factor MYB SUCROSE 1 (MYBS1) and activated MYBS1 interacts with the TA box at the promoter region of a-Amylase (α-Amy) genes. MYBS2 competes with MYBS1 for the promoter binding of α-Amy gene, and SnRK1A-INTERACTING NEGATIVE REGULATORs (SKIN1/2) negatively regulate the expression of MYBS1 and a-Amy3 (Zhang et al. 2009; Chen et al. 2019). α-Amylase hydrolyses seed starch to provide sugars serving as substrate for fermentation-mediated energy production to support anaerobic germination and anaerobic seedling development underwater. CIPK15 also contributes to the upregulated expression of ADH genes (Lee et al. 2009; Miro and Ismail 2013), which is required to maintain the regeneration of NAD+ in anaerobic respiration. The coupling of SnRK1A-dependent starch catabolism and anaerobic metabolism supplies energy for the elongation of underwater coleoptiles (Lee et al. 2014).
The regulation of anaerobic germination and anaerobic seedling development is a quantitative trait and 11 significant marker-associated sites have been discovered through a genome-wide associated study of 273 Japonica rice varieties (Nghi et al. 2019). Based on RNA-Seq analysis, a total number of 26 genes associated with the establishment of cell membrane and cell wall during embryonic sheath elongation have been identified (Hsu and Tung 2017). However, further investigation is still required to pinpoint the regulatory mechanisms governing this feature particularly to flooding-tolerant genotypes. In molecular breeding, the discovery of novel QTLs for anaerobic tolerance during germination aids in the understanding of the genetic and molecular bases of anaerobic tolerance and then these potent QTLs can be used for molecular marker-assisted selection in agriculture.
Aerenchyma formation and O2 diffusion
Aerenchyma forms a low-resistance pathway for gas diffusion between roots and shoot with O2 diffusion downwards and CO2 and CH4 diffusing in the opposite direction. Aerenchyma formation in rice is a result of programmed cell death in roots, stem and leaf tissues, and programmed cell death has been studied in great detail (see Nishiuchi et al. 2012 for a thorough review on the topic). When soil flooding kicks in, O2 initially present in the soil pores is soon consumed by plant roots and soil microbes rendering the soil anoxic after a few days (Ponnamperuma 1972). Therefore, the only source of O2 to the roots is diffusion of molecular O2 from shoot to root via internal gas spaces (Drew et al. 2000; Colmer 2003a; Yamauchi et al. 2018). The development of aerenchyma in rice tissues greatly reduces the resistance to long-distance O2 diffusion and in combination with the barrier to radial O2 loss (see next section) oxygenation of root tissues is sustained under soil flooding (Fig. 1). Rice forms two types of aerenchyma; i.e. constitutive aerenchyma that is also formed in drained soils, and inducible aerenchyma forming as a response to soil flooding (Yukiyoshi and Karahara 2014; Yamauchi et al. 2016).
Aerenchyma is constitutively formed in rice under aerobic, drained conditions. The molecular mechanism behind constitutive aerenchyma formation has long been a puzzle, and only recently it was discovered that formation of constitutive aerenchyma is mediated by auxin (Yamauchi et al. 2019). Auxin/indole-3-acetic acid protein (AUX/IAA; IAA) and auxin response factor (ARF)-mediated signalling are essential both for aerenchyma and lateral root formation. ARF-dependent transcriptional regulation is repressed by IAAs. IAA13 belongs to the IAA family, and lack of IAA13 (iaa13 mutant) results in a phenotype of reduced aerenchyma and lateral root formation (Yamauchi et al. 2019). ARF19 belongs to the ARF family, and it was further identified as the target of IAA13, and LBD1-8 (a protein containing a lateral organ boundary domain, LBD) was subsequently identified as the target of ARF19 (Yamauchi et al. 2019). IAA13, ARF19 and LBD1-8 are all highly expressed in the root cortex, indicating that they are involved in co-regulating the formation of constitutive aerenchyma. Importantly, overexpression of LBD1-8 in the iaa13 background fully restored the formation of constitutive aerenchyma, and auxin transport inhibitors prevented the formation of aerenchyma, while the application of natural auxin reversed the inhibitory effect (Yamauchi et al. 2019). The current evidence indicates that constitutive aerenchyma formation is under the control of auxin signalling via AUX/IAA and ARF module (Yamauchi et al. 2019). Interestingly, recent research implies that there is a direct link between constitutive aerenchyma and plant tolerance to waterlogging. Despite sharing the same potential to form inducible aerenchyma, maize (Zea mays) and wheat (Triticum aestivum) both have relatively little waterlogging tolerance probably due to the absence of constitutive aerenchyma, which is thought to facilitate oxygenation of root tissues at the onset of soil flooding and prior to the formation of inducible aerenchyma (Colmer and Voesenek 2009; Yamauchi et al. 2018).
Formation of inducible aerenchyma under waterlogged conditions is mainly controlled by the ethylene and ROS signalling pathways (Yamauchi et al. 2017). There is always a low, constitutive production of ethylene in rice roots, but in a drained soil ethylene is lost to the rhizosphere via radial diffusion. However during waterlogging, outward diffusion is impeded and ethylene accumulates in the tissue where it triggers programmed cell death as well as an increased ethylene production (Yamauchi et al. 2018). In contrast, ROS are mainly produced by NADPH oxidase and respiratory burst oxidase homologues (RBOH), triggering signal transduction in different processes in plants (Mhamdi and Van Breusegem 2018; Mittler et al. 2022). It has recently been shown that the RBOH subtype (RBOHH) plays an important role in ethylene-induced root aerenchyma formation (Yamauchi et al. 2017). In cortex cells of rice roots, hypoxia induces the expression of the CDPK5 and CDPK13, which encode calcium-dependent protein kinases, and the co-expression of RBOHH with CDPK5 or CDPK13 results in ROS accumulation in infiltrated tobacco (Nicotiana tabacum) leaves (Yamauchi et al. 2017, 2018). However, inhibition of RBOH activity or cytosolic calcium influx abolishes the aerenchyma formation induced by ethylene, and knocking out RBOHH reduces the accumulation of ROS and induces the formation of aerenchyma in rice roots (Yamauchi et al. 2017). These results indicate that under hypoxic conditions, RBOHH-mediated ROS production is crucial for ethylene-induced root aerenchyma formation through CDPK5 and CDPK13 in rice (Yamauchi et al. 2017).
In addition to the root, rice plants also form aerenchyma in the internode during partial or complete submergence. Aerenchyma formation in the internode also facilitates O2 diffusion from shoot to root and thereby enhances flood tolerance. The aerenchyma is ubiquitously present in each internode of the deepwater rice cv. Pin Gaew 56 (PG56) (Steffens et al. 2011) with pronounced developmental gradients of inducible aerenchyma formation from old to younger internodes; the nodes do not form aerenchyma (Steffens et al. 2011). Two lowland rice types also exhibit aerenchyma development at the internode, albeit to a lesser degree (Steffens et al. 2011). Treatment with the ethylene-releasing compound ethephon or submergence both promoted aerenchyma formation in different rice genotypes (Steffens et al. 2011), and anatomical analysis indicated that pre-aerenchymal cells contain less starch, no chloroplasts and have thinner cell walls (Steffens et al. 2011). In addition, high levels of singlet O2 and H2O2 were observed in these pre-aerenchymal cells as compared with other parenchymal cells. Interestingly, the formation of singlet O2 and H2O2 promoted by ethephon is essential for the formation of aerenchyma. This is further supported by the enhanced aerenchyma formation in the rice mutant of the H2O2 scavenger -MT2b (Steffens et al. 2011).
Formation of a root barrier to radial O2 loss
Constitutive as well as inducible aerenchyma are key traits for root growth in flooded soils, but without the inducible barrier to radial O2 loss, maximum root length is significantly restricted. Even with aerenchyma constituting 50% of the root cross-sectional area, the root of rice would not grow much longer than 150 mm (see modelling of maximum root length in Pedersen et al. 2021). In order to grow longer roots, O2 must be retained inside the root tissue in order to diffuse all the way to the root tip, since cell divisions and continuous root extension cannot take place in the absence of molecular O2 (Armstrong and Webb 1985). Fortunately, rice roots can form a barrier to radial O2 loss in the proximal parts of the mature adventitious roots to prevent O2 loss to the anoxic rhizosphere (Fig. 1) (Colmer 2003b).
In rice, the root barrier to radial O2 loss is inducible. Paddy rice growing in non-flooded soils do not form a barrier to radial O2 loss, but the formation is triggered as soon as the soil becomes waterlogged (Colmer et al. 1998; Colmer 2003b; Ejiri et al. 2021). The functional properties; i.e. high resistance to O2 diffusion in the outer part of the root can be observed already after 6 h of exposure to anoxia and after 24 h, the barrier is complete (Shiono et al. 2011). The environmental signalling for barrier formation is surprisingly not related to low O2, high CO2 or high ethylene (Colmer et al. 2006), which would otherwise characterise an anoxic soil. Instead, it has been shown that H2S (Peralta Ogorek et al. 2023a), Fe2+ (Mongon et al. 2014) and low-molecular-weight carboxylic acids (Colmer et al. 2019) can all act as environmental signals for barrier formation. These chemical compounds are all produced by anaerobic bacteria in anoxic soils, indicating that the barrier formation relies on functional anoxia of the microbial community in the rhizosphere.
Although the induction of the barrier to radial O2 loss is swift and functionally in place within 24 h of exposure to soil flooding, cell wall modifications of the outer part of the root are not yet detectable with histochemical staining. Cell wall depositions of suberin and/or lignin in the outer part of the root are thought to form the functional biochemical components of the barrier (Schreiber 1999; Kotula et al. 2009; Watanabe et al. 2017). Suberin is mainly deposited in the exodermal cell walls (Kotula et al. 2009; Nishiuchi et al. 2021), whereas lignin is mainly found in the sclerenchyma (Armstrong 2000; Soukup et al. 2007). The molecular mechanisms controlling barrier formation have not yet been fully elucidated. However, laser microdissection enabled isolation of the outer part of the root and subsequent tissue-specific transcriptome analysis showed that flooding resulted in upregulation of two genes involved in the suberin biosynthesis (cytochrome P450 (OsCYP86B3) and ABC transporter (OsABCG5)) suggesting that these molecular regulators are essential for the development of the barrier (Shiono et al. 2014). This study also showed that the expression of different transcription factors, including WRKY, NAC and MYB, increased under conditions of soil flooding (Shiono et al. 2014), and these transcription factors might directly or indirectly contribute to the development of radial O2 loss barriers. Given the lack of relevant rice mutants, functional studies of these genes associated with barrier development still need investigation. There are also studies indicating that lignin plays an important role in the formation of a barrier to radial O2 loss (Kotula et al. 2009; Shiono et al. 2011; Abiko et al. 2012). A very recent study showed that lignin-related genes were upregulated during barrier formation as a response to external H2S application with 11 genes involved in the phenylpropanoid and/or lignin metabolic processes (Peralta Ogorek et al. 2023a). Moreover, genes involved in the biosynthesis of lignin were also upregulated as a response to low molecular carboxylic acids and the subsequent formation of a barrier to radial O2 loss (Colmer et al. 2019). Nevertheless, the roles of suberin and lignin in barrier formation have not yet been fully elucidated.
It has very recently been shown that the formation of the barrier to radial O2 loss relies on ABA signal transduction. The application of the ABA biosynthesis inhibitor FLU prevented the formation of the barrier, as well as in the rice mutant, osaba1 (Shiono et al. 2022). An impaired ROL barrier was observed in the rice mutant with a defective ABA biosynthesis gene (osaba1), and the barrier was made fully functional by adding exogenous ABA to the root medium. These findings suggest that ABA is an inducer of suberin lamellae formation as visualised by more pronounced suberin depositions in the exodermis (Shiono et al. 2022). In order to better understand how the barrier is regulated at the molecular level, it thus seems appropriate to look into these ABA-related genes.
In addition to the primary role of the barrier to radial O2 loss; i.e. maintaining high O2 status of the root tissues, the barrier also has additional functions in roots of rice. Gas diffusion is generally restricted by the barrier as shown for H2 and water vapour (Peralta Ogorek et al. 2021) and also H2S (Peralta Ogorek et al. 2023a). Moreover, apoplastic movements of H2O (Song et al. 2022), Na+ (Krishnamurthy et al. 2011) and Fe2+ (Jimenez et al. 2021) are also greatly restricted by the barrier and therefore, this trait can be considered a jack of all trades with its multiple functional roles (Peralta Ogorek et al. 2023b).
An often-overlooked trait also restricting radial O2 loss from roots under soil flooding is a low surface area to volume ratio. The thicker the root, the smaller the relative surface from which O2 can be lost to the anoxic rhizosphere, and therefore a thick root will lose less O2 compared with a thin root if the concentration gradient from tissue to environment is the same (Pedersen et al. 2021). Paddy rice forms thick adventitious roots as a response to soil flooding (Lorbiecke and Sauter 1999), and thick roots in combination with a tight barrier to radial O2 loss is thus an excellent combination of root traits serving to conserve O2 within the cortical tissues.
Aquatic adventitious root growth as response to partial submergence
Adventitious root development is a key trait conferring flood tolerance since these roots replace the impaired soil root system during submergence. Even if the existing soil root system has high amounts of cortical aerenchyma, molecular diffusion of O2 can be too slow to satisfy tissue demand when the distance is long as would be the case if a large portion of the shoot is submerged into water. However, the primordia of adventitious root growth are constitutively formed at the node as part of the normal developmental process (Lorbiecke and Sauter 1999; Steffens and Rasmussen 2016). During flooding, entrapped ethylene in the stem tissues promotes the formation of adventitious roots at the nodes (Fig. 1). In rice, the epidermal cell death above the root primordia is coordinated with adventitious root growth (Steffens and Sauter 2005), both of which are mediated by ethylene, ROS and mechanical signalling (Steffens and Sauter 2009; Steffens et al. 2012). During flooding, the expression of the ethylene biosynthesis genes, ACO1 and EOL1, are highly induced in the epidermal cells above the adventitious root tips, and ethylene accumulation stimulates NADPH oxidase activity and elevates biosynthesis of H2O2 (Steffens and Sauter 2009; Steffens et al. 2012). Meanwhile, the expression of the H2O2 scavenger gene, MT2b, is inhibited, further promoting ethylene and H2O2 signalling transduction (Steffens and Sauter 2009). Ethylene also promotes the production of ROS in the adventitious root primordia, and mechanical signals and ROS are both required for the emergence of adventitious roots as mechanical signals locally provide spatial information triggering programmed cell death of epidermal cells (Steffens et al. 2012). While GA alone is not functional, it acts synergistically with ethylene to induce the formation of adventitious roots (Steffens et al. 2006), but adventitious root growth induced by ethylene is suppressed by exogenous application of ABA (Steffens et al. 2006).
The lack of O2 in flooded soils damages the soil roots and eventually they become dysfunctional and perish. However, the new adventitious roots developed by deepwater rice as responses to partial submergence are primarily formed in the floodwater and are therefore referred to as aquatic adventitious roots. These roots are capable of obtaining O2 from the floodwater since they do not form a barrier to radial O2 loss (the barrier is very weak) rendering these roots permeable to O2 (Lin et al. 2021). Deepwater rice (Lin et al. 2023) and paddy rice (Inouye and Mochizuki 1980) form two types of adventitious roots differing in morphology and timing of emergence. First, relatively thin roots emerge as fast as 3 days after exposure to partial submergence, and these continue to grow for about 2 weeks, reaching a maximum length of 200 mm (Lin et al. 2023). Second, another thicker type then forms some days later, reaching 350 mm and continues to grow for 6–8 weeks. This second type can continue to form from new or existing primordia following periods of recurrent flooding, whereas the first thinner type only grows during the first flood event and dies if they become de-submerged (Lin et al. 2023). The upregulation of key genes between thin and thick adventitious roots indicates a common contribution in activating meristems in aquatic adventitious roots, enhancing their response to flooding. Morphological and anatomical analyses indicate that thick adventitious are more suitable for long-term floods than the thinner, but the exact importance for water and nutrient uptake of these two types of roots awaits further investigation (Lin et al. 2023).
Underwater photosynthesis during submergence
The terrestrial leaves of rice can photosynthesise under water although at a much lower rate than in air. The lower photosynthetic rate is due to the extremely slow gas diffusion in water resulting in CO2 limitation of photosynthesis and possibly also photorespiration caused by internal build-up of photosynthetically produced O2 (Colmer et al. 2011). Both processes restrict underwater photosynthesis in rice to maximum rates of about 25% of that attained in air, but the achieved photosynthetic rates during partial or complete submergence are normally below 10% of those in air (Winkel et al. 2016), and consequently, the production of organic carbon is limited. However, the molecular O2 produced in underwater photosynthesis can diffuse over long distances internally via the well-developed aerenchyma providing a low-resistance pathway (Pedersen et al. 2021). Therefore, molecular O2 can sustain aerobic processes in tissues as far away as in the root tips (Yamauchi et al. 2018).
A crucial leaf trait enabling underwater photosynthesis is the superhydrophobic leaf cuticle that retains a thin gas film during partial or complete submergence (Fig. 1). Leaf gas films are visible as a silvery sheen from the submerged parts of the leaves (Raskin and Kende 1983), and they prevent flooding of the stomata, which are positioned in the grooves both on the ad- and abaxial side of the plicate leaves (Lauridsen et al. 2014). Hydrophobicity of rice leaves is the result of three leaf traits: (1) at the macro level by the plicate leaf structure, where the deep grooves prevent water from wetting the bottom of the grooves; (2) at the micro level where the leaf papillae add extra 3D structure to the leaf surface; and (3) at the nano level caused by the wax platelets forming the cuticle surface (Bhushan et al. 2009). In addition to preventing flooding of the stomata and the sub-stomatal cavities, the leaf gas films present a large surface area for gas exchange with the surrounding floodwater (Verboven et al. 2014). Once CO2 is trapped inside the gas film, it can quickly diffuse to the nearest stomata and enter the leaf; the diffusion inside the gas film is 10 000-fold faster than in the floodwater. Similarly, O2 produced in photosynthesis can swiftly diffuse into the gas film and further dissolve in the floodwater via the vast surface area presented by the gas film, and both processes contribute to establishing photosynthetic rates, which are about 5-fold higher in the presence of leaf gas films than without at environmentally relevant CO2 concentrations in the floodwater (Pedersen et al. 2009).
Regrettably, the superhydrophobic features of the leaf cuticle are gradually lost over a submergence event and therefore the leaf gas films also disappear. Four genotypes of completely submerged rice all lost their leaf gas films within 4–7 days of submergence under field conditions (Winkel et al. 2014). Following loss of gas films, underwater net photosynthesis also declined significantly, and chlorophyll senescence accelerated. In this study, gas film retention time was not related to the SUB1 locus, which was represented in two of the four tested genotypes (FR13A in which the SUB1 locus occurs naturally and Swarna-SUB1 where SUB1 had been introgressed) (Winkel et al. 2014). In the interim, a later study found that leaf gas film thickness was significantly higher in SUB1 genotypes than genotypes not containing the SUB1 locus, and it seemed related to lower expression of LGF1 gene (Kurokawa et al. 2018) in the latter (Chakraborty et al. 2021). For rice submerged in a field situation, the benefits of superhydrophobic leaves and the associated leaf gas films as related to underwater photosynthesis and respiration are restricted to about 1 week of complete submergence, whereas under controlled laboratory conditions with submergence into clean artificial floodwater, the gas films can persist longer (Pedersen et al. 2009).
Perspectives
Since cultivated rice was domesticated from wild ancestors that were true wetland plants, Oryza sativa (Asian rice) and Oryza glaberrima (African rice) both possess root traits enabling growth in waterlogged soils. However, higher aerenchyma formation (constitutive as well as inducible), a large cortex to stele ratio, and an even tighter barrier to radial O2 loss could all improve growth in flooded paddy soils and even holds the potential to reduce greenhouse gas emissions (Jimenez and Pedersen 2023). Importantly, it was recently shown that some of these root traits (e.g. the barrier to radial O2 loss and thick adventitious roots) formed during soil flooding also prime the root system of rice for a subsequent drought (Song et al. 2023; Peralta Ogorek et al. 2023c) a situation that often occurs in the farmer’s field. Multiple genes or QTLs underlying the control of these key root traits can be introgressed into modern high-yielding cultivars via pyramidisation producing novel cultivars that can withstand multiple abiotic stresses, but his approach requires that the genes or QTLs are known (Lin et al. 2022), so much more research focusing on genetic regulation of quantitative root traits are needed.
In deeper floods resulting in partial or complete submergence, focus needs to be more on shoot traits enabling growth and survival during deep flood events. We therefore suggest further investigating the genes in the two QTLs regulating adventitious root growth in deepwater rice. The functions of both types of aquatic adventitious roots with respect to water and nutrient uptake during partial submergence have to be further studied to justify introducing aquatic adventitious roots as a flood tolerance trait in rice cultivars for areas experiencing shallow flooding causing partial submergence of paddy rice for months (Kuanar et al. 2017). Similarly, much more focus is needed on the superhydrophobic properties of the rice cuticle and why hydrophobicity, and therefore also the beneficial leaf gas films, are lost after only a few days of submergence (Winkel et al. 2014). It is interesting that initial leaf gas film thickness is linked to SUB1 (Chakraborty et al. 2021), but the mechanism remains unknown. We propose to investigate the role of leaf morphology since research has shown that the vast majority of the gas volume is retained within the grooves of the plicate leaves (Lauridsen et al. 2014), and therefore leaves with deeper grooves and steeper angles within each groove may retain gas films longer than leaves with a more flattened surfaces.
In this review, we have summarised the many root and shoot traits in rice conferring tolerance to waterlogging or submergence. However, unlike rice, the majority of the world’s crop plants are very susceptible to different types of flooding, and therefore more research and integration are needed to apply this knowledge from rice to improve flood tolerance in other crops such as maize, wheat, barley (Hordeum vulgare) and soybean (Glycine max).
Data availability
Data sets supporting the analyses of this review are made available in the original papers.
Conflicts of interest
Author Ole Pedersen is Associate Editor of Functional Plant Biology. To mitigate this potential conflict of interest they were blinded from the review process. The authors declare no other conflicts of interest.
Declaration of funding
Funding by the Natural Science Foundation of Jiangsu Province (grant no. BK20230568 to CL), the National Natural Science Foundation of China (grant no. 32301870 to CL), the China Scholarship Council (CL and DL), Kiel Life Science, ZMB Young Scientists Grant (CL), by the Priority Academic Program Development of Jiangsu Higher Education Institutions to CL, by Outstanding Ph.D. program in Yangzhou (grant no. YZLYJFJH2022YXBS147 to CL) and by the General Project of Basic Scientific Research to colleges and universities in Jiangsu Province (grant no. 22KJB210019 to CL) and Danida through ‘Climate-smart African rice’ (grant no. 19-03-KU, OP) is greatly acknowledged.
Author contributions
CL and OP conceptualised the reviews, and ZZ, XWS and DL designed the illustration. CL and OP wrote the manuscript with the contribution from ZZ, XWS and DL. All authors approved the final submission.
References
Abiko T, Kotula L, Shiono K, Malik AI, Colmer TD, Nakazono M (2012) Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant, Cell & Environment 35(9), 1618-1630.
| Crossref | Google Scholar | PubMed |
Alpi A, Beevers H (1983) Effects of O2 concentration on rice seedlings. Plant Physiology 71(1), 30-34.
| Crossref | Google Scholar | PubMed |
Alpuerto JB, Hussain RMF, Fukao T (2016) The key regulator of submergence tolerance, SUB1A, promotes photosynthetic and metabolic recovery from submergence damage in rice leaves. Plant, Cell & Environment 39(3), 672-684.
| Crossref | Google Scholar | PubMed |
Angaji SA, Septiningsih EM, Mackill DJ, Ismail AM (2010) QTLs associated with tolerance of flooding during germination in rice (Oryza sativa L.). Euphytica 172(2), 159-168.
| Crossref | Google Scholar |
Armstrong W (2000) Oxygen distribution in wetland plant roots and permeability barriers to gas-exchange with the rhizosphere: a microelectrode and modelling study with Phragmites australis. Annals of Botany 86(3), 687-703.
| Crossref | Google Scholar |
Armstrong W, Webb T (1985) A critical oxygen pressure for root extension in rice. Journal of Experimental Botany 36(10), 1573-1582.
| Crossref | Google Scholar |
Bailey-Serres J, Chang R (2005) Sensing and signalling in response to oxygen deprivation in plants and other organisms. Annals of Botany 96(4), 507-518.
| Crossref | Google Scholar | PubMed |
Bailey-Serres J, Fukao T, Ronald P, Ismail A, Heuer S, Mackill D (2010) Submergence tolerant rice: SUB1’s journey from landrace to modern cultivar. Rice 3(2–3), 138-147.
| Crossref | Google Scholar |
Bailey-Serres J, Fukao T, Gibbs DJ, Holdsworth MJ, Lee SC, Licausi F, Perata P, Voesenek LACJ, van Dongen JT (2012) Making sense of low oxygen sensing. Trends in Plant Science 17(3), 129-138.
| Crossref | Google Scholar | PubMed |
Baltazar MD, Ignacio JCI, Thomson MJ, Ismail AM, Mendioro MS, Septiningsih EM (2019) QTL mapping for tolerance to anaerobic germination in rice from IR64 and the aus landrace Kharsu 80A. Breeding Science 69(2), 227-233.
| Crossref | Google Scholar | PubMed |
Barding GA, Jr, Fukao T, Beni S, Bailey-Serres J, Larive CK (2012) Differential metabolic regulation governed by the rice SUB1A gene during submergence stress and identification of alanylglycine by 1H NMR spectroscopy. Journal of Proteome Research 11(1), 320-330.
| Crossref | Google Scholar | PubMed |
Bhushan B, Jung YC, Koch K (2009) Micro-, nano- and hierarchical structures for superhydrophobicity, self-cleaning and low adhesion. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 367(1894), 1631-1672.
| Crossref | Google Scholar | PubMed |
Bleecker AB, Schuette JL, Kende H (1986) Anatomical analysis of growth and developmental patterns in the internode of deepwater rice. Planta 169(4), 490-497.
| Crossref | Google Scholar | PubMed |
Catling D (1992) ‘Rice in deep water.’ (Palgrave Macmillan: London, UK) https://doi.org/10.1007/978-1-349-12309-4
Chakraborty K, Guru A, Jena P, Ray S, Guhey A, Chattopadhyay K, Sarkar RK (2021) Rice with SUB1 QTL possesses greater initial leaf gas film thickness leading to delayed perception of submergence stress. Annals of Botany 127(2), 251-265.
| Crossref | Google Scholar | PubMed |
Chen Y-S, David Ho T-H, Liu L, Lee DH, Lee C-H, Chen Y-R, Lin S-Y, Lu C-A, Yu S-M (2019) Sugar starvation-regulated MYBS2 and 14-3-3 protein interactions enhance plant growth, stress tolerance, and grain weight in rice. Proceedings of the National Academy of Sciences of the United States of America 116(43), 21925-21935.
| Crossref | Google Scholar | PubMed |
Choi D, Lee Y, Cho H-T, Kende H (2003) Regulation of expansin gene expression affects growth and development in transgenic rice plants. The Plant Cell 15(6), 1386-1398.
| Crossref | Google Scholar | PubMed |
Colmer TD (2003a) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26(1), 17-36.
| Crossref | Google Scholar |
Colmer TD (2003b) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Annals of Botany 91(2), 301-309.
| Crossref | Google Scholar |
Colmer TD, Pedersen O (2008) Underwater photosynthesis and respiration in leaves of submerged wetland plants: gas films improve CO2 and O2 exchange. New Phytologist 177(4), 918-926.
| Crossref | Google Scholar | PubMed |
Colmer TD, Voesenek LACJ (2009) Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology 36(8), 665-681.
| Crossref | Google Scholar | PubMed |
Colmer TD, Gibberd MR, Wiengweera A, Tinh TK (1998) The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany 49(325), 1431-1436.
| Crossref | Google Scholar |
Colmer TD, Cox MCH, Voesenek LACJ (2006) Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytologist 170(4), 767-778.
| Crossref | Google Scholar | PubMed |
Colmer TD, Winkel A, Pedersen O (2011) A perspective on underwater photosynthesis in submerged terrestrial wetland plants. AoB Plants 2011, plr030.
| Crossref | Google Scholar |
Colmer TD, Kotula L, Malik AI, Takahashi H, Konnerup D, Nakazono M, Pedersen O (2019) Rice acclimation to soil flooding: low concentrations of organic acids can trigger a barrier to radial oxygen loss in roots. Plant, Cell & Environment 42(7), 2183-2197.
| Crossref | Google Scholar | PubMed |
Das KK, Sarkar RK, Ismail AM (2005) Elongation ability and non-structural carbohydrate levels in relation to submergence tolerance in rice. Plant Science 168(1), 131-136.
| Crossref | Google Scholar |
Drew MC, He C-J, Morgan PW (2000) Programmed cell death and aerenchyma formation in roots. Trends in Plant Science 5(3), 123-127.
| Crossref | Google Scholar | PubMed |
Ejiri M, Fukao T, Miyashita T, Shiono K (2021) A barrier to radial oxygen loss helps the root system cope with waterlogging-induced hypoxia. Breeding Science 71(1), 40-50.
| Crossref | Google Scholar | PubMed |
Ella ES, Setter TL (1999) Importance of seed carbohydrates in rice seedling establishment under anoxia. Acta Horticulturae 504, 209-218.
| Crossref | Google Scholar |
Figueroa CM, Lunn JE (2016) A tale of two sugars: trehalose 6-phosphate and sucrose. Plant Physiology 172(1), 7-27.
| Crossref | Google Scholar | PubMed |
Fukao T, Bailey-Serres J (2008) Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proceedings of the National Academy of Sciences of the United States of America 105(43), 16814-16819.
| Crossref | Google Scholar | PubMed |
Fukao T, Xiong L (2013) Genetic mechanisms conferring adaptation to submergence and drought in rice: simple or complex? Current Opinion in Plant Biology 16, 196-204.
| Crossref | Google Scholar |
Fukao T, Xu K, Ronald PC, Bailey-Serres J (2006) A variable cluster of ethylene response factor-like genes regulates metabolic and developmental acclimation responses to submergence in rice. The Plant Cell 18(8), 2021-2034.
| Crossref | Google Scholar | PubMed |
Fukao T, Yeung E, Bailey-Serres J (2011) The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. The Plant Cell 23(1), 412-427.
| Crossref | Google Scholar | PubMed |
Fukao T, Barrera-Figueroa BE, Juntawong P, Pena-Castro JM (2019) Submergence and waterlogging stress in plants: a review highlighting research opportunities and understudied aspects. Frontiers in Plant Science 10, 340.
| Crossref | Google Scholar | PubMed |
Gibbs J, Morrell S, Valdez A, Setter TL, Greenway H (2000) Regulation of alcoholic fermentation in coleoptiles of two rice cultivars differing in tolerance to anoxia. Journal of Experimental Botany 51(345), 785-796.
| Crossref | Google Scholar | PubMed |
Gibbs DJ, Lee SC, Isa NM, Gramuglia S, Fukao T, Bassel GW, Correia CS, Corbineau F, Theodoulou FL, Bailey-Serres J, Holdsworth MJ (2011) Homeostatic response to hypoxia is regulated by the N-end rule pathway in plants. Nature 479(7373), 415-418.
| Crossref | Google Scholar | PubMed |
Gu X-Y, Liu T, Feng J, Suttle JC, Gibbons J (2010) The qSD12 underlying gene promotes abscisic acid accumulation in early developing seeds to induce primary dormancy in rice. Plant Molecular Biology 73(1–2), 97-104.
| Crossref | Google Scholar | PubMed |
Hattori Y, Nagai K, Furukawa S, Song X-J, Kawano R, Sakakibara H, Wu J, Matsumoto T, Yoshimura A, Kitano H, Matsuoka M, Mori H, Ashikari M (2009) The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature 460(7258), 1026-1030.
| Crossref | Google Scholar | PubMed |
Hsu S-K, Tung C-W (2017) RNA-Seq analysis of diverse rice genotypes to identify the genes controlling coleoptile growth during submerged germination. Frontiers in Plant Science 8, 762.
| Crossref | Google Scholar | PubMed |
Inouye J, Mochizuki T (1980) Emergence of crown roots from the elongated culm in several floating rice varieties under submerged conditions. Japanese Journal of Tropical Agriculture 24, 125-131.
| Google Scholar |
Ismail AM, Ella ES, Vergara GV, Mackill DJ (2009) Mechanisms associated with tolerance to flooding during germination and early seedling growth in rice (Oryza sativa). Annals of Botany 103(2), 197-209.
| Crossref | Google Scholar | PubMed |
Ismail AM, Johnson DE, Ella ES, Vergara GV, Baltazar AM (2012) Adaptation to flooding during emergence and seedling growth in rice and weeds, and implications for crop establishment. AoB Plants 2012, pls019.
| Crossref | Google Scholar |
Ismail AM, Singh US, Singh S, Dar MH, Mackill DJ (2013) The contribution of submergence-tolerant (Sub1) rice varieties to food security in flood-prone rainfed lowland areas in Asia. Field Crops Research 152, 83-93.
| Crossref | Google Scholar |
Jimenez JdlC, Pedersen O (2023) Mitigation of greenhouse gas emissions from rice via manipulation of key root traits. Rice 16(1), 24.
| Crossref | Google Scholar | PubMed |
Jimenez JdlC, Clode PL, Signorelli S, Veneklaas EJ, Colmer TD, Kotula L (2021) The barrier to radial oxygen loss impedes the apoplastic entry of iron into the roots of Urochloa humidicola. Journal of Experimental Botany 72(8), 3279-3293.
| Crossref | Google Scholar | PubMed |
Jung K-H, Seo Y-S, Walia H, Cao P, Fukao T, Canlas PE, Amonpant F, Bailey-Serres J, Ronald PC (2010) The submergence tolerance regulator Sub1A mediates stress-responsive expression of AP2/ERF transcription factors. Plant Physiology 152(3), 1674-1692.
| Crossref | Google Scholar | PubMed |
Kende H, van der Knaap E, Cho H-T (1998) Deepwater rice: a model plant to study stem elongation. Plant Physiology 118(4), 1105-1110.
| Crossref | Google Scholar | PubMed |
Kotula L, Ranathunge K, Schreiber L, Steudle E (2009) Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. Journal of Experimental Botany 60(7), 2155-2167.
| Crossref | Google Scholar | PubMed |
Kretzschmar T, Pelayo MAF, Trijatmiko KR, Gabunada LFM, Alam R, Jimenez R, Mendioro MS, Slamet-Loedin IH, Sreenivasulu N, Bailey-Serres J, Ismail AM, Mackill DJ, Septiningsih EM (2015) A trehalose-6-phosphate phosphatase enhances anaerobic germination tolerance in rice. Nature Plants 1, 15124.
| Crossref | Google Scholar | PubMed |
Krishnamurthy P, Ranathunge K, Nayak S, Schreiber L, Mathew MK (2011) Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). Journal of Experimental Botany 62(12), 4215-4228.
| Crossref | Google Scholar | PubMed |
Kuanar SR, Ray A, Sethi SK, Chattopadhyay K, Sarkar RK (2017) Physiological basis of stagnant flooding tolerance in rice. Rice Science 24(2), 73-84.
| Crossref | Google Scholar |
Kuroha T, Nagai K, Gamuyao R, Wang DR, Furuta T, Nakamori M, Kitaoka T, Adachi K, Minami A, Mori Y, Mashiguchi K, Seto Y, Yamaguchi S, Kojima M, Sakakibara H, Wu J, Ebana K, Mitsuda N, Ohme-Takagi M, Yanagisawa S, Yamasaki M, Yokoyama R, Nishitani K, Mochizuki T, Tamiya G, McCouch SR, Ashikari M (2018) Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science 361(6398), 181-186.
| Crossref | Google Scholar | PubMed |
Kurokawa Y, Nagai K, Huan PD, Shimazaki K, Qu H, Mori Y, Toda Y, Kuroha T, Hayashi N, Aiga S, Itoh JI, Yoshimura A, Sasaki-Sekimoto Y, Ohta H, Shimojima M, Malik AI, Pedersen O, Colmer TD, Ashikari M (2018) Rice leaf hydrophobicity and gas films are conferred by a wax synthesis gene (LGF1) and contribute to flood tolerance. New Phytologist 218(4), 1558-1569.
| Crossref | Google Scholar | PubMed |
Lauridsen T, Glavina K, Colmer TD, Winkel A, Irvine S, Lefmann K, Feidenhans’l R, Pedersen O (2014) Visualisation by high resolution synchrotron X-ray phase contrast micro-tomography of gas films on submerged superhydrophobic leaves. Journal of Structural Biology 188(1), 61-70.
| Crossref | Google Scholar | PubMed |
Lee T-M, Lin Y-H (1995) Changes in soluble and cell wall-bound peroxidase activities with growth in anoxia-treated rice (Oryza sativa L.) coleoptiles and roots. Plant Science 106(1), 1-7.
| Crossref | Google Scholar |
Lee K-W, Chen P-W, Lu C-A, Chen S, Ho T-HD, Yu S-M (2009) Coordinated responses to oxygen and sugar deficiency allow rice seedlings to tolerate flooding. Science Signaling 2(91), ra61.
| Crossref | Google Scholar |
Lee K-W, Chen PW, Yu S-M (2014) Metabolic adaptation to sugar/O2 deficiency for anaerobic germination and seedling growth in rice. Plant, Cell & Environment 37(10), 2234-2244.
| Crossref | Google Scholar | PubMed |
Lin C-C, Chao Y-T, Chen W-C, Ho H-Y, Chou M-Y, Li Y-R, Wu Y-L, Yang H-A, Hsieh H, Lin C-S, Wu F-H, Chou S-J, Jen H-C, Huang Y-H, Irene D, Wu W-J, Wu J-L, Gibbs DJ, Ho M-C, Shih M-C (2019) Regulatory cascade involving transcriptional and N-end rule pathways in rice under submergence. Proceedings of the National Academy of Sciences of the United States of America 116(8), 3300-3309.
| Crossref | Google Scholar | PubMed |
Lin C, Ogorek LLP, Pedersen O, Sauter M (2021) Oxygen in the air and oxygen dissolved in the floodwater both sustain growth of aquatic adventitious roots in rice. Journal of Experimental Botany 72(5), 1879-1890.
| Crossref | Google Scholar | PubMed |
Lin C, Zhu T, Ogorek LLP, Wang Y, Sauter M, Pedersen O (2022) The pyramiding of three key root traits aid breeding of flood-tolerant rice. Plants 11(15), 2033.
| Crossref | Google Scholar |
Lin C, Ogorek LLP, Liu D, Pedersen O, Sauter M (2023) A quantitative trait locus conferring flood tolerance to deepwater rice regulates the formation of two distinct types of aquatic adventitious roots. New Phytologist 238(4), 1403-1419.
| Crossref | Google Scholar | PubMed |
Ling J, Ming-Yu H, Chun-Ming W, Jian-Min WJ (2004) Quantitative trait loci and epistatic analysis of seed anoxia germinability in rice (Oryza sativa). Rice Science 11, 238-244.
| Google Scholar |
Locke AM, Barding GA, Jr, Sathnur S, Larive CK, Bailey-Serres J (2018) Rice SUB1A constrains remodelling of the transcriptome and metabolome during submergence to facilitate post-submergence recovery. Plant, Cell & Environment 41(4), 721-736.
| Crossref | Google Scholar | PubMed |
Lorbiecke R, Sauter M (1999) Adventitious root growth and cell-cycle induction in deepwater rice. Plant Physiology 119(1), 21-30.
| Crossref | Google Scholar | PubMed |
Magneschi L, Kudahettige RL, Alpi A, Perata P (2009) Comparative analysis of anoxic coleoptile elongation in rice varieties: relationship between coleoptile length and carbohydrate levels, fermentative metabolism and anaerobic gene expression. Plant Biology 11(4), 561-573.
| Crossref | Google Scholar | PubMed |
Mhamdi A, Van Breusegem F (2018) Reactive oxygen species in plant development. Development 145(15), dev164376.
| Crossref | Google Scholar |
Miro B, Ismail AM (2013) Tolerance of anaerobic conditions caused by flooding during germination and early growth in rice (Oryza sativa L.). Frontiers in Plant Science 4, 269.
| Crossref | Google Scholar | PubMed |
Miro B, Longkumer T, Entila FD, Kohli A, Ismail AM (2017) Rice seed germination underwater: morpho-physiological responses and the bases of differential expression of alcoholic fermentation enzymes. Frontiers in Plant Science 8, 1857.
| Crossref | Google Scholar |
Mittler R, Zandalinas SI, Fichman Y, Van Breusegem F (2022) Reactive oxygen species signalling in plant stress responses. Nature Reviews Molecular Cell Biology 23(10), 663-679.
| Crossref | Google Scholar | PubMed |
Mongon J, Konnerup D, Colmer TD, Rerkasem B (2014) Responses of rice to Fe2+ in aerated and stagnant conditions: growth, root porosity and radial oxygen loss barrier. Functional Plant Biology 41(9), 922-929.
| Crossref | Google Scholar | PubMed |
Nagai K, Ashikari M (2023) Molecular mechanism of internode elongation in rice. Breeding Science 73(2), 108-116.
| Crossref | Google Scholar | PubMed |
Nagai K, Kuroha T, Ayano M, Kurokawa Y, Angeles-Shim RB, Shim J-H, Yasui H, Yoshimura A, Ashikari M (2012) Two novel QTLs regulate internode elongation in deepwater rice during the early vegetative stage. Breeding Science 62(2), 178-185.
| Crossref | Google Scholar | PubMed |
Nagai K, Mori Y, Ishikawa S, Furuta T, Gamuyao R, Niimi Y, Hobo T, Fukuda M, Kojima M, Takebayashi Y, Fukushima A, Himuro Y, Kobayashi M, Ackley W, Hisano H, Sato K, Yoshida A, Wu J, Sakakibara H, Sato Y, Tsuji H, Akagi T, Ashikari M (2020) Antagonistic regulation of the gibberellic acid response during stem growth in rice. Nature 584(7819), 109-114.
| Crossref | Google Scholar | PubMed |
Nghi KN, Tondelli A, Vale G, Tagliani A, Mare C, Perata P, Pucciariello C (2019) Dissection of coleoptile elongation in japonica rice under submergence through integrated genome-wide association mapping and transcriptional analyses. Plant, Cell & Environment 42(6), 1832-1846.
| Crossref | Google Scholar | PubMed |
Nishiuchi S, Yamauchi T, Takahashi H, Kotula L, Nakazono M (2012) Mechanisms for coping with submergence and waterlogging in rice. Rice 5, 2.
| Crossref | Google Scholar |
Nishiuchi S, Watanabe K, Sato S, Takahashi H, Nakazono M (2021) Expression analysis of genes for cytochrome P450 CYP86 and glycerol-3-phosphate acyltransferase related to suberin biosynthesis in rice roots under stagnant deoxygenated conditions. Plant Root 15(0), 19-35.
| Crossref | Google Scholar |
Pedersen O, Rich SM, Colmer TD (2009) Surviving floods: leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. The Plant Journal 58(1), 147-156.
| Crossref | Google Scholar | PubMed |
Pedersen O, Perata P, Voesenek LACJ (2017) Flooding and low oxygen responses in plants. Functional Plant Biology 44(9), iii-vi.
| Crossref | Google Scholar |
Pedersen O, Sauter M, Colmer TD, Nakazono M (2021) Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytologist 229(1), 42-49.
| Crossref | Google Scholar |
Peralta Ogorek LL, Pellegrini E, Pedersen O (2021) Novel functions of the root barrier to radial oxygen loss – radial diffusion resistance to H2 and water vapour. New Phytologist 231(4), 1365-1376.
| Crossref | Google Scholar | PubMed |
Peralta Ogorek LL, Takahashi H, Nakazono M, Pedersen O (2023a) The barrier to radial oxygen loss protects roots against hydrogen sulphide intrusion and its toxic effect. New Phytologist 238(5), 1825-1837.
| Crossref | Google Scholar | PubMed |
Peralta Ogorek LL, Jiménez JdlC, Visser EJW, Takahashi H, Nakazono M, Shabala S, Pedersen O (2023b) Outer apoplastic barriers in roots: prospects for abiotic stress tolerance. Functional Plant Biology
| Crossref | Google Scholar |
Peralta Ogorek LL, Song Z, Pellegrini E, Liu F, Tomasella M, Nardini A, Pedersen O (2023c) Root acclimations to soil flooding prime rice (Oryza sativa L.) for conditions of water deficit. Plant and Soil
| Crossref | Google Scholar |
Ponnamperuma FN (1972) The chemistry of submerged soils. Advances in Agronomy 24, 29-96.
| Crossref | Google Scholar |
Ramon M, Dang TVT, Broeckx T, Hulsmans S, Crepin N, Sheen J, Rolland F (2019) Default activation and nuclear translocation of the plant cellular energy sensor SnRK1 regulate metabolic stress responses and development. The Plant Cell 31(7), 1614-1632.
| Crossref | Google Scholar | PubMed |
Raskin I, Kende H (1983) How does deep water rice solve its aeration problem. Plant Physiology 72(2), 447-454.
| Crossref | Google Scholar | PubMed |
Raskin I, Kende H (1984a) Regulation of growth in stem sections of deep-water rice. Planta 160(1), 66-72.
| Crossref | Google Scholar | PubMed |
Raskin I, Kende H (1984b) Role of gibberellin in the growth response of submerged deep water rice. Plant Physiology 76(4), 947-950.
| Crossref | Google Scholar | PubMed |
Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M (2002) Green revolution: a mutant gibberellin-synthesis gene in rice. Nature 416(6882), 701-702.
| Crossref | Google Scholar | PubMed |
Sauter M (2013) Root responses to flooding. Current Opinion in Plant Biology 16(3), 282-286.
| Crossref | Google Scholar | PubMed |
Schmitz AJ, Folsom JJ, Jikamaru Y, Ronald P, Walia H (2013) SUB1A-mediated submergence tolerance response in rice involves differential regulation of the brassinosteroid pathway. New Phytologist 198(4), 1060-1070.
| Crossref | Google Scholar | PubMed |
Schreiber L (1999) Review article. Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. Journal of Experimental Botany 50(337), 1267-1280.
| Crossref | Google Scholar |
Shiono K, Ogawa S, Yamazaki S, Isoda H, Fujimura T, Nakazono M, Colmer TD (2011) Contrasting dynamics of radial O2-loss barrier induction and aerenchyma formation in rice roots of two lengths. Annals of Botany 107(1), 89-99.
| Crossref | Google Scholar | PubMed |
Shiono K, Yamauchi T, Yamazaki S, Mohanty B, Malik AI, Nagamura Y, Nishizawa NK, Tsutsumi N, Colmer TD, Nakazono M (2014) Microarray analysis of laser-microdissected tissues indicates the biosynthesis of suberin in the outer part of roots during formation of a barrier to radial oxygen loss in rice (Oryza sativa). Journal of Experimental Botany 65(17), 4795-4806.
| Crossref | Google Scholar | PubMed |
Shiono K, Yoshikawa M, Kreszies T, Yamada S, Hojo Y, Matsuura T, Mori IC, Schreiber L, Yoshioka T (2022) Abscisic acid is required for exodermal suberization to form a barrier to radial oxygen loss in the adventitious roots of rice (Oryza sativa). New Phytologist 233(2), 655-669.
| Crossref | Google Scholar | PubMed |
Singh P, Sinha AK (2016) A positive feedback loop governed by SUB1A1 interaction with MITOGEN-ACTIVATED PROTEIN KINASE3 imparts submergence tolerance in rice. The Plant Cell 28(5), 1127-1143.
| Crossref | Google Scholar | PubMed |
Song Z, Zonta F, Ogorek LLP, Bastegaard VK, Herzog M, Pellegrini E, Pedersen O (2022) The quantitative importance of key root traits for radial water loss under low water potential. Plant and Soil 482(1-2), 567-584.
| Crossref | Google Scholar |
Song Z, Zonta F, Peralta Ogorek LL, Bastegaard VK, Herzog M, Pellegrini E, Pedersen O (2023) The quantitative importance of key root traits for radial water loss under low water potential. Plant and Soil 482, 567-584.
| Crossref | Google Scholar |
Soukup A, Armstrong W, Schreiber L, Franke R, Votrubova O (2007) Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytologist 173(2), 264-278.
| Crossref | Google Scholar | PubMed |
Steffens B, Rasmussen A (2016) The physiology of adventitious roots. Plant Physiology 170(2), 603-617.
| Crossref | Google Scholar | PubMed |
Steffens B, Sauter M (2005) Epidermal cell death in rice is regulated by ethylene, gibberellin, and abscisic acid. Plant Physiology 139(2), 713-721.
| Crossref | Google Scholar | PubMed |
Steffens B, Sauter M (2009) Epidermal cell death in rice is confined to cells with a distinct molecular identity and is mediated by ethylene and H2O2 through an autoamplified signal pathway. The Plant Cell 21(1), 184-196.
| Crossref | Google Scholar | PubMed |
Steffens B, Wang J, Sauter M (2006) Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 223(3), 604-612.
| Crossref | Google Scholar | PubMed |
Steffens B, Geske T, Sauter M (2011) Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytologist 190(2), 369-378.
| Crossref | Google Scholar | PubMed |
Steffens B, Kovalev A, Gorb SN, Sauter M (2012) Emerging roots alter epidermal cell fate through mechanical and reactive oxygen species signaling. The Plant Cell 24(8), 3296-3306.
| Crossref | Google Scholar | PubMed |
Tamang B, Fukao T (2015) Plant adaptation to multiple stresses during submergence and following desubmergence. International Journal of Molecular Sciences 16(12), 30164-30180.
| Crossref | Google Scholar | PubMed |
Tong S, Kjaer JE, Peralta Ogorek LL, Pellegrini E, Song Z, Pedersen O, Herzog M (2023) Responses of key root traits in the genus Oryza to soil flooding mimicked by stagnant, deoxygenated nutrient solution. Journal of Experimental Botany 74(6), 2112-2126.
| Crossref | Google Scholar | PubMed |
Tuong TP, Singh AK, Siopongco JD, Wade LJ (2015) Constraints to high yield of dry-seeded rice in the rainy season of a humid tropic environment. Plant Production Science 3(2), 164-172.
| Crossref | Google Scholar |
van der Knaap E, Kim JH, Kende H (2000) A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth. Plant Physiology 122(3), 695-704.
| Crossref | Google Scholar | PubMed |
Verboven P, Pedersen O, Ho QT, Nicolai BM, Colmer TD (2014) The mechanism of improved aeration due to gas films on leaves of submerged rice. Plant, Cell & Environment 37(10), 2433-2452.
| Crossref | Google Scholar | PubMed |
Voesenek LACJ, Bailey-Serres J (2009) Plant biology: genetics of high-rise rice. Nature 460(7258), 959-960.
| Crossref | Google Scholar | PubMed |
Voesenek LACJ, Bailey-Serres J (2015) Flood adaptive traits and processes: an overview. New Phytologist 206(1), 57-73.
| Crossref | Google Scholar | PubMed |
Watanabe K, Takahashi H, Sato S, Nishiuchi S, Omori F, Malik AI, Colmer TD, Mano Y, Nakazono M (2017) A major locus involved in the formation of the radial oxygen loss barrier in adventitious roots of teosinte Zea nicaraguensis is located on the short-arm of chromosome 3. Plant, Cell & Environment 40(2), 304-316.
| Crossref | Google Scholar | PubMed |
Waters I, Morrell S, Greenway H, Colmer TD (1991) Effects of anoxia on wheat seedlings. Journal of Experimental Botany 42(11), 1437-1447.
| Crossref | Google Scholar |
Winkel A, Pedersen O, Ella E, Ismail AM, Colmer TD (2014) Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes. Journal of Experimental Botany 65(12), 3225-3233.
| Crossref | Google Scholar | PubMed |
Winkel A, Visser EJW, Colmer TD, Brodersen KP, Voesenek LACJ, Sand-Jensen K, Pedersen O (2016) Leaf gas films, underwater photosynthesis and plant species distributions in a flood gradient. Plant, Cell & Environment 39(7), 1537-1548.
| Crossref | Google Scholar | PubMed |
Xu K, Xu X, Fukao T, Canlas P, Maghirang-Rodriguez R, Heuer S, Ismail AM, Bailey-Serres J, Ronald PC, Mackill DJ (2006) Sub1A is an ethylene-response-factor-like gene that confers submergence tolerance to rice. Nature 442(7103), 705-708.
| Crossref | Google Scholar | PubMed |
Yadav UP, Ivakov A, Feil R, Duan GY, Walther D, Giavalisco P, Piques M, Carillo P, Hubberten H-M, Stitt M, Lunn JE (2014) The sucrose-trehalose 6-phosphate (Tre6P) nexus: specificity and mechanisms of sucrose signalling by Tre6P. Journal of Experimental Botany 65(4), 1051-1068.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Tanaka A, Mori H, Takamure I, Kato K, Nakazono M (2016) Ethylene-dependent aerenchyma formation in adventitious roots is regulated differently in rice and maize. Plant, Cell & Environment 39(10), 2145-2157.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Yoshioka M, Fukazawa A, Mori H, Nishizawa NK, Tsutsumi N, Yoshioka H, Nakazono M (2017) An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. The Plant Cell 29(4), 775-790.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Colmer TD, Pedersen O, Nakazono M (2018) Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiology 176(2), 1118-1130.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Tanaka A, Inahashi H, Nishizawa NK, Tsutsumi N, Inukai Y, Nakazono M (2019) Fine control of aerenchyma and lateral root development through AUX/IAA- and ARF-dependent auxin signaling. Proceedings of the National Academy of Sciences of the United States of America 116(41), 20770-20775.
| Crossref | Google Scholar | PubMed |
Yim H-K, Lim M-N, Lee S-E, Lim J, Lee Y, Hwang Y-S (2012) Hexokinase-mediated sugar signaling controls expression of the calcineurin B-like interacting protein kinase 15 gene and is perturbed by oxidative phosphorylation inhibition. Journal of Plant Physiology 169(15), 1551-1558.
| Crossref | Google Scholar | PubMed |
Yu X, Zhou L, Zhang J, Yu H, Xiong F, Wang Z (2015) Comparison of starch granule development and physicochemical properties of starches in wheat pericarp and endosperm. Journal of the Science of Food and Agriculture 95(1), 148-157.
| Crossref | Google Scholar | PubMed |
Yu S-M, Lee H-T, Lo S-F, Ho T-HD (2021) How does rice cope with too little oxygen during its early life? New Phytologist 229(1), 36-41.
| Crossref | Google Scholar | PubMed |
Yukiyoshi K, Karahara I (2014) Role of ethylene signalling in the formation of constitutive aerenchyma in primary roots of rice. AoB Plants 6, plu043.
| Crossref | Google Scholar |
Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ (2009) Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiology 149(4), 1860-1871.
| Crossref | Google Scholar | PubMed |


