Outer apoplastic barriers in roots: prospects for abiotic stress tolerance
Lucas León Peralta Ogorek A B * , Juan de la Cruz Jiménez
A B * , Juan de la Cruz Jiménez  A , Eric J. W. Visser
A , Eric J. W. Visser  C , Hirokazu Takahashi
C , Hirokazu Takahashi  D , Mikio Nakazono
D , Mikio Nakazono  D E , Sergey Shabala
D E , Sergey Shabala  E F and Ole Pedersen
E F and Ole Pedersen  A E *
A E *
A
B
C
D
E
F
Handling Editor: Rana Munns
Abstract
Floods and droughts are becoming more frequent as a result of climate change and it is imperative to find ways to enhance the resilience of staple crops to abiotic stresses. This is crucial to sustain food production during unfavourable conditions. Here, we analyse the current knowledge about suberised and lignified outer apoplastic barriers, focusing on the functional roles of the barrier to radial O2 loss formed as a response to soil flooding and we discuss whether this trait also provides resilience to multiple abiotic stresses. The barrier is composed of suberin and lignin depositions in the exodermal and/or sclerenchyma cell walls. In addition to the important role during soil flooding, the barrier can also restrict radial water loss, prevent phytotoxin intrusion, salt intrusion and the main components of the barrier can impede invasion of pathogens in the root. However, more research is needed to fully unravel the induction pathway of the outer apoplastic barriers and to address potential trade-offs such as reduced nutrient or water uptake. Nevertheless, we suggest that the outer apoplastic barriers might act as a jack of all trades providing tolerance to multiple abiotic and/or biotic stressors.
Keywords: abiotic stress, cell walls, drought tolerance, lignin, nutrient acquisition, oxygen deficiency, suberin, salinity stress, toxins, waterlogging.
Introduction
Climate change is increasing the frequency and intensity of extreme weather events limiting crop production and reducing food supply worldwide (IPCC 2019). Of these, floods, drought and soil salinity are considered to be major ones and account for ~US$130 billion p.a. losses in crop and pasture productivity (Razzaq et al. 2021). Moreover, water supply for agricultural uses becomes increasingly scarce with those mainly affected being in the Global South (Rijsberman 2006) and the quality of irrigation water is gradually deteriorating (Liu et al. 2020), leading to land salinisation. Increasing the crops’ resilience to abiotic stresses is therefore utterly important (Lynch 2007; Palmgren et al. 2015). Is there a root trait that can be a ‘silver bullet’ for these abiotic stresses? The present paper focuses on the outer apoplastic barriers, which have been shown to be a ‘jack of all trades’ as these provide tolerance to: (1) soil flooding, (2) water deficit (as under drought and saline conditions) and (3) protects roots from soil phytotoxins (e.g. in contaminated soils). This new broad term proposed here encompasses the various apoplastic barriers formed in the outer parts of the root, such as the exodermis and sclerenchyma layers, to respond to different environmental cues. The term ‘outer’ implies the location of these apoplastic barriers in the periphery of the root tissues rather than in the central parts (endodermis). We describe the environmental signals related to the barrier formation, present difficulties to unravel its induction pathway and discuss whether this root trait could be the single solution to the multiple abiotic stresses challenging crop production. Flooding literature constitutes the backbone of our review since most research on the outer apoplastic barriers is based on the so called ‘barrier to radial O2 loss’ (ROL) formed as a response to soil flooding. Lastly, we suggest that the term ‘outer apoplastic barriers’ is a more fitting new name rather than barrier to radial O2 loss, given all the additional functions these provide besides preventing radial O2 loss.
Contrasting water availability impact key soil properties
Excessive water results in profound changes in physical, chemical, and biological properties of the soil causing acclimation in key root anatomical traits. The exchange of gases between the soil and atmosphere is extremely slow in flooded soils because gas diffusion in water is 10 000-fold slower than in air (Armstrong 1979). Flooded soils are therefore typically anoxic or severely hypoxic with a low redox potential, since O2 consumption by roots and soil microorganisms is faster than resupply of O2 from the atmosphere (Ponnamperuma 1972). Lack of O2 leads to impaired root respiration, nutrient uptake and root growth due to a significant decline in ATP production (Gibbs and Greenway 2003; Colmer et al. 2015). Tissue anoxia in the roots not only halts root elongation (Armstrong and Webb 1985) but can also cause root apex death (Armstrong et al. 1991). In addition, anaerobic microorganisms use alternative electron acceptors such as Fe2O2 or SO42− in the absence of O2 for their metabolism (Laanbroek 1990), leading to the production of reduced iron (Fe2+) or sulfides, which can reach toxic concentrations for plant growth (Armstrong et al. 1991).
On the other end of the spectrum from flooding is water deficit. Low soil water potential occurs under drought conditions and is also observed in the presence of high amounts of dissolved salts in the rhizosphere (hence, prompting salinity being called a ‘physiological drought’) (Ma et al. 2020). Low soil water potential increases the tensile forces that retain water with the soil particles, limiting water and nutrient uptake by roots. Soil desiccation also reduces soil porosity, as the soil particles are pulled closer together, making the soil harder and restricting root elongation (Bengough et al. 2006). Since compacted soils, i.e. low porosity soils, restrict root elongation, the volume of soil explored is reduced and thus access to potential resources is impaired (Bengough et al. 2011). As the soils become drier, water uptake can be further restricted due to the loss of root–soil contact caused by root shrinkage, when the cortical tissue contracts (Carminati et al. 2009). At the physiological level, low soil water potential triggers stomata closure in leaves, thus reducing their ability to assimilate CO2 (hence, causing yield penalties). Nutrients delivery to the root surface is compromised (due to reduction in both mass flow and diffusion) and reduced transpiration rates caused by stomatal closure affect nutrient transport to the shoot. In addition, low soil water potential severely reduces the mineralisation of nitrogen and carbon, leading to changes in the soil microbial communities as these nutrients become limited (Yahdjian et al. 2006; Zhang et al. 2019). Consequently, the microbial activity of the soil changes, causing soil nutrient deficiencies since the recycling of nitrogen, carbon and phosphorous is diminished (Sardans and Peñuelas 2005).
Root acclimations to contrasting water availability
Aerenchyma formation and changes in root thickness
Root acclimation to drought or flooding share some key anatomical features. Both flooding and water deficit can induce the formation of large gas filled spaces (aerenchyma) as a result of programmed cell death (Fig. 1). In such scenarios, aerenchyma formation reduces: (1) the metabolic maintenance cost of the root, (2) the amount of water in root tissues and (3) the amount of C, N and P allocated to roots (Zhu et al. 2010; Yamauchi et al. 2021). While in flooded conditions, aerenchyma formation enables effective O2 diffusion from the above water tissues down to the roots and saves water and organic carbon during drought conditions, i.e. longer roots can be formed with the same investment in water and carbon.
Root acclimations to contrasting water availability. Root cross sections before (left side) and after (right side) acclimating to either soil flooding or water deficit. The enhancement of aerenchyma formation (right side) is the result of programmed cell death in order to reduce the maintenance cost (water, energy, nutrients) of the root and to improve gas diffusion longitudinally through the tissues. In this conceptual diagram, the increased root thickness is driven by the enlargement of the cortex to allow for more space to form aerenchyma and to increase the mechanical strength of the root. Ex, exodermis; Scl, sclerenchyma (black arrows); Co, cortex; En, endodermis; Ae, aerenchyma (gas-filled spaces); St, stele. Cross-section images from Peralta Ogorek (2022).

In addition to aerenchyma, root thickness also increases during flooding or drought conditions (Fig. 1). Soil flooding can induce the enlargement of the cortex to enable larger cross-sectional aerenchymatous tissues, which further facilitates diffusional supply of molecular O2 to the root (Yamauchi et al. 2019). Thicker roots also have a reduced radial O2 loss to the anoxic soils due to the lower surface area to volume ratio, improving the oxygen status of root tissues (Pedersen et al. 2021). Interestingly, root thickening can also be induced during drought to increase the mechanical strength of the roots and the capability to penetrate harder soils (Yu et al. 1995; Bengough et al. 2011).
Endodermis, exodermis and sclerenchyma function and development
The endodermis, the innermost cell layer of the cortex, develops Casparian bands (Enstone et al. 2002). Casparian bands are cell wall modifications composed mainly by lignin and suberin (these two biopolymers are introduced in the following section), creating an apoplastic barrier. Casparian bands can mature between 0.1 and 10 mm within the root tip, depending on the species (Ma and Peterson 2003). The blockage of the apoplastic pathway imposed by the endodermis restricts the free movement of ions into the stele, forcing them to traverse the selective symplastic pathway (Enstone et al. 2002). In addition, it has been suggested that the endodermis also prevents the backflow of ions accumulated in the inner root tissues (Ranathunge et al. 2011a). With time, some roots will develop suberin lamella acting as a secondary wall in certain endodermal cells, whereas other cells lack suberin lamella and act passage cells (Ma and Peterson 2003). The function of the suberin lamella is attributed to further regulate water movement. (Enstone et al. 2002).
The exodermis and sclerenchyma cell layers are found in the outer parts of the root. The exodermis is formed in most species when the hypodermis (peripheral cortical cells) develops Casparian bands in anticlinal cell walls and suberin lamellae start to form quicker than in the endodermis, sometimes even before the Casparian bands (Hose et al. 2001). Being an apoplastic barrier, the exodermis blocks the free passage of ions and water much like the apoplastic barrier of the endodermis. However, the exodermis forms further behind the root tip compared to the endodermis (Ma and Peterson 2003). Therefore, the endodermis starts restricting free flow of ions and water in much younger root tissues than the exodermis (Hose et al. 2001). In addition, the exodermis tends to be more discontinuous than the endodermis (Lux et al. 2004). In contrast, beneficial functions of the exodermis may be attributed under stress conditions (Hose et al. 2001). Other peripheral cells may also develop differently than the exodermis, forming sclerenchyma cell layers that are heavily lignified, but formation of sclerenchyma mostly occurs in monocotyledonous plants (Lux et al. 2004). The sclerenchyma is associated with mechanical functions of the root, preventing bending, stretching and other physical stresses (Schneider et al. 2021).
The degree of suberisation and lignification, i.e. how much suberin and lignin is deposited in the cell walls, is regulated by tissue age and environmental stresses such as drought, flooding, salt stress, pathogen invasion, etc. (Enstone et al. 2002; Ma and Peterson 2003; Ranathunge et al. 2011a). More detailed information on the progressive and tissue age-dependant suberisation and lignification has been the focus of other reviews (Hose et al. 2001; Kreszies et al. 2018; Serra and Geldner 2022). In this paper, we focus on the environmental cues that enhance suberin and lignin depositions in these peripheral cell layers (exodermis, sclerenchyma), forming outer apoplastic barriers, and their various functions under stress conditions.
The various suberin and lignin biopolymers involved in cell wall modifictions
The formation of these outer apoplastic barriers in roots coincides with histochemical visualisations of enhanced suberin and lignin depositions in cell walls of the outer layers of roots (i.e. exodermis and sclerenchyma (Kotula et al. 2009; Meyer et al. 2011, Ranathunge et al. 2011b; Abiko et al. 2012; Jiménez et al. 2019, 2021). Suberin and lignin are biopolymers; suberin polymer contains polyaliphatic and polyaromatic domains, where ω-hydroxycarboxylic acids, α,ω-diacids, fatty acids and primary alcohols are the main aliphatic monomers, whereas hydroxycinnamic acids are the main components of the aromatic domain (Kolattukudy 1984). Lignin polymers are derived mainly from three hydroxycinnamyl alcohol monomers (p-coumaryl, coniferyl and sinapyl alcohols; Boerjan et al. 2003). Suberin and lignin depositions are part of the outer apoplastic barriers restricting the axial bidirectional movement of ions, gases and compounds within roots and/or between roots and the rhizosphere (Hose et al. 2001; Enstone et al. 2002; Ma and Peterson 2003; Ranathunge et al. 2011a), although to our knowledge the specific functions of each polymer has not yet been clarified. Overall, roots can induce similar responses in contrasting evironmental conditions (e.g. high or low soil water content), illustrating that the same root trait can be beneficial in contrasting environmental settings.
Suberised and lignified outer apoplastic barriers: the barrier to radial O2 loss
Root responses to flooding include the formation of an apoplastic barrier in the outer part of the root to impede radial O2 loss (Fig. 2). In flooded soils, the formation of such barriers impedes radial O2 loss from roots to the rhizosphere, thereby enhancing internal, longitudinal O2 diffusion from the shoot to the roots, and thus improving root respiration, nutrient uptake and root penetration into anoxic soils (Colmer 2003a). Interestingly, the barrier to radial O2 loss has also been shown to greatly restrict radial loss of water from roots, indicating a role in drought tolerance for this root trait (Peralta Ogorek et al. 2021; Song et al. 2023). While the formation of such barrier can limit water uptake to root regions where the barrier is not formed, it also prevents the backflow of water from the root to the soil (Ranathunge et al. 2011a) thus limiting water loss (Brunner et al. 2015). In some species (e.g. rice (Oryza spp.) and Hordeum marinum), the barrier can be inducible (Colmer 2003b), but in others (e.g. in wild rice, the genera of Echinochloa and Urochloa), the barrier is constitutively expressed (Ejiri and Shiono 2019; Jiménez et al. 2019). In the following sections, we refer to the barrier to radial O2 loss as an ‘outer apoplastic barrier’.
Functions of outer apoplastic barriers. The loss of O2 (blue arrows) occurs closer to the root–shoot junction in the absence (left side) of barrier to radial O2 loss (outer apoplastic barrier). Once the barrier is formed (right side), radial O2 loss takes place closer to the root apex as the barrier enhances longitudinal diffusion of O2 in combination with enhanced aerenchyma formation. The figure summarises several alternative functions of the barrier (restricted Fe2+, H2S, Na+ and Cl− intrusion, restricted radial water loss). Question marks indicate that the barrier might play a role against organic acids intrusion or pathogen invasion, but these functions are currently untested. Figure adapted from Yamauchi et al. (2018).
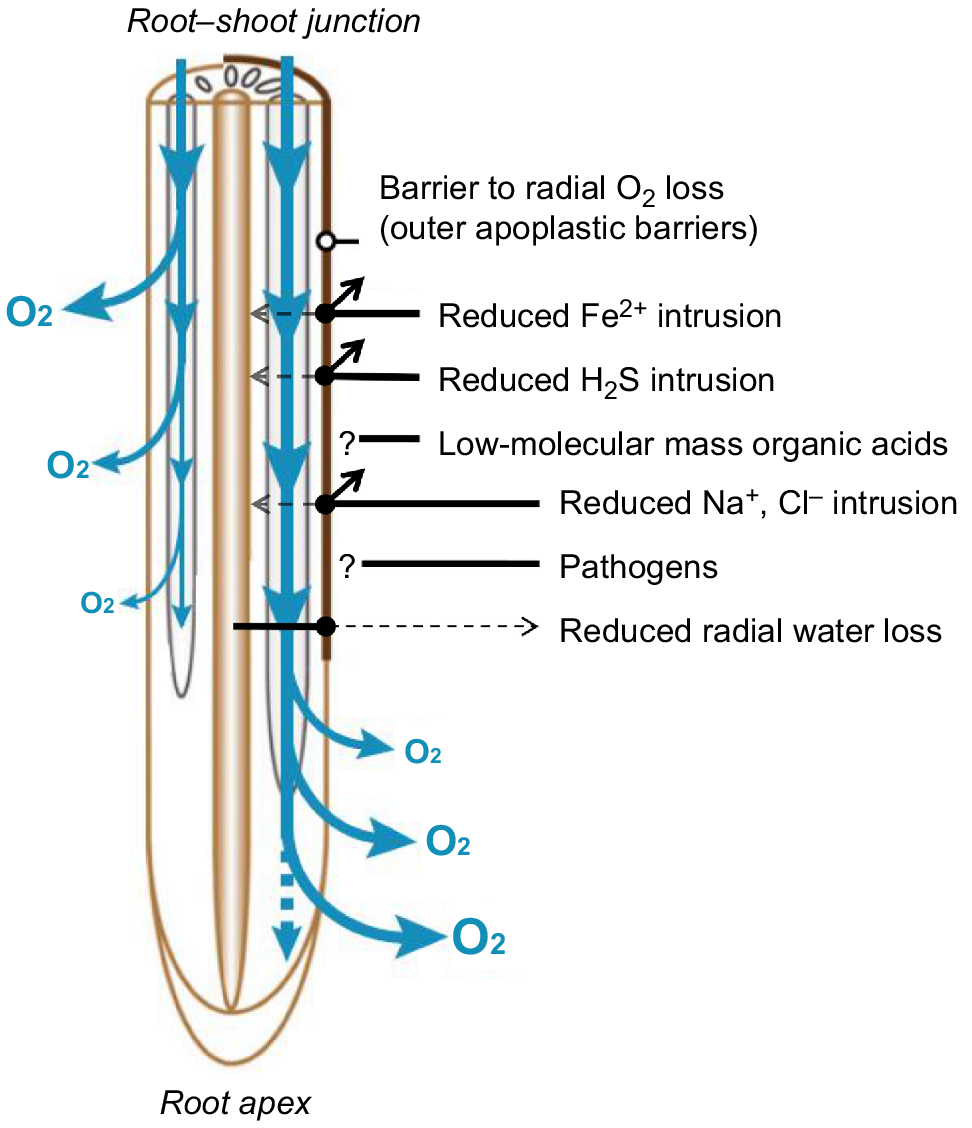
Alternative functions of outer apoplastic barriers
The outer apoplastic barriers not only restricts O2 diffusion, but also restricts intrusion of phytotoxins into the roots that are produced by facultative or obligate anaerobes during soil flooding. It has been widely hypothesised that the barriers can prevent intrusion of H2S (Armstrong and Armstrong 2005; Soukup et al. 2007; Nishiuchi et al. 2012) and this has only recently been experimentally tested. Experimental setups using H2S microsensors revealed that the apparent permeance to H2S of the outer parts of the root was reduced by almost 99% by the outer apoplastic barriers (Peralta Ogorek et al. 2023). The protective role was demonstrated at the physiological level; root tissue respiration was unaffected in roots with outer apoplastic barriers by external H2S concentrations up to 500 μM (cytochrome c oxidase is inhibited already in the range of 1–10 μM H2S; Fenchel and Finlay 1995). Moreover, H2S intrusion at the root–shoot junction was greatly restricted by the presence of the outer apoplastic barriers, indicating that H2S diffusion to the shoot of intact plants is also restricted, demonstrating the importance of this trait not only for the roots, but also at the whole plant level (Peralta Ogorek et al. 2023). Besides H2S, the outer apoplastic barriers also restrict the diffusion of water vapour and hydrogen gas (Peralta Ogorek et al. 2021), suggesting that suberised and lignified apoplastic barriers might be capable of restricting the diffusion of gases in general.
Very recently, the outer apoplastic barriers have been proposed to restrict venting of greenhouse gases from rice (Jimenez and Pedersen 2023), where methane (CH4) and nitrous oxide (N2O) emissions from paddy rice contribute with 8 and 10%, respectively, to global anthropogenic emissions (Saunois et al. 2020; Wang et al. 2020). The idea is that tight outer apoplastic barriers would restrict radial diffusion of greenhouse gases produced in the soil into the roots, and thereby reduce plant-mediated diffusion with subsequent reduction in emission via the shoot (Jimenez and Pedersen 2023).
Besides gases, intrusion of toxic reduced iron (Fe2+) from the anoxic soil is also prevented in areas where fully suberised lamellae have developed in the root exodermis (Jiménez et al. 2021). Interestingly, a 1.5-fold increase in the amount of hypodermal suberin and a 2-fold increase in lignin content were reported in Cd-treated roots (Schreiber et al. 1999), suggesting a role of these cell wall components in root adaptation to heavy metal toxicity. Even stronger evidence exists for the essential role of apoplastic barriers in salinity tolerance, and reinforcement of apoplastic barriers (present in the outer part of the root or endodermis) has been nominated as key targets for breeding of salt tolerant crops (Cui et al. 2021). Such a breeding strategy is justified by a significant reduction in radial apoplastic movements of Na+ and Cl− by the outer apoplastic barriers and a subsequent reduction in translocation to the shoot of rice (Ranathunge et al. 2011b).
The biochemical composition of the exodermal cell walls significantly restricts radial loss of water from the roots. Species with a root exodermis showed reduced radial water loss when compared to species without exodermis (Taleisnik et al. 1999). In addition, Iris germanica roots can form a multiseriated exodermis under dry conditions restricting water permeability (Meyer et al. 2011). Rice roots possess an exodermis that restricts radial water loss to a certain extent. However, if suberised and lignified outer apoplastic barriers are formed, radial water loss from roots is further restricted by 93%, indicating a potential role in drought tolerance for this root trait (Song et al. 2023). In rice, the outer apoplastic barriers can limit water uptake to root regions where the barriers are not formed, i.e. short lateral roots (Noorrohmah et al. 2020), and prevent the backflow of water from the root to the soil (Ranathunge et al. 2011a), thus limiting water loss (Brunner et al. 2015). Accordingly, root hydraulic conductance in rice is only positively correlated to the number of short laterals and not other types of roots (Watanabe et al. 2020). In a drought situation, the topsoil will be significantly drier than the deeper soil, and in such conditions suberised and lignified outer barriers in proximal roots would restrict radial loss to the topsoil of water taken up from deeper layers of the soil.
Suberin, a key component of these apoplastic barriers, has been shown to serve an important role against pathogen invasion. Roots with suberin depositions containing higher aliphatic domains provided resistance to fungal invasion, while those with higher aromatic domains were relevant against bacterial infection (Lulai and Corsini 1998; Ranathunge et al. 2011a). In addition, increased amounts of constitutive suberin depositions in roots of soybean were correlated with higher resistance to Phytophthora sojae causing stem and root rot, compared to roots with less suberin (Thomas et al. 2007). Thus, there is growing evidence that suberised outer apoplastic barriers also provide benefits against soil phytotoxins and pathogens.
Lignin, another key component of apoplastic barriers, has been shown to increase the mechanical strength for root penetration in hard soils. Roots with multiseriate sclerenchyma had higher lignin concentrations and improved root penetration in hard soils (Schneider et al. 2021). These studies suggest that the presence of outer apoplastic barriers, by enhancing tissue suberisation and lignification, may provide tolerance to pathogens or even mechanical strength to penetrate hard soils.
In summary, the outer apoplastic barriers and/or their components provide a wide variety of benefits against several abiotic and potentially biotic stressors (Fig. 2), making these a strong focal point in the process of improving crop resilience in a changing environment.
Environmental signals induce the formation of outer apoplastic barriers
The formation of outer apoplastic barriers can be induced by several environmental cues. As mentioned in the previous sections, soil flooding triggers the formation of outer apoplastic barriers functioning as a barrier to radial O2 loss (Colmer 2003a). Interestingly, it is not the lack of O2 nor the accumulation of CO2 or the gaseous hormone ethylene that induces the formation of such barriers (Colmer et al. 2006). In rice, the outer apoplastic barriers restrict radial O2 loss and can be induced in stagnant deoxygenated nutrient solutions (Colmer et al. 1998), but the chemical component(s) acting as a signal for barrier formation in hydroponic culture has not yet been identified.
Several studies have instead focused on soil phytotoxins produced in anoxic soils as chemical candidates involved in signalling for barrier formation (Fig. 2). Phytotoxins such as low-molecular mass organic acids, which are produced by anaerobic bacteria, have been shown to induce the barrier at low, sub-toxic concentrations (Kotula et al. 2014; Colmer et al. 2019) and at comparatively higher concentrations where these compounds are already toxic (Armstrong and Armstrong 2001). Similarly for reduced iron, which is also toxic to roots (Becker and Asch 2005), supplying high concentrations of Fe2+ to aerated nutrient solutions that mimic well-oxygenated soils induced outer apoplastic barriers in rice (Mongon et al. 2014). Outer apoplastic barriers can also be induced in rice by sub-toxic concentrations of H2S (Peralta Ogorek et al. 2023), which is produced by sulfate-reducing bacteria and is highly toxic to plants (Raven and Scrimgeour 1997). Moreover, outer apoplastic barriers can be further enhanced in rice using higher and severely toxic concentrations of H2S (Armstrong and Armstrong 2005). Therefore, the formation of outer apoplastic barriers seems tightly linked to the presence of phytotoxins, but, surprisingly, also to conditions of water-deficit, where such phytotoxins would not be found in the soil. At present, environmental signals for the formation of outer apoplastic barriers under water deficit have not been identified (Song et al. 2023).
Interestingly, some studies demonstrated that the extent of suberisation and lignification of root tissues is strongly influenced by soil nutrient stress. Accelerated biosynthesis of lignin was reported in rice under conditions of high ammonium concentrations (Ranathunge et al. 2016), and lignin biosynthesis genes were also highly upregulated in apple (Malus spp.) rootstock under low N conditions (Wang et al. 2023).
Genetic regulation of the formation of outer apoplastic barriers
The development of outer apoplastic barriers have been shown to be beneficial for plants growing in flooded or drought conditions. However, the molecular mechanisms and genetic regulations controlling this root trait remain largely unknown, and only studied on the barrier to radial O2 loss. Conventional hybridisation of wild-type flood tolerant species with their flood sensitive relatives and subsequent pyramidisation of quantitative trait loci (QTL) for formation of an outer apoplastic barrier have been a key strategy for gene discovery and development of plants with barriers (Mano and Omori 2013). Successful attempts to hybridise wild-type flood tolerant species with their agriculturally important crop relatives have been done for maize (Zea spp.) and wheat (Triticum aestivum). QTL mapping from flood sensitive maize × flood tolerant Zea nicaraguensis progenies revealed that a segment of the short-arm of chromosome 3 of the Z. nicaraguensis conferred barrier formation to the maize inbred line (Watanabe et al. 2017). In addition, amphiploids developed through wide hybridisation of flood sensitive wheat × flood tolerant Hordeum marinum, showed an outer apoplastic barrier when grown in low O2 conditions, in comparison to no barrier formation of the wheat donor lines (Malik et al. 2011).
The use of laser capture microdissection technologies and cell type specific gene expression tools are fundamental to achieve the resolution needed to understand the genetic signature encoding the development of outer apoplastic barriers (cf. Nakazono et al. 2003; Reynoso et al. 2022). Gene expression profiles from isolated hypodermal/exodermal roots of rice suggest that WRKY, AP2, MYB and NAC transcription factors regulate suberin biosynthesis in rice roots grown in anoxic conditions (Shiono et al. 2014), whereas MYB and NAC were key transcription factors modulating an upregulated pattern for suberin biosynthesis in exodermal cells of rice roots grown under water deficit conditions (Reynoso et al. 2022). Gene expression profiles indicated that 128 genes were significantly up- or downregulated in the hypodermal/exodermal layers of rice roots during formation of an outer apoplastic barrier, where several genes associated with suberin biosynthesis were strongly upregulated (Shiono et al. 2014). Most of those genes that were upregulated during barrier formation were also highly enhanced by exogenous abscisic acid (ABA), suggesting that ABA is an inducer of exodermal suberin lamellae in rice roots (Shiono et al. 2022). However, the signal(s) that triggers an ABA, or other signalling network, to form an outer apoplastic barrier in roots is not known. Interestingly, gene expression analyses during barrier formation induced by low concentrations of H2S revealed a significant upregulation of genes related to lignin (Peralta Ogorek et al. 2023). During exposure to comparatively high concentrations of organic acids, target genes also related to lignin biosynthesis (cinnamyl alcohol dehydrogenase, Os01g0528800 (CADL) and cinnamoyl CoA reductase, Os02g0811800 (OsCCR10), respectively) were significantly upregulated (Colmer et al. 2019). The prevalence of either suberin or lignin during the formation of the outer apoplastic barriers suggest that the environmental conditions, which the roots are subjected to, might influence synthesis of these biopolymers.
Progress in understanding the regulatory factors and genes associated with suberisation and/or lignification of hypodermal/exodermal cells in roots forming barriers remains slow. Major difficulties to achieve such progress include: (1) the formation of such barriers is developmentally complex and cell-type specific, (2) inter- and intraspecific differences in suberin and/or lignin monomeric composition of the exodermis cell walls, (3) outer apoplastic barriers can be a constitutive or inducible, (4) outer apoplastic barriers can be triggered by different environmental conditions and (5) formation of outer apoplastic barriers is dependent of root tissue age/development.
Trade-offs of outer apoplastic barriers
Can the outer apoplastic barriers, including the barrier to radial O2 loss, be a ‘silver bullet’ for abiotic stress tolerance in crops – at least, for flood, drought, and salinity tolerance? Will the increased suberisation/lignification of plant roots come with potential penalties for plant nutrient acquisition? What are the possible trade-offs, and can they be accepted?
Radial uptake of essential nutrients in plants occurs via two parallel pathways: apoplastic and symplastic. In broad terms, the apoplastic transport will be affected by the presence of two barriers: an external one (in the exodermis); and an internal one (in the endodermis) (Schreiber et al. 1999; Barberon and Geldner 2014). Both suberin and lignin can play a major role in establishing an apoplastic transport barrier in exodermis as well as in endodermis (Schreiber et al. 1999; Naseer et al. 2012; Reyt et al. 2021). Also, while most of the knowledge comes from studies on the endodermis, Casparian strips can form in the hypodermis (Reinhardt and Rost 1995; Enstone and Peterson 1998). Hence, some analogies between deposition of suberin and/or lignin in exo- and endodermis can likely be made.
An increase in exodermal and endodermal suberin deposition affected root radial transport properties, with a 2–3 -fold decrease in root hydraulic conductivity reported in Zea mays (Zimmermann and Steudle 1998). Similarly, in Arabidopsis thaliana, horst mutants, which have delayed suberin lamellae formation and lower suberin content in the endodermis had higher hydraulic conductivity compared with the wild-type (Höfer et al. 2008). However, the esb1 mutant characterised by enhanced root suberisation likely in the endodermis as Arabidopsis roots lack an exodermis, displayed increased water use efficiency (Baxter et al. 2009; Hosmani et al. 2013). As for essential nutrients, modulation of suberin-degrading enzymes in Arabidopsis demonstrated that ectopic suberisation in endodermal cells limits Ca transport and delivery to the shoot (Kamiya et al. 2015). However, while an ectopic suberisation in esb1 mutant was associated with a decreased accumulation of Ca, Mn and Zn, it also increased content of K and Mo, revealing the complex relationship between the extent of root suberisation and selective nutrient uptake/transport. Similarly, the sgn3 Arabidopsis mutant with a defective Casparian band in the endodermis caused by alterations in lignin biosynthesis shows increased Mg but reduced Zn and K phenotype (Pfister et al. 2014). In barley, less suberin in the endodermis favoured the inwards radial transport of Ca but negatively affected K concentration in the stele (Chen et al. 2019).
Of specific interest is an impact of suberisation on plant adaptive responses to salinity. In Pongamia pinnata roots, increased exodermal suberin deposition was associated with decreased Na+ uptake (Marriboina and Attipalli 2020), and in Arabidopsis reducing the extent of endodermal suberisation by overexpressing cutinase (CDEF1) led to increased Na+ accumulation and salt-sensitive phenotype (Barberon et al. 2016). Moreover, Na+ and Cl− permeabilities were restricted in rice roots that developed outer apoplastic barriers in conditions mimicking soil flooding, but such barriers did not reduce the root hydraulic conductivity (Ranathunge et al. 2011b). Nevertheless, some plants use Na+ as a ‘cheap osmotic’ solute. This phenomenon is observed not only for halophytic species but also for many salt tolerant crops (Chen et al. 2007; Shabala 2013; Puniran-Hartley et al. 2014; Rawat et al. 2022). Hence, the effectiveness of the mechanisms preventing sodium flow into the plants will likely depend on the importance of sodium exclusion for salt tolerance of different plant species. Sodium accumulation in vacuoles is used for osmotic adjustment, thereby maintaining the gradient of water potential between roots and external solution. Preventing Na+ uptake by apoplastic barriers may therefore potentially increase the carbon cost for plant osmotic adjustment by forcing plants to rely on de novo synthesis of organic osmolytes, which comes with high energy cost (Munns et al. 2020).
One of the anatomical hallmarks of rice is the presence of so-called ‘bypass flow’, originating from the breakage of the integrity of Casparian band by lateral roots (Flam-Shepherd et al. 2018; Ishikawa and Shabala 2019). As a result, the SOS-mediated mechanism for Na+ exclusion that is considered to be the major mechanism for preventing Na+ toxicity (Zhao et al. 2020) is not efficient in rice, as the presence of the apoplastic bypass flow may result in a futile cycle, depleting metabolic energy but not achieving a significant reduction in Na+ influx. Tight outer apoplastic barriers may, however, overcome this limitation. Indeed, the salt tolerant rice cultivar Pokkali is capable of forming strong suberised barriers in both exo- and endodermis, and it is correlated with reduced Na+ accumulation in the shoot (Krishnamurthy et al. 2009). In addition, when exposed to high Na+ concentrations, the lateral root density increases without creating a significant bypass flow for Na+ (Krishnamurthy et al. 2011). It would be interesting to test whether the enhanced suberisation caused by Na+ would also restrict radial O2 loss, as salt stress can often occur together with soil flooding (Colmer and Flowers 2008), although it would likely be the case since the barrier to radial O2 loss reduced the root permeability to Na+ and Cl− (Ranathunge et al. 2011b). Regardless, these studies clearly indicate that despite the potential breakage of the apoplastic barrier integrity caused by lateral root emergence creating a window for Na+ intrusion, this effect is compensated by enhanced suberisation of exo- and endodermis.
So, what about other species? Will the benefits from reducing Na+ uptake by forming outer apoplastic barriers overcome possible issues with uptake of essential nutrients? Can potentially detrimental effects of suberisation of the apoplast be compensated for by increased activity of transporters of these elements? Or will the hydraulic conductivity in other species be negatively affected by the presence of outer apoplastic barriers, ultimately impacting nutrient uptake and shoot growth? While the explicit answers to these questions are yet to come, a possible solution could be in turning our attention towards the symplastic pathway of nutrient acquisition and, specifically, using tissue-specific promoters for preferential expression of key nutrient transporters in the root hairs. For example, accumulation of mRNA for the LeNrtl-2 nitrate transporter was restricted exclusively to root hairs, while LeNrtl-1 transcripts were detected in both root hairs and other root tissues (Lauter et al. 1996). Thus, the possibility of engineering plants with a dominant symplastic pathways of nutrient uptake is certainly plausible.
Conclusions and perspectives
The term ‘barrier to radial O2 loss’ was coined several decades ago, but an extensive array of functions beyond that of restricting radial O2 loss have been discovered since then. Therefore, a more appropriate name for this apoplastic and gas-tight barrier in the periphery of the root is ‘outer apoplastic barriers’.
The outer apoplastic barriers are indeed a ‘jack of all trades’ as these provide tolerance to soil flooding, drought, and salinity stress, and protect roots from various soil phytotoxins as well. However, more attention needs to be placed on the possible trade-offs of the barriers, particularly across species, before this trait can be used to improve abiotic stress tolerance in crops sensitive to soil flooding, salinity, or drought. Moreover, we also propose to further unravel the genetic network involved during the barrier formation in the outer parts of the root with particular emphasis on species forming constitutive outer apoplastic barriers such as in Urochloa humidicola (Jiménez et al. 2019) or wild rice relatives (Ejiri et al. 2020). This would help reducing the complexity of studying this root trait present in species with inducible barriers.
Conflicts of interest
Authors JJ, SS and OP are Editors of Functional Plant Biology. To mitigate this potential conflict of interest they were blinded from the review process. The authors declare no other conflicts of interest.
Declaration of funding
Financial support provided by EU Horizon 2020 Talent program (LLPO), the Danish International Development Agency, DANIDA (grant no. 19-03-KU to OP), the Independent Research Fund Denmark (grant no. 8021-00120B to LLPO and OP), EMBO Postdoctoral Fellowships (EMBO ALTF 619-2022 to LLPO) and the Marie Skłodowska-Curie Postdoctoral Fellowships (grant no. 101061962, SmartRoots to JdlCJ).
Acknowledgements
We thank Dr. Bipin Pandey for comments on the manuscript, and the constructive comments by the referees are much appreciated. Open access publishing facilitated by The University of Western Australia, as part of the CSIRO Publishing – The University of Western Australia agreement via the Council of Australian University Librarians.
References
Abiko T, Kotula L, Shiono K, Malik AI, Colmer TD, Nakazono M (2012) Enhanced formation of aerenchyma and induction of a barrier to radial oxygen loss in adventitious roots of Zea nicaraguensis contribute to its waterlogging tolerance as compared with maize (Zea mays ssp. mays). Plant, Cell & Environment 35(9), 1618-1630.
| Crossref | Google Scholar | PubMed |
Armstrong J, Armstrong W (2001) Rice and Phragmites: effects of organic acids on growth, root permeability, and radial oxygen loss to the rhizosphere. American Journal of Botany 88(8), 1359-1370.
| Crossref | Google Scholar | PubMed |
Armstrong J, Armstrong W (2005) Rice: sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Annals of Botany 96(4), 625-638.
| Crossref | Google Scholar | PubMed |
Armstrong W, Webb T (1985) A critical oxygen pressure for root extension in rice. Journal of Experimental Botany 36(10), 1573-1582.
| Crossref | Google Scholar |
Armstrong W, Justin SHFW, Beckett PM, Lythe S (1991) Root adaptation to soil waterlogging. Aquatic Botany 39(1–2), 57-73.
| Crossref | Google Scholar |
Barberon M, Geldner N (2014) Radial transport of nutrients: the plant root as a polarized epithelium. Plant Physiology 166(2), 528-537.
| Crossref | Google Scholar | PubMed |
Barberon M, Vermeer JEM, De Bellis D, Wang P, Naseer S, Andersen TG, Humbel BM, Nawrath C, Takano J, Salt DE, et al. (2016) Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164(3), 447-459.
| Crossref | Google Scholar | PubMed |
Baxter I, Hosmani PS, Rus A, Lahner B, Borevitz JO, Muthukumar B, Mickelbart MV, Schreiber L, Franke RB, Salt DE (2009) Root suberin forms an extracellular barrier that affects water relations and mineral nutrition in Arabidopsis. PLoS Genetics 5(5), e1000492.
| Crossref | Google Scholar | PubMed |
Becker M, Asch F (2005) Iron toxicity in rice—conditions and management concepts. Journal of Plant Nutrition and Soil Science 168(4), 558-573.
| Crossref | Google Scholar |
Bengough AG, Bransby MF, Hans J, McKenna SJ, Roberts TJ, Valentine TA (2006) Root responses to soil physical conditions; growth dynamics from field to cell. Journal of Experimental Botany 57(2), 437-447.
| Crossref | Google Scholar | PubMed |
Bengough AG, McKenzie BM, Hallett PD, Valentine TA (2011) Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. Journal of Experimental Botany 62(1), 59-68.
| Crossref | Google Scholar | PubMed |
Boerjan W, Ralph J, Baucher M (2003) Lignin biosynthesis. Annual Review of Plant Biology 54, 519-546.
| Crossref | Google Scholar | PubMed |
Brunner I, Herzog C, Dawes MA, Arend M, Sperisen C (2015) How tree roots respond to drought. Frontiers in Plant Science 6, 547.
| Crossref | Google Scholar | PubMed |
Carminati A, Vetterlein D, Weller U, Vogel H-J, Oswald SE (2009) When roots lose contact. Vadose Zone Journal 8(3), 805-809.
| Crossref | Google Scholar |
Chen Z, Cuin TA, Zhou M, Twomey A, Naidu BP, Shabala S (2007) Compatible solute accumulation and stress-mitigating effects in barley genotypes contrasting in their salt tolerance. Journal of Experimental Botany 58(15–16), 4245-4255.
| Crossref | Google Scholar | PubMed |
Chen A, Husted S, Salt DE, Schjoerring JK, Persson DP (2019) The intensity of manganese deficiency strongly affects root endodermal suberization and ion homeostasis. Plant Physiology 181(2), 729-742.
| Crossref | Google Scholar | PubMed |
Colmer TD (2003a) Aerenchyma and an inducible barrier to radial oxygen loss facilitate root aeration in upland, paddy and deep-water rice (Oryza sativa L.). Annals of Botany 91, 301-309.
| Crossref | Google Scholar |
Colmer TD (2003b) Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26(1), 17-36.
| Crossref | Google Scholar |
Colmer TD, Flowers TJ (2008) Flooding tolerance in halophytes. New Phytologist 179(4), 964-974.
| Crossref | Google Scholar | PubMed |
Colmer TD, Gibberd MR, Wiengweera A, Tinh TK (1998) The barrier to radial oxygen loss from roots of rice (Oryza sativa L.) is induced by growth in stagnant solution. Journal of Experimental Botany 49(325), 1431-1436.
| Crossref | Google Scholar |
Colmer TD, Cox MCH, Voesenek LACJ (2006) Root aeration in rice (Oryza sativa): evaluation of oxygen, carbon dioxide, and ethylene as possible regulators of root acclimatizations. New Phytologist 170(4), 767-778.
| Crossref | Google Scholar | PubMed |
Colmer TD, Atwell BJ, Ismail AM, Pedersen O, Shabala S, Sorrell BK, Voesenek LACJ (2015) Waterlogging and submergence. In ‘Plants in action’. 2nd edn. (Eds R Munns, S Schmidt, C Beveridge). (Australian Society of Plant Scientists, New Zealand Society of Plant Biologists, and New Zealand Institute of Agricultural and Horticultural Science). Available at https://www.rseco.org/content/chapter-18-waterlogging-and-submergence.html
Colmer TD, Kotula L, Malik AI, Takahashi H, Konnerup D, Nakazono M, Pedersen O (2019) Rice acclimation to soil flooding: low concentrations of organic acids can trigger a barrier to radial oxygen loss in roots. Plant, Cell & Environment 42(7), 2183-2197.
| Crossref | Google Scholar | PubMed |
Cui B, Liu R, Flowers TJ, Song J (2021) Casparian bands and suberin lamellae: key targets for breeding salt tolerant crops? Environmental and Experimental Botany 191, 104600.
| Crossref | Google Scholar |
Ejiri M, Shiono K (2019) Prevention of radial oxygen loss is associated with exodermal suberin along adventitious roots of annual wild species of Echinochloa. Frontiers in Plant Science 10, 254.
| Crossref | Google Scholar | PubMed |
Ejiri M, Sawazaki Y, Shiono K (2020) Some accessions of amazonian wild rice (Oryza glumaepatula) constitutively form a barrier to radial oxygen loss along adventitious roots under aerated conditions. Plants (Basel) 9(7), 880.
| Crossref | Google Scholar |
Enstone DE, Peterson CA (1998) Effects of exposure to humid air on epidermal viability and suberin deposition in maize (Zea mays L.) roots. Plant, Cell & Environment 21(8), 837-844.
| Crossref | Google Scholar |
Enstone DE, Peterson CA, Ma F (2002) Root endodermis and exodermis: structure, function, and responses to the environment. Journal of Plant Growth Regulation 21(4), 335-351.
| Crossref | Google Scholar |
Flam-Shepherd R, Huynh WQ, Coskun D, Hamam AM, Britto DT, Kronzucker HJ (2018) Membrane fluxes, bypass flows, and sodium stress in rice: the influence of silicon. Journal of Experimental Botany 69(7), 1679-1692.
| Crossref | Google Scholar | PubMed |
Gibbs J, Greenway H (2003) Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30(1), 1-47.
| Crossref | Google Scholar | PubMed |
Höfer R, Briesen I, Beck M, Pinot F, Schreiber L, Franke R (2008) The Arabidopsis cytochrome P450 CYP86A1 encodes a fatty acid ω-hydroxylase involved in suberin monomer biosynthesis. Journal of Experimental Botany 59(9), 2347-2360.
| Crossref | Google Scholar | PubMed |
Hose E, Clarkson DT, Steudle E, Schreiber L, Hartung W (2001) The exodermis: a variable apoplastic barrier. Journal of Experimental Botany 52(365), 2245-2264.
| Crossref | Google Scholar | PubMed |
Hosmani PS, Kamiya T, Danku J, Naseer S, Geldner N, Guerinot ML, Salt DE (2013) Dirigent domain-containing protein is part of the machinery required for formation of the lignin-based Casparian strip in the root. Proceedings of the National Academy of Sciences 110(35), 14498-14503.
| Crossref | Google Scholar | PubMed |
IPCC (2019) Climate Change and Land: an IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. (Eds PR Shukla, E Calvo Buendia, V Masson-Delmotte, H-O. Pörtner, DC Roberts, P Zhai, R Slade, S Connors, R van Diemen, M Ferrat, E Haughey, S Luz, S Neogi, M Pathak, J Petzold, J Portugal Pereira, P Vyas, E Huntley, K Kissick, M Belkacemi, J Malley). (Intergovernmental Panel on Climate Change: Geneva Switzerland). Available at https://www.ipcc.ch/site/assets/uploads/2019/11/SRCCL-Full-Report-Compiled-191128.pdf
Ishikawa T, Shabala S (2019) Control of xylem Na+ loading and transport to the shoot in rice and barley as a determinant of differential salinity stress tolerance. Physiologia Plantarum 165(3), 619-631.
| Crossref | Google Scholar | PubMed |
Jimenez JdlC, Pedersen O (2023) Mitigation of greenhouse gas emissions from Rice via manipulation of key root traits. Rice 16(1), 24.
| Crossref | Google Scholar | PubMed |
Jiménez JdlC, Kotula L, Veneklaas EJ, Colmer TD (2019) Root-zone hypoxia reduces growth of the tropical forage grass Urochloa humidicola in high-nutrient but not low-nutrient conditions. Annals of Botany 124(6), 1019-1032.
| Crossref | Google Scholar |
Jiménez JdlC, Clode PL, Signorelli S, Veneklaas EJ, Colmer TD, Kotula L (2021) The barrier to radial oxygen loss impedes the apoplastic entry of iron into the roots of Urochloa humidicola. Journal of Experimental Botany 72(8), 3279-3293.
| Crossref | Google Scholar |
Kamiya T, Borghi M, Wang P, Danku JMC, Kalmbach L, Hosmani PS, Naseer S, Fujiwara T, Geldner N, Salt DE (2015) The MYB36 transcription factor orchestrates Casparian strip formation. Proceedings of the National Academy of Sciences 112(33), 10533-10538.
| Crossref | Google Scholar | PubMed |
Kolattukudy PE (1984) Biochemistry and function of cutin and suberin. Canadian Journal of Botany 62(12), 2918-2933.
| Crossref | Google Scholar |
Kotula L, Ranathunge K, Steudle E (2009) Apoplastic barriers effectively block oxygen permeability across outer cell layers of rice roots under deoxygenated conditions: roles of apoplastic pores and of respiration. New Phytologist 184(4), 909-917.
| Crossref | Google Scholar | PubMed |
Kotula L, Colmer TD, Nakazono M (2014) Effects of organic acids on the formation of the barrier to radial oxygen loss in roots of Hordeum marinum. Functional Plant Biology 41(2), 187-202.
| Crossref | Google Scholar | PubMed |
Kreszies T, Schreiber L, Ranathunge K (2018) Suberized transport barriers in Arabidopsis, barley and rice roots: from the model plant to crop species. Journal of Plant Physiology 227, 75-83.
| Crossref | Google Scholar | PubMed |
Krishnamurthy P, Ranathunge K, Franke R, Prakash HS, Schreiber L, Mathew MK (2009) The role of root apoplastic transport barriers in salt tolerance of rice (Oryza sativa L.). Planta 230(1), 119-134.
| Crossref | Google Scholar | PubMed |
Krishnamurthy P, Ranathunge K, Nayak S, Schreiber L, Mathew MK (2011) Root apoplastic barriers block Na+ transport to shoots in rice (Oryza sativa L.). Journal of Experimental Botany 62(12), 4215-4228.
| Crossref | Google Scholar | PubMed |
Laanbroek HJ (1990) Bacterial cycling of minerals that affect plant growth in waterlogged soils: a review. Aquatic Botany 38(1), 109-125.
| Crossref | Google Scholar |
Lauter FR, Ninnemann O, Bucher M, Riesmeier JW, Frommer WB (1996) Preferential expression of an ammonium transporter and of two putative nitrate transporters in root hairs of tomato. Proceedings of the National Academy of Sciences 93(15), 8139-8144.
| Crossref | Google Scholar | PubMed |
Liu M, Pan T, Allakhverdiev SI, Yu M, Shabala S (2020) Crop halophytism: an environmentally sustainable solution for global food security. Trends in Plant Science 25(7), 630-634.
| Crossref | Google Scholar | PubMed |
Lulai EC, Corsini DL (1998) Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiological and Molecular Plant Pathology 53(4), 209-222.
| Crossref | Google Scholar |
Lux A, Luxová M, Abe J, Morita S (2004) Root cortex: structural and functional variability and responses to environmental stress. Root Research 13(3), 117-131.
| Crossref | Google Scholar |
Lynch JP (2007) Roots of the second green revolution. Australian Journal of Botany 55(5), 493-512.
| Crossref | Google Scholar |
Ma F, Peterson CA (2003) Current insights into the development, structure, and chemistry of the endodermis and exodermis of roots. Canadian Journal of Botany 81(5), 405-421.
| Crossref | Google Scholar |
Ma Y, Dias MC, Freitas H (2020) Drought and salinity stress responses and microbe-induced tolerance in plants. Frontiers in Plant Science 11, 591911.
| Crossref | Google Scholar | PubMed |
Malik AI, Islam AKMR, Colmer TD (2011) Transfer of the barrier to radial oxygen loss in roots of Hordeum marinum to wheat (Triticum aestivum): evaluation of four H. marinum-wheat amphiploids. New Phytologist 190(2), 499-508.
| Crossref | Google Scholar | PubMed |
Mano Y, Omori F (2013) Flooding tolerance in interspecific introgression lines containing chromosome segments from teosinte (Zea nicaraguensis) in maize (Zea mays subsp. mays). Annals of Botany 112(6), 1125-1139.
| Crossref | Google Scholar | PubMed |
Marriboina S, Attipalli RR (2020) Hydrophobic cell-wall barriers and vacuolar sequestration of Na+ ions are among the key mechanisms conferring high salinity tolerance in a biofuel tree species, Pongamia pinnata L. pierre. Environmental and Experimental Botany 171, 103949.
| Crossref | Google Scholar |
Meyer CJ, Peterson CA, Steudle E (2011) Permeability of Iris germanica’s multiseriate exodermis to water, NaCl, and ethanol. Journal of Experimental Botany 62(6), 1911-1926.
| Crossref | Google Scholar | PubMed |
Mongon J, Konnerup D, Colmer TD, Rerkasem B (2014) Responses of rice to Fe2+ in aerated and stagnant conditions: growth, root porosity and radial oxygen loss barrier. Functional Plant Biology 41(9), 922-929.
| Crossref | Google Scholar | PubMed |
Munns R, Day DA, Fricke W, Watt M, Arsova B, Barkla BJ, Bose J, Byrt CS, Chen Z-H, Foster KJ, et al. (2020) Energy costs of salt tolerance in crop plants. New Phytologist 225(3), 1072-1090.
| Crossref | Google Scholar | PubMed |
Nakazono M, Qiu F, Borsuk LA, Schnable PS (2003) Laser-capture microdissection, a tool for the global analysis of gene expression in specific plant cell types: identification of genes expressed differentially in epidermal cells or vascular tissues of maize. The Plant Cell 15(3), 583-596.
| Crossref | Google Scholar | PubMed |
Naseer S, Lee Y, Lapierre C, Franke R, Nawrath C, Geldner N (2012) Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proceedings of the National Academy of Sciences 109(25), 10101-10106.
| Crossref | Google Scholar |
Nishiuchi S, Yamauchi T, Takahashi H, Kotula L, Nakazono M (2012) Mechanisms for coping with submergence and waterlogging in rice. Rice 5(1), 2.
| Crossref | Google Scholar | PubMed |
Noorrohmah S, Takahashi H, Nakazono M (2020) Formation of a barrier to radial oxygen loss in L-type lateral roots of rice. Plant Root 14, 33-41.
| Crossref | Google Scholar |
Palmgren MG, Edenbrandt AK, Vedel SE, Andersen MM, Landes X, Osterberg JT, Falhof J, Olsen LI, Christensen SB, Sandoe P, et al. (2015) Are we ready for back-to-nature crop breeding? Trends in Plant Science 20(3), 155-164.
| Crossref | Google Scholar | PubMed |
Pedersen O, Sauter M, Colmer TD, Nakazono M (2021) Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytologist 229(1), 42-49.
| Crossref | Google Scholar | PubMed |
Peralta Ogorek LL, Pellegrini E, Pedersen O (2021) Novel functions of the root barrier to radial oxygen loss – radial diffusion resistance to H2 and water vapour. New Phytologist 231(4), 1365-1376.
| Crossref | Google Scholar | PubMed |
Peralta Ogorek LL, Takahashi H, Nakazono M, Pedersen O (2023) The barrier to radial oxygen loss protects roots against hydrogen sulphide intrusion and its toxic effect. New Phytologist 238(5), 1825-1837.
| Crossref | Google Scholar | PubMed |
Pfister A, Barberon M, Alassimone J, Kalmbach L, Lee Y, Vermeer JEM, Yamazaki M, Li G, Maurel C, Takano J, et al. (2014) A receptor-like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. eLife 3, e03115.
| Crossref | Google Scholar | PubMed |
Puniran-Hartley N, Hartley J, Shabala L, Shabala S (2014) Salinity-induced accumulation of organic osmolytes in barley and wheat leaves correlates with increased oxidative stress tolerance: in planta evidence for cross-tolerance. Plant Physiology and Biochemistry 83, 32-39.
| Crossref | Google Scholar | PubMed |
Ranathunge K, Schreiber L, Franke R (2011a) Suberin research in the genomics era – new interest for an old polymer. Plant Science 180(3), 399-413.
| Crossref | Google Scholar | PubMed |
Ranathunge K, Lin J, Steudle E, Schreiber L (2011b) Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCl permeabilities in rice (Oryza sativa L.) roots. Plant, Cell & Environment 34(8), 1223-1240.
| Crossref | Google Scholar | PubMed |
Ranathunge K, Schreiber L, Bi Y-M, Rothstein SJ (2016) Ammonium-induced architectural and anatomical changes with altered suberin and lignin levels significantly change water and solute permeabilities of rice (Oryza sativa L.) roots. Planta 243(1), 231-249.
| Crossref | Google Scholar | PubMed |
Raven JA, Scrimgeour CM (1997) The influence of anoxia on plants of saline habitats with special reference to the sulphur cycle. Annals of Botany 79(suppl 1), 79-86.
| Crossref | Google Scholar |
Rawat N, Wungrampha S, Singla-Pareek SL, Yu M, Shabala S, Pareek A (2022) Rewilding staple crops for the lost halophytism: toward sustainability and profitability of agricultural production systems. Molecular Plant 15(1), 45-64.
| Crossref | Google Scholar | PubMed |
Razzaq A, Wani SH, Saleem F, Yu M, Zhou M, Shabala S, Foyer C (2021) Rewilding crops for climate resilience: economic analysis and de novo domestication strategies. Journal of Experimental Botany 72(18), 6123-6139.
| Crossref | Google Scholar | PubMed |
Reinhardt DH, Rost TL (1995) Salinity accelerates endodermal development and induces an exodermis in cotton seedling roots. Environmental and Experimental Botany 35(4), 563-574.
| Crossref | Google Scholar |
Reynoso MA, Borowsky AT, Pauluzzi GC, Yeung E, Zhang J, Formentin E, Velasco J, Cabanlit S, Duvenjian C, Prior MJ, et al. (2022) Gene regulatory networks shape developmental plasticity of root cell types under water extremes in rice. Developmental Cell 57(9), 1177-1192.e6.
| Crossref | Google Scholar | PubMed |
Reyt G, Ramakrishna P, Salas-González I, Fujita S, Love A, Tiemessen D, Lapierre C, Morreel K, Calvo-Polanco M, Flis P, Geldner N, Boursiac Y, Boerjan W, George MW, Castrillo G, Salt DE (2021) Two chemically distinct root lignin barriers control solute and water balance. Nature Communications 12(1), 2320.
| Crossref | Google Scholar | PubMed |
Rijsberman FR (2006) Water scarcity: fact or fiction? Agricultural Water Management 80(1–3), 5-22.
| Crossref | Google Scholar |
Sardans J, Peñuelas J (2005) Drought decreases soil enzyme activity in a Mediterranean Quercus ilex L. forest. Soil Biology and Biochemistry 37(3), 455-461.
| Crossref | Google Scholar |
Saunois M, Stavert AR, Poulter B, Bousquet P, Canadell JG, Jackson RB, Raymond PA, Dlugokencky EJ, Houweling S, Patra PK, et al. (2020) The global methane budget 2000–2017. Earth System Science Data 12(3), 1561-1623.
| Crossref | Google Scholar |
Schreiber L, Hartmann K, Skrabs M, Zeier J (1999) Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. Journal of Experimental Botany 50(337), 1267-1280.
| Crossref | Google Scholar |
Schneider HM, Strock CF, Hanlon MT, Vanhees DJ, Perkins AC, Ajmera IB, Sidhu JS, Mooney SJ, Brown KM, Lynch JP (2021) Multiseriate cortical sclerenchyma enhance root penetration in compacted soils. Proceedings of the National Academy of Sciences 118(6), e2012087118.
| Crossref | Google Scholar | PubMed |
Serra O, Geldner N (2022) The making of suberin. New Phytologist 235(3), 848-866.
| Crossref | Google Scholar | PubMed |
Shabala S (2013) Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Annals of Botany 112(7), 1209-1221.
| Crossref | Google Scholar | PubMed |
Shiono K, Yamauchi T, Yamazaki S, Mohanty B, Malik AI, Nagamura Y, Nishizawa NK, Tsutsumi N, Colmer TD, Nakazono M (2014) Microarray analysis of laser-microdissected tissues indicates the biosynthesis of suberin in the outer part of roots during formation of a barrier to radial oxygen loss in rice (Oryza sativa). Journal of Experimental Botany 65(17), 4795-4806.
| Crossref | Google Scholar | PubMed |
Shiono K, Yoshikawa M, Kreszies T, Yamada S, Hojo Y, Matsuura T, Mori IC, Schreiber L, Yoshioka T (2022) Abscisic acid is required for exodermal suberization to form a barrier to radial oxygen loss in the adventitious roots of rice (Oryza sativa). New Phytologist 233(2), 655-669.
| Crossref | Google Scholar | PubMed |
Song Z, Zonta F, Ogorek LLP, Bastegaard VK, Herzog M, Pellegrini E, Pedersen O (2023) The quantitative importance of key root traits for radial water loss under low water potential. Plant and Soil 482(1–2), 567-584.
| Crossref | Google Scholar |
Soukup A, Armstrong W, Schreiber L, Franke R, Votrubova O (2007) Apoplastic barriers to radial oxygen loss and solute penetration: a chemical and functional comparison of the exodermis of two wetland species, Phragmites australis and Glyceria maxima. New Phytologist 173(2), 264-278.
| Crossref | Google Scholar | PubMed |
Taleisnik E, Peyrano G, Cordoba A, Arias C (1999) Water retention capacity in root segments differing in the degree of exodermis development. Annals of Botany 83(1), 19-27.
| Crossref | Google Scholar |
Thomas R, Fang X, Ranathunge K, Anderson TR, Peterson CA, Bernards MA (2007) Soybean root suberin: anatomical distribution, chemical composition, and relationship to partial resistance to Phytophthora sojae. Plant Physiology 144(1), 299-311.
| Crossref | Google Scholar | PubMed |
Wang Q, Zhou F, Shang Z, Ciais P, Winiwarter W, Jackson RB, Tubiello FN, Janssens-Maenhout G, Tian H, Cui X, et al. (2020) Data-driven estimates of global nitrous oxide emissions from croplands. National Science Review 7(2), 441-452.
| Crossref | Google Scholar | PubMed |
Wang X, Chai X, Gao B, Deng C, Gunther CS, Wu T, Zhang X, Xu X, Han Z, Wang Y (2023) Multi-omics analysis reveals the mechanism of bHLH130 responding to low-nitrogen stress of apple rootstock. Plant Physiology 191(2), 1305-1323.
| Crossref | Google Scholar | PubMed |
Watanabe K, Takahashi H, Sato S, Nishiuchi S, Omori F, Malik AI, Colmer TD, Mano Y, Nakazono M (2017) A major locus involved in the formation of the radial oxygen loss barrier in adventitious roots of teosinte Zea nicaraguensis is located on the short-arm of chromosome 3. Plant, Cell & Environment 40(2), 304-316.
| Crossref | Google Scholar | PubMed |
Watanabe Y, Kabuki T, Kakehashi T, Kano-Nakata M, Mitsuya S, Yamauchi A (2020) Morphological and histological differences among three types of component roots and their differential contribution to water uptake in the rice root system. Plant Production Science 23(2), 191-201.
| Crossref | Google Scholar |
Yahdjian L, Sala OE, Austin AT (2006) Differential controls of water input on litter decomposition and nitrogen dynamics in the Patagonian steppe. Ecosystems 9(1), 128-141.
| Crossref | Google Scholar |
Yamauchi T, Colmer TD, Pedersen O, Nakazono M (2018) Regulation of root traits for internal aeration and tolerance to soil waterlogging-flooding stress. Plant Physiology 176(2), 1118-1130.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Abe F, Tsutsumi N, Nakazono M (2019) Root cortex provides a venue for gas-space formation and is essential for plant adaptation to waterlogging. Frontiers in Plant Science 10, 259.
| Crossref | Google Scholar | PubMed |
Yamauchi T, Pedersen O, Nakazono M, Tsutsumi N (2021) Key root traits of Poaceae for adaptation to soil water gradients. New Phytologist 229(6), 3133-3140.
| Crossref | Google Scholar | PubMed |
Yu L-X, Ray JD, O’Toole JC, Nguyen HT (1995) Use of wax-petrolatum layers for screening rice root penetration. Crop Science 35(3), 684-687.
| Crossref | Google Scholar |
Zhang Q, Shao M, Jia X, Wei X (2019) Changes in soil physical and chemical properties after short drought stress in semi-humid forests. Geoderma 338, 170-177.
| Crossref | Google Scholar |
Zhao C, Zhang H, Song C, Zhu J-K, Shabala S (2020) Mechanisms of plant responses and adaptation to soil salinity. The Innovation 1(1), 100017.
| Crossref | Google Scholar | PubMed |
Zhu J, Brown KM, Lynch JP (2010) Root cortical aerenchyma improves the drought tolerance of maize (Zea mays L.). Plant, Cell & Environment 33(5), 740-749.
| Crossref | Google Scholar | PubMed |
Zimmermann HM, Steudle E (1998) Apoplastic transport across young maize roots: effect of the exodermis. Planta 206, 7-19.
| Crossref | Google Scholar |


