Short-term elevated temperature and CO2 promote photosynthetic induction in the C3 plant Glycine max, but not in the C4 plant Amaranthus tricolor
Tianyu Zheng A , Yuan Yu A and Huixing Kang A *
A , Yuan Yu A and Huixing Kang A *
A Department of Ecology, College of Urban and Environmental Sciences, and Key Laboratory for Earth Surface Processes of the Ministry of Education, Peking University, Beijing 100871, China.
Functional Plant Biology 49(11) 995-1007 https://doi.org/10.1071/FP21363
Submitted: 24 December 2021 Accepted: 10 July 2022 Published: 1 August 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial 4.0 International License (CC BY-NC)
Abstract
The continuous increases of atmospheric temperature and CO2 concentration will impact global photosynthesis. However, there are few studies considering the interaction of elevated temperature (eT) and elevated CO2 (eCO2) on dynamic photosynthesis, particularly for C4 species. We examine dynamic photosynthesis under four different temperature and [CO2] treatments: (1) 400 ppm × 28°C (CT); (2) 400 ppm × 33°C (CT+); (3) 800 ppm × 28°C (C+T); and (4) 800 ppm × 33°C (C+T+). In Glycine max L., the time required to reach 50% (T50%A) and 90% (T90%A) of full photosynthetic induction was smaller under the CT+, C+T, and C+T+ treatments than those under the CT treatment. In Amaranthus tricolor L., however, neither T50%A nor T90%A was not significantly affected by eT or eCO2. In comparison with the CT treatment, the achieved carbon gain was increased by 58.3% (CT+), 112% (C+T), and 136.6% (C+T+) in G. max and was increased by 17.1% (CT+), 2.6% (C+T) and 56.9% (C+T+) in A. tricolor. The increases of achieved carbon gain in G. max were attributable to both improved photosynthetic induction efficiency (IE) and enhanced steady-state photosynthesis, whereas those in A. tricolor were attributable to enhanced steady-state photosynthesis.
Keywords: C4 photosynthesis, climate change, dynamic photosynthesis, fluctuating light, lightfleck, Rubisco activase, soybean, stomatal conductance.
Introduction
Atmospheric CO2 concentration has increased by half since the Industrial Revolution, and the annual global mean temperature has also risen by more than 0.6°C since 1950 (IPCC 2014). A large volume of studies have reported that elevated CO2 concentration (eCO2) enhances photosynthetic capacity (Drake et al. 1997; Ainsworth and Long 2005; Ainsworth and Rogers 2007), whereas the effects of elevated temperature (eT) on photosynthetic capacity are complex, depending on both the initial leaf temperature and the degree of warming (Dusenge et al. 2019). However, the stress of eCO2 and warming does not occur in isolation. Under climate change, the two stresses occur simultaneously (Norby and Luo 2004; Luo et al. 2008). Photosynthetic responses to the two stresses may be synergistic in some circumstances, but antagonistic in others (Dieleman et al. 2012; Smith and Dukes 2013). To project photosynthesis in a future of climate change, studies on the interactive effects of eT and eCO2 on photosynthesis are urgently needed (Xu et al. 2013, 2014).
Most studies on photosynthesis were carried out under constant light conditions, where light was well controlled. However, in natural environments, light is constantly fluctuating, leading to fluctuations in operating leaf photosynthesis (Chazdon and Pearcy 1986; Pearcy 1990; Pearcy et al. 1996; Tang 1997). Investigating photosynthesis in fluctuating light; i.e. dynamic photosynthesis, will improve our understanding of photosynthesis in natural environments.
There have been some studies addressing the effects of eT or eCO2 on dynamic photosynthesis in C3 plants. Below the temperature optimum point, short-term eT can promote photosynthetic induction (the time course of photosynthetic rate in response to a sudden increase in light intensity) by reducing the biochemical limitation of RuBP (ribulose-1,5-bisphosphate) regeneration and Rubisco activation (Kaiser et al. 2015). In contrast, elevating temperature above the temperature optimum point may inhibit photosynthetic induction by depressing Rubisco activation (Leakey et al. 2003; Kang et al. 2020). Short-term eCO2 can promote photosynthetic induction by reducing the biochemical limitation of Rubisco activation and stomatal limitation (Tomimatsu and Tang 2016; Kaiser et al. 2017a, 2017b; Tomimatsu et al. 2019; Kang et al. 2021).
As far as we know, there has been no study addressing the effects of the interaction of eT and eCO2 on dynamic photosynthesis. Elevating temperature decreases the solubility of CO2 in water but eCO2 can compensate this factor (Jordan and Ogren 1984; Foyer et al. 2009). Another source of uncertainty regarding the interaction of eT and eCO2 results from the inconsistent results about the effects of eT and eCO2 on stomatal limitation in previous studies (Naumburg et al. 2001; Leakey et al. 2002, 2003; Tomimatsu and Tang 2012; Kaiser et al. 2017a). Elevating CO2 reduces stomatal limitation, whereas eT could increase or reduce stomatal limitation (Kaiser et al. 2015, 2017a; Wachendorf and Küppers 2017b), probably due to the trade-off between carbon gain and evaporative cooling (Moore et al. 2021). Elevating temperature generally increases post-illumination CO2 fixation and post-illumination CO2 burst at low and medium leaf temperature (Sun et al. 1999; Foyer et al. 2009), but eCO2 decreases post-illumination CO2 burst by inhibiting photorespiration (Leakey et al. 2002). Therefore, studies are needed to address the uncertainty of the effects of the interaction of eT and eCO2 on dynamic photosynthesis.
About 3% of the Earth’s angiosperm species utilise the C4 photosynthetic pathway, yet C4 plants contribute about 25% of the net terrestrial primary productivity on Earth (Sage 2004). There have been many studies on the steady-state photosynthesis in C4 plants (Furbank et al. 1990; Sage and Kubien 2007; Sage and Zhu 2011; Sage et al. 2012; Long and Spence 2013; von Caemmerer 2021), but rare on their dynamic photosynthesis (Furbank and Walker 1985; Horton and Neufeld 1998; Pignon et al. 2021; Wang et al. 2021). Kubásek et al. (2013) have shown that C4 plants lost more carbon in fluctuating light than in steady light compared with C3 plants, which they assumed was a result of an increase of bundle sheath cells (BSC) leakiness to CO2 in C4 plants in fluctuating light. However, Stitt and Zhu (2014) proposed that the two-cell Kranz system of C4 photosynthesis ensures large pools of metabolites that could drive diffusion between BSCs and mesophyll cells (MC) for buffering ATP and NADPH, which may provide powerful protection against fluctuating light intensities. Recently, Li et al. (2021) have proposed that the accumulation and diffusion of metabolites in C4 plants from MCs to BSCs take more time, which may delay the photosynthetic induction. Therefore, the differences between C3 and C4 plants in their dynamic photosynthesis are still under debate.
In this study, we characterised photosynthetic responses to the simulated changes of light intensity in a C3 species and a C4 species under four different treatments, aiming to address: (1) the effects of the interaction of short-term eT and eCO2 on dynamic photosynthesis; and (2) the differences between C3 and C4 plants in their dynamic photosynthetic responses to eT and eCO2. We hypothesised that: (1) the short-term eT and eCO2 would promote photosynthetic induction and enhance carbon gain; and (2) the promotion of photosynthetic induction and the enhancement of carbon gain were greater for C3 plants than for C4 plants.
Methods and materials
Plant materials
The seeds of the C3 species, Glycine max L. and the C4 species, Amaranthus tricolor L. (NAD-ME subtype) were sown in pots (the circular radius was 5 cm and the height was 14 cm) filled with composite soil. Each of the two species was grown within a growth chamber (E-36L1, Percival, Perry, Iowa, USA). After germination, seedlings were thinned. The plants were watered with distilled water regularly and supplied with 5 mL full concentration formula nutrient solution (nitrogen 17 g/L, phosphorus anhydride 17 g/L, potassium oxide 17 g/L, organic matter 25 g/L, and amino acid 12 g/L) every 3–5 days. These ensure that all plants can obtain sufficient nutrients and water, which helps reduce the degree of growth limitation (Poorter et al. 2012). Growth environments was kept constant and consistent throughout the experiment in both growth chambers. Photosynthetic photon flux density (PPFD) incident on the top of both canopies was about 600 μmol m−2 s−1. The photoperiod was 14 h. Day/night air temperature was set to 28/22°C, [CO2] was the same as ambient CO2 concentration and relative humidity was maintained at 70%.
Leaf gas exchange measurements
Leaf gas exchange was measured on plants 25 days after germination using a Li-6800 portable photosynthesis system (LI-COR Biosciences, Lincoln, NE, USA) equipped with a Li-6800-01 fluorometer (90% red and 10% blue) on the most recently fully expanded leaves (n = 4). The measured plants were moved to another growth chamber 60 min before the start of the measurement to acclimate to the target temperature and [CO2] in advance. The measured leaves were enclosed in the Li-6800 leaf chamber, and were first acclimated in the chamber to a PPFD of 100 μmol m−2 s−1 until steady-state net assimilation rate (A) and stomatal conductance for H2O (gsw) were visibly reached, after which PPFD was raised to 600 μmol m−2 s−1 and kept steady for 60 min. Then, PPFD was decreased to 100 μmol m−2 s−1 until A reached steady states again. Gas exchange parameters, including A, gsw, intercellular CO2 concentration (Ci), and transpiration rate (E), were logged every second. To avoid any swinging from correctional changes in temperature or relative humidity, the temperature of the heat exchanger (Texchg) was controlled. All measurements were repeated under four different combinations of temperature and CO2 concentration: (1) 400 ppm × 28°C, denoted as CT; (2) 400 ppm × 33°C, denoted as CT+; (3) 800 ppm × 28°C, denoted as C+T; and (4) 800 ppm × 33°C, denoted as C+T+. The vapour pressure difference between leaf and ambient air ranged from 0.9 kPa to 1.1 kPa at 28°C and from 1.1 kPa to 1.5 kPa at 33°C.
Data analysis
Steady-state A, gsw, and Ci reached at each PPFD level were calculated by averaging the single values over the last minute of each period; T50%A and T90%A were defined as the time required to reach 50% and 90% of the differences between A100 and A600. We calculated the induction state (IS) after Chazdon and Pearcy (1986):
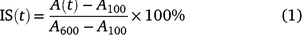
where A(t) is the transient A at time t.
For C3 plants, under Rubisco limitation, A(t) can be calculated after Farquhar et al. (1980):
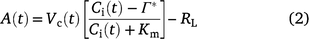
where Vc(t) and Ci(t) is Vc and Ci at time t, respectively. Km is the Michaelis–Menten constants of Rubisco and Γ* is the CO2 compensation point, they were both taken from Bernacchi et al. (2003). RL is mitochondrial respiration rate in the light and assumed to be 40% of dark respiration rate between 25 and 35°C (Way et al. 2019).
The time courses of Vc(t) were then fitted to the model proposed by Woodrow and Mott (1989), and we use Vc(t) to replace A(t) after correction of Ci(t).
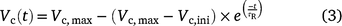
where Vc,ini and Vc,max are the initial and maximum Vc(t) after correction to 25°C (Bernacchi et al. 2003), respectively; τR is the apparent time constant of Rubisco activation.
The achieved carbon gain (ACG) and ideal carbon gain (ICG) were calculated after Kang et al. (2021):
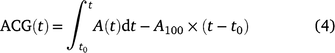
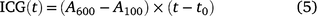
where t0 is the time when PPFD was increased. The ratio of ACG to ICG is photosynthetic induction efficiency (IE), which is calculated after Yanhong et al. (1994):
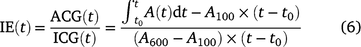
Eqn 4 indicates that ACG during photosynthetic induction can be decomposed into ICG, which is only influenced by steady-state A; and IE, which is influenced mostly by the time course of photosynthetic induction. Short-term eT and eCO2 can influence ACG via changes in ICG and/or those in IE. We assumed that changes in IE reflected the responses of photosynthetic induction to different treatments per se.
Post-illumination carbon gain (PICG) due to the simulated lightfleck was calculated as follows:
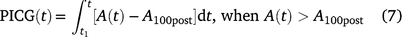
where t1 is the time when PPFD was decreased, A100post is the steady-state A at the end of post-illumination period.
Intrinsic water use efficiency (iWUE) was calculated by dividing the transient A by the transient gs (Farquhar and Sharkey 1982):

Statistical analysis
To determine the effects of measurement temperature and CO2 concentration ([CO2]) on gas exchange parameters for two species, when the requirement of the normality and homogeneity of variances were met, we used two-way ANOVA with temperature and [CO2] as the main factors and temperature × [CO2] as interaction, and Duncan test was used for post hoc multiple comparisons. When the requirement of the normality and homogeneity of variances were not met, we used a Kruskal–Wallis test to perform the same analysis. All tests were conducted using SPSS Statistics ver. 18.0 (IBM Corp., Armonk, NY, USA) and R ver. 3.6.1.
Results
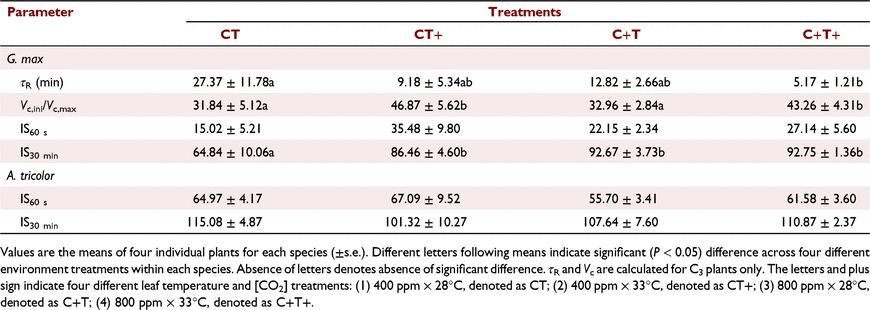
Steady-state photosynthesis under different temperature and CO2 treatments
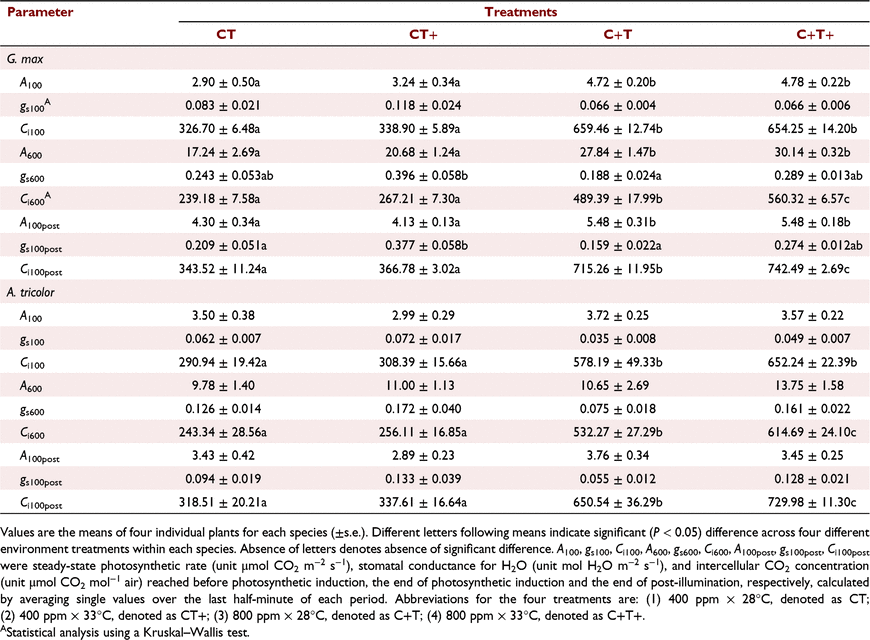
In G. max, the mean A600 was increased by 20.0% and 61.5% under the CT+ and C+T treatments compared to the CT treatment. However, the mean A600 was increased by 74.8% under the C+T+ treatment, which was less than the sum of the effects of eT and eCO2 alone (Table 1). In A. tricolor, the mean A100 and A600 were not significantly affected by eT, eCO2 or their interaction (Table 1).
|
Dynamic photosynthesis under different temperature and CO2 treatments
In comparison with CT treatment, the transient A in G. max were higher under the other three treatments, and evident difference was observed in the first minute of photosynthetic induction (Figs 1a, 2a). The transient IS was highest under the CT+ treatment during the first 5 min of induction; after that, IS was much higher under the C+T and C+T+ treatments (Fig. 1c). In A. tricolor, the transient A was almost the same in the first minute between all treatments; after that, the transient A was highest under the C+T+ treatment and still similar in the other three treatments (Figs 1b, 2b). The transient IS was always similar between all treatments (Fig. 1d). Bars for s.e. in Figs 1 and 2 were omitted for visual clarity. In addition, iWUE was higher under the C+T and C+T+ than other two treatments during photosynthetic induction in both species (Fig. S1).
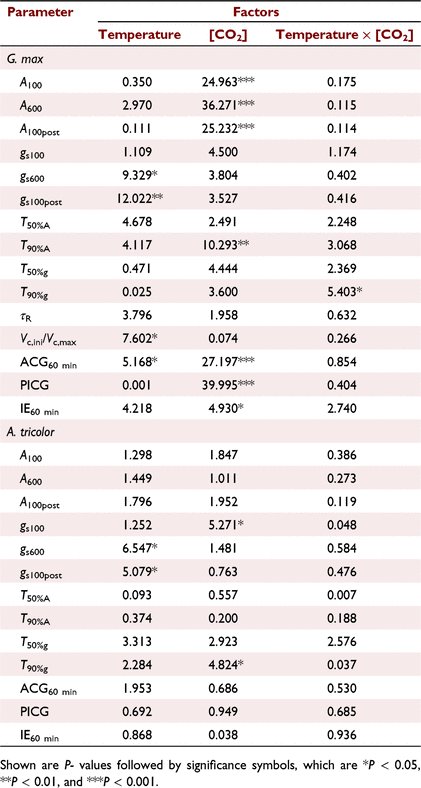
In comparison with CT treatment, the mean T50%A, T90%A, τR, T50%g and T90%g in G. max were decreased under the other three treatments. However, effects on the mean T50%A, T90%A and τR under C+T+ treatment was less than the sum of the effects of eT and eCO2 alone (Fig. 3a, b), while effects on the mean T50%g and T90%g under C+T+ treatment was smaller than the effects of eCO2 alone (Fig. 3c, d). In A. tricolor, the mean T50%A and T90%A did not differ significantly between treatments (Fig. 3a, b). The mean T50%g and T90%g still had no difference between the CT, CT+ and C+T treatments, but significant higher under the C+T+ treatment (Fig. 3c, d).
ACG and IE
In G. max, ACG60 min was increased by 58.3% and 111.9% under the CT+ and C+T treatments compared to the CT treatment. However, ACG60 min was increased by 136.4% under the C+T+ treatment, which was less than the sum of the effects of eT and eCO2 alone (Fig. 4a). In A. tricolor, ACG60 min under the CT treatment was also less than the other three treatments, but the promotion effect on the C+T+ treatment was larger than the sum of the effects of eT and eCO2 alone (Fig. 4b). In comparison with CT treatment, PICG in G. max and A. tricolor were higher under the other three treatments, but the effects on the C+T+ treatment was less than the effects of eT alone (Fig. 4c, d).
In G. max, ACG and IE were higher under the other three treatments than those under the CT treatment (Fig. 5a) and ACG were always highest under the C+T+ treatment at different time points (Fig. 5c). In A. tricolor, IE did not differ significantly between all treatments at any time (Fig. 5b, d), despite there were changes in ACG60 min in A. tricolor (Fig. 4b).
To further assess the contribution of ACG and IE on increasing ACG60 min, we estimated the potential ICG60 min by assuming that eT and eCO2 had no effect on photosynthetic induction (equivalent to no changes in IE). In comparison with CT treatment, the increase in ICG60 min enhanced ACG60 min by 16.9%, 55.3% and 73.1% in G. max and also enhanced ACG60 min by 27.7%, 11.4% and 64.5% in A. tricolor under the other three treatments (Fig. 6a, b). Then, we assumed no effects of eT and eCO2 on steady-state A (equivalent to no changes in ICG) to assess the separate contribution of IE. Compared to the CT treatment, the increase in IE60 min increased ACG60 min by 31.5%, 31.4% and 34.3% in G. max under the other three treatments, but decreased ACG60 min by 6.5%, 5.4% and 3.5% in A. tricolor (Fig. 6a, b). In summary, ACG60 min in G. max was affected by both IE60 min and ICG60 min. eT had a similar effect on IE60 min as eCO2 but a lower effect on ICG60 min than eCO2 (Fig. 6a). However, ACG60 min in A. tricolor was only affected by ICG60 min and the effect of eT on it was higher than that of eCO2 (Fig. 6b).
The correlations between photosynthetic steady-state and induction parameters
We assessed the correlations between steady-state and induction parameters using Pearson’s coefficients. In G. max, ACG60 min was not only positively correlated with A100 (P < 0.001), A600 (P < 0.001) and IE60 min (P < 0.001), but also negatively related to T50%A (P < 0.001), T90%A (P < 0.001) and T50%g (P < 0.01) (Fig. 7a). But in A. tricolor, ACG60 min was only positively correlated with A600 (P < 0.001); not related to T50%A, T90%A, T50%g and IE60 min (Fig. 7b).
Discussion
Differential effects of eT and eCO2 on dynamic photosynthesis in G. max
In G. max, both eT and eCO2 promoted photosynthetic induction, but their effects were differential: eT alone imposed influences on the early stage (the first 5 min) of induction, whereas eCO2 alone imposed significant influences on the late stage of induction (Fig. 1a, c). It is reported that among 193 genes related to photosynthesis in the KEGG database, expression of only nine genes changed significantly after sudden increases in irradiation received by rice (Oryza sativa L.) eaves (Adachi et al. 2019). This result indicates that few genes are involved in photosynthetic responses to sudden changes in light. We focused on the physiological responses of photosynthetic induction to short-term eT and eCO2.
Photosynthetic induction at the early stage is widely assumed to be limited by the time lags in biochemical processes, especially RuBP regeneration and Rubisco activation (Pearcy et al. 1996; Tomimatsu and Tang 2016). We found moderately eT decrease the T50%A and τR in this study (Fig. 3a; Table 2). Kaiser et al. (2015) reported a parabolic relationship between photosynthesis induction rate and temperature, with the fastest induction occurring at about 30°C. Such a parabolic relationship would be related to the activation rate of Rca (Rubisco activase) on Rubisco (CarmoSilva and Salvucci 2011). Elevating CO2 reduces biochemical limitation by accelerating the Rubisco activation (a smaller τR), which may be ascribed to CO2-stimulated Rca upregulation (Zhao et al. 2019). However, in this study, eT had a greater impact on the transient IS than eCO2 in the first 5 min (Fig. 1c). At a longer timescale (∼60 min), photosynthetic induction is mainly limited by diffusional limitation (Way and Pearcy 2012; Kaiser et al. 2015; Lawson and Vialet-Chabrand 2019). Diffusional limitation can be alleviated more rapidly by higher initial gs and faster stomata opening (McAusland et al. 2016; Wachendorf and Küppers 2017a). However, effects of eT and eCO2 on gs and stomata opening rate are conflicting between studies; increases (von Caemmerer and Evans 2015; Urban et al. 2017), decreases, (Sage and Sharkey 1987) or no changes (von Caemmerer and Evans 2015) of gs under eT have been reported before. Elevating CO2 generally reduces gs but its effect on stomatal opening can be positive (Naumburg et al. 2001; Leakey et al. 2002; Kaiser et al. 2017a) or negative (Tomimatsu and Tang 2012). In this study, both eT and eCO2 reduced diffusional limitation by accelerating stomata opening (Fig. 3c), without significant influences on the initial gs (Table 1).
Both eT and eCO2 enhanced carbon gain in fluctuating light by improving photosynthetic induction efficiency and photosynthetic capacity in G. max. The enhancement of ACG under eT was mainly attributable to the improved IE, while that under eCO2 was mainly attributable to the improved photosynthetic capacity. The effects of eCO2 were consistent with Kang et al. (2021), where ICG had a larger effect than IE on ACG during induction in wheat (Triticum aestivum L.) and rice.
Minor effects of eT, eCO2, or their interaction on dynamic photosynthesis in A. tricolor
In A. tricolor, photosynthetic induction was not significantly affected by eT, eCO2 or their interaction (Table 3, Fig. 3b, d). In particular, during the first minute of photosynthetic induction, the time course of transient A under each of the four treatments almost overlapped in A. tricolor (Fig. 2b), indicating that factors insensitive to both temperature and [CO2] are dominating photosynthesis during this time. This result is consistent with the report on photosynthetic induction at various temperatures and [CO2] in maize (Ireland et al. 1984). Rubisco activity and stomatal conductance (Fig. 2d) change little during this period (Kaiser et al. 2015; Tomimatsu and Tang 2016), thus we propose that the enhancement of photosynthetic rate is associated with the build-up of metabolite concentration gradients. In C4 photosynthesis, atmospheric CO2 is first assimilated as C4 acids in a C4 cycle before entering the Calvin-Benson cycle, or referred to as C3 cycle (Bräutigam and Weber 2011). The C4 and C3 cycle is coordinated by intracellular transport of C4 acids and C3 metabolites (Furbank et al. 2000), or light regulation of key enzymes (Furbank et al. 1997). Light regulation of the key enzymes takes minutes and should impose relatively small limitation on photosynthesis during the initial stage of the induction (Usuda et al. 1984). Intracellular transport of C4 acids and C3 metabolites is widely believed to occur by symplastic diffusion and require metabolite concentration gradients between the compartments (Sowiński et al. 2008). Isotopic labeling experiments have revealed the establishment of metabolite pools and the concentration gradients during induction (Moore and Edwards 1986a, 1986b). C4 species have low levels of RuBP and its precursors even at high light (Borghi et al. 2022), but maintain high levels of C4 acids at both low and high light (Moore and Edwards 1986a). Such large pools of C4 acids facilitate a fast build-up of sufficient metabolite concentration gradients (Wang et al. 2021) and thereby provide additional abilities to buffer the redox and energy status against fluctuating environments (Stitt and Zhu 2014).
|
Different from G. max, the increases of ACG in A. tricolor were almost independent of the changes of IE, but were mainly attributable to the enhancement of ideal carbon gain (ICG) (Fig. 6b). The enhancement of ICG in A. tricolor was less than G. max. The transient iWUE of C4 plants were not significantly different from those of C3 plants under C+T+ treatment (see Supplementary Fig. S1). These findings suggest C3 plants will benefit more from the simultaneous eT and eCO2 under the background of future climate change than C4 plants.
The effects of the interaction of eT and eCO2 on dynamic photosynthesis
The effects of simultaneously eT and eCO2 on T50%A, T90%A, ACG60 min and IE60 min in G. max were lower than the sum of the sole effect of eT and eCO2, indicating that the effects of eT and eCO2 on photosynthetic induction were partially offset. We hypothesised that this finding was a result of an offset in the effects of eT and eCO2 on stomatal behaviour. Elevating CO2 decreased the gs600 but promoted the rate of the increases in gs during induction, both of which shortened the time required for stomatal opening (Fig. 1e). The effects of eCO2 on stomatal behaviour observed in this study were consistent with previous reports (Kaiser et al. 2017b). In contrast, eT increased the gs600 and promoted the rate of the increases in gs during induction; yet the effect of the latter dominated over that of the former. We were not clear on the mechanism that underlies the increases of gs600 under eT. An increases of the guard cell metabolic activity or of the evaporative demand under eT could drive the increases of gs600. The interaction of eT and eCO2 had no influences on T50%A, T90%A and IE60 min in A. tricolor.
The effects of simultaneously eT and eCO2 on A600 in G. max were in close proximity to the sum of the sole effect of eT and eCO2 and the effects of simultaneously eT and eCO2 on A600 in A. tricolor are higher than the sum of the sole effect of eT and eCO2. These findings are different from some studies, where eT inhibited the positive effect of eCO2 on steady-state photosynthetic rate and photosynthetic efficiency (Lambreva et al. 2005; Cai et al. 2016). In contrast, Sage and Kubien (2003) found that the effects of eCO2 on C3 and C4 photosynthesis were greater at warmer than at cooler temperatures. Some studies have also found the enhancement of photosynthesis by eCO2 is larger at higher temperature (Long 1991; Morison and Lawlor 1999). That is because eCO2 suppresses photorespiration and mitochondrial respiration in C3 plants, expanding the photosynthetic thermal optimum range (Long 1991; Way et al. 2015).
Low steady-state photosynthetic rate in A. tricolor
In general, the steady state photosynthetic rate of C4 plants is higher than that of C3 plants. A lower A600 in A. tricolor than that in G. max in this study may result from the low growth light intensity. Due to biochemical and energetic requirement (Furbank et al. 1990; Ubierna et al. 2011), C4 plants are more suitable for growing in high light. Photosynthetic rate was reduced to a greater extent in low light in the six C4 grasses relative to the two C3 species, and C4 grasses also tended to have a lower stomatal conductance and stomatal aperture than C3 species (Israel et al. 2022).
However, C4 plants grown in low light or medium light still have a large metabolites pool because BSC leakiness was found to be similar for C4 plants grown in different light intensity (Pengelly et al. 2010; Bellasio and Griffiths 2014; Ma et al. 2017). Above researches suggest that the CCM is still robust and the biochemical efficiency of the C4 cycle does not decrease for C4 plants grown in low light or medium light. Therefore, the low growth light intensity may have little influences on the effects of eT, eCO2, and their interaction on photosynthetic induction in A. tricolor observed in our study.
Conclusion
By examining dynamic photosynthesis under four different temperature and [CO2] treatments, this study showed that for G.max, the T50%A and T90%A were significantly affected by eT and eCO2; whereas for A. tricolor, they were almost unaffected by eT or eCO2. This study suggests that the effects of eT and eCO2 on photosynthetic induction were partially offset in C3 plants and greater enhancement of photosynthesis in fluctuating light for C3 plants than for C4 plants in a warming and CO2-enriched future. More research is needed to address how the interaction of eT and eCO2 influences dynamic photosynthesis in future experiments.
Supplementary material
Supplementary material is available online.
Data availability
The original contributions presented in the study are included in the article and Supplementary material, further enquiries can be directed to the corresponding author.
Conflicts of interest
Author has no conflicts of interest.
Declaration of funding
This study was supported by the Key Research of Plant Functional Ecology Program of Peking University (No. 7101302307), National Natural Science Foundation of China (Grant No. 41530533), and Key Laboratory of College of Urban and Environmental Sciences (No. 7100602014).
Acknowledgements
We thank Yanhong Tang, Xinran Ke and Yan Zhang for their comments on the manuscript.
References
Adachi S, Tanaka Y, Miyagi A, Kashima M, Tezuka A, Toya Y, Kobayashi S, Ohkubo S, Shimizu H, Kawai-Yamada M, Sage RF, Nagano AJ, Yamori W (2019) High-yielding rice Takanari has superior photosynthetic response to a commercial rice Koshihikari under fluctuating light. Journal of Experimental Botany 70, 5287–5297.| High-yielding rice Takanari has superior photosynthetic response to a commercial rice Koshihikari under fluctuating light.Crossref | GoogleScholarGoogle Scholar |
Ainsworth EA, Long SP (2005) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351–372.
| What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2.Crossref | GoogleScholarGoogle Scholar |
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell & Environment 30, 258–270.
| The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions.Crossref | GoogleScholarGoogle Scholar |
Bellasio C, Griffiths H (2014) Acclimation to low light by C4 maize: implications for bundle sheath leakiness. Plant, Cell & Environment 37, 1046–1058.
| Acclimation to low light by C4 maize: implications for bundle sheath leakiness.Crossref | GoogleScholarGoogle Scholar |
Bernacchi CJ, Pimentel C, Long SP (2003) In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis. Plant, Cell & Environment 26, 1419–1430.
| In vivo temperature response functions of parameters required to model RuBP-limited photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Borghi GL, Arrivault S, Günther M, Barbosa Medeiros D, Dell’Aversana E, Fusco GM, Carillo P, Ludwig M, Fernie AR, Lunn JE, Stitt M (2022) Metabolic profiles in C3, C3–C4 intermediate, C4-like, and C4 species in the genus Flaveria. Journal of Experimental Botany 73, 1581–1601.
| Metabolic profiles in C3, C3–C4 intermediate, C4-like, and C4 species in the genus Flaveria.Crossref | GoogleScholarGoogle Scholar |
Bräutigam A, Weber APM (2011) Chapter 11 Transport processes: Connecting the reactions of C4 photosynthesis. In ‘C4 photosynthesis and related CO2 concentrating mechanisms.’ (Eds AS Raghavendra, RF Sage.) pp. 199–219. (Springer Netherlands: Dordrecht)
Cai C, Yin X, He S, Jiang W, Si C, Struik PC, Luo W, Li G, Xie Y, Xiong Y, Pan G (2016) Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments. Global Change Biology 22, 856–874.
| Responses of wheat and rice to factorial combinations of ambient and elevated CO2 and temperature in FACE experiments.Crossref | GoogleScholarGoogle Scholar |
Carmo-Silva AE, Salvucci ME (2011) The activity of Rubisco’s molecular chaperone, Rubisco activase, in leaf extracts. Photosynthesis Research 108, 143–155.
| The activity of Rubisco’s molecular chaperone, Rubisco activase, in leaf extracts.Crossref | GoogleScholarGoogle Scholar |
Chazdon RL, Pearcy RW (1986) Photosynthetic responses to light variation in rainforest species. Oecologia 69, 524–531.
| Photosynthetic responses to light variation in rainforest species.Crossref | GoogleScholarGoogle Scholar |
Dieleman WIJ, Vicca S, Dijkstra FA, Hagedorn F, Hovenden MJ, Larsen KS, Morgan JA, Volder A, Beier C, Dukes JS, King J, Leuzinger S, Linder S, Luo Y, Oren R, De Angelis P, Tingey D, Hoosbeek MR, Janssens IA (2012) Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature. Global Change Biology 18, 2681–2693.
| Simple additive effects are rare: a quantitative review of plant biomass and soil process responses to combined manipulations of CO2 and temperature.Crossref | GoogleScholarGoogle Scholar |
Drake BG, Gonzàlez-Meler MA, Long SP (1997) MORE EFFICIENT PLANTS: a consequence of rising atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology 48, 609–639.
| MORE EFFICIENT PLANTS: a consequence of rising atmospheric CO2?Crossref | GoogleScholarGoogle Scholar |
Dusenge ME, Duarte AG, Way DA (2019) Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration. New Phytologist 221, 32–49.
| Plant carbon metabolism and climate change: elevated CO2 and temperature impacts on photosynthesis, photorespiration and respiration.Crossref | GoogleScholarGoogle Scholar |
Farquhar GD, Sharkey TD (1982) Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33, 317–345.
| Stomatal conductance and photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90.
| A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species.Crossref | GoogleScholarGoogle Scholar |
Foyer CH, Bloom AJ, Queval G, Noctor G (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annual Review of Plant Biology 60, 455–484.
| Photorespiratory metabolism: genes, mutants, energetics, and redox signaling.Crossref | GoogleScholarGoogle Scholar |
Furbank RT, Walker DA (1985) Photosynthetic induction in C4 leaves. Planta 163, 75–83.
| Photosynthetic induction in C4 leaves.Crossref | GoogleScholarGoogle Scholar |
Furbank RT, Jenkins CLD, Hatch MD (1990) C4 photosynthesis: quantum requirement, C4 and overcycling and Q-Cycle involvement. Functional Plant Biology 17, 1–7.
| C4 photosynthesis: quantum requirement, C4 and overcycling and Q-Cycle involvement.Crossref | GoogleScholarGoogle Scholar |
Furbank RT, Chitty JA, Jenkins CLD, Taylor WC, Trevanion SJ, Caemmerer Sv, Ashton AR (1997) Genetic manipulation of key photosynthetic enzymes in the C4 plant Flaveria bidentis. Functional Plant Biology 24, 477–485.
Furbank RT, Hatch MD, Jenkins CLD (2000) C4 photosynthesis: mechanism and regulation. In ‘Photosynthesis: physiology and metabolism’. (Eds RC Leegood, TD Sharkey, S von Caemmerer) pp. 435–457. (Springer Netherlands: Dordrecht)
Horton JL, Neufeld HS (1998) Photosynthetic responses of Microstegium vimineum (Trin.) A. Camus, a shade-tolerant, C4 grass, to variable light environments. Oecologia 114, 11–19.
| Photosynthetic responses of Microstegium vimineum (Trin.) A. Camus, a shade-tolerant, C4 grass, to variable light environments.Crossref | GoogleScholarGoogle Scholar |
IPCC (2014) IPCC fifth assessment report (AR5) observed climate change impacts database. (NASA Socioeconomic Data and Applications Center (SEDAC): Palisades, NY)
Ireland CR, Long SP, Baker NR (1984) The relationship between carbon dioxide fixation and chlorophyll a fluorescence during induction of photosynthesis in maize leaves at different temperatures and carbon dioxide concentrations. Planta 160, 550–558.
| The relationship between carbon dioxide fixation and chlorophyll a fluorescence during induction of photosynthesis in maize leaves at different temperatures and carbon dioxide concentrations.Crossref | GoogleScholarGoogle Scholar |
Israel WK, Watson-Lazowski A, Chen Z-H, Ghannoum O (2022) High intrinsic water use efficiency is underpinned by high stomatal aperture and guard cell potassium flux in C3 and C4 grasses grown at glacial CO2 and low light. Journal of Experimental Botany 73, 1546–1565.
| High intrinsic water use efficiency is underpinned by high stomatal aperture and guard cell potassium flux in C3 and C4 grasses grown at glacial CO2 and low light.Crossref | GoogleScholarGoogle Scholar |
Jordan DB, Ogren WL (1984) The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase. Planta 161, 308–313.
| The CO2/O2 specificity of ribulose 1,5-bisphosphate carboxylase/oxygenase.Crossref | GoogleScholarGoogle Scholar |
Kaiser E, Morales A, Harbinson J, Kromdijk J, Heuvelink E, Marcelis LFM (2015) Dynamic photosynthesis in different environmental conditions. Journal of Experimental Botany 66, 2415–2426.
| Dynamic photosynthesis in different environmental conditions.Crossref | GoogleScholarGoogle Scholar |
Kaiser E, Kromdijk J, Harbinson J, Heuvelink E, Marcelis LFM (2017a) Photosynthetic induction and its diffusional, carboxylation and electron transport processes as affected by CO2 partial pressure, temperature, air humidity and blue irradiance. Annals of Botany 119, 191–205.
| Photosynthetic induction and its diffusional, carboxylation and electron transport processes as affected by CO2 partial pressure, temperature, air humidity and blue irradiance.Crossref | GoogleScholarGoogle Scholar |
Kaiser E, Zhou D, Heuvelink E, Harbinson J, Morales A, Marcelis LFM (2017b) Elevated CO2 increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state. Journal of Experimental Botany 68, 5629–5640.
| Elevated CO2 increases photosynthesis in fluctuating irradiance regardless of photosynthetic induction state.Crossref | GoogleScholarGoogle Scholar |
Kang H-X, Zhu X-G, Yamori W, Tang Y-H (2020) Concurrent increases in leaf temperature with light accelerate photosynthetic induction in tropical tree seedlings. Frontiers in Plant Science 11, 1216
| Concurrent increases in leaf temperature with light accelerate photosynthetic induction in tropical tree seedlings.Crossref | GoogleScholarGoogle Scholar |
Kang H, Zhu T, Zhang Y, Ke X, Sun W, Hu Z, Zhu X, Shen H, Huang Y, Tang Y (2021) Elevated CO2 enhances dynamic photosynthesis in rice and wheat. Frontiers in Plant Science 12, 727374
| Elevated CO2 enhances dynamic photosynthesis in rice and wheat.Crossref | GoogleScholarGoogle Scholar |
Kubásek J, Urban O, Šantrůčke J (2013) C4 plants use fluctuating light less efficiently than do C3 plants: a study of growth, photosynthesis and carbon isotope discrimination. Physiologia Plantarum 149, 528–539.
| C4 plants use fluctuating light less efficiently than do C3 plants: a study of growth, photosynthesis and carbon isotope discrimination.Crossref | GoogleScholarGoogle Scholar |
Lambreva M, Stoyanova-Koleva D, Baldjiev G, Tsonev T (2005) Early acclimation changes in the photosynthetic apparatus of bean plants during short-term exposure to elevated CO2 concentration under high temperature and light intensity. Agriculture, Ecosystems & Environment 106, 219–232.
| Early acclimation changes in the photosynthetic apparatus of bean plants during short-term exposure to elevated CO2 concentration under high temperature and light intensity.Crossref | GoogleScholarGoogle Scholar |
Lawson T, Vialet-Chabrand S (2019) Speedy stomata, photosynthesis and plant water use efficiency. New Phytologist 221, 93–98.
| Speedy stomata, photosynthesis and plant water use efficiency.Crossref | GoogleScholarGoogle Scholar |
Leakey ADB, Press MC, Scholes JD, Watling JR (2002) Relative enhancement of photosynthesis and growth at elevated CO2 is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling. Plant, Cell & Environment 25, 1701–1714.
| Relative enhancement of photosynthesis and growth at elevated CO2 is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling.Crossref | GoogleScholarGoogle Scholar |
Leakey ADB, Press MC, Scholes JD (2003) High-temperature inhibition of photosynthesis is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling. Plant, Cell & Environment 26, 1681–1690.
| High-temperature inhibition of photosynthesis is greater under sunflecks than uniform irradiance in a tropical rain forest tree seedling.Crossref | GoogleScholarGoogle Scholar |
Li Y-T, Luo J, Liu P, Zhang Z-S (2021) C4 species utilize fluctuating light less efficiently than C3 species. Plant Physiology 187, 1288–1291.
| C4 species utilize fluctuating light less efficiently than C3 species.Crossref | GoogleScholarGoogle Scholar |
Long SP (1991) Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated? Plant, Cell & Environment 14, 729–739.
| Modification of the response of photosynthetic productivity to rising temperature by atmospheric CO2 concentrations: has its importance been underestimated?Crossref | GoogleScholarGoogle Scholar |
Long SP, Spence AK (2013) Toward cool C4 crops. Annual Review of Plant Biology 64, 701–722.
| Toward cool C4 crops.Crossref | GoogleScholarGoogle Scholar |
Luo Y, Gerten D, Le Maire G, Parton WJ, Weng E, Zhou X, Keough C, Beier C, Ciais P, Cramer W, Dukes JS, Emmett B, Hanson PJ, Knapp A, Linder S, Nepstad D, Rustad L (2008) Modeled interactive effects of precipitation, temperature, and [CO2] on ecosystem carbon and water dynamics in different climatic zones. Global Change Biology 14, 1986–1999.
| Modeled interactive effects of precipitation, temperature, and [CO2] on ecosystem carbon and water dynamics in different climatic zones.Crossref | GoogleScholarGoogle Scholar |
Ma J-Y, Sun W, Koteyeva NK, Voznesenskaya E, Stutz SS, Gandin A, Smith-Moritz AM, Heazlewood JL, Cousins AB (2017) Influence of light and nitrogen on the photosynthetic efficiency in the C4 plant Miscanthus × giganteus. Photosynthesis Research 131, 1–13.
| Influence of light and nitrogen on the photosynthetic efficiency in the C4 plant Miscanthus × giganteus.Crossref | GoogleScholarGoogle Scholar |
McAusland L, Vialet-Chabrand S, Davey P, Baker NR, Brendel O, Lawson T (2016) Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency. New Phytologist 211, 1209–1220.
| Effects of kinetics of light-induced stomatal responses on photosynthesis and water-use efficiency.Crossref | GoogleScholarGoogle Scholar |
Moore BD, Edwards GE (1986a) Photosynthetic induction in a C4 dicot, Flaveria trinervia: I. Initial products of 14CO2 assimilation and levels of whole leaf C4 metabolites. Plant Physiology 81, 663–668.
| Photosynthetic induction in a C4 dicot, Flaveria trinervia: I. Initial products of 14CO2 assimilation and levels of whole leaf C4 metabolites.Crossref | GoogleScholarGoogle Scholar |
Moore BD, Edwards GE (1986b) Photosynthetic induction in a C4 dicot, Flaveria trinervia: II. Metabolism of products of 14CO2 fixation after different illumination times. Plant Physiology 81, 669–673.
| Photosynthetic induction in a C4 dicot, Flaveria trinervia: II. Metabolism of products of 14CO2 fixation after different illumination times.Crossref | GoogleScholarGoogle Scholar |
Moore CE, Meacham-Hensold K, Lemonnier P, Slattery RA, Benjamin C, Bernacchi CJ, Lawson T, Cavanagh AP (2021) The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems. Journal of Experimental Botany 72, 2822–2844.
| The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems.Crossref | GoogleScholarGoogle Scholar |
Morison JIL, Lawlor DW (1999) Interactions between increasing CO2 concentration and temperature on plant growth. Plant, Cell & Environment 22, 659–682.
| Interactions between increasing CO2 concentration and temperature on plant growth.Crossref | GoogleScholarGoogle Scholar |
Naumburg E, Ellsworth DS, Katul GG (2001) Modeling dynamic understory photosynthesis of contrasting species in ambient and elevated carbon dioxide. Oecologia 126, 487–499.
| Modeling dynamic understory photosynthesis of contrasting species in ambient and elevated carbon dioxide.Crossref | GoogleScholarGoogle Scholar |
Norby RJ, Luo Y (2004) Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world. New Phytologist 162, 281–293.
| Evaluating ecosystem responses to rising atmospheric CO2 and global warming in a multi-factor world.Crossref | GoogleScholarGoogle Scholar |
Pearcy RW (1990) Sunflecks and photosynthesis in plant canopies. Annual Review of Plant Physiology and Plant Molecular Biology 41, 421–453.
| Sunflecks and photosynthesis in plant canopies.Crossref | GoogleScholarGoogle Scholar |
Pearcy RW, Krall JP, Sassenrath-Cole GF (1996) Photosynthesis in fluctuating light environments. In ‘Photosynthesis and the environment’. (Ed. NR Baker) pp. 321–346. (Springer Netherlands: Dordrecht)
Pengelly JJL, Sirault XRR, Tazoe Y, Evans JR, Furbank RT, von Caemmerer S (2010) Growth of the C4 dicot Flaveria bidentis: photosynthetic acclimation to low light through shifts in leaf anatomy and biochemistry. Journal of Experimental Botany 61, 4109–4122.
| Growth of the C4 dicot Flaveria bidentis: photosynthetic acclimation to low light through shifts in leaf anatomy and biochemistry.Crossref | GoogleScholarGoogle Scholar |
Pignon CP, Leakey ADB, Long SP, Kromdijk J (2021) Drivers of natural variation in water-use efficiency under fluctuating light are promising targets for improvement in sorghum. Frontiers in Plant Science 12, 627432
| Drivers of natural variation in water-use efficiency under fluctuating light are promising targets for improvement in sorghum.Crossref | GoogleScholarGoogle Scholar |
Poorter H, Fiorani F, Stitt M, Schurr U, Finck A, Gibon Y, Usadel B, Munns R, Atkin OK, Tardieu F, Pons TL (2012) The art of growing plants for experimental purposes: a practical guide for the plant biologist. Functional Plant Biology 39, 821–838.
| The art of growing plants for experimental purposes: a practical guide for the plant biologist.Crossref | GoogleScholarGoogle Scholar |
Sage RF (2004) The evolution of C4 photosynthesis. New Phytologist 161, 341–370.
| The evolution of C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Sage RF, Kubien DS (2003) Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants. Photosynthesis Research 77, 209–225.
| Quo vadis C4? An ecophysiological perspective on global change and the future of C4 plants.Crossref | GoogleScholarGoogle Scholar |
Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant, Cell & Environment 30, 1086–1106.
| The temperature response of C3 and C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Sage RF, Sharkey TD (1987) The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field grown plants. Plant Physiology 84, 658–664.
| The effect of temperature on the occurrence of O2 and CO2 insensitive photosynthesis in field grown plants.Crossref | GoogleScholarGoogle Scholar |
Sage RF, Zhu X-G (2011) Exploiting the engine of C4 photosynthesis. Journal of Experimental Botany 62, 2989–3000.
| Exploiting the engine of C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Annual Review of Plant Biology 63, 19–47.
| Photorespiration and the evolution of C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Smith NG, Dukes JS (2013) Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2. Global Change Biology 19, 45–63.
| Plant respiration and photosynthesis in global-scale models: incorporating acclimation to temperature and CO2.Crossref | GoogleScholarGoogle Scholar |
Sowiński P, Szczepanik J, Minchin PEH (2008) On the mechanism of C4 photosynthesis intermediate exchange between Kranz mesophyll and bundle sheath cells in grasses. Journal of Experimental Botany 59, 1137–1147.
| On the mechanism of C4 photosynthesis intermediate exchange between Kranz mesophyll and bundle sheath cells in grasses.Crossref | GoogleScholarGoogle Scholar |
Stitt M, Zhu X-G (2014) The large pools of metabolites involved in intercellular metabolite shuttles in C4 photosynthesis provide enormous flexibility and robustness in a fluctuating light environment. Plant, Cell & Environment 37, 1985–1988.
| The large pools of metabolites involved in intercellular metabolite shuttles in C4 photosynthesis provide enormous flexibility and robustness in a fluctuating light environment.Crossref | GoogleScholarGoogle Scholar |
Sun J, Edwards GE, Okita TW (1999) Feedback inhibition of photosynthesis in rice measured by O2 dependent transients. Photosynthesis Research 59, 187–200.
| Feedback inhibition of photosynthesis in rice measured by O2 dependent transients.Crossref | GoogleScholarGoogle Scholar |
Tang Y (1997) Light: natural, abiotic factors in plant ecophysiology. In ‘Plant ecophysiology’. (Ed. MNV Prasad) pp. 3–40. (John Wiley & Sons, Inc)
Tomimatsu H, Tang Y (2012) Elevated CO2 differentially affects photosynthetic induction response in two Populus species with different stomatal behavior. Oecologia 169, 869–878.
| Elevated CO2 differentially affects photosynthetic induction response in two Populus species with different stomatal behavior.Crossref | GoogleScholarGoogle Scholar |
Tomimatsu H, Tang Y (2016) Effects of high CO2 levels on dynamic photosynthesis: carbon gain, mechanisms, and environmental interactions. Journal of Plant Research 129, 365–377.
| Effects of high CO2 levels on dynamic photosynthesis: carbon gain, mechanisms, and environmental interactions.Crossref | GoogleScholarGoogle Scholar |
Tomimatsu H, Sakata T, Fukayama H, Tang Y (2019) Short-term effects of high CO2 accelerate photosynthetic induction in Populus koreana × trichocarpa with always-open stomata regardless of phenotypic changes in high CO2 growth conditions. Tree Physiology 39, 474–483.
| Short-term effects of high CO2 accelerate photosynthetic induction in Populus koreana × trichocarpa with always-open stomata regardless of phenotypic changes in high CO2 growth conditions.Crossref | GoogleScholarGoogle Scholar |
Ubierna N, Sun W, Cousins AB (2011) The efficiency of C4 photosynthesis under low light conditions: assumptions and calculations with CO2 isotope discrimination. Journal of Experimental Botany 62, 3119–3134.
| The efficiency of C4 photosynthesis under low light conditions: assumptions and calculations with CO2 isotope discrimination.Crossref | GoogleScholarGoogle Scholar |
Urban J, Ingwers MW, McGuire MA, Teskey RO (2017) Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra. Journal of Experimental Botany 68, 1757–1767.
| Increase in leaf temperature opens stomata and decouples net photosynthesis from stomatal conductance in Pinus taeda and Populus deltoides x nigra.Crossref | GoogleScholarGoogle Scholar |
Usuda H, Ku MSB, Edwards GE (1984) Activation of NADP-malate dehydrogenase, pyruvate, Pi dikinase, and fructose 1,6-bisphosphatase in relation to photosynthetic rate in maize. Plant Physiology 76, 238–243.
| Activation of NADP-malate dehydrogenase, pyruvate, Pi dikinase, and fructose 1,6-bisphosphatase in relation to photosynthetic rate in maize.Crossref | GoogleScholarGoogle Scholar |
von Caemmerer S (2021) Updating the steady-state model of C4 photosynthesis. Journal of Experimental Botany 72, 6003–6017.
| Updating the steady-state model of C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar |
von Caemmerer S, Evans JR (2015) Temperature responses of mesophyll conductance differ greatly between species. Plant, Cell & Environment 38, 629–637.
| Temperature responses of mesophyll conductance differ greatly between species.Crossref | GoogleScholarGoogle Scholar |
Wachendorf M, Küppers M (2017a) The effect of initial stomatal opening on the dynamics of biochemical and overall photosynthetic induction. Trees 31, 981–995.
| The effect of initial stomatal opening on the dynamics of biochemical and overall photosynthetic induction.Crossref | GoogleScholarGoogle Scholar |
Wachendorf M, Küppers M (2017b) Effects of leaf temperature on initial stomatal opening and their roles in overall and biochemical photosynthetic induction. Trees 31, 1667–1681.
| Effects of leaf temperature on initial stomatal opening and their roles in overall and biochemical photosynthetic induction.Crossref | GoogleScholarGoogle Scholar |
Wang Y, Chan KX, Long SP (2021) Towards a dynamic photosynthesis model to guide yield improvement in C4 crops. The Plant Journal 107, 343–359.
| Towards a dynamic photosynthesis model to guide yield improvement in C4 crops.Crossref | GoogleScholarGoogle Scholar |
Way DA, Pearcy RW (2012) Sunflecks in trees and forests: from photosynthetic physiology to global change biology. Tree Physiology 32, 1066–1081.
| Sunflecks in trees and forests: from photosynthetic physiology to global change biology.Crossref | GoogleScholarGoogle Scholar |
Way DA, Oren R, Kroner Y (2015) The space-time continuum: the effects of elevated CO2 and temperature on trees and the importance of scaling. Plant, Cell & Environment 38, 991–1007.
| The space-time continuum: the effects of elevated CO2 and temperature on trees and the importance of scaling.Crossref | GoogleScholarGoogle Scholar |
Way DA, Aspinwall MJ, Drake JE, Crous KY, Campany CE, Ghannoum O, Tissue DT, Tjoelker MG (2019) Responses of respiration in the light to warming in field-grown trees: a comparison of the thermal sensitivity of the Kok and Laisk methods. New Phytologist 222, 132–143.
| Responses of respiration in the light to warming in field-grown trees: a comparison of the thermal sensitivity of the Kok and Laisk methods.Crossref | GoogleScholarGoogle Scholar |
Woodrow IE, Mott KA (1989) Rate limitation of non-steady-state photosynthesis by ribulose-1,5-bisphosphate carboxylase in Spinach. Functional Plant Biology 16, 487–500.
| Rate limitation of non-steady-state photosynthesis by ribulose-1,5-bisphosphate carboxylase in Spinach.Crossref | GoogleScholarGoogle Scholar |
Xu Z, Shimizu H, Yagasaki Y, Ito S, Zheng Y, Zhou G (2013) Interactive effects of elevated CO2, drought, and warming on plants. Journal of Plant Growth Regulation 32, 692–707.
| Interactive effects of elevated CO2, drought, and warming on plants.Crossref | GoogleScholarGoogle Scholar |
Xu Z, Shimizu H, Ito S, Yagasaki Y, Zou C, Zhou G, Zheng Y (2014) Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland. Planta 239, 421–435.
| Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis and peroxidation in dominant species from North China grassland.Crossref | GoogleScholarGoogle Scholar |
Yanhong T, Hiroshi K, Mitsumasa S, Izumi W (1994) Characteristics of transient photosynthesis in Quercus serrata seedlings grown under lightfleck and constant light regimes. Oecologia 100, 463–469.
| Characteristics of transient photosynthesis in Quercus serrata seedlings grown under lightfleck and constant light regimes.Crossref | GoogleScholarGoogle Scholar |
Zhao X, Li W-F, Wang Y, Ma Z-H, Yang S-J, Zhou Q, Mao J, Chen B-H (2019) Elevated CO2 concentration promotes photosynthesis of grape (Vitis vinifera L. cv. ‘Pinot noir’) plantlet in vitro by regulating RbcS and Rca revealed by proteomic and transcriptomic profiles. BMC Plant Biology 19, 42
| Elevated CO2 concentration promotes photosynthesis of grape (Vitis vinifera L. cv. ‘Pinot noir’) plantlet in vitro by regulating RbcS and Rca revealed by proteomic and transcriptomic profiles.Crossref | GoogleScholarGoogle Scholar |