Variations in leaf and stem traits across two elevations in subtropical forests
Liwei Zhu A B C , Yaxing Zhang A , Huiying Ye A , Yanqiong Li A , Weiting Hu A , Jie Du A and Ping Zhao A B C *
A B C , Yaxing Zhang A , Huiying Ye A , Yanqiong Li A , Weiting Hu A , Jie Du A and Ping Zhao A B C *
A Key Laboratory of Vegetation Restoration and Management of Degraded Ecosystems, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China.
B Center of Plant Ecology, Core Botanical Gardens, Chinese Academy of Sciences, Guangzhou 510650, China.
C Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou 510650, China.
Functional Plant Biology 49(4) 319-332 https://doi.org/10.1071/FP21220
Submitted: 26 July 2021 Accepted: 18 January 2022 Published: 15 February 2022
© 2022 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
Understanding the variations in plant traits across elevations may provide valuable insights into the species structure and function of forests and their responses to climate change. To explore the patterns of trait variation across elevations, we analysed 14 leaf and stem traits associated with resource acquisition and stress tolerance in Schima superba Gardner & Champion, Castanopsis chinensis (Sprengel) Hance, and Pinus massoniana Lambert trees at two elevations in a subtropical forest in southern China. Wood density increased, whereas crown width, leaf water potential at 0700 hours (ΨL-0700), and leaf δ18O decreased in high-elevation plants. Vessel diameter, daily maximum sap flux density, leaf δ13C, and leaf C and N concentrations per unit mass were comparable across elevations. We found species-specific variations in specific leaf area, midday leaf water potential, and leaf P concentration across elevations. Decreasing crown width with increasing elevation was associated with decreasing leaf δ18O and ΨL-0700, suggesting that higher stomatal conductance may moderate the loss of carbon assimilation. We elucidated the adaptive strategies of plants in response to environmental change, and showed that physiological traits varied in coordination with structural traits. Future studies incorporating multi-dimensional trait analyses can improve our understanding of the responses of forest ecosystems to climate change and global warming.
Keywords: elevational gradient, leaf δ13C, leaf δ18O, leaf water potential, sap flux density, southern China, specific leaf area, wood density.
Introduction
According to the IPCC special report (IPCC 2018), in 2017, anthropogenic activities had led to an increase in global average air temperature to 1°C above the pre-industrial levels. Global climate warming is expected to continue during the 21st century, and the strongest warming of ocean waters is projected to occur in tropical and subtropical regions (IPCC 2014). Plants in different climatic regions are known to exhibit differing levels of temperature sensitivity, and tropical tree species generally have narrower thermal tolerance than temperate species (Cunningham and Read 2002). In the last five decades, the annual air temperature in the Dinghushan Biosphere Reserve, China, has increased by approximately 1°C. This area, where our study site is located, has a subtropical climate (Zhou et al. 2011). Reports on the effects of warming on plant growth and survival are inconsistent. However, warming is generally expected to enhance plant growth in cold regions, but reduce biomass productivity in subtropical areas. This discrepancy is ascribed to the differences in plant physiological responses in different geographical regions (van Mantgem et al. 2009; Liu et al. 2013; León-Sánchez et al. 2016; Li et al. 2016; Wang et al. 2017; Wu et al. 2019; Lie et al. 2021). Therefore, understanding the functional responses of plants to environmental gradients is important for accurately predicting forest dynamics under climate change.
The gradual changes in temperature along altitudinal gradients offer an excellent natural laboratory to investigate the impacts of climate warming on the structuring and functioning of forest ecosystems (Körner 2007; Thomas 2011; De Frenne et al. 2013). Functional traits can indicate plant fitness and influence organismal performance across differing environmental conditions (McGill et al. 2006; Westoby and Wright 2006). Spatial variations in plant traits across elevations may show similar trends associated with the effects of climate warming (Dunne et al. 2004; Fukami and Wardle 2005). Previous studies have reported that plants tend to exhibit acquisitive resource use strategies (higher specific leaf area, SLA; and lower wood density, WD) in favourable environments and more conservative strategies (higher WD, lower SLA, and lower maximum tree height) under stressful conditions (Poorter et al. 2008; Muscarella et al. 2016). Compared to lower elevations, higher elevations generally offer more stressful conditions including decreasing atmospheric pressure and temperature, increasing radiation, and shallow soils due to high precipitation and runoff that restrict plant growth (Tateno et al. 2004; Körner 2007; Thomas 2011; De Bello et al. 2012; Ding et al. 2019; Umaña and Swenson 2019). Leaf mass per unit area (LMA, the inverse of SLA) and leaf N concentration on an area basis (Narea) increase with elevation. These responses indicate the stress tolerance strategies of plants that accumulate more resources (such as carbon investments) on a per-leaf basis under abiotic constraints (Cordell et al. 1999; Hulshof et al. 2013; Read et al. 2014). Species at higher elevations have a smaller leaf area and higher WD and water use efficiency, which were associated with increased longevity in response to resource limitation (Huxman et al. 2008; Hernández-Calderón et al. 2014). Apart from the aforementioned spectrum of leaf and stem economics associated with carbon and nutrient use, leaf water economy is also an essential factor for assessing the diversity of resource use strategies (Prieto et al. 2018). Moreover, carbon and oxygen isotope ratios related to water use economy can also reflect more direct physiological responses to the environment. Of these, the former is correlated with photosynthesis and stomatal conductance, whereas the latter is inversely proportional to stomatal conductance (Cernusak et al. 2002, 2007; Weigt et al. 2018). Analysing the δ18O levels in organic matter can help us understand the stomatal control of leaf water loss (Flanagan and Farquhar 2014; Sánchez-Bragado et al. 2016). Carbon assimilation and stomatal conductance have been shown to increase with elevation due to greater water availability (Richardson and Berlyn 2002; Van de Water et al. 2002; Adams and Kolb 2004; McDowell et al. 2008; Bresson et al. 2011). The increase in δ13C with elevation is explained by increasing stomatal conductance and more photosynthetic discrimination due to decreasing temperatures and increasing light intensity and soil moisture (Körner et al. 1991; Beerling et al. 1996; Sun et al. 1996; Pan et al. 2016). However, Wang et al. (2010) reported that δ13C levels do not always increase with elevation. Moreover, Reed and Loik (2016) reported an increase in plant water potential with elevation due to increasing soil moisture. Thus, plant morphological traits (such as SLA and WD) tend to reflect conservative resource use strategies, whereas their physiological traits (such as stomatal conductance and water potential) reflect acquisitive resource use strategies as a response to high elevation. Using field experimental studies to investigate these contrasting responses to environmental changes in plant morphological and physiological traits can help uncover the adaptive mechanisms of plants under changing environmental conditions.
Exploring the changes in correlations among plant traits along elevational gradients is an important task in ecology, and could help elucidate the mechanisms of resource exploitation by plants, which are currently unclear (Sterck et al. 2011; Umaña and Swenson 2019; Dusenge et al. 2021; Mujawamariya et al. 2021). Few studies have investigated intraspecific trait variations across environmental gradients; however, the results may have significant implications in predicting community structure and function under environmental changes (Violle et al. 2012; Siefert et al. 2015; Des Roches et al. 2018). Recently, Umaña and Swenson (2019) have emphasised the importance of defining intraspecific variations in functional traits along elevational gradients for improving our understanding of species dynamics and responses to environmental changes. Therefore, in the present study, we asked the following questions: (1) how do the morphological and physiological traits of trees vary across elevations in subtropical forests? and (2) what are the underlying factors governing traits shifts across elevations? To answer these questions, we measured 14 leaf and stem traits related to resource acquisition and construction cost in trees at two elevations in a subtropical forest in south China. We analysed these data to elucidate the major ecophysiological strategies of trees at two elevations.
Materials and methods
Study site
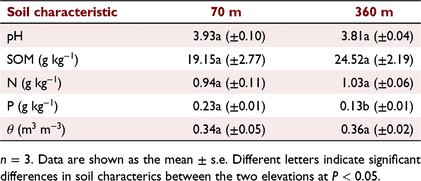
This study was conducted in the Dinghushan Biosphere Reserve located in the Guangdong Province of southern China (23°10′N, 112°10′E). This region has a monsoonal climate with a mean annual rainfall and mean annual air temperature of 1927 mm and 21°C, respectively. Approximately 75% of the annual rainfall occurs during March–August (Lu et al. 2010). We conducted our research in two 80-year-old coniferous and broad-leaved mixed forests (70 m and 360 m above sea level, respectively). These forests are at a mid-succession stage, and are dominated by broadleaf tree species (such as Schima superba and Castanopsis chinensis) and coniferous tree species (such as Pinus massoniana) (Zheng et al. 2020). The understorey layer has high species diversity and contains tree seedlings, shrubs, and herbaceous plants of various species Litsea rotundifolia var. oblongifolia (Nees) Allen, Psychotria rubra (Lour.) Poir, Cratoxylum cochinchinense (Lour.) Blume, Ficus variolosa Lindl. ex Benth., Rhodomyrtus tomentosa (Ait.) Hassk., Ardisia quinquegona Blume, Lophatherum gracile Brongn., Gahnia tristis Nees, Dicranopteris pedata (Houttuyn) Nakaike and Adiantum capillus-veneris L. The soil is a loam soil, and its characteristics are in Table 1. To avoid the effects of differences in water content, the field experiment was conducted in June 2019, when both elevations received sufficient rainfall.
|
Measurement of environmental variables
Soil water content (θ, m3 m−3) was measured at seven randomly selected locations at each elevation using a TDR 300 sensor (Spectrum Technologies, Inc., Illinois, USA). Three soil samples at a depth of 20 cm were collected at each sampling point and stored in sealed bags for the measurement of soil physiochemical properties. Soil organic matter (SOM) levels were measured using the potassium dichromate volumetric method. Total nitrogen (N) and total phosphorus (P) levels in the soil were measured with the Kjeldahl method, and soil pH was measured using a pH meter (HANNA, pH211, Italy). Solar radiation, air temperature (T) and air relative humidity (RH) were automatically monitored using the WatchDog 2700 station (Spectrum Technologies, Inc., Illinois, USA). Vapour pressure deficit (VPD, KPa) was calculated according to the following empirical formula (Campbell and Norman 1998):
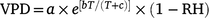
where a, b and c are constants with values of 0.611, 17.502 and 240.97, respectively; T is the air temperature; and RH is the air relative humidity.
Measurement of plant morphological and physiological traits
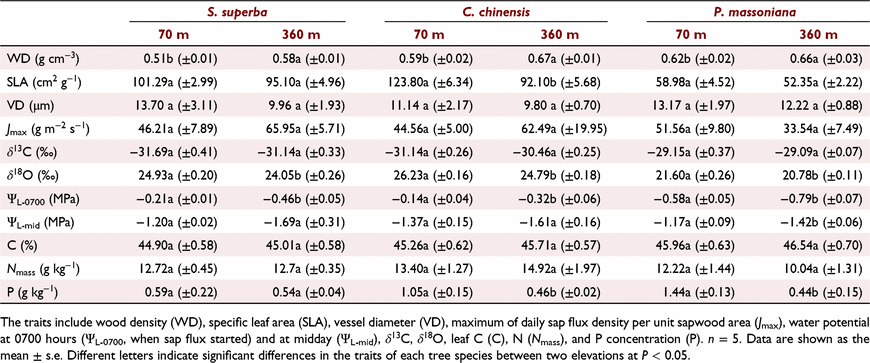
We selected five individuals per species for trait measurements. The diameter at breast height (DBH) and tree height (H) were measured using a tape and a VERTEX IV dendrometer (Haglöf Inc., Langsele, Sweden), respectively. Crown width (Cw) was calculated as the mean of four perpendicular crown radii measured in each cardinal direction. We collected 2 cm long cores from the stem of each sample tree at a height of 1.3 m above the ground using an increment borer (Haglöf Inc., Langsele, Sweden). The barks of the cores were removed, and the cores were dried at 65°C to a constant weight. Core volumes were calculated as the volumes of cylinders with 2 cm in length and 0.515 cm in diameter. Wood density (WD) was calculated as the dry mass divided by the respective core volume. Additionally, stem cores (∼1 cm in length) were collected at a height of 1.3 m using the increment borer. The bark was removed and the cores were immediately placed in glass bottles with 50% ethanol. These were embedded in paraffin and radial sections were prepared for further structural observation. Each sample was photographed at 50× magnification using a digital microscopy, and vessel diameters were measured using the CaseViewer 2.4 software (3DHISTECH Ltd., Budapest, Hungary). Several vessels were randomly selected from 3–5 samples of each species for the measurement of vessel diameter (VD, μm). Several fully expanded sun-exposed leaves were used for leaf area measurements using a leaf area meter (LI-3000C, LI-COR, Inc., Nebraska, USA), and the leaves were mixed and dried for dry mass determination. SLA was calculated by dividing the leaf area by leaf dry mass. Total C concentrations in leaves were measured through the oxidation of potassium dichromate and titration with an Fe2+ solution using an isotope ratio mass spectrometer (Thermo Fisher Scientific, Inc., Bremen, Germany). Total N (Nmass) and P concentrations of leaves were measured by micro-Kjeldahl digestion followed by indophenol blue and Mo-Sb colorimetric methods using a UV-8000 spectrophotometer (Metash Instruments Corp., Shanghai, China). Carbon and oxygen isotope ratios in leaf organic matter were determined using the DELTA V Advantage isotope ratio mass spectrometer (Thermo Fisher Scientific, Inc.) and Finnigan Delta V Advantage isotope ratio mass spectrometer (Thermo Fisher Scientific, Inc., MA, USA), respectively. The isotopic values are reported using the delta notation and are relative to PDB standard for carbon and the VSMOW standard for oxygen (δ13C and δ18O, respectively).
Monitoring of stem sap flux density
A pair of Granier thermal dissipation probes (length, 20 mm) were vertically installed into the stem xylem at 1.3 m above the ground for each sample tree (Granier 1987) according to the methods of Zhu et al. (2016). The temperature differences between the two probes were recorded using a data logger (Delta-T Devices Ltd., Cambridge, UK). Sap flux density per unit sapwood area (Js, g m−2 s−1) was calculated according to the Granier equation. The normalised Js was calculated by dividing the instantaneous Js by the daily maximal Js. For each tree species, the normalised Js was used to compare the sensitivity of sap flux to VPD in trees at the two elevations.
Measurement of leaf water potential
From each sample tree, we collected 3–5 branches with several leaves exposed to the sun using a 20 m long pole pruner. Leaf water potential was measured at 0700 hours (when sap flux started, ΨL-0700) and at midday (1200 hours) (ΨL-mid) on 28 and 30 June 2019 using a pressure chamber (1000, PMS Instrument Co., Corvallis, OR, USA). The lowest ΨL-mid was used as a proxy of drought tolerance.
Statistical analyses
Statistical analyses were performed using the SPSS 25 software (IBM, Inc., New York, USA). The environmental variables at the two elevations were compared using paired-samples t-tests at a significance level of P < 0.05. Differences in soil properties and the leaf and stem traits of each tree species between the two elevations were analysed by independent-sample t-tests at P < 0.05. The responses of normalised Js to VPD were compared between the two elevations using a UNIANOVA analysis. Multivariate associations between leaf and stem traits were analysed with a principal component analysis (PCA) across two elevations.
Results
Environmental variables
We compared the means of environmental variables in June 2019. Solar radiation was higher but T and VPD were lower at 360 m (high elevation) than at 70 m (low elevation) (Fig. 1; P < 0.05). The mean T was 26.84 ± 0.04°C and 25.66 ± 0.04°C at the 70 m and 360 m sites, respectively (Sheet 1 in Supplementary Information). Soil pH, SOM, N and θ were not significantly different between the two elevations; however, soil P was lower at 360 m than at 70 m (Table 1, P < 0.05).
Variations in leaf and stem traits with increasing elevation
When the morphological trait data were collated for three tree species, DBH and H were not significantly different between the two elevations, but Cw was lower at 360 m than at 70 m (Table 2, n = 15, P < 0.05). The daily maximal sap flux density per unit sapwood area (Jmax) of each tree species did not differ significantly between the two elevations (Table 3, P > 0.05). Based on the Js values at VPD > 1 kPa, the slope of the linear regression between normalised Js and VPD values was higher at 360 m than at 70 m for both S. superba and C. chinensis. For P. massoniana, the normalised Js was not related to VPD at 70 m (Fig. 2). These results indicate the higher sensitivity of water transport rate to VPD in plants at a higher elevation.

|
|
Leaf δ13C, leaf C and Nmass values for the three tree species did not differ significantly between the two elevations (Table 3; P > 0.05). In C. chinensis, the SLA was lower at 360 m than at 70 m. The SLA of the other two tree species did not differ significantly between elevations; however, their mean SLA was relatively low at 360 m, indicating high leaf construction costs at high elevations. Leaf P levels were lower at 360 m than at 70 m for C. chinensis and P. massoniana, but were similar between elevations for S. superba. Stem WD was higher at high elevation for all three tree species, indicating higher stem construction costs. Although VD was smaller at 360 m than at 70 m for all three species, the differences were not statistically significant. Both leaf δ18O and ΨL-0700 values were lower at 360 m than at 70 m for all three tree species (P < 0.05). Only P. massoniana, a gymnosperm, exhibited lower ΨL-mid values at high elevation, which likely indicates its higher drought tolerance.
In the PCA, the first two principal components explained 77.4% of the total variation (Fig. 3). The first axis explained 51.6% of the variation and mainly separated species according to traits associated with resources acquisition; that is, species with high leaf δ18O, ΨL-0700, and SLA were placed at high axis values and those with high leaf δ13C and C were placed at low axis values. The second axis explained an additional 25.8% of the variation. Species separation along the second axis was determined by traits associated with drought resistance; that is, species with high VD and ΨL-mid were at high axis values and those with high WD were at low axis values. Leaf-specific traits (including SLA, Nmass, δ18O, ΨL-0700, δ13C, and C) characterised the first PCA axis, where SLA, Nmass, and δ18O had an opposite influence compared to the other two traits. The second PCA axis was determined by wood-specific traits (including VD, ΨL-mid and WD), where WD seemed to have a trade-off relationship with VD.
Leaf and stem traits in relation to crown width across elevations
Cw was positively related to leaf δ18O and ΨL-0700 (Fig. 4), indicating that the decrease in Cw across elevations was mainly caused by decreasing leaf δ18O and ΨL-0700.
Discussion
PCA results revealed that the first and second axes represented the major ecological strategies of trees related to resource acquisition and stress resistance, respectively. In the present study, changes in leaf and stem traits with increasing elevation reflected an ability to resist drought. The decreases in leaf δ18O (indicating increased stomatal conductance) and ΨL-0700 were associated with a decrease in crown width at high elevation. Although WD increased at high elevation, it could not account for the variations in structural parameters. In the following sections, we discuss our results in more detail and explore their implications in trait-based ecology.
Variations in leaf and stem traits across two elevations
SLA decreased significantly at high elevation only in C. chinensis, and did not vary significantly in S. superba and P. massoniana. The statistically non-significant variation in SLA across elevations was inconsistent with the results from previous studies showing that SLA is more variable than other traits (Messier et al. 2010; Fajardo and Piper 2011; Umaña and Swenson 2019). SLA is a highly plastic trait, and can adjust to a range of environmental conditions during plant growth (Sultan 2000; Poorter et al. 2009). Trees exhibit a more conservative resource use strategy at high elevations. This is typically characterised by a lower SLA and thicker leaves with lower light-capturing area per unit biomass investment, indicating an adaptation to high radiation and low CO2 concentrations (Kao and Chang 2001; Körner 2007; Poorter et al. 2009; Homeier et al. 2010; Hernández-Calderón et al. 2014; Read et al. 2014; Ding et al. 2019; Umaña and Swenson 2019). In Metrosideros polymorpha Gaud., SLA varies approximately four-fold along elevation gradients, and the decrease in SLA with increasing elevation is mainly ascribed to decreasing temperatures and changes in water availability at high elevation (Milla et al. 2009; Poorter et al. 2009; Scheepens et al. 2010).
Multiple abiotic factors are known to covary with elevation, which may complicate the patterns of variation in plant traits (Körner 2007; Umaña and Swenson 2019). The strong association between SLA and leaf nutrient concentrations (Wright et al. 2004; Fyllas et al. 2009; Baraloto et al. 2010) suggested that the change in SLA across elevations may have been caused by environmental variables other that soil fertility conditions in this study. This is indicated by the fact that SLA did not decrease with lower leaf P concentrations at high elevation. Moreover, limited water availability can positively or negatively influence SLA, and this can be quantified by decreasing growth and leaf structure investments (Silva et al. 2004; Aspelmeier and Leuschner 2006). Therefore, when investigating the impacts of warming on vegetation, it is important to avoid the confounding effects of soil water availability on plant traits along an elevation gradient (Mujawamariya et al. 2018, 2021). The reduced abiotic differences between elevations during the rainy season may also lead to the lack of a clear relationship between SLA and elevation (Swenson et al. 2006; Gotsch et al. 2010). We found that soil moisture was comparable between the two elevations during the study period, which could partly explain the week relationship between SLA and elevation in the present study. However, a wider range of elevation gradients needs to be considered when investigating the specific reasons for variations in SLA across elevations.
Leaf chemical traits have been recognised to be mainly mediated by soil fertility (Asner and Martin 2016). In the present study, soil P concentration decreased significantly at high elevation, and leaf P levels were correspondingly lower in C. chinensis and P. massoniana. Leaf P increases with elevation in fertile sites, but does not vary in infertile sites (Asner and Martin 2016). This suggests that the high elevation site in this study may have been P-limited, which is also supported by the leaf N:P being >14 at this site (data not shown). Suppressed foliar P levels in low-fertility soil are correlated with increased investment in leaf structure and defence (such as the increased LMA) and decreased photosynthetic pigment (Asner et al. 2014). Ding et al. (2019) found that leaf N and P concentrations per unit mass decreased with increasing elevations, and ascribed this to the conservative resource use strategies of evergreen trees at high elevation. In this study, we did not find any changes in Nmass with elevation. This is congruent with the results of Read et al. (2014), who ascribed the lack of responses in Nmass to the other constraints varying independently of elevation. For instance, soil N availability, which is strongly associated with leaf N, showed no global variation with elevation (Körner 2007). Acquisitive species tend to show higher SLA and Nmass but lower Narea (Shipley et al. 2006). In this study, we found an increase in Narea and a decrease in SLA with increasing elevation only for C. chinensis (Sheet 2 in Supplementary Information). This partially corroborates the notion that dominant tree species exhibit conservative strategies at high elevations. Alternatively, the increase in leaf N content at high elevation may have resulted from the lower temperature at higher elevations (Körner 1999; Weih and Karlsson 2001). Leaf N content is more concentrated under cold conditions due to reduced growth (Körner and Larcher 1988; Morecroft et al. 1992), and high elevation plants typically show restricted growth due to low temperatures and a shorter growing season. Moreover, N uptake by the roots of these plants is not as limited, resulting in higher leaf N content (Hultine and Marshall 2000; Pop et al. 2000). However, the air temperature was >20°C at the high elevation site in our study, which may not have been low enough to influence leaf N content. Decreased leaf size may also cause an increase in leaf N with altitude (Cordell et al. 1998, 1999). This, combined with the relatively stable SLA across elevations, may explain the lack of variation in leaf N in our study.
High WD and low SLA values indicate a trade-off between resource allocation to metabolic production and growth rate (Sterck et al. 2006). Tree species with high LMA and WD dominated the high elevations. These traits are associated with increased longevity and improved carbon acquisition via more efficient photosynthesis (due to a higher capacity for RuBP (primary acceptor of CO2 in the Calvin cycle RuBP) regeneration at low temperatures) (Huxman et al. 2008; Hernández-Calderón et al. 2014), therefore compensating for a decreased growth rate. A high WD is typically associated with higher conductive safety and greater mechanical support, and may confer increased resistance to stem breakage by wind and other extrinsic forces (Stratton et al. 2000; ter Steege and Hammond 2001; Chave et al. 2009). However, some studies have reported a lack of change in WD along elevational gradients. This could be due to the conflicting effects of mechanical support and hydraulic functions caused by multiple abiotic factors across elevations (Fajardo and Piper 2011; Siefert et al. 2015; Fajardo 2016; Umaña and Swenson 2019). For instance, some studies have attributed the low intraspecific variation in WD across elevations to the mixed impacts of variation in precipitation and canopy height along elevational gradients (Siefert et al. 2015; Fajardo 2016; Umaña and Swenson 2019). This inconsistency with our results may be partly due to the comparable tree heights across elevations in our study. A higher sapwood density at high elevations is associated with a reduction in conduit number and size (Gričar et al. 2005; Hoch and Körner 2005; Rossi et al. 2008). A decrease in VD with increasing elevation is generally considered to confer increased safety from embolism caused by freeze–thaw cycles (Noshiro and Suzuki 1995; Fisher et al. 2007; Jiménez-Noriega et al. 2017; Pandey et al. 2021). WD is also affected by the thickness and width of conduit wall and fibre structures (Hacke et al. 2001, 2005). In the present study, the conduit diameter did not significantly differ across elevations. However, the increase in WD may have resulted from changes in conduit wall or fibre traits, which can maintain efficient water transport within the xylem system while offering biotic and abiotic safety. In this study, ΨL-0700 was found to be related to other plant traits. ΨL-0700 may indicate the amount of water stored within the plants for use in transpiration, which have not been reported by any studies to date. This was demonstrated by the higher WD at high elevations in the present study, suggesting lower water content of the xylem. Several studies have reported the correlations between minimum leaf water potential (ΨL-mid) and other plant functional traits (Bucci et al. 2004; Santiago et al. 2004; Chave et al. 2009), as ΨL-mid is strongly linked with plant rooting depth. In this study, the soil moisture was comparable between the two elevations, which suggested an unlimited water supply at both sites. This may explain the decoupling of the relationship between plant traits and root depth (and therefore, ΨL-mid). Furthermore, we observed that ΨL-0700 (associated with tree height) was correlated with plant traits (leaf δ18O and WD; Sheet 3 in Supplementary Information). This suggests that ΨL-0700 is an important trait with potentially profound ecological implications in the intraspecific responses to environmental change, and deserves thorough investigation in future studies.
Since an increase of leaf δ18O is associated with a decrease in stomatal conductance (Sheshshayee et al. 2005; Barbour 2007; Cernusak et al. 2009; Cabrera-Bosquet et al. 2011), we propose that a smaller leaf δ18O indicates higher stomatal conductance at high elevation in this study. The elevation-related intraspecific variations in leaf δ18O were also reflected in the responses of the normalised Js to VPD, which was more sensitive to water demand at high elevation. Similarly, a higher stomatal sensitivity to VPD at high elevation has been reported in temperate mountain forests (Jung et al. 2014). An increase in maximum stomatal conductance with elevation facilitates more carbon assimilation under lower CO2 partial pressure (Bresson et al. 2011). Apart from stomatal behaviour, changes in the ratio of leaf area to sapwood area (AL:AS) can also regulate the efficiency of water transpiration in trees (Fischer et al. 2002; Franks et al. 2007; Martínez-Vilalta and Garcia-Forner 2017). A decrease in AL:AS may increase water supply to leaves and prevent a decline in canopy conductance (Monserud and Marshall 2001; Fischer et al. 2002). Moreover, the decreased crown width and invariant DBH in this study may indicate a larger sapwood area per unit leaf area at the high elevation site. This further suggests an improved capacity for water supply through stem to leaves in the high elevation plants. Denser wood is known to be associated with low leaf area per stem area (Ackerly 2004; Wright et al. 2006). This finding supports our aforementioned suggestion, as we found an increase in WD at the high elevation site. Comparable sap flux densities per unit sapwood area and smaller AL:AS values may also lead to a higher transpiration rate per unit leaf area, which is implied by the higher stomatal conductance of plants at high elevation. Previous studies have confirmed that an increase in leaf δ13C with increasing elevation is attributable to the decrease in stomatal conductance, an increase in carboxylation efficiency (caused by a higher N content or leaf mass per area), and higher internal resistance (Morecroft et al. 1992; Hultine and Marshall 2000). The decrease in leaf δ18O (that is, higher stomatal conductance) at high elevation was expected to result in a decrease in leaf δ13C. However, the leaf δ13C remained constant across the two elevations in the present study. In addition, we found a negative correlation between leaf δ18O and δ13C (data not shown; Pearson correlation coefficient, −0.727, P < 0.01), indicating that photosynthetic capacity drives variations in leaf δ13C (Flanagan and Farquhar 2014). Therefore, we suggest that increased photosynthetic capacity, rather than stomatal conductance, likely played a crucial role in maintaining constant leaf δ13C across the two elevations in the present study.
Several studies have reported that photosynthetic capacity increases along elevational gradients (Girardin et al. 2010; Bresson et al. 2011; Fan et al. 2011; Huasco et al. 2014). Environmental factors such as temperature and solar radiation, rather than soil properties, regulate photosynthetic capacity and elevated CO2 concentrations can reduce photosynthesis capacity (Ainsworth and Rogers 2007; Smith et al. 2019). Thus, in our experiment, the increased solar radiation and the reduced atmospheric CO2 concentration likely contributed to the enhanced photosynthetic capacity of plants at higher elevation. Woodruff et al. (2007) reported that high photosynthetic rates were associated with increasing transpiration rates in the tree canopy. In our study, the sap flux density per unit sapwood area did not change at high elevation, and the crown width decreased in plants at the high elevation site. Furthermore, an increased transpiration rate per unit leaf area may be associated with the high photosynthetic capacity in high elevation plants. As CO2 concentrations decrease with elevation, the sensitivity of photosynthesis to stomatal conductance increases (Zhang et al. 1993; Niinemets 2002; Premoli and Brewer 2007; Reed and Loik 2016). Consequently, an increase in stomatal conductance with elevation may improve photosynthetic capacity by favouring CO2 diffusion, thus counterbalancing the diffusive limitations caused by morphological adaptations (such as an increase in LMA) (Niinemets 2002; Gago et al. 2019). Therefore, we suggest that a trade-off between CO2 uptake and hydraulic risk (associated with WD) due to stomatal apertures may have profound implications for the functional fitness of plants to environmental changes.
Plant life history theory suggests that species that exhibit rapid resource exploitation can be characterised by high SLA, hydraulic conductance, photosynthetic rates, and stomatal conductance, and low wood density (Reich et al. 1997; Wright et al. 2004; Díaz et al. 2016). However, Augustine and Reinhardt (2019) found differing levels of plasticity among morphological and physiological traits in first-year conifer seedlings exposed to water stress. In this study, we found evidence for conservative resource use strategies (including high WD and low leaf P) as well as acquisitive resource-use strategies (including improved leaf-level stomatal conductance, indicated by variations in leaf δ18O) in plants at high elevation. Grady et al. (2013) indicated that the relationships among leaf economic traits may not apply to adaptive variation at the intraspecific level. They suggested that a higher leaf area to sapwood area ratio could increase whole-canopy photosynthesis, thus compensating for low leaf-level photosynthesis. This is consistent with our findings; that is, the decrease in leaf-level photosynthetic capacity in low elevation plants does not necessarily indicate a low whole-tree carbon assimilation rate. This is because a larger canopy width as a surrogate of leaf area may compensate for the loss of leaf-level photosynthetic capacity. Moreover, a decrease in respiration coupled with the photosynthetic capacity at low elevation likely favoured carbon fixation within plants (Dusenge et al. 2021; Mujawamariya et al. 2021). Therefore, it is necessary to investigate these traits at different plant tissue levels in order to thoroughly elucidate the intraspecific adaptive mechanisms to changing environments.
Responses of the canopy width to trait variations across elevations
Umaña and Swenson (2019) proposed a structural spectrum at the canopy level, ranging from ‘cheap’ leaves (high SLA, low construction cost) comprising a bigger crown to leaves with low SLA comprising smaller crowns. Consistent with this, we observed a smaller crown width at high elevation, although the differences in SLA across elevations were species-specific. Normand et al. (2008) proposed two main hypotheses to explain the changes in tree canopy structure with elevation. (1) First, the hydraulic limitation theory suggests that water-related environmental stress along elevational gradients would affect canopy expansion. The plant canopy has high water demand; therefore, changes in its structure and function could moderate water transport through the stems from the roots under adverse conditions (McDowell et al. 2002; Martínez-Vilalta et al. 2009; Gebrekirstos et al. 2011; Rosas et al. 2019). The height and total leaf area of plants were constrained as a response to the stressful climate at high elevation (Dierig et al. 2006; Ahmad et al. 2016, 2018; Leitold et al. 2018). Smith et al. (2019) reported that over the dry season, leaf area decreased in low canopy surfaces due to low soil water availability. In the present study, the lower water potential in the morning in high elevation plants likely indicated higher daily levels of water tension within the xylem. Therefore, the increased stomatal conductance and decreased crown width likely ensured sufficient water supply to leaves. (2) Second, the demand for mechanical support with changing environments modifies tree allometry (Henry and Aarssen 1999; Osunkoya et al. 2007; Normand et al. 2008). The climate features at high elevation include high wind exposure, which damages the upright plants (Premoli and Brewer 2007; Poorter et al. 2009; Wang et al. 2010). Species with lighter wood grow faster and reach the canopy faster in order to acquire more light resources (Enquist et al. 1999); however, this is not necessary for plants at high elevations that receive high solar radiation. Therefore, a high wood density, combined with a small canopy width, can promote whole-plant mechanical support and resistance to stem breakage from extrinsic forces such as wind (ter Steege and Hammond 2001).
Conclusions
Some physiological traits of leaves and stems such as leaf δ18O and water potential showed plasticity across two elevations in response to environmental stresses. A decrease in leaf δ18O resulted in an increase in stomatal conductance, thus assuring water transport efficiency within the xylem. Combined with a larger WD, this likely helped protect the xylem from cavitation under the more negative water potentials at high elevations. The leaf δ13C remained constant and stomatal conductance increased, which probably helped maintain net carbon assimilation for the high tissue construction cost (high LMA and WD) at high elevation. Thereby, stomatal activity could play a central role in regulating the ecophysiological responses of plants to changing environments. In addition to the widely studied leaf and stem economics spectrum, measurements of other physiological traits such as leaf water potential, stomatal conductance, and transpiration rate should also be investigated along environmental gradients in future studies. Such investigations can improve our understanding of resource use strategies in forest ecosystems under changing environmental conditions.
Supplementary material
Supplementary material is available online.
Data availability
The datasets used in this paper are available from the first author on reasonable request.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This study was funded by the National Natural Science Foundation of China (31770646) and Guangdong Basic and Applied Basic Research Foundation (2021A1515012486).
References
Ackerly D (2004) Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance. Ecological Monographs 74, 25–44.| Functional strategies of chaparral shrubs in relation to seasonal water deficit and disturbance.Crossref | GoogleScholarGoogle Scholar |
Adams HD, Kolb TE (2004) Drought responses of conifers in ecotone forests of northern Arizona: tree ring growth and leaf δ13C. Oecologia 140, 217–225.
| Drought responses of conifers in ecotone forests of northern Arizona: tree ring growth and leaf δ13C.Crossref | GoogleScholarGoogle Scholar | 15148600PubMed |
Ahmad KS, Hameed M, Ahmad F, Sadia B (2016) Edaphic factors as major determinants of plant distribution of temperate Himalayan grasses. Pakistan Journal of Botany 48, 567–573.
Ahmad KS, Hameed M, Hamid A, Nawaz F, Kiani BH, Ahmad MSA, Deng JB, Ahmad F, Hussain I, Fatima S (2018) Beating cold by being tough: impact of elevation on leaf characteristics in Phleum himalaicum Mez. endemic to Himalaya. Acta Physiologiae Plantarum 40, 56
| Beating cold by being tough: impact of elevation on leaf characteristics in Phleum himalaicum Mez. endemic to Himalaya.Crossref | GoogleScholarGoogle Scholar |
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant, Cell & Environment 30, 258–270.
| The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions.Crossref | GoogleScholarGoogle Scholar |
Asner GP, Martin RE (2016) Convergent elevation trends in canopy chemical traits of tropical forests. Global Change Biology 22, 2216–2227.
| Convergent elevation trends in canopy chemical traits of tropical forests.Crossref | GoogleScholarGoogle Scholar | 26582427PubMed |
Asner GP, Martin RE, Tupayachi R, Anderson CB, Sinca F, Carranza-Jiménez L, Martinez P (2014) Amazonian functional diversity from forest canopy chemical assembly. Proceedings of the National Academy of Sciences of the United States of America 111, 5604–5609.
| Amazonian functional diversity from forest canopy chemical assembly.Crossref | GoogleScholarGoogle Scholar | 24591585PubMed |
Aspelmeier S, Leuschner C (2006) Genotypic variation in drought responses of silver birch (Betula pendula Roth): leaf and root morphology and carbon partitioning. Trees 20, 42–52.
| Genotypic variation in drought responses of silver birch (Betula pendula Roth): leaf and root morphology and carbon partitioning.Crossref | GoogleScholarGoogle Scholar |
Augustine SP, Reinhardt K (2019) Differences in morphological and physiological plasticity in two species of first-year conifer seedlings exposed to drought result in distinct survivorship patterns. Tree Physiology 39, 1446–1460.
| Differences in morphological and physiological plasticity in two species of first-year conifer seedlings exposed to drought result in distinct survivorship patterns.Crossref | GoogleScholarGoogle Scholar | 31181151PubMed |
Baraloto C, Timothy Paine CE, Poorter L, Beauchene J, Bonal D, Domenach A-M, Hérault B, Patiño S, Roggy J-C, Chave J (2010) Decoupled leaf and stem economics in rain forest trees. Ecology Letters 13, 1338–1347.
| Decoupled leaf and stem economics in rain forest trees.Crossref | GoogleScholarGoogle Scholar | 20807232PubMed |
Barbour MM (2007) Stable oxygen isotope composition of plant tissue: a review. Functional Plant Biology 34, 83–94.
| Stable oxygen isotope composition of plant tissue: a review.Crossref | GoogleScholarGoogle Scholar | 32689335PubMed |
Beerling DJ, Heath J, Woodward FI, Mansfield TA (1996) Drought–CO2 interactions in trees: observations and mechanisms. New Phytologist 134, 235–242.
| Drought–CO2 interactions in trees: observations and mechanisms.Crossref | GoogleScholarGoogle Scholar |
Bresson CC, Vitasse Y, Kremer A, Delzon S (2011) To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech? Tree Physiology 31, 1164–1174.
| To what extent is altitudinal variation of functional traits driven by genetic adaptation in European oak and beech?Crossref | GoogleScholarGoogle Scholar | 21908436PubMed |
Bucci SJ, Goldstein G, Meinzer FC, Scholz FG, Franco AC, Bustamante M (2004) Functional convergence in hydraulic architecture and water relations of tropical savanna trees: from leaf to whole plant. Tree Physiology 24, 891–899.
| Functional convergence in hydraulic architecture and water relations of tropical savanna trees: from leaf to whole plant.Crossref | GoogleScholarGoogle Scholar | 15172839PubMed |
Cabrera-Bosquet L, Albrizio R, Nogués S, Araus JL (2011) Dual Δ13C/δ18O response to water and nitrogen availability and its relationship with yield in field-grown durum wheat. Plant, Cell & Environment 34, 418–433.
| Dual Δ13C/δ18O response to water and nitrogen availability and its relationship with yield in field-grown durum wheat.Crossref | GoogleScholarGoogle Scholar |
Campbell GS, Norman JM (1998) ‘An introduction to environmental biophysics’. (Springer)
Cernusak LA, Pate JS, Farquhar GD (2002) Diurnal variation in the stable isotope composition of water and dry matter in fruiting Lupinus angustifolius under field conditions. Plant, Cell & Environment 25, 893–907.
| Diurnal variation in the stable isotope composition of water and dry matter in fruiting Lupinus angustifolius under field conditions.Crossref | GoogleScholarGoogle Scholar |
Cernusak LA, Winter K, Aranda J, Turner BL, Marshall JD (2007) Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility. Journal of Experimental Botany 58, 3549–3566.
| Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility.Crossref | GoogleScholarGoogle Scholar | 18057036PubMed |
Cernusak LA, Winter K, Turner BL (2009) Physiological and isotopic (δ13C and δ18O) responses of three tropical tree species to water and nutrient availability. Plant, Cell & Environment 32, 1441–1455.
| Physiological and isotopic (δ13C and δ18O) responses of three tropical tree species to water and nutrient availability.Crossref | GoogleScholarGoogle Scholar |
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecology Letters 12, 351–366.
| Towards a worldwide wood economics spectrum.Crossref | GoogleScholarGoogle Scholar | 19243406PubMed |
Cordell S, Goldstein G, Mueller-Dombois D, Webb D, Vitousek PM (1998) Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along altitudinal gradient: the role of phenotypic plasticity. Oecologia 113, 188–196.
| Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along altitudinal gradient: the role of phenotypic plasticity.Crossref | GoogleScholarGoogle Scholar | 28308196PubMed |
Cordell S, Goldstein G, Meinzer FC, Handley LL (1999) Allocation of nitrogen and carbon in leaves of Metrosideros polymorpha regulates carboxylation capacity and δ13C along an altitudinal gradient. Functional Ecology 13, 811–818.
| Allocation of nitrogen and carbon in leaves of Metrosideros polymorpha regulates carboxylation capacity and δ13C along an altitudinal gradient.Crossref | GoogleScholarGoogle Scholar |
Cunningham S, Read J (2002) Comparison of temperate and tropical rainforest tree species: photosynthetic responses to growth temperature. Oecologia 133, 112–119.
| Comparison of temperate and tropical rainforest tree species: photosynthetic responses to growth temperature.Crossref | GoogleScholarGoogle Scholar | 28547297PubMed |
De Bello F, Lavorel S, Lavergne S, Albert CH, Boulangeat I, Mazel F, Thuiller W (2012) Hierarchical effects of environmental filters on the functional structures of plant communities: a case study in the French Alps. Ecography 36, 393–402.
| Hierarchical effects of environmental filters on the functional structures of plant communities: a case study in the French Alps.Crossref | GoogleScholarGoogle Scholar |
De Frenne P, Graae BJ, Rodríguez-Sánchez F, Kolb A, Chabrerie O, Decocq G, De Kort H, De Schrijver A, Diekmann M, Eriksson O, Gruwez R, Hermy M, Lenoir J, Plue J, Coomes DA, Verheyen K (2013) Latitudinal gradients as natural laboratories to infer species’ response to temperature. Journal of Ecology 101, 784–795.
| Latitudinal gradients as natural laboratories to infer species’ response to temperature.Crossref | GoogleScholarGoogle Scholar |
Des Roches S, Post DM, Turley NE, Bailey JK, Hendry AP, Kinnison MT, Schweitzer JA, Palkovacs EP (2018) The ecological importance of intraspecific variation. Nature Ecology & Evolution 2, 57–64.
| The ecological importance of intraspecific variation.Crossref | GoogleScholarGoogle Scholar |
Díaz S, Kattge J, Cornelissen JHC, Wright IJ, Lavorel S, Dray S, Reu B, Kleyer M, Wirth C, Prentice IC, Garnier E, Bönisch G, Westoby M, Poorter H, Reich PB, Moles AT, Dickie J, Gillison AN, Zanne AE, Chave J, Wright SJ, Sheremet’ev SN, Jactel H, Baraloto C, Cerabolini B, Pierce S, Shipley B, Kirkup D, Casanoves F, Joswig JS, Günther A, Faclzuk V, Rüger N, Mahecha MD, Gorné LD (2016) The global spectrum of plant form and function. Nature 529, 167–171.
| The global spectrum of plant form and function.Crossref | GoogleScholarGoogle Scholar | 26700811PubMed |
Dierig DA, Adama NR, Mackey BE, Dahlquist GH, Coffelt TA (2006) Temperature and elevation effects on plant growth, development, and seed production of two Lesquerella species. Industrial Crops and Products 24, 17–25.
| Temperature and elevation effects on plant growth, development, and seed production of two Lesquerella species.Crossref | GoogleScholarGoogle Scholar |
Ding Y, Zang RG, Lu XH, Huang JH, Xu Y (2019) The effect of environmental filtering on variation in functional diversity along a tropical elevational gradient. Journal of Vegetation Science 30, 973–983.
| The effect of environmental filtering on variation in functional diversity along a tropical elevational gradient.Crossref | GoogleScholarGoogle Scholar |
Dunne JA, Saleska SR, Fischer ML, Harte J (2004) Integrating experimental and gradient methods in ecological climate change research. Ecology 85, 904–916.
| Integrating experimental and gradient methods in ecological climate change research.Crossref | GoogleScholarGoogle Scholar |
Dusenge ME, Wittemann M, Mujawamariya M, Ntawuhiganayo EB, Zibera E, Ntirugulirwa B, Way DA, Nsabimana D, Udling J, Wallin G (2021) Limited thermal acclimation of photosynthesis in tropical montane tree species. Global Change Biology 27, 4860–4878.
| Limited thermal acclimation of photosynthesis in tropical montane tree species.Crossref | GoogleScholarGoogle Scholar | 34233063PubMed |
Enquist BJ, West GB, Charnov EL, Brown JH (1999) Allometric scaling of production and life-history variation in vascular plants. Nature 401, 907–911.
| Allometric scaling of production and life-history variation in vascular plants.Crossref | GoogleScholarGoogle Scholar |
Fajardo A (2016) Wood density is a poor predictor of competitive ability among individuals of the same species. Forest Ecology and Management 372, 217–225.
| Wood density is a poor predictor of competitive ability among individuals of the same species.Crossref | GoogleScholarGoogle Scholar |
Fajardo A, Piper FI (2011) Intraspecific trait variation and covariation in a widespread tree species (Nothofagus pumilio) in southern Chile. New Phytologist 189, 259–271.
| Intraspecific trait variation and covariation in a widespread tree species (Nothofagus pumilio) in southern Chile.Crossref | GoogleScholarGoogle Scholar |
Fan Y, Zhong Z, Zhang X (2011) Determination of photosynthetic parameters Vcmax and Jmax for a C3 plant (spring hulless barley) at two altitudes on the Tibetan Plateau. Agricultural and Forest Meteorology 151, 1481–1487.
| Determination of photosynthetic parameters Vcmax and Jmax for a C3 plant (spring hulless barley) at two altitudes on the Tibetan Plateau.Crossref | GoogleScholarGoogle Scholar |
Fischer DG, Kolb TE, Dewald LE (2002) Changes in whole-tree water relations during ontogeny of Pinus flexilis and Pinus ponderosa in a high-elevation meadow. Tree Physiology 22, 675–685.
| Changes in whole-tree water relations during ontogeny of Pinus flexilis and Pinus ponderosa in a high-elevation meadow.Crossref | GoogleScholarGoogle Scholar | 12091149PubMed |
Fisher JB, Goldstein G, Jones TJ, Cordell S (2007) Wood vessel diameter is related to elevation and genotype in the Hawaiian tree Metrosideros polymorpha (Myrtaceae). American Journal of Botany 94, 709–715.
| Wood vessel diameter is related to elevation and genotype in the Hawaiian tree Metrosideros polymorpha (Myrtaceae).Crossref | GoogleScholarGoogle Scholar | 21636440PubMed |
Flanagan LB, Farquhar GD (2014) Variation in the carbon and oxygen isotope composition of plant biomass and its relationship to water-use efficiency at the leaf- and ecosystem-scales in a northern Great Plains grassland. Plant, Cell & Environment 37, 425–438.
| Variation in the carbon and oxygen isotope composition of plant biomass and its relationship to water-use efficiency at the leaf- and ecosystem-scales in a northern Great Plains grassland.Crossref | GoogleScholarGoogle Scholar |
Franks GD, Cernusak LA, Barnes B (2007) Heavy water fractionation during transpiration. Plant Physiology 143, 11–18.
| Heavy water fractionation during transpiration.Crossref | GoogleScholarGoogle Scholar |
Fukami T, Wardle DA (2005) Long-term ecological dynamics: reciprocal insights from natural and anthropogenic gradients. Proceedings of the Royal Society B: Biological Sciences 272, 2105–2115.
| Long-term ecological dynamics: reciprocal insights from natural and anthropogenic gradients.Crossref | GoogleScholarGoogle Scholar | 16191623PubMed |
Fyllas NM, Patiño S, Baker TR, Bielefeld Nardoto G, Martinelli LA, Quesada CA, et al. (2009) Basin-wide variations in foliar properties of Amazonian forest: phylogeny, soils and climate. Biogeosciences 6, 2677–2708.
| Basin-wide variations in foliar properties of Amazonian forest: phylogeny, soils and climate.Crossref | GoogleScholarGoogle Scholar |
Gago J, Carriquí M, Nadal M, Clemente-Moreno MJ, Coopman RE, Fernie AR, Flexas J (2019) Photosynthesis optimized across land plant phylogeny. Trends in Plant Science 24, 947–958.
| Photosynthesis optimized across land plant phylogeny.Crossref | GoogleScholarGoogle Scholar | 31362860PubMed |
Gebrekirstos A, van Noordwijk M, Neufeldt H, Mitlöhner R (2011) Relationships of stable carbon isotopes, plant water potential and growth: an approach to assess water use efficiency and growth strategies of dry land agroforestry species. Trees 25, 95–102.
| Relationships of stable carbon isotopes, plant water potential and growth: an approach to assess water use efficiency and growth strategies of dry land agroforestry species.Crossref | GoogleScholarGoogle Scholar |
Girardin CAJ, Malhi Y, Aragão LECO, Mamani M, Huasco WH, Durand L, Feeley KJ, Rapp J, Silva-Espejo JE, Silman M, Salinas N, Whittaker RJ (2010) Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes. Global Change Biology 16, 3176–3192.
| Net primary productivity allocation and cycling of carbon along a tropical forest elevational transect in the Peruvian Andes.Crossref | GoogleScholarGoogle Scholar |
Gotsch SG, Powers JS, Lerdau MT (2010) Leaf traits and water relations of 12 evergreen species in Costa Rican wet and dry forests: patterns of intra-specific variation across forests and seasons. Plant Ecology 211, 133–146.
| Leaf traits and water relations of 12 evergreen species in Costa Rican wet and dry forests: patterns of intra-specific variation across forests and seasons.Crossref | GoogleScholarGoogle Scholar |
Grady KC, Laughlin DC, Ferrier SM, Kolb TE, Hart SC, Allan GJ, Whitham TG (2013) Conservative leaf economic traits correlate with fast growth of genotypes of a foundation riparian species near the thermal maximum extent of its geographic range. Functional Ecology 27, 428–438.
| Conservative leaf economic traits correlate with fast growth of genotypes of a foundation riparian species near the thermal maximum extent of its geographic range.Crossref | GoogleScholarGoogle Scholar |
Granier A (1987) Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements. Tree Physiology 3, 309–320.
| Evaluation of transpiration in a Douglas-fir stand by means of sap flow measurements.Crossref | GoogleScholarGoogle Scholar | 14975915PubMed |
Gričar J, Čufar K, Oven P, Schmitt U (2005) Differentiation of terminal latewood tracheids in Silver fir trees during autumn. Annals of Botany 95, 959–965.
| Differentiation of terminal latewood tracheids in Silver fir trees during autumn.Crossref | GoogleScholarGoogle Scholar | 15760912PubMed |
Hacke UG, Sperry JS, Pockeman WT, Davis SD, McCulloch KA (2001) Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure. Oecologia 126, 457–461.
| Trends in wood density and structure are linked to prevention of xylem implosion by negative pressure.Crossref | GoogleScholarGoogle Scholar | 28547229PubMed |
Hacke UG, Sperry JS, Pitterman J (2005) Efficiency versus safety trade offs for water conduction in angiosperm vessels versus gymnosperm tracheids. In ‘Vascular transport in plants’. (Eds NM Holbrook, MA Zwieniecki) pp. 333–353. (Elsevier Academic Press: London, UK)
Henry HAL, Aarssen LW (1999) The interpretation of stem diameter–height allometry in trees: biomechanical constraints, neighbour effects, or biased regressions. Ecology Letters 2, 89–97.
| The interpretation of stem diameter–height allometry in trees: biomechanical constraints, neighbour effects, or biased regressions.Crossref | GoogleScholarGoogle Scholar |
Hernández-Calderón E, Méndez-Alonzo R, Martínez-Cruz J, González-Rodríguez A, Oyama K (2014) Altitudinal changes in tree leaf and stem functional diversity in a semi-tropical mountain. Journal of Vegetation Science 25, 955–966.
| Altitudinal changes in tree leaf and stem functional diversity in a semi-tropical mountain.Crossref | GoogleScholarGoogle Scholar |
Hoch G, Körner CC (2005) Growth, demography and carbon relations of Polylepis trees at the world’ s highest treeline. Functional Ecology 19, 941–951.
| Growth, demography and carbon relations of Polylepis trees at the world’ s highest treeline.Crossref | GoogleScholarGoogle Scholar |
Homeier J, Breckle S-W, Günter S, Rollenbeck RT, Leuschner C (2010) Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich ecuadorian montane rain forest. Biotropica 42, 140–148.
| Tree diversity, forest structure and productivity along altitudinal and topographical gradients in a species-rich ecuadorian montane rain forest.Crossref | GoogleScholarGoogle Scholar |
Huasco WH, Girardin CAJ, Doughty CE, Metcalfe DB, Baca LD, Silva-Espejo JE, Cabrera DG, Aragão LEOC, Davila AR, Marthews TR, Huaraca-Quispe LP, Alzamora-Taype I, Mora LE, Farfá-Rios W, Cabrera KG, Halladay K, Salinas-Revilla N, Silman MR, Meir P, Malhi Y (2014) Seasonal production, allocation and cycling of carbon in two mid-elevation tropical montane forest plots in the Peruvian Andes. Plant Ecology & Diversity 7, 125–142.
| Seasonal production, allocation and cycling of carbon in two mid-elevation tropical montane forest plots in the Peruvian Andes.Crossref | GoogleScholarGoogle Scholar |
Hulshof CM, Violle C, Spasojevic MJ, McGill B, Damschen E, Harrison S, Enquist BJ (2013) Intra-specific and inter-specific variation in specific leaf area reveal the importance of abiotic and biotic drivers of species diversity across elevation and latitude. Journal of Vegetation Science 24, 921–931.
| Intra-specific and inter-specific variation in specific leaf area reveal the importance of abiotic and biotic drivers of species diversity across elevation and latitude.Crossref | GoogleScholarGoogle Scholar |
Hultine KR, Marshall JD (2000) Altitude trends in conifer leaf morphology and stable carbon isotope composition. Oecologia 123, 32–40.
| Altitude trends in conifer leaf morphology and stable carbon isotope composition.Crossref | GoogleScholarGoogle Scholar | 28308741PubMed |
Huxman TE, Barron-Gafford G, Gerst KL, Angert AL, Tyler AP, Venable DL (2008) Photosynthetic resource-use efficiency and demographic variability in desert annual plants. Ecology 89, 1554–1563.
| Photosynthetic resource-use efficiency and demographic variability in desert annual plants.Crossref | GoogleScholarGoogle Scholar | 18589520PubMed |
IPCC (2014) Climate Change 2014: synthesis report. Contribution of Working Groups I, II, and III to the fifth assessment report of the intergovernmental panel on Climate Change [Core Writing Team, (Eds PK Pachauri, LA Meyer) p. 151. (IPCC, Geneva, Switzerland)
IPCC (2018) Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty. [Eds V Masson-Delmotte, P Zhai, HO Pörtner, D Roberts, J Skea, PR Shukla, A Pirani, W Moufouma-Okia, C Péan, R Pidcock, JBR Matthews, Y Chen, X Zhou, MI Gomis, E Lonnoy, T Maycock, M Tignor, T Waterfield] (IPCC)
Jiménez-Noriega PMS, Terrazas T, López-Mata L, Sánchez-González A, Vibrans H (2017) Anatomical variation of five plant species along an elevation gradient in Mexico City basin within the Trans-Mexican Volcanic Belt, Mexico. Journal of Mountain Science 14, 2182–2199.
| Anatomical variation of five plant species along an elevation gradient in Mexico City basin within the Trans-Mexican Volcanic Belt, Mexico.Crossref | GoogleScholarGoogle Scholar |
Jung E-Y, Otieno D, Kwon H, Berger S, Hauer M, Tenhunen J (2014) Influence of elevation on canopy transpiration of temperate deciduous forests in a complex mountainous terrain of South Korea. Plant and Soil 378, 153–172.
| Influence of elevation on canopy transpiration of temperate deciduous forests in a complex mountainous terrain of South Korea.Crossref | GoogleScholarGoogle Scholar |
Kao W-Y, Chang K-W (2001) Altitudinal trends in photosynthetic rate and leaf characteristics of Miscanthus populations from central Taiwan. Australian Journal of Botany 49, 509–514.
| Altitudinal trends in photosynthetic rate and leaf characteristics of Miscanthus populations from central Taiwan.Crossref | GoogleScholarGoogle Scholar |
Körner C (1999) ‘Alpine plant life’. p. 338. (Springer: Berlin)
Körner C (2007) The use of ‘altitude’ in ecological research. Trends in Ecology & Evolution 22, 569–574.
| The use of ‘altitude’ in ecological research.Crossref | GoogleScholarGoogle Scholar |
Körner CH, Larcher W (1988) Plant life in cold climates. In ‘Plant and temperature’. Vol. 42. Symposium of experimental biology. (Eds SF Long, FI Woodward) pp. 25–57. (The Company of Biologists Ltd.: Cambridge, MA, USA)
Körner C, Farquhar GD, Wong SC (1991) Carbon isotope discrimination by plants follows latitudinal and altitudinal trends. Oecologia 88, 30–40.
| Carbon isotope discrimination by plants follows latitudinal and altitudinal trends.Crossref | GoogleScholarGoogle Scholar | 28312728PubMed |
Leitold V, Morton DC, Longo M, dos-Santos MN, Keller M, Scaranello M (2018) EI Niño drought increased canopy turnover in Amazon forests. New Phytologist 219, 959–971.
| EI Niño drought increased canopy turnover in Amazon forests.Crossref | GoogleScholarGoogle Scholar |
León-Sánchez L, Nicolás E, Nortes PA, Maestre FT, Querejeta JI (2016) Photosynthesis and growth reduction with warming are driven by nonstomatal limitations in a Mediterranean semi-arid shrub. Ecology and Evolution 6, 2725–2738.
| Photosynthesis and growth reduction with warming are driven by nonstomatal limitations in a Mediterranean semi-arid shrub.Crossref | GoogleScholarGoogle Scholar | 27066247PubMed |
Li YY, Liu JX, Zhou GY, Huang WJ, Duan HL (2016) Warming effects on photosynthesis of subtropical tree species: a translocation experiment along an altitudinal gradient. Scientific Report 6, 24895
| Warming effects on photosynthesis of subtropical tree species: a translocation experiment along an altitudinal gradient.Crossref | GoogleScholarGoogle Scholar |
Lie ZY, Huang WJ, Liu XJ, Zhou GY, Yan JH, Li YL, Huang CM, Wu T, Fang X, Zhao MD, Liu SZ, Chu GW, Kadowaki K, Pan XP, Liu JX (2021) Warming leads to more closed nitrogen cycling in nitrogen-rich tropical forests. Global Change Biology 27, 664–674.
| Warming leads to more closed nitrogen cycling in nitrogen-rich tropical forests.Crossref | GoogleScholarGoogle Scholar |
Liu HY, Williams AP, Allen CD, Guo DL, Wu XC, Anenkhonov OA, Liang EY, Sandanov DV, Yin Y, Qi ZH, Badmaeva NK (2013) Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia. Global Change Biology 19, 2500–2510.
| Rapid warming accelerates tree growth decline in semi-arid forests of Inner Asia.Crossref | GoogleScholarGoogle Scholar |
Lu XK, Mo JM, Gilliam FS, Zhou GY, Fang YT (2010) Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest. Global Change Biology 16, 2688–2700.
| Effects of experimental nitrogen additions on plant diversity in an old-growth tropical forest.Crossref | GoogleScholarGoogle Scholar |
Martínez-Vilalta J, Garcia-Forner N (2017) Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept. Plant, Cell & Environment 40, 962–976.
| Water potential regulation, stomatal behaviour and hydraulic transport under drought: deconstructing the iso/anisohydric concept.Crossref | GoogleScholarGoogle Scholar |
Martínez-Vilalta J, Cochard H, Mencuccini M, Sterck F, Herrero A, Korhonen JFJ, Llorens P, Nikinmaa E, Nolè A, Poyatos R, Ripullone F, Sass-Klaassen U, Zweifel R (2009) Hydraulic adjustment of Scots pine across Europe. New Phytologist 184, 353–364.
| Hydraulic adjustment of Scots pine across Europe.Crossref | GoogleScholarGoogle Scholar |
McDowell N, Barnard H, Bond BJ, Hinckley T, Hubbard RM, Ishii H, Köstner B, Magnani F, Marshall J, Meinzer F, Phillips N, Ryan M, Whitehead D (2002) The relationship between tree height and leaf area: sapwood area ratio. Oecologia 132, 12–20.
| The relationship between tree height and leaf area: sapwood area ratio.Crossref | GoogleScholarGoogle Scholar | 28547290PubMed |
McDowell NG, White S, Pockman WT (2008) Transpiration and stomatal conductance across a steep climate gradient in the southern Rocky Mountains. Ecohydrology 1, 193–204.
| Transpiration and stomatal conductance across a steep climate gradient in the southern Rocky Mountains.Crossref | GoogleScholarGoogle Scholar |
McGill BJ, Enquist BJ, Weiher E, Westoby M (2006) Rebuilding community ecology from functional traits. Trends in Ecology & Evolution 21, 178–185.
| Rebuilding community ecology from functional traits.Crossref | GoogleScholarGoogle Scholar |
Messier J, McGill BJ, Lechowicz MJ (2010) How do traits vary across ecological scales? A case for trait-based ecology. Ecology Letters 13, 838–848.
| How do traits vary across ecological scales? A case for trait-based ecology.Crossref | GoogleScholarGoogle Scholar | 20482582PubMed |
Milla R, Giménez-Benavides L, Escudero A, Reich PB (2009) Intra- and interspecific performance in growth and reproduction increase with altitude: a case study with two Saxigraga species from northern Spain. Functional Ecology 23, 111–118.
| Intra- and interspecific performance in growth and reproduction increase with altitude: a case study with two Saxigraga species from northern Spain.Crossref | GoogleScholarGoogle Scholar |
Monserud RA, Marshall JD (2001) Time-series analysis of δ13C from tree rings. I. Time trends and autocorrelation. Tree Physiology 21, 1087–1102.
| Time-series analysis of δ13C from tree rings. I. Time trends and autocorrelation.Crossref | GoogleScholarGoogle Scholar | 11581016PubMed |
Morecroft MD, Woodward FI, Marris RH (1992) Altitudinal trends in leaf nutrient contents, leaf size and delta13C of Alchemilla alpine. Functional Ecology 6, 730–740.
| Altitudinal trends in leaf nutrient contents, leaf size and delta13C of Alchemilla alpine.Crossref | GoogleScholarGoogle Scholar |
Mujawamariya M, Manishimwe A, Ntirugulirwa B, Zibera E, Ganszky D, Bahati EN, Nyirambangutse B, Nsabimana D, Wallin G, Uddling J (2018) Climate sensitivity of tropical trees along an elevation gradient in Rwanda. Forests 9, 647
| Climate sensitivity of tropical trees along an elevation gradient in Rwanda.Crossref | GoogleScholarGoogle Scholar |
Mujawamariya M, Wittemann M, Manishimwe A, Nyirambangutse B, Zibera E, Nsabimana D, Wallin G, Uddling J, Dusenge ME (2021) Complete or overcompensatory thermal acclimation of leaf dark respiration in African tropical trees. New Phytologist 229, 2548–2561.
| Complete or overcompensatory thermal acclimation of leaf dark respiration in African tropical trees.Crossref | GoogleScholarGoogle Scholar |
Muscarella R, Uriarte M, Erickson DL, Swenson NG, Kress WJ, Zimmerman JK (2016) Variation of tropical forest assembly processes across regional environmental gradients. Perspectives in Plant Ecology, Evolution and Systematics 23, 52–62.
| Variation of tropical forest assembly processes across regional environmental gradients.Crossref | GoogleScholarGoogle Scholar |
Niinemets Ü (2002) Stomatal conductance alone does not explain the decline in foliar photosynthetic rates with increasing tree age and size in Picea abies and Pinus sylvestris. Tree Physiology 22, 515–535.
| Stomatal conductance alone does not explain the decline in foliar photosynthetic rates with increasing tree age and size in Picea abies and Pinus sylvestris.Crossref | GoogleScholarGoogle Scholar | 12045025PubMed |
Normand F, Bissery C, Damour G, Lauri P-E (2008) Hydraulic and mechanical stem properties affect leaf-stem allometry in mango cultivars. New Phytologist 178, 590–602.
| Hydraulic and mechanical stem properties affect leaf-stem allometry in mango cultivars.Crossref | GoogleScholarGoogle Scholar |
Noshiro S, Suzuki M (1995) Ecological wood anatomy of Nepalese Rhododendron (Ericaceae). 2. Intraspecific variation. Journal of Plant Research 108, 217–233.
| Ecological wood anatomy of Nepalese Rhododendron (Ericaceae). 2. Intraspecific variation.Crossref | GoogleScholarGoogle Scholar |
Osunkoya OO, Omar-Ali K, Amit N, Dyan J, Daud DS, Sheng TK (2007) Comparative height-crown allometry and mechanical design in 22 tree species of Kuala Belalong rainforest, Brunei, Borneo. American Journal of Botany 94, 1951–1962.
| Comparative height-crown allometry and mechanical design in 22 tree species of Kuala Belalong rainforest, Brunei, Borneo.Crossref | GoogleScholarGoogle Scholar | 21636390PubMed |
Pan S, Zhang WP, Zhao MS, Li Y, Xu SS, Wang GX (2016) Altitude patterns of leaf carbon isotope composition in a subtropical monsoon forest. Polish Journal of Ecology 64, 178–188.
| Altitude patterns of leaf carbon isotope composition in a subtropical monsoon forest.Crossref | GoogleScholarGoogle Scholar |
Pandey M, Pathak ML, Shrestha BB (2021) Morphological and wood anatomical traits of Rhododendron lepidotum Wall ex G. Don along the elevation gradients in Nepal Himalayas. Arctic, Antarctic, and Alpine Research 53, 35–47.
| Morphological and wood anatomical traits of Rhododendron lepidotum Wall ex G. Don along the elevation gradients in Nepal Himalayas.Crossref | GoogleScholarGoogle Scholar |
Poorter L, Wright SJ, Paz H, Ackerly DD, Condit R, Ibarra-Manríques G, Harms KE, Licona JC, Martínez-Ramos M, Mazer SJ, Muller-Landau HC, Peña-Claros M, Webb CO, Wright IJ (2008) Are functional traits good predictors of demographic rates? Evidence from five neotropical forests. Ecology 89, 1908–1920.
| Are functional traits good predictors of demographic rates? Evidence from five neotropical forests.Crossref | GoogleScholarGoogle Scholar | 18705377PubMed |
Poorter H, Niinemets Ü, Poorter L, Wright IJ, Villar R (2009) Caused and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytologist 182, 565–588.
| Caused and consequences of variation in leaf mass per area (LMA): a meta-analysis.Crossref | GoogleScholarGoogle Scholar |
Pop EW, Oberbauer SF, Starr G (2000) Predicting vegetative bud break in two arctic deciduous shrub species, Salix pulchra and Betula nana. Oecologia 124, 176–184.
| Predicting vegetative bud break in two arctic deciduous shrub species, Salix pulchra and Betula nana.Crossref | GoogleScholarGoogle Scholar | 28308177PubMed |
Premoli AC, Brewer CA (2007) Environmental v. genetically driven variation in ecophysiological traits of Nothofagus pumilio from contrasting elevations. Australian Journal of Botany 55, 585–591.
| Environmental v. genetically driven variation in ecophysiological traits of Nothofagus pumilio from contrasting elevations.Crossref | GoogleScholarGoogle Scholar |
Prieto I, Querejeta JI, Segrestin J, Volaire F, Roumet C (2018) Leaf carbon and oxygen isotopes are coordinated with the leaf economics spectrum in Mediterranean rangeland species. Functional Ecology 32, 612–625.
| Leaf carbon and oxygen isotopes are coordinated with the leaf economics spectrum in Mediterranean rangeland species.Crossref | GoogleScholarGoogle Scholar |
Reed CC, Loik ME (2016) Water relations and photosynthesis along an elevation gradient for Artemisia tridentata during an historic drought. Oecologia 181, 65–76.
| Water relations and photosynthesis along an elevation gradient for Artemisia tridentata during an historic drought.Crossref | GoogleScholarGoogle Scholar | 26822944PubMed |
Read QD, Moorhead LC, Swenson NG, Bailey JK, Snaders NJ (2014) Convergent effects of elevation on functional leaf traits within and among species. Functional Ecology 28, 37–45.
| Convergent effects of elevation on functional leaf traits within and among species.Crossref | GoogleScholarGoogle Scholar |
Reich PB, Walters MB, Ellsworth DS (1997) From tropics to tundra: global convergence in plant functioning. Proceedings of the National Academy of Sciences of the United States of America 94, 13730–13734.
| From tropics to tundra: global convergence in plant functioning.Crossref | GoogleScholarGoogle Scholar | 9391094PubMed |
Richardson AD, Berlyn GP (2002) Spectral reflectance and photosynthetic properties of Betula papyrifera (Betulaceae) leaves along an elevational gradient on Mt. Mansfield, Vermont, USA. American Journal of Botany 89, 88–94.
| Spectral reflectance and photosynthetic properties of Betula papyrifera (Betulaceae) leaves along an elevational gradient on Mt. Mansfield, Vermont, USA.Crossref | GoogleScholarGoogle Scholar | 21669715PubMed |
Rosas T, Mencuccini M, Barba J, Cochard H, Saura-Mas S, Martínez-Vilalta J (2019) Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient. New Phytologist 223, 632–646.
| Adjustments and coordination of hydraulic, leaf and stem traits along a water availability gradient.Crossref | GoogleScholarGoogle Scholar |
Rossi S, Deslauriers A, Griçar J, Seo JW, Rathgeber CBK, Anfodillo T, Morin H, Levanic T, Oven P, Jalkanen R (2008) Critical temperatures for xylogenesis in conifers of cold climates. Global Ecology and Biogeography 17, 696–707.
| Critical temperatures for xylogenesis in conifers of cold climates.Crossref | GoogleScholarGoogle Scholar |
Sánchez-Bragado R, Araus JL, Scheerer U, Cairns JE, Rennenberg H, Ferrio JP (2016) Factors preventing the performance of oxygen isotope ratios as indicators of grain yield in maize. Planta 243, 355–368.
| Factors preventing the performance of oxygen isotope ratios as indicators of grain yield in maize.Crossref | GoogleScholarGoogle Scholar | 26424228PubMed |
Santiago LS, Goldstein G, Meinzer FC, Fisher JB, Machado K, Woodruff D, Jones T (2004) Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees. Oecologia 140, 543–550.
| Leaf photosynthetic traits scale with hydraulic conductivity and wood density in Panamanian forest canopy trees.Crossref | GoogleScholarGoogle Scholar | 15232729PubMed |
Scheepens JF, Frein ES, Stöcklin J (2010) Genotypic and environmental variation in specific leaf area in a widespread Alpine plant after transplantation to different altitudes. Oecologia 164, 141–150.
| Genotypic and environmental variation in specific leaf area in a widespread Alpine plant after transplantation to different altitudes.Crossref | GoogleScholarGoogle Scholar | 20461412PubMed |
Sheshshayee MS, Bindumadhava H, Ramesh R, Prasad TG, Lakshminarayana MR, Udayakumar M (2005) Oxygen isotope enrichment (Δ18O) as a measure of time-averaged transpiration rate. Journal of Experimental Botany 56, 3033–3039.
| Oxygen isotope enrichment (Δ18O) as a measure of time-averaged transpiration rate.Crossref | GoogleScholarGoogle Scholar | 16263911PubMed |
Shipley B, Lechowicz MJ, Wright I, Reich PB (2006) Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 87, 535–541.
| Fundamental trade-offs generating the worldwide leaf economics spectrum.Crossref | GoogleScholarGoogle Scholar | 16602282PubMed |
Siefert A, Violle C, Chalmandrier L, Albert CH, Taudiere A, Fajardo A, Aarssen LW, Baraloto C, Carlucci MB, Cianciaruso MV, Dantas VD, de Bello F, Duarte LDS, Fonseca CR, Freschet GT, Gaucherand S, Gross N, Hikosaka K, Jackson B, Jung V, Kamiyama C, Katabuchi M, Kembel SW, Kichenin E, Kraft NJB, Lagerstrom A, LeBousse-Pinguet Y, Li YZ, Mason N, Messier J, Nakashizuka T, Overton JM, Peltzer DA, Perez-Ramos IM, Pillar VD, Prentice HC, Richardson S, Sasaki T, Schamp BS, Schob C, Shipley B, Sundqvist M, Sykes MT, Vandewalle M, Wardle DA (2015) A global meta-analysis of the relative extent of intraspecific trait variation in plant communities. Ecology Letters 18, 1406–1419.
| A global meta-analysis of the relative extent of intraspecific trait variation in plant communities.Crossref | GoogleScholarGoogle Scholar | 26415616PubMed |
Silva FCE, Shvaleva A, Maroco JP, Almeida MH, Chaves MM, Pereira JS (2004) Response to water stress in two Eucalptus globulus clones differing in drought tolerance. Tree Physiology 24, 1165–1172.
| Response to water stress in two Eucalptus globulus clones differing in drought tolerance.Crossref | GoogleScholarGoogle Scholar |
Smith MN, Stark SC, Taylor TC, Ferreira ML, de Oliveira E, Restrepo-Coupe N, Chen S, Woodcock T, dos Santos DB, Alves LF, Figueira M, de Camargo PB, de Oliveria RC, Aragão LEOC, Falk DA, McMahon SM, Huxman TE, Saleska SR (2019) Seasonal and drought-related changes in leaf area profiles depend on height and light environment in an Amazon forest. New Phytologist 222, 1284–1297.
| Seasonal and drought-related changes in leaf area profiles depend on height and light environment in an Amazon forest.Crossref | GoogleScholarGoogle Scholar |
Sterck FJ, Poorter L, Schieving F (2006) Leaf traits determine the growth-survival trade-off across rain forest tree species. The American Naturalist 167, 758–765.
| Leaf traits determine the growth-survival trade-off across rain forest tree species.Crossref | GoogleScholarGoogle Scholar | 16671019PubMed |
Sterck F, Markesteijn L, Schieving F, Poorter L (2011) Functional traits determine trade-offs and niches in a tropical forest community. Proceedings of the National Academy of Sciences of the United States of America 108, 20627–20632.
| Functional traits determine trade-offs and niches in a tropical forest community.Crossref | GoogleScholarGoogle Scholar | 22106283PubMed |
Stratton L, Goldstein G, Meinzer FC (2000) Stem water storage capacity and efficiency of water transport: their functional significance in a Hawaiian dry forest. Plant, Cell & Environment 23, 99–106.
| Stem water storage capacity and efficiency of water transport: their functional significance in a Hawaiian dry forest.Crossref | GoogleScholarGoogle Scholar |
Swenson NG, Enquist BJ, Pither J, Thompson J, Zimmerman JK (2006) The problem and promise of scale dependency in community phylogenetics. Ecology 87, 2418–2424.
| The problem and promise of scale dependency in community phylogenetics.Crossref | GoogleScholarGoogle Scholar | 17089650PubMed |
Sun ZJ, Livingston NJ, Guy RD, Ethier GJ (1996) Stable carbon isotopes as indicators of increased water use efficiency and productivity in white spruce (Picea glauca (Moench) Voss) seedlings. Plant, Cell & Environment 19, 887–894.
| Stable carbon isotopes as indicators of increased water use efficiency and productivity in white spruce (Picea glauca (Moench) Voss) seedlings.Crossref | GoogleScholarGoogle Scholar |
Sultan SE (2000) Phenotypic plasticity for plant development, function and life history. Trends in Plant Science 5, 537–542.
| Phenotypic plasticity for plant development, function and life history.Crossref | GoogleScholarGoogle Scholar | 11120476PubMed |
Tateno R, Hishi T, Takeda H (2004) Above- and belowground biomass and net primary production in a cool-temperate deciduous forest in relation to topographical changes in soil nitrogen. Forest Ecology and Management 193, 297–306.
| Above- and belowground biomass and net primary production in a cool-temperate deciduous forest in relation to topographical changes in soil nitrogen.Crossref | GoogleScholarGoogle Scholar |
ter Steege H, Hammond DS (2001) Character convergence, diversity, and disturbance in tropical rain forest in Guyana. Ecology 82, 3197–3212.
| Character convergence, diversity, and disturbance in tropical rain forest in Guyana.Crossref | GoogleScholarGoogle Scholar |
Thomas SC (2011) Genetic vs. phenotypic responses of trees to altitude. Tree Physiology 31, 1161–1163.
| Genetic vs. phenotypic responses of trees to altitude.Crossref | GoogleScholarGoogle Scholar | 22084019PubMed |
Umaña MN, Swenson NG (2019) Intraspecific variation in traits and tree growth along an elevational gradient in a subtropical forest. Oecologia 191, 153–164.
| Intraspecific variation in traits and tree growth along an elevational gradient in a subtropical forest.Crossref | GoogleScholarGoogle Scholar | 31367911PubMed |
Van de Water PK, Leavitt SW, Betancourt JL (2002) Leaf δ13C variability with elevation, slope aspect, and precipitation in the southwest United States. Oecologia 132, 332–343.
| Leaf δ13C variability with elevation, slope aspect, and precipitation in the southwest United States.Crossref | GoogleScholarGoogle Scholar | 28547410PubMed |
van Mantgem PJ, Stephenson NL, Byrne JC, Daniels LD, Franklin JF, Fulé PZ, Harmon ME, Larson AJ, Smith JM, Taylor AH, Veblen TT (2009) Widespread increase of tree mortality rates in the western United States. Science 323, 521–524.
| Widespread increase of tree mortality rates in the western United States.Crossref | GoogleScholarGoogle Scholar | 19164752PubMed |
Violle C, Enquist BJ, McGill BJ, McGill BJ, Jiang L, Albert CH, Hulshof C, Jung V, Messier J (2012) The return of the variance: intraspecific variability in community ecology. Trends in Ecology & Evolution 27, 244–252.
| The return of the variance: intraspecific variability in community ecology.Crossref | GoogleScholarGoogle Scholar |
Wang GA, Zhou LP, Liu M, Han JM, Guo JH, Faiia A, Su F (2010) Altitudinal trends of leaf δ13C follow different patterns across a mountainous terrain in north China characterized by a temperate semi-humid climate. Rapid Communications in Mass Spectrometry 24, 1557–1564.
| Altitudinal trends of leaf δ13C follow different patterns across a mountainous terrain in north China characterized by a temperate semi-humid climate.Crossref | GoogleScholarGoogle Scholar |
Wang WZ, Jia M, Wang GX, Zhu WZ, McDowell NG (2017) Rapid warming forces contrasting growth trends of subalpine fir (Abies fabri) at higher- and lower-elevations in the eastern Tibetan Plateau. Forest Ecology and Management 402, 135–144.
| Rapid warming forces contrasting growth trends of subalpine fir (Abies fabri) at higher- and lower-elevations in the eastern Tibetan Plateau.Crossref | GoogleScholarGoogle Scholar |
Weigt RB, Streit K, Saurer M, Siegwolf RTW (2018) The influence of increasing temperature and CO2 concentration on recent growth of old-growth larch: contrasting responses at leaf and stem processes derived from tree-ring width and stable isotopes. Tree Physiology 38, 706–720.
| The influence of increasing temperature and CO2 concentration on recent growth of old-growth larch: contrasting responses at leaf and stem processes derived from tree-ring width and stable isotopes.Crossref | GoogleScholarGoogle Scholar | 29194509PubMed |
Weih M, Karlsson PS (2001) Growth response of Mountain birch to air and soil temperature: is increasing leaf-nitrogen content an acclimation to lower air temperature? New Phytologist 150, 147–155.
| Growth response of Mountain birch to air and soil temperature: is increasing leaf-nitrogen content an acclimation to lower air temperature?Crossref | GoogleScholarGoogle Scholar |
Westoby M, Wright IJ (2006) Land-plant ecology on the basis of functional traits. Trends in Ecology & Evolution 21, 261–268.
| Land-plant ecology on the basis of functional traits.Crossref | GoogleScholarGoogle Scholar |
Woodruff DR, McCulloh KA, Warren JM, Meinzer FC, Lachenbruch B (2007) Impacts of tree height on leaf hydraulic architecture and stomatal control in Douglas-fir. Plant, Cell & Environment 30, 559–569.
| Impacts of tree height on leaf hydraulic architecture and stomatal control in Douglas-fir.Crossref | GoogleScholarGoogle Scholar |
Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom PK, Gulias J, Hikosaka K, Lamont BB, Lee T, Lee W, Lusk C, Midgley JJ, Navas ML, Niinemets Ü, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov VI, Roumet C, Thomas SC, Tjoelker MG, Veneklaas EJ, Villar R (2004) The worldwide leaf economics spectrum. Nature 428, 821–827.
| The worldwide leaf economics spectrum.Crossref | GoogleScholarGoogle Scholar | 15103368PubMed |
Wright IJ, Leishman MR, Read C, Westoby M (2006) Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees. Functional Plant Biology 33, 407–419.
| Gradients of light availability and leaf traits with leaf age and canopy position in 28 Australian shrubs and trees.Crossref | GoogleScholarGoogle Scholar | 32689248PubMed |
Wu T, Qu C, Li YY, Li X, Zhou GY, Liu SZ, Chu GW, Meng Z, Lie ZY, Liu JX (2019) Warming effects on leaf nutrients and plant growth in tropical forests. Plant Ecology 220, 663–674.
| Warming effects on leaf nutrients and plant growth in tropical forests.Crossref | GoogleScholarGoogle Scholar |
Zhang J, Marshall JD, Jaquish BC (1993) Genetic differentiation in carbon isotope discrimination and gas exchange in Pseudotsuga menziesii: a common-garden experiment. Oecologia 93, 80–87.
| Genetic differentiation in carbon isotope discrimination and gas exchange in Pseudotsuga menziesii: a common-garden experiment.Crossref | GoogleScholarGoogle Scholar | 28313778PubMed |
Zheng M, Chen H, Li D, Luo Y, Mo J (2020) Substrate stoichiometry determines nitrogen fixation throughout succession in southern Chinese forests. Ecology Letters 23, 336–347.
| Substrate stoichiometry determines nitrogen fixation throughout succession in southern Chinese forests.Crossref | GoogleScholarGoogle Scholar | 31802606PubMed |
Zhou G, Wei X, Xu Y, Liu S, Huang Y, Yan J, Zhang D, Zhang Q, Liu J, Meng Z, Wang C, Chu G, Liu S, Tang X, Liu X (2011) Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China. Global Change Biology 17, 3736–3746.
| Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China.Crossref | GoogleScholarGoogle Scholar |
Zhu LW, Hu YT, Zhao XH, Zeng XM, Zhao P, Zhang ZZ (2016) The impact of drought on sap flow of coocurring Liquidambar formosana Hance and Quercus variabilis Blume in a temperate forest, Central China. Ecohydrology 10, e1828
| The impact of drought on sap flow of coocurring Liquidambar formosana Hance and Quercus variabilis Blume in a temperate forest, Central China.Crossref | GoogleScholarGoogle Scholar |


