The mechanisms underlying melatonin improved soybean seedling growth at different nitrogen levels
Huamei Wang A , Chunyuan Ren A , Liang Cao A , Xijun Jin A , Mengxue Wang A , Mingcong Zhang A , Qiang Zhao A , He Li A , Yuxian Zhang A B * and Gaobo Yu A *
A , Chunyuan Ren A , Liang Cao A , Xijun Jin A , Mengxue Wang A , Mingcong Zhang A , Qiang Zhao A , He Li A , Yuxian Zhang A B * and Gaobo Yu A *
A College of Agronomy, Heilongjiang Bayi Agricultural University, Daqing 163319, China.
B National Coarse Cereals Engineering Research Center, Daqing 163000, China.
Functional Plant Biology 48(12) 1225-1240 https://doi.org/10.1071/FP21154
Submitted: 3 March 2021 Accepted: 25 August 2021 Published: 11 October 2021
© 2021 The Author(s) (or their employer(s)). Published by CSIRO Publishing. This is an open access article distributed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License (CC BY-NC-ND)
Abstract
To investigate the function of melatonin (MT) on nitrogen uptake and metabolism in soybean, six groups of treatments, with and without 100 μM melatonin were conducted at low, normal, and high nitrogen levels (1.5, 7.5, and 15 mM, respectively). The related indexes of nitrogen metabolism and the antioxidant system of seedlings were measured and analysed. Results indicated that MT could enhance the level of nitrogen metabolism by upregulating the coding genes of enzymes related to nitrogen metabolism and increasing total nitrogen content, especially under low nitrogen levels. Under high nitrogen conditions, the addition of MT not only accelerated ammonium assimilation and utilisation by enhancing the activity of glutamine synthetase involved in ammonium assimilation, but also reduced the extent of membrane lipid peroxidation to alleviate the degree of damage by improving the activity of antioxidant enzymes. In addition, MT enhanced soybean growth with positive effects in morphological changes at different nitrogen levels, including significantly increased stem diameter, total leaf area, and root nodule number, and biomass accumulation. Finally, biomass accumulation increased under low, normal, and high nitrogen levels by 9.80%, 14.06%, and 11.44%, respectively. The results suggested that MT could enhance the soybean tolerance to low and excessive N treatments.
Keywords: crop physiology, legumes, melanin, nitrogen inappropriate, nitrogen metabolism, nitrogen nutrition, nitrogen regulation, soybean.
Introduction
Nitrogen is an essential nutrient element since its availability is closely related to crop growth, yield, and stress responses (Arun et al. 2016). As a nitrogen-loving crop, soybean is highly dependent on nitrogen nutrition (Sinclair and de Wit 1975; Wang et al. 2017). Although soybean has the unique ability of facilitating symbiotic nitrogen fixation with root nodules under low soil nitrogen conditions, it cannot always achieve the high nitrogen demand of soybean (Harper 1974; Ray et al. 2006; Cafaro La Menza et al. 2017), especially because the N fixation ability of root nodules is weak, and the source of nitrogen mainly dependent on exogenous application (Bergersen 1958). Under conditions of nitrogen deficiency, nitrogen metabolism in plants will be reduced, leading to the decline of other physiological metabolic processes such as photosynthesis and inhibiting overall growth and development (Takahashi et al. 2005). In addition, nitrogen restriction inhibits yield potential. According to the soybean yield potential, the model of nitrogen requirement to simulate total nitrogen supply has been used (Tamagno et al. 2017). The results showed that the yield of zero-N treatment was significantly lower than that of full-N treatment by 11% (Cafaro La Menza et al. 2017). However, if nitrogen fertiliser application is too great, the excess ammonium accumulation will cause toxicity, resulting in an interference in physiological and metabolic processes and yield reduction (Guan et al. 2016). A study on the effect of high nitrogen on wheat also demonstrated that the application of superabundant nitrogen fertiliser led to excessive accumulation of reactive oxygen species (ROS), aggravated the degree of membrane lipid peroxidation, significantly interfered with metabolic activities in the cell, and finally resulted in the reduction of wheat yield (Kong et al. 2017). Additionally, it may also increase the risk of large-scale plant lodging and is not conducive to the symbiotic nitrogen fixation function of soybean (Ciampitti and Salvagiotti 2018). Therefore, excessive supply of nitrogen fertiliser will reduce the utilisation rate of nitrogen fertiliser, pare off the yield, and cause environmental pollution (Xu et al. 2012; Yong et al. 2018). In addition, the high nitrogen concentration in the soil could be suitable for increasing the possibility of infection by pathogens such as soybean root rot (Liu et al. 2016a). Therefore, either a lack or an excess of nitrogen is not beneficial to the growth and development of crops.
To reduce the effect of nitrogen deficiency or excessive application on soybean during cultivation, it is necessary to find a method to solve this issue. Currently, many researchers are attempting to improve the biological yield of soybean under nitrogen stress through the cultivation of new varieties tolerant to nitrogen stress (Nawaz et al. 2018; Xie et al. 2019). Additionally, a large number of experiments have been conducted to enhance the utilisation of nitrogen fertiliser and increase yield by optimising nitrogen management (Du et al. 2020), using crop rotation and intercropping, or improving field management measures (Grassini et al. 2014; Gavili et al. 2019; Raza et al. 2019). In recent years, some researchers have begun to pay attention to the regulatory effects of plant growth regulators on crop growth and development under different nitrogen concentrations (Du et al. 2020). Melatonin, a newly discovered type of plant growth regulator with a similar effect to auxin (Josefa et al. 2004; Arnao and Hernández-Ruiz 2019), has been widely confirmed to promote root development (Liang et al. 2017; Chen et al. 2018), benefit plant growth (Byeon and Back 2014), delay leaf senescence (Park and Back 2012), and increase yield significantly (Liu et al. 2016b; Hu et al. 2018).
In addition, melatonin can function a an antioxidant capacity. In recent years, melatonin has been widely used as a protective agent against multiple stresses including unfavourable salt concentrations, ultraviolet radiation, toxic chemicals, extreme temperature, osmotic stress, pathogen infection, and nutritional defects (Zhang et al. 2017a; Fan et al. 2018; Bawa et al. 2020; Mohamed et al. 2020; Zhang et al. 2020). Under abiotic stress including drought, the alleviation effect of melatonin on plant growth may also be attributed to its regulation of carbon and nitrogen metabolism (Cao et al. 2019; Zou et al. 2020). Some studies have pointed out that melatonin could play a role in plants by enhancing cell division, promoting photosynthesis and carbohydrate synthesis, and other carbon metabolic pathways (Wei et al. 2015). Furthermore, a study on the application of melatonin under the condition of nitrogen deficiency confirmed that melatonin could increase biological yield by participating in the pathway of nitrogen uptake and assimilation of wheat (Qiao et al. 2019). However, under excessive nitrogen supply conditions, the use of melatonin could also effectively inhibit the accumulation of inorganic nitrogen ions in plant leaves (Zhou et al. 2019). A study on the molecular regulation mechanism of exogenous hormone in Malus under nutritional stress has explored that melatonin could not only promote the expression of antioxidant enzyme-related genes, but also regulate the expression of potassium channel protein genes by regulating ROS signalling and activating CBL1-CIPK23 pathway, thus promoting plant nutrient absorption (Li et al. 2016).
Nevertheless, little has been known about how melatonin influences soybean growth under different nitrogen levels including the detailed regulatory mechanism of melatonin on nitrogen absorption and metabolism of soybean under different nitrogen levels. Further, it is unknown whether the regulatory pathway of melatonin is different between conditions of nitrogen deficiency and excess. The small root nodules could usually be seen on the main root after Vegetative 3 stage, indicating that the nitrogen required for Vegetative 1 (V1) stage mainly depends on exogenous supply (Purcell et al. 2014; Zeng et al. 2019). Therefore, sand culture treatments of soybean at seedling stage were conducted at low, normal, and high nitrogen levels (1.5, 7.5, and 15 mM, respectively), with and without 100 μM melatonin application at V1 stage, to investigate the effect of nitrogen metabolism and soybean growth under different nitrogen levels. Thus, an innovative solution and theoretical basis to alleviate the effect of inappropriate nitrogen use on soybean seedlings’ growth in the process of cultivation were provided.
Materials and methods
Plant materials and experimental treatments
Pots with a diameter of 20 cm and a height of 17 cm was used in the study. A layer of yarn net was laid on the pot bottom to prevent the roots from growing out of the basin. Then each pot was filled with 6 kg of river sand that had been previously washed once with tap water and twice with distilled water. Soybean variety ‘Suinong 26’ was used as experimental material. Six full seeds, uniform in colour and size, were sown in each pot, and then three uniform seedlings were retained in each pot at the Vegetative-Cotyledon (VC) stage. The tested plants were cultivated in the outdoor research base of the National Coarse Cereals Engineering Research Center in Daqing City, Heilongjiang Province, China (46°59′N, 125°17′E), during mid-June. The study area has a temperate monsoon climate.
From sowing to full expansion of the opposite leaves, 500 mL distilled water was sprinkled once a day to each plant. After the opposite leaves were unfolded, they were divided into three groups. Once a day, 500 mL 1/2 Hoagland nutrient solution was provided to the plants, with ammonium sulfate as the only nitrogen source (see Supplementary Table S1 available at the journal website for the formula of other required nutrients) (Guan et al. 2016). Corresponding to low, normal, and high nitrogen levels, the nitrogen concentrations in the nutrient solution were 1.5 mM, 7.5 mM (corresponding nitrogen concentration in 1/2 Hoagland’s nutrient solution), and 15 mM, respectively (Xia et al. 2017). To avoid salt accumulation in sand culture, 500 mL tap water was slowly drenched twice every 3 days. When soybean reached V1 stage, each treatment was randomly divided into two groups to spray with 100 μM melatonin or distilled water, respectively, for 3 days at 9:00 pm. Six treatments were obtained: (1) LN (low nitrogen); (2) LN + MT (low nitrogen + 100 μM melatonin); (3) CK (normal nitrogen); (4) CK + MT (normal nitrogen + 100 μM melatonin); (5) HN (high nitrogen); (6) HN + MT (high nitrogen + 100 μM melatonin).
After melatonin application for 24 h, three samples of functional leaves and three samples of roots were immediately placed in liquid nitrogen and then stored in a freezer at −80°C for gene expression analysis. The whole plant samples were taken at 9:00 am on the 6th, 12th, 18th, and 24th day after melatonin spraying to determine morphology, dry matter, total nitrogen, and urea content. The functional leaf samples were directly determined or wrapped in tin foil and stored in a freezer at −20°C for corresponding physiological and biochemical analysis.
Determination method of index
Measurement of morphological index and the accumulation of dry matter
The entire plant was washed with distilled water, and the plant height, the root length, the stem diameter, and the number of root nodules per plant were measured by conventional methods (Li et al. 2018). Further, the total leaf area of a single plant was measured by the Yaxin-1241 leaf area meter. Then the root, stem, and leaf were dried at 105°C for 0.5 h to inactivate the enzyme and then dried to constant weight at 80°C for about 48 h. The dried samples will be utilised to determine the dry matter content.
Measurement of nitrogen assimilating enzymes activity
Nitrate reductase (NR: EC 1.7.1.3) activity was measured according to the method of Sherrard and Dalling (1979). Four samples of 0.5 g fresh, functional leaves were weighed quickly for each treatment. One leaf was taken as a control, and the other three were used for activity determination. First, 1 mL 30% (m/v) TCA was added to the control, followed by 5 mL phosphate buffer solution (0.1 M, pH 7.5). Second, 5 mL potassium nitrate solution (0.2 M) was added to each bottle to avoid light for 30 min and incubated at 25°C for 30 min. Subsequently, 1 mL 30% (m/v) TCA was added to stop the reaction in all bottles except in the control. The 2 mL of the reaction solution and 8 mL of nitric acid reagent were added to the test tube, and the colour was developed at room temperature for 20 min. The optical density of the supernatant was measured at 540 nm.
The determination method for glutamine synthetase (GS: EC 6.3.1.2) enzyme activity was according to Zhang et al. (2016). We ground 0.1 g samples into a powder with liquid nitrogen, added an 8 mL extract (100 mM Tris–HCl, 0.5 mM EDTA, 5 mM β-mercaptoethanol, pH 7.5) to it, and then centrifuged in 15 000g at 4°C for 20 min. The supernatant was used to determine the enzyme activity. A mixture of 1.6 mL of the reaction solution and 0.6 mL of the enzyme extract was incubated in a 25°C water bath for 5 min and 0.2 mL of hydroxylamine reagent was added to start the reaction. After 15 min of incubation, 1 mL of FeCl3 reagent was added to terminate the reaction. The mixed solution was centrifuged in 15 000g for 10 min, and the optical density of the supernatant was measured at 540 nm.
The method for preparation of the extract to determine glutamate synthase (GOGAT: EC 1.4.7.1) and glutamate dehydrogenase (GDH:EC 1.4.1.2) activity was the same as that of GS. GOGAT activity was measured with l-glutamine to initiate the reaction, and one extinction value was measured every 30 s at 340 nm, 10 consecutive times. The enzyme activity was measured when the optical density decreased steadily. GDH activity was determined by adding 0.1 mL enzyme solution to activate the reaction. The decreasing value in absorbance after 3 min was recorded at 340 nm (Lin and Kao 1996; Singh et al. 2020).
Measurement of antioxidant indexes
The above frozen samples were used to determine the activities of superoxide dismutase (SOD), guaiacol peroxidase (GPX) and catalase (CAT) by the nitrogen blue tetrazolium reduction, guaiacol, and ultraviolet absorption methods, respectively (Zhang et al. 2017a). The content of malondialdehyde (MDA) formed due to membrane lipid peroxidation was determined by thiobarbituric acid heating colorimetry (Nakano and Asada 1981).
Measurement of inorganic nitrogen content
Fresh leaf samples (1 g) were macerated in liquid nitrogen, and the metabolites were extracted for 24 h with 10 mL of a methanol:chloroform:water (12:5:3, v:v:v) solution. After centrifugation at 2000g for 30 min, one volume of chloroform and 1.5 volume of water were added to each of the four volumes of the obtained supernatant. After phase separation over a 24 h period, the aqueous phase was collected. The resulted extract was concentrated for 15 h at 37°C and then used for biochemical analysis (Oliveira et al. 2013). NO3− was determined spectrophotometrically at 410 nm by the salicylic acid method described by Cataldo et al. (1975). The determination of NO2− was determined according to the method described by Hageman and Reed (1980).
Measurement of total nitrogen and ureide content
Nitrogen concentrations of plant tissues were measured using the Kjeldahl method. The dried samples of root, stem, and leaf were ground, and subsamples weighing about 0.1 g were digested with H2SO4–H2O2. Then the nitrogen content was determined using a Kjeldahl nitrogen meter (Zhang et al. 2016). The content of ureide in the root, stem, and leaf of the soybean tissue was determined according to the method used by Zhang et al. (2018).
Total RNA extraction and gene expression (qRT-PCR) analysis
Total RNA of functional leaf or root tip sample (50–100 mg) was extracted using an Axygen reagent kit (AxyPrep) and reverse-transcribed according to manufacturer's instructions (Prime Script RT Enzyme Mix I for TB Green qPCR, Takara). The gene-specific primers were designed with Primer Premier 5.0 (Table S2), and qRT-PCR was performed using a 10 μL mixture (5 μL TB Green Premix Ex Taq II; 3 μL distilled water; 0.5 μL PCR Forward Primer; 0.5 μL PCR Reverse Primer; 1 μL cDNA) according to manufacturer instructions (CFX96 Real-Time PCR Detection System, Takara). A two-step PCR method was used with the following conditions: pre-denaturation at 95°C for 30 s, 40 cycles at 95°C for 5 s, and 60°C for 30 s. The specialist software Bio-Rad iQ5 was used to collect data. Actin (gene ID: Glyma18g52780) was adopted as an internal reference for the normalisation of gene expression. In the qRT-PCR analysis, samples of two plants from per pot treated with melatonin for 24 h were mixed as a single replicate, three pots per treatment were considered as three biological replicates. Each biological replicate had three technical replicates.
Three repetitive values were recorded in the determination process of the above physiological, biochemical, and morphological indexes. Each value was the average value of three mixed samples in the same container.
Statistical analyses
The experiment was conducted in a completely randomised design. The data obtained were analysed by Microsoft Excel 2016. l.s.d. and Duncan’s multiple range test (P ≤ 0.05) were performed using the statistical program SPSS 20.0. Finally, charts including the principal component analysis (PCA) biplot were formed with Origin 2017 software.
Results
Effects of exogenous melatonin on plant morphological parameters
According to the phenotype analysis of four time points (Fig. 1, Table S3), it was evident that, compared with the control, the plant height, stem diameter, total leaf area, and dry matter accumulation of seedlings were inhibited under low nitrogen level. The growth of seedlings under high nitrogen levels was also significantly inhibited. On the 24th day after treatment began, the dry matter accumulation at a high nitrogen level was significantly less than that of the control by 18.41%. The application of melatonin promoted the growth of seedlings in different degrees under different nitrogen concentrations. With the establishment of plant morphology, it was observed that on the 24th day after melatonin treatment, the stem diameter and root length of seedlings increased significantly at all nitrogen levels. However, there was no significant difference in plant height between melatonin sprayed and unsprayed melatonin treatments. In addition, the application of melatonin significantly increased the total leaf area per plant at low N and normal N levels.
The results of the root nodule analysis indicated that melatonin promoted root nodule growth and increased the average dry weight of root nodules per plant under low nitrogen levels. In contrast, the average dry weight of a single root nodule increased significantly under high nitrogen levels with melatonin. From the analysis of the dry weight of the aboveground and underground parts, it was concluded that the application of melatonin further increased the dry matter accumulation of all parts under low and normal nitrogen levels. However, it mainly promoted root development under high nitrogen treatment so that the root:shoot ratio increased by 10.99% compared to that under HN. Finally, compared to soybeans without melatonin treatment, the total dry matter accumulation per plant of soybeans exposed to melatonin at low N, normal N, and high N levels increased significantly by 9.80%, 14.06%, and 11.44%, respectively.
Effects of exogenous melatonin on the activity of enzymes related to nitrogen assimilation
On the whole, the activities of enzymes related to N metabolism were significantly lower than those of control due to low N level. As the N level was higher than control, the activities of these enzymes tended to be enhanced in a short period. However, with the extension of high N supply time, the activities of these enzymes decreased gradually compared with the previous high N level. On the 24th day after treatment began, no significant difference was shown observed in the activities of the GS and GOGAT between the high N and control group, and the GDH activity in high N group was lower than that of control (Fig. 2d). Notably, the effects of melatonin on different enzymes activities were distinct.
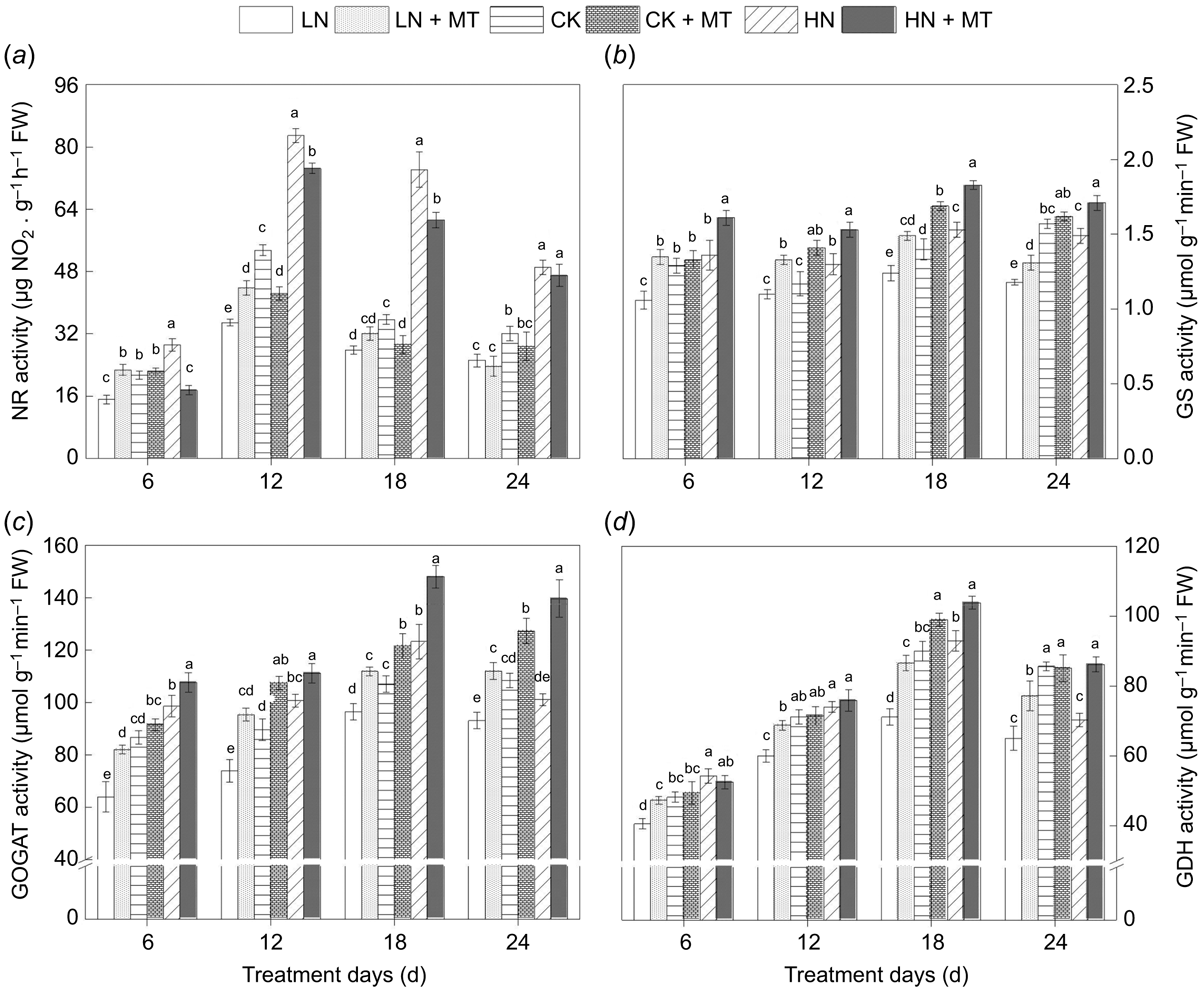
|
Compared with low N alone, melatonin significantly increased the NR activity under low N levels at 6th and 12th day after spraying, which improved by 50.08% and 25.74%, respectively. Nevertheless, the activity was inhibited after melatonin treatment in the control and high N group. There were no apparent differences in NR activity between two groups with and without melatonin on the 24th day after melatonin (Fig. 2a).
Through the analysis of GS activity (Fig. 2b), it could be concluded that melatonin could enhance its activity at various N levels, which were higher than that without melatonin. When treatment days of melatonin increased to 12, the activity of GS in the LN + melatonin group was even higher than the control. Compared with the control, the CK + melatonin group was also significantly increased by 20.80% and 20.84%, respectively, on the 12th and 18th day after melatonin treatment. Notably, the promotion effect of melatonin under high N was the most obvious on the 18th day after spraying, which was 19.05% higher than that of high N alone. The contribution of melatonin to GOGAT and GDH activity was closely similar to its effect on GS (Fig. 2c, d). These enzymes activity of leaf with melatonin under low N level was remarkably improved. In addition, this promoting effect was evident on the 12th day after melatonin treatment in the groups fed with normal N and high N.
Effects of exogenous melatonin on the activity of antioxidant enzymes and the content of malondialdehyde
As a major indicator of the degree of membrane lipid peroxidation, the content of MDA was higher than that of the control (Fig. 3d) in the early stage of high N treatment. The application of melatonin could significantly promote SOD, GPX, and CAT activities under high N levels (Fig. 3a–c), and decreased the content of MDA. In the high N treatment, MDA content returned to the control level after melatonin spraying. However, compared with the control, there was rarely a significant distinction in antioxidant enzymes activity and MDA content between the low N and the control.
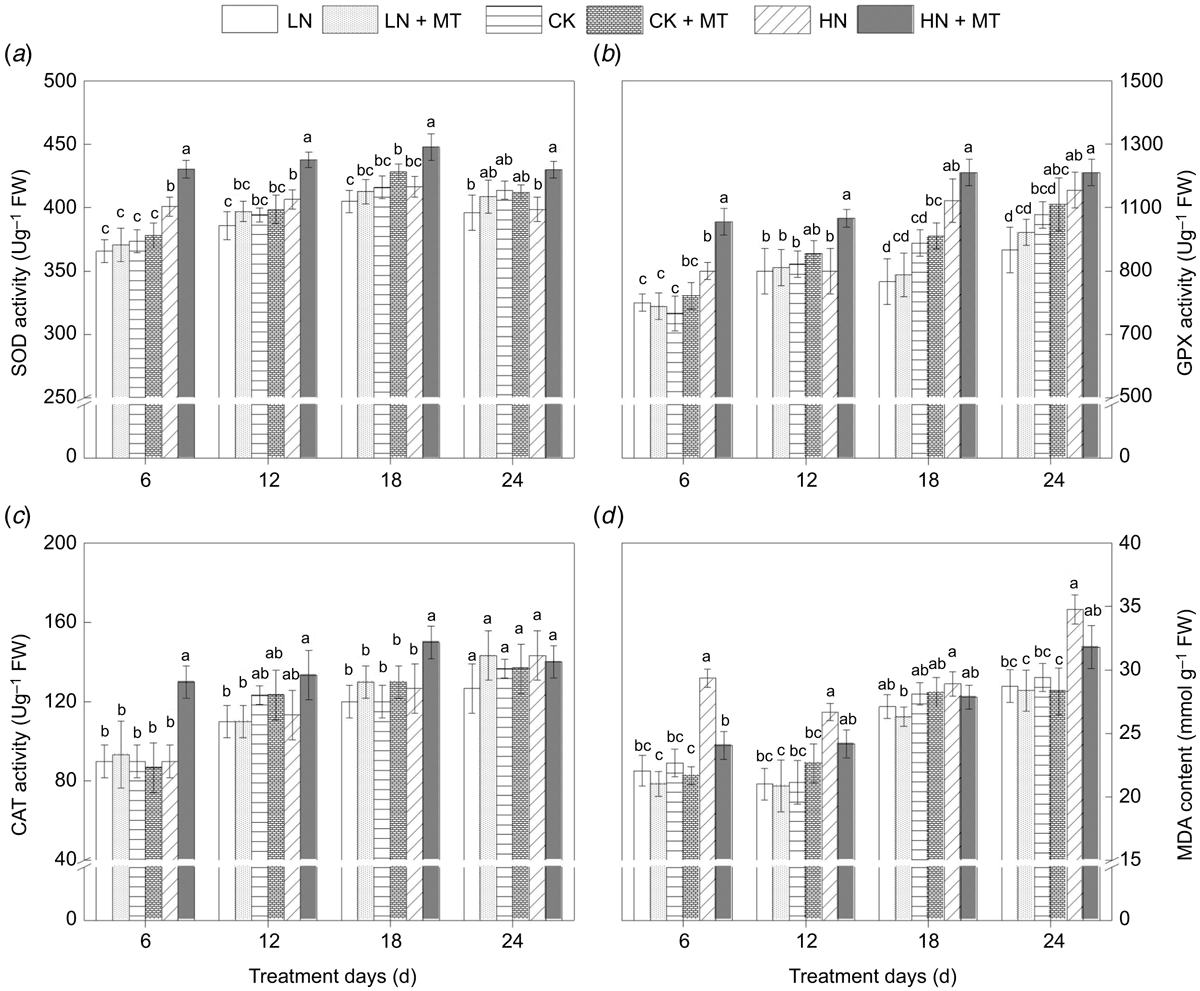
|
Effect of exogenous melatonin on nitrate and nitrite content in functional leaves
Combined with the analysis of nitrate-nitrogen (Fig. 4a) and nitrite content (Fig. 4b) in functional leaves, it was found that levels of the two inorganic nitrogens were significantly lower than those of the control at low N levels. However, the application of melatonin at this nitrogen level significantly promoted the absorption of nitrate. Thus, nitrite accumulation increased, decreasing the difference between the low N treatment and the control (Fig. 4a). However, under both normal N and high N concentrations, the application of melatonin showed negative feedback regulation on the accumulation of nitrate, and the content of nitrite in leaves treated with melatonin was generally lower. The inhibitory effect on nitrate-nitrogen was significant on the 12th and 24th days after melatonin treatment.
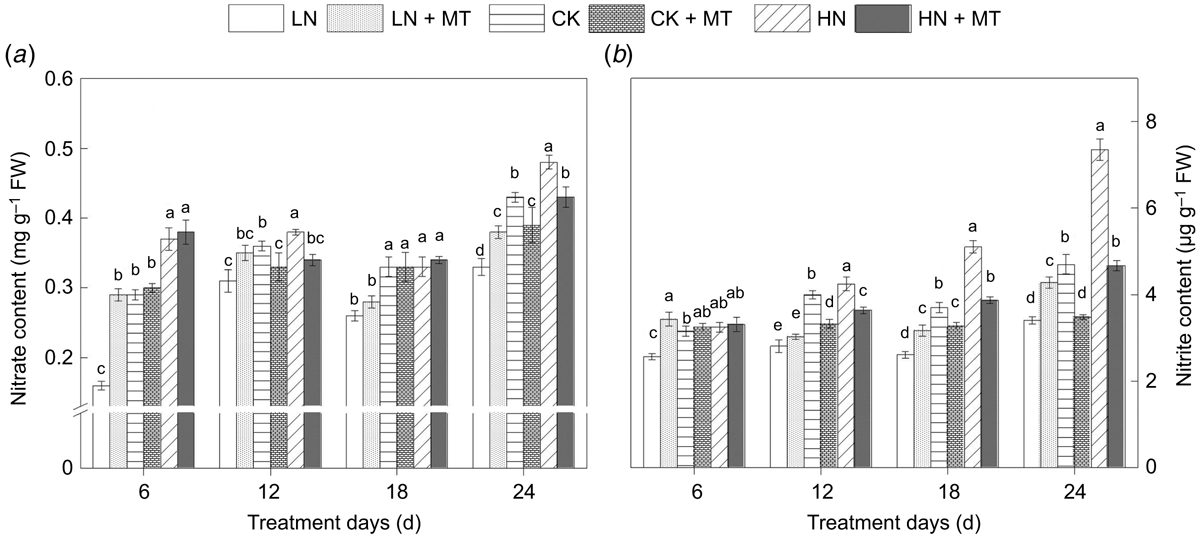
|
Effects of exogenous melatonin on total nitrogen content of each tissue
The nitrogen content of soybean leaves, stems, and roots increased with the incrase in exogenous N concentration (Fig. 5). Results demonstrated that melatonin modulated the nitrogen accumulation under low N and normal N levels, mainly reflected in leaves and roots. Nevertheless, there was rare discrimination in nitrogen content at high N levels between the two groups with or without melatonin.
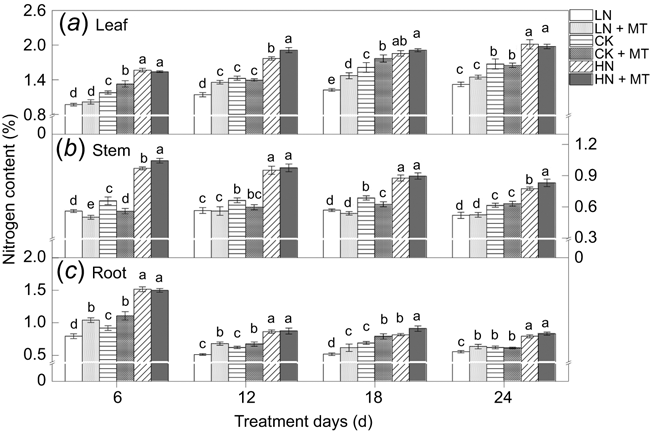
|
Effects of exogenous melatonin on ureide content of each tissue
It can be seen that the ureide content of the soybean seedling stage was very limited, but that it increased with the enhancement of nitrogen fixation ability (Fig. 6). Melatonin played a key role in promoting the accumulation of ureide content in various tissues of soybean at low N concentrations. Further analysis concluded that the addition of N supply would inhibit ureide accumulation, alleviating effectively with melatonin. The contribution of melatonin to acylurea accumulation at normal N and high N levels was mainly reflected in stems and roots.
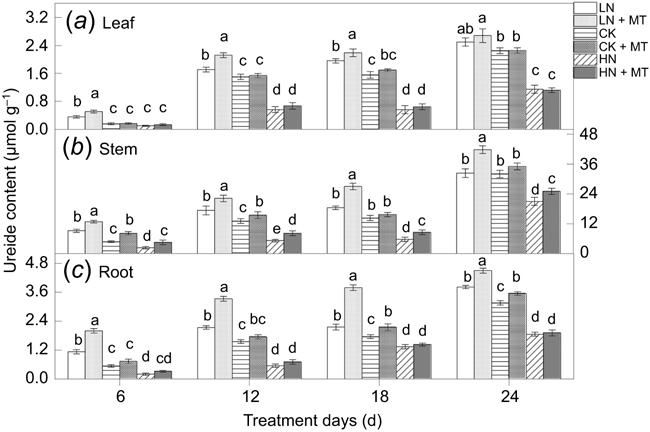
|
Effects of exogenous melatonin on the expression of genes related to nitrogen metabolism
To investigate the molecular mechanism for melatonin-induced nitrogen stress tolerance, we examined the effects of melatonin on gene expression related to nitrogen metabolism (Fig. 7). Through qRT-PCR, it was found that under low nitrogen treatment, the gene expression of the ammonium transporter gene (AMT1, AMT2), nitrite reductase gene (NiR), glutamine synthetase gene (GS1β), glutamate synthase gene (GOGAT), and amino acid transport gene (AAT) in leaves and roots was significantly upregulated under the stimulation of melatonin. In addition, the nitrate reductase gene (NR2), which catalysed the reduction of nitrate to nitrite, was significantly upregulated in leaves. Under high N conditions, melatonin application stimulated the upregulation of genes (AMT1, AMT2, NiR, GS1β, GOGAT, AS, AAT) involved in nitrogen assimilation, which was mainly reflected in roots. However, the expression of NR2 was regulated by melatonin negative feedback in both leaves and roots under this condition. In addition, Gmduf-cbs and Gmdof1.4 genes were also significant, and their expression levels are all upregulated compared with treatments without melatonin spraying at three nitrogen levels.
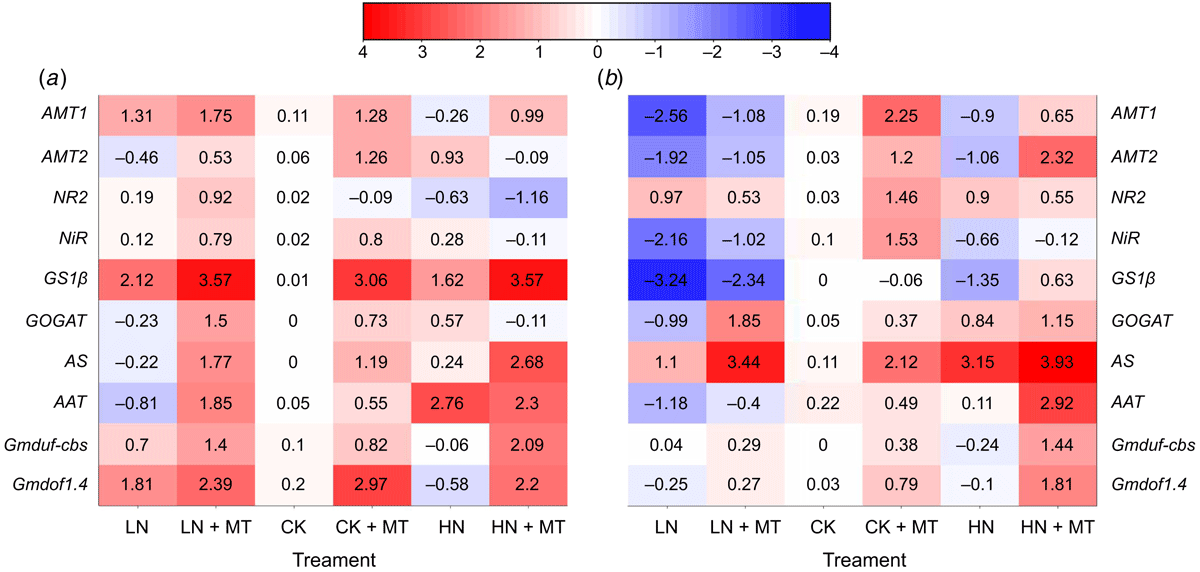
|
Effect of exogenous melatonin on all the parameters studied under different nitrogen levels
Different treatments and determination indicators were merged in a single PCA biplot graph to visualise further (Fig. 8). The PCA biplot for 6th, 12th, 18th and 24th day after melatonin treatment explained 70.9%, 71.4%, 72.8%, and 68.7% of the total variability, respectively. Among them, PC1 accounted for 50.2%, 51.5%, 47.5%, and 38.3%, respectively, and PC2 accounted for 20.7%, 19.9%, 25.3%, and 30.4%, respectively. Moreover, from the score plot of different treatments at four sampling time points, it could be observed that the distance between LN + MT and CK was much smaller than that between LN and CK, and similarly, the distance between HN + MT and CK was much less than the distance between HN and CK. In addition, the factor loading plot reflects that most of the relevant indicators of analysis were discriminating traits that could significantly distinguish the differences between treatments, i.e. their vectors on the biplot were all relatively long. Therefore, the index studied could reflect that melatonin treatment was beneficial in reducing the gap between the control and low or high nitrogen conditions on plant development level and nitrogen metabolism.
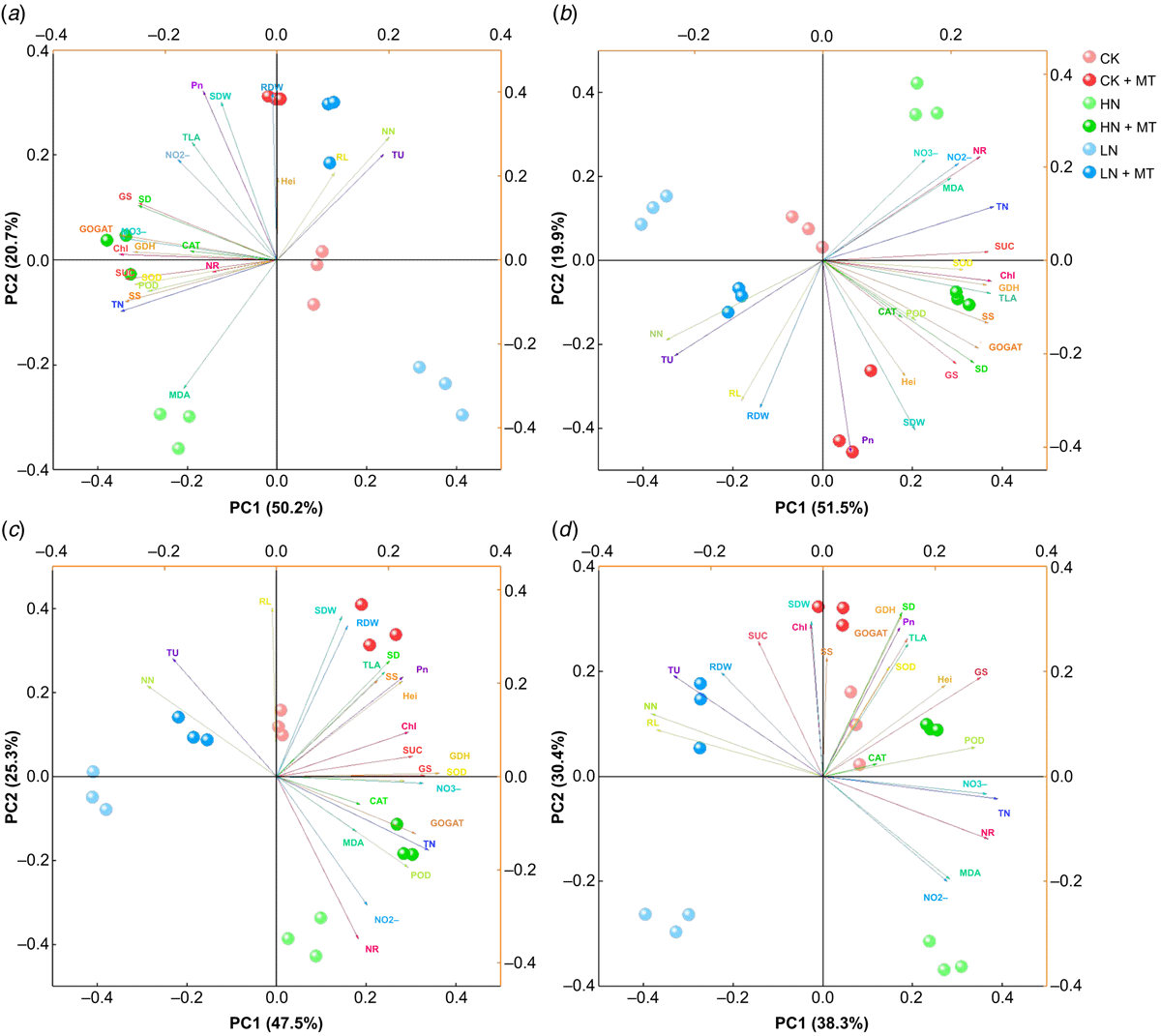
|
Discussion
Melatonin is a pleiotropic molecule with many diverse actions in plants (Shi et al. 2015; Arnao and Hernández-Ruiz 2019). Although it had been confirmed that exogenous melatonin has a certain regulatory effect on nitrogen metabolism under many treatment conditions (Turk and Erdal 2015; Arora and Bhatla 2017; Zhang et al. 2017b), there was not enough data to prove whether exogenous melatonin could enhance the nitrogen adaptability of soybean by regulating nitrogen cycles. Therefore, our study wis the first to demonstrate the mechanism of melatonin-regulated nitrogen metabolism in soybean under different nitrogen levels.
Under low N levels, the nitrogen metabolism of soybean was inhibited; however, the application of melatonin could significantly accelerate the absorption and utilisation of nitrogen by seedlings under this condition. Our study found that the expression of ammonium transporter genes (AMT1, AMT2) was significantly upregulated at this nitrogen level after melatonin was applied. It has been well documented that AMT1 and AMT2 proteins play a critical role in ammonium uptake and root-to-shoot translocation, respectively (Gu et al. 2013; Giehl et al. 2017; Chen et al. 2019). Therefore, melatonin could stimulate ammonium uptake of seedlings to compensate for the nitrogen demand of seedlings. However, because NR is greatly affected by light intensity, some studies have pointed out that its activity would be significantly inhibited in dark conditions (Shaha 2003; Creighton et al. 2017). Therefore, most of the nitrate absorbed by the root was transported to its leaves and then assimilated by NR, as reported by many studies (Timpo and Neyra 1983; Pinto et al. 2014). Additionally, our study proved that the upregulation effect of melatonin on NR gene expression was mainly in functional leaves. Further, it was also found that the accumulation of nitrate-nitrogen in functional leaves returned to the control level 6–12 days after melatonin treatment. However, the activity of nitrate reductase, which catalysed its reduction, significantly increased. This fully reflected that melatonin was also beneficial to nitrate absorption by soybean at low N levels (Fig. 4). Previous studies on wheat under low N stress showed that melatonin-treated wheat maintained higher nitrate levels and total nitrogen content. The activities of NR and GS were elevated (Qiao et al. 2019), which was similar to that observed in our study. The results of this study summarised that: (1) melatonin was beneficial to nitrogen absorption by soybeans at low nitrogen levels; (2) melatonin could promote nitrogen assimilation by upregulating the expression of enzyme-encoding genes, such as glutaminase and glutamate synthase, which were involved in ammonium assimilation; and (3) melatonin enhanced the activity of the aforementioned enzymes. All kinds of amino acids synthesised by the nitrogen metabolism pathway will be transported to specific sites through the amino acid transporter in the cell (Cheng et al. 2016). Therefore, the results of upregulation of melatonin-stimulated amino acid transporter gene (Glyma11g11310) in this study also indicated that melatonin played a unique role in enhancing nitrogen use efficiency.
In this study, the effect of high nitrogen treatment on soybean seedlings was consistent with the result that excessive nitrogen supply was harmful to plant growth, as demonstrated by Kong et al. (2017). Previous studies have pointed out that this adverse effect was likely caused by oxidative stress from the excessive accumulation of ROS (Skopelitis et al. 2006). In this study, the analysis of MDA content revealed that it was found that its content at high N levels was significantly higher than that of the control (Fig. 3d). It was clear that the excessive accumulation of MDA was closely correlated with the toxicity caused by excessive nitrogen application (Bi et al. 2007). As a free radical scavenger, melatonin has been proved to significantly promote the activity of antioxidant enzymes at high N levels (Fig. 3a–c), similar to the response mechanism of melatonin in alleviating nitrogen stress summarised by previous studies (Wang et al. 2016). This is consistent with the view that melatonin plays a significant role in plant stress signals (Gong et al. 2017). And it was considered a key component in the center of the redox network from which the different biochemical, cellular, and physiological responses were controlled (Arnao and Hernández-Ruiz 2018, 2019). Therefore, melatonin is predicted to alleviate the harmful effects of high N levels on soybean growth by promoting the activities of antioxidant enzymes, inhibiting the accumulation of RNS and ROS, thus reducing the degree of membrane lipid peroxidation.
In addition, in recent years, proteins containing cystathionine-β-synthase (CBS) domains have been considered to be related to abiotic stress resistance. This family plays an essential role in plant responses to abiotic and abiotic stresses (Hao et al. 2011). Among them, there are many stress signaling-related sites in the upstream promoter region of Gmduf-cbs gene. The upregulation of this gene has been proved to alleviate nitrogen stress (Kushwaha et al. 2009; Hao et al. 2021). Therefore, the result of significant upregulation of Gmduf-cbs gene after melatonin application in this study provided a novel opinion for promoting soybean nitrogen adaptability. Still, the specific response pathway is necessary to be determined.
This study also revealed that under the condition of excessive nitrogen supply, exogenous melatonin was also beneficial to prevent the excessive accumulation of ammonium ions in mesophyll and root cells. Due to excessive nitrogen supply, plant development was significantly inhibited, mainly in the root (Chen et al. 2021). Therefore, in this study, it was observed that the expression of key genes about nitrogen metabolism was alleviated in melatonin treatment, and the upregulation effect was more evident in the root. The expression of GS and other genes that catalyse further assimilation of ammonium ions were increased, and the protein activity of GS also enhanced, which were consistent with the regulation pathway of soybean under low N condition. Zhang et al. (2017b) also concluded a similar conclusion in the mechanism of melatonin alleviating excessive nitrogen stress of cucumber. Moreover, melatonin was confirmed to significantly reduce nitrate accumulation in lettuce leaves and roots (Zhou et al. 2019). In this study, in addition to the above similar conclusions, it was also observed that melatonin could significantly inhibit the accumulation of nitrite in soybean functional leaves, thus alleviating the toxicity caused by excess nitrogen.
The results also showed that melatonin significantly upregulated the expression of Gmdof1.4 gene, a member of Dof1 family, under three nitrogen concentrations. Members of the DOF family were known to be involved in the tolerance of plants to nitrogen stress and improve plant growth. It has been suggested that genes carrying DOF domain function were related to the co-expression of organic acid metabolising enzymes (Yanagisawa et al. 2004; Hao et al. 2011). Therefore, it may be predicted that melatonin may enhance nitrogen metabolism by activating the organic acid pathway to produce the carbon skeleton needed for nitrogen assimilation (Massange-Sánchez et al. 2016).
Based on the hypothesis that melatonin promoted carbon metabolism, through the determination of chlorophyll content and net photosynthetic rate in functional leaves, it was confirmed that the photosynthetic capacity was indeed improved with melatonin treatment (Supplementary Fig. S1A, B). And compared with the control without melatonin treatment, the accumulation of soluble sugar of photosynthate also increased at all nitrogen levels (Fig. S1C, D). Previous studies had shown that melatonin could directly stimulate gene expression involved in photosynthesis (Wei et al. 2015). In addition, Zhang et al. (2018) had found that melatonin could potentially increase soybean PSII efficiency, improve leaf area index, and increase dry matter accumulation under drought stress. And Erdal (2019) had also confirmed that melatonin could significantly promote C and N metabolism in maize, and the intuitive phenomenon was reflected in better morphological parameters. The coordination between carbon and nitrogen metabolism underlies the growth process in higher plants (Duan et al. 2018). Nitrogen metabolism provides a variety of amino acids for carbon metabolism, while photosynthetic reactions are involved in the synthesis, regulation, and the maintenance of the enzymes of nitrogen assimilation pathway, cellular respiration, in particular, the citric acid cycle, provides carbon skeletons for amino acid biosynthesis (Lawlor 2002). Therefore, the promotion of melatonin on soybean growth under an unsuitable nitrogen supply level is attributed to the promotion of nitrogen metabolism and its direct stimulation of the carbon metabolism pathway.
In this study, the analysis of plant morphological parameters also confirmed the positive effect of melatonin on the growth and development of soybean. It mainly manifested in the recovery of growth when the plant was treated with nitrogen deficiency or excess (Fig. 1, Table S3). It is worth while noting that melatonin could significantly alleviate the growth inhibition of soybean nodules, especially at high N levels. Since melatonin has been proved to significantly promote the effect on all root morphogenetic processes, it was believed that the improvement of nodules development might be related to the restoration of root growth (Erland and Saxena 2018). As one of the main storage forms of nitrogen, ureide is mainly produced by nodule fixation. And in the results of our study, the accumulation of ureide increased in varying degrees after melatonin treatment, which also proved the conclusion that melatonin is beneficial to root nodules. However, it needs to be researched deeply on the specific regulatory pathway of melatonin in promoting nodule growth.
In short, as shown in Fig. 9, nitrogen deficiency could directly limit the activity of nitrogen assimilation enzymes, reduce the synthesis of chlorophyll, limit photosynthetic capacity, thus affect plant dry matter accumulation and make plants yellowing and dwarfing. Melatonin could effectively promote nitrogen assimilation enzyme activity at low nitrogen levels, enhance nitrogen absorption and utilisation, increase chlorophyll content, and enhance photosynthetic carbon metabolism to alleviate the effects of insufficient nitrogen supply on soybean growth and yield. However, under high N levels, although the ability of nitrogen assimilation was enhanced quickly, the nitrogen fixation ability of root nodules was significantly inhibited. High N treatment led to increased membrane lipid peroxidation products and oxidative stress in plants (Skopelitis et al. 2006). As a result, other physiological and metabolic activities were disturbed, affecting the formation of soybean yield (Arnao and Hernández-Ruiz 2018, 2019). Nevertheless, the application of melatonin could enhance the assimilation of ammonium nitrogen, reduce the accumulation of inorganic nitrogen, alleviate the stress response caused by high nitrogen, and increase the formation of yield by increasing the activity of the antioxidant enzyme in the case of excessive nitrogen application.
Conclusion
This study confirmed that melatonin can promote the growth of soybean seedlings under different nitrogen concentrations. However, the regulatory effect of melatonin on various nitrogen levels of soybean was reflected in the different metabolic regulation pathways. At low N levels, it not only stimulated nitrogen absorption but also induced the upregulation of genes expression involved in nitrogen assimilation (AMT1, AMT2, NR, NiR, GS, GOGAT, AS, AAT) and the activity of several enzymes (NR, GS, GOGAT, GDH). Under high N levels, it was primarily conducive to the further assimilation of catalytic ammonium to reduce the accumulation of inorganic nitrogen in cells. It could significantly enhance the activity of antioxidant enzymes (SOD, GPX, CAT) to alleviate oxidative stress and other physiological damage. Results confirmed the effects of melatonin on the growth and nitrogen metabolism of soybean. They support an original idea and theoretical basis for understanding regulation of nitrogen use, and point to possible methods for use in remediation of insufficient or excessive nitrogen fertiliser application in soybean.
Supplementary material
Supplementary material is available
Data availability
The data that support this study are available in the article and accompanying online supplementary material.
Conflicts of interest
The authors declare no conflicts of interest.
Declaration of funding
This work was supported by the China Agriculture Research System of MOF and MARA (No. CARS-04-PS16), the Key Scientific Research Projects of Heilongjiang Farmsand Land Reclamation Administration (No. HKKY190206-1), the Postdoctoral Scientific Research Developmental Fund of Heilongjiang (No. LBH-Q20052), the technology innovation team of soybean high quality and efficient cultivation (No. 2042110005) and the Research Initiation Plan for Talent Introduction (No. XYB202011). However, the supporting source was not involved in preparing the data or manuscript or the decision to submit for publication.
Acknowledgements
We thank our colleagues Bin Qin, Mingyao Wang, Shan Jiang for their assistance in the experiment process. In addition, we sincerely thank reviewers and the editor for their comments that helped to improve an earlier version of this manuscript.
References
Arnao MB, Hernández-Ruiz J (2018) Melatonin and its relationship to plant hormones. Annals of Botany 121, 195–207.| Melatonin and its relationship to plant hormones.Crossref | GoogleScholarGoogle Scholar | 29069281PubMed |
Arnao MB, Hernández-Ruiz J (2019) Melatonin: a new plant hormone and/or a plant master regulator? Trends in Plant Science 24, 38–48.
| Melatonin: a new plant hormone and/or a plant master regulator?Crossref | GoogleScholarGoogle Scholar | 30446305PubMed |
Arora D, Bhatla SC (2017) Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radical Biology and Medicine 106, 315–328.
| Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD.Crossref | GoogleScholarGoogle Scholar | 28254544PubMed |
Arun M, Radhakrishnan R, Ai TN, Naing AH, Lee IJ, Kim CK (2016) Nitrogenous compounds enhance the growth of petunia and reprogram biochemical changes against the adverse effect of salinity. The Journal of Horticultural Science and Biotechnology 91, 562–572.
| Nitrogenous compounds enhance the growth of petunia and reprogram biochemical changes against the adverse effect of salinity.Crossref | GoogleScholarGoogle Scholar |
Bawa G, Feng L, Shi J, Chen G, Cheng Y, Luo J, Wu W, Ngoke B, Cheng P, Tang Z, Pu T, Liu J, Liu W, Yong T, Du J, Yang W, Wang X (2020) Evidence that melatonin promotes soybean seedlings growth from low-temperature stress by mediating plant mineral elements and genes involved in the antioxidant pathway. Functional Plant Biology 47, 815
| Evidence that melatonin promotes soybean seedlings growth from low-temperature stress by mediating plant mineral elements and genes involved in the antioxidant pathway.Crossref | GoogleScholarGoogle Scholar | 32553087PubMed |
Bergersen FJ (1958) The bacterial component of soybean root nodules; changes in respiratory activity, cell dry weight and nucleic acid content with increasing nodule age. Journal of General Microbiology 19, 312–323.
| The bacterial component of soybean root nodules; changes in respiratory activity, cell dry weight and nucleic acid content with increasing nodule age.Crossref | GoogleScholarGoogle Scholar | 13587897PubMed |
Bi YM, Wang RL, Zhu T, Rothstein SJ (2007) Global transcription profiling reveals differential responses to chronic nitrogen stress and putative nitrogen regulatory components in Arabidopsis. BMC Genomics 8, 281
| Global transcription profiling reveals differential responses to chronic nitrogen stress and putative nitrogen regulatory components in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 17705847PubMed |
Byeon Y, Back K (2014) An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield. Journal of Pineal Research 56, 408–414.
| An increase in melatonin in transgenic rice causes pleiotropic phenotypes, including enhanced seedling growth, delayed flowering, and low grain yield.Crossref | GoogleScholarGoogle Scholar | 24571270PubMed |
Cafaro La Menza N, Monzon JP, Specht JE, Grassini P (2017) Is soybean yield limited by nitrogen supply? Field Crops Research 213, 204–212.
| Is soybean yield limited by nitrogen supply?Crossref | GoogleScholarGoogle Scholar |
Cao L, Jin XJ, Zhang YX (2019) Melatonin confers drought stress tolerance in soybean (Glycine max L.) by modulating photosynthesis, osmolytes, and reactive oxygen metabolism. Photosynthetica 57, 812–819.
| Melatonin confers drought stress tolerance in soybean (Glycine max L.) by modulating photosynthesis, osmolytes, and reactive oxygen metabolism.Crossref | GoogleScholarGoogle Scholar |
Cataldo DA, Maroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Communications in Soil Science and Plant Analysis 6, 71–80.
| Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid.Crossref | GoogleScholarGoogle Scholar |
Chen YE, Mao JJ, Sun LQ, Huang B, Ding CB, Gu Y, Liao JQ, Hu C, Zhang ZW, Yuan S, Yuan M (2018) Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiologia Plantarum 164, 349–363.
| Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity.Crossref | GoogleScholarGoogle Scholar | 29633289PubMed |
Chen Q, Wu W, Zhao T, Tan W, Tian J, Liang C (2019) Complex gene regulation underlying mineral nutrient homeostasis in soybean root response to acidity stress. Genes 10, 402
| Complex gene regulation underlying mineral nutrient homeostasis in soybean root response to acidity stress.Crossref | GoogleScholarGoogle Scholar |
Chen J, Wang Z, Liu S, Zhang S, Ge C, Shen Q, Ma H, Zhang X, Dong H, Zhao X, Liu R, Pang C (2021) Nitrogen stress inhibits root growth by regulating cell wall and hormone changes in cotton (Gossypium hirsutum L.). Journal of Agronomy and Crop Science 1–18.
| Nitrogen stress inhibits root growth by regulating cell wall and hormone changes in cotton (Gossypium hirsutum L.).Crossref | GoogleScholarGoogle Scholar |
Cheng L, Yuan HY, Ren R, Zhao SQ, Han YP, Zhou QY, Ke DX, Wang YX, Wang L (2016) Genome-wide identification, classification, and expression analysis of amino acid transporter gene family in Glycine Max. Frontiers in Plant Science 7, 515
| Genome-wide identification, classification, and expression analysis of amino acid transporter gene family in Glycine Max.Crossref | GoogleScholarGoogle Scholar | 27148336PubMed |
Ciampitti IA, Salvagiotti F (2018) New insights into soybean biological nitrogen fixation. Agronomy Journal 110, 1185–1196.
| New insights into soybean biological nitrogen fixation.Crossref | GoogleScholarGoogle Scholar |
Creighton MT, Sanmartín M, Kataya ARA, Averkina IO, Heidari B, Nemie-Feyissa D, Sánchez-Serrano JJ, Lillo C (2017) Light regulation of nitrate reductase by catalytic subunits of protein phosphatase 2A. Planta 246, 701–710.
| Light regulation of nitrate reductase by catalytic subunits of protein phosphatase 2A.Crossref | GoogleScholarGoogle Scholar | 28656346PubMed |
Du Q, Zhou L, Chen P, Liu X, Song C, Yang F, Wang X, Liu W, Sun X, Du J, Liu J, Shu K, Yang W, Yong T (2020) Relay-intercropping soybean with maize maintains soil fertility and increases nitrogen recovery efficiency by reducing nitrogen input. The Crop Journal 8, 140–152.
| Relay-intercropping soybean with maize maintains soil fertility and increases nitrogen recovery efficiency by reducing nitrogen input.Crossref | GoogleScholarGoogle Scholar |
Duan W, Wang Q, Zhang H, Xie B, Li A, Hou F, Dong S, Wang B, Qin Z, Zhang L (2018) Comparative study on carbon–nitrogen metabolism and endogenous hormone contents in normal and overgrown sweetpotato. South African Journal of Botany 115, 199–207.
| Comparative study on carbon–nitrogen metabolism and endogenous hormone contents in normal and overgrown sweetpotato.Crossref | GoogleScholarGoogle Scholar |
Erdal S (2019) Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms. Plant Cell Reports 38, 1001–1012.
| Melatonin promotes plant growth by maintaining integration and coordination between carbon and nitrogen metabolisms.Crossref | GoogleScholarGoogle Scholar | 31069499PubMed |
Erland LAE, Saxena PK (2018) Melatonin in plant morphogenesis. In Vitro Cellular & Developmental Biology-Plant 54, 3–24.
| Melatonin in plant morphogenesis.Crossref | GoogleScholarGoogle Scholar |
Fan J, Xie Y, Zhang Z, Chen L (2018) Melatonin: a multifunctional factor in plants. International Journal of Molecular Sciences 19, 1528
| Melatonin: a multifunctional factor in plants.Crossref | GoogleScholarGoogle Scholar |
Gavili E, Moosavi AA, Zahedifar M (2019) Integrated effects of cattle manure-derived biochar and soil moisture conditions on soil chemical characteristics and soybean yield. Archives of Agronomy and Soil Science 65, 1758–1774.
| Integrated effects of cattle manure-derived biochar and soil moisture conditions on soil chemical characteristics and soybean yield.Crossref | GoogleScholarGoogle Scholar |
Giehl RFH, Laginha AM, Duan F, Rentsch D, Yuan L, von Wirén N (2017) A critical role of AMT2;1 in root-to-shoot translocation of ammonium in Arabidopsis. Molecular Plant 10, 1449–1460.
| A critical role of AMT2;1 in root-to-shoot translocation of ammonium in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 29032248PubMed |
Gong B, Yan Y, Wen D, Shi Q (2017) Hydrogen peroxide produced by NADPH oxidase: a novel downstream signaling pathway in melatonin-induced stress tolerance in Solanum lycopersicum. Physiologia Plantarum 160, 396–409.
| Hydrogen peroxide produced by NADPH oxidase: a novel downstream signaling pathway in melatonin-induced stress tolerance in Solanum lycopersicum.Crossref | GoogleScholarGoogle Scholar | 28464254PubMed |
Grassini P, Specht JE, Tollenaar M, Ciampitti I, Cassman KG (2014) High-yield maize-soybean cropping systems in the US Corn Belt. In ‘Crop physiology’, 2nd edn. pp. 17–41. (Academic Press: Cambridge, MA, USA)
Gu R, Duan F, An X, Zhang F, von Wirén N, Yuan L (2013) Characterization of AMT-mediated high-affinity ammonium uptake in roots of maize (Zea mays L.). Plant and Cell Physiology 54, 1515–1524.
| Characterization of AMT-mediated high-affinity ammonium uptake in roots of maize (Zea mays L.).Crossref | GoogleScholarGoogle Scholar | 23832511PubMed |
Guan M, de Bang TC, Pedersen C, Schjoerring JK (2016) Cytosolic glutamine synthetase Gln1;2 is the main isozyme contributing to GS1 activity and can be up-regulated to relieve ammonium toxicity. Plant Physiology 171, 1921–1933.
| Cytosolic glutamine synthetase Gln1;2 is the main isozyme contributing to GS1 activity and can be up-regulated to relieve ammonium toxicity.Crossref | GoogleScholarGoogle Scholar | 27231101PubMed |
Hageman RH, Reed AJ (1980) Nitrate reductase from higher plants. Methods in Enzymology 69, 270–280.
| Nitrate reductase from higher plants.Crossref | GoogleScholarGoogle Scholar |
Hao Q, Yang Y, Shan Z, Chen H, Zhang C, Chen L, Yuan S, Zhang X, Chen S, Yang Z, Qiu D, Zhou X (2021) Genome-wide investigation and expression profiling under abiotic stresses of a soybean unknown function (DUF21) and cystathionine-β-synthase (CBS) domain-containing protein family. Biochemical Genetics 59, 83–113.
| Genome-wide investigation and expression profiling under abiotic stresses of a soybean unknown function (DUF21) and cystathionine-β-synthase (CBS) domain-containing protein family.Crossref | GoogleScholarGoogle Scholar | 32778975PubMed |
Hao QN, Zhou XA, Sha AH, Wang C, Zhou R, Chen SL (2011) Identification of genes associated with nitrogen-use efficiency by genome-wide transcriptional analysis of two soybean genotypes. BMC Genomics 12, 525
| Identification of genes associated with nitrogen-use efficiency by genome-wide transcriptional analysis of two soybean genotypes.Crossref | GoogleScholarGoogle Scholar | 22029603PubMed |
Harper JE (1974) Soil and symbiotic nitrogen requirements for optimum soybean production. Crop Science 14, 255–260.
| Soil and symbiotic nitrogen requirements for optimum soybean production.Crossref | GoogleScholarGoogle Scholar |
Hernández-Ruiz J, Antonio C, Arnao MB (2004) Melatonin: a growth-stimulating compound present in lupin tissues. Planta 220, 140–144.
| Melatonin: a growth-stimulating compound present in lupin tissues.Crossref | GoogleScholarGoogle Scholar | 15232696PubMed |
Hu W, Tie W, Ou W, Yan Y, Kong H, Zuo J, Ding X, Ding Z, Liu Y, Wu C, Guo Y, Shi H, Li K, Guo A (2018) Crosstalk between calcium and melatonin affects postharvest physiological deterioration and quality loss in cassava. Postharvest Biology and Technology 140, 42–49.
| Crosstalk between calcium and melatonin affects postharvest physiological deterioration and quality loss in cassava.Crossref | GoogleScholarGoogle Scholar |
Kong L, Xie Y, Hu L, Si J, Wang Z (2017) Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses. Scientific Reports 7, 43363
| Excessive nitrogen application dampens antioxidant capacity and grain filling in wheat as revealed by metabolic and physiological analyses.Crossref | GoogleScholarGoogle Scholar | 28233811PubMed |
Kushwaha HR, Singh AK, Sopory SK, Singla-Pareek SL, Pareek A (2009) Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation. BMC Genomics 10, 200
| Genome wide expression analysis of CBS domain containing proteins in Arabidopsis thaliana (L.) Heynh and Oryza sativa L. reveals their developmental and stress regulation.Crossref | GoogleScholarGoogle Scholar | 19400948PubMed |
Lawlor DW (2002) Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems. Journal of Experimental Botany 53, 773–787.
| Carbon and nitrogen assimilation in relation to yield: mechanisms are the key to understanding production systems.Crossref | GoogleScholarGoogle Scholar | 11912221PubMed |
Li C, Liang B, Chang C, Wei Z, Zhou S, Ma F (2016) Exogenous melatonin improved potassium content in Malus under different stress conditions. Journal of Pineal Research 61, 218–229.
| Exogenous melatonin improved potassium content in Malus under different stress conditions.Crossref | GoogleScholarGoogle Scholar | 27145234PubMed |
Li M, Xu J, Wang X, Fu H, Zhao M, Wang H, Shi L (2018) Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress. Journal of Plant Physiology 229, 132–141.
| Photosynthetic characteristics and metabolic analyses of two soybean genotypes revealed adaptive strategies to low-nitrogen stress.Crossref | GoogleScholarGoogle Scholar | 30081252PubMed |
Liang C, Li A, Yu H, Li W, Liang C, Guo S, Zhang R, Chu C (2017) Melatonin regulates root architecture by modulating auxin response in rice. Frontiers in Plant Science 8, 134
| Melatonin regulates root architecture by modulating auxin response in rice.Crossref | GoogleScholarGoogle Scholar | 28223997PubMed |
Lin CC, Kao CH (1996) Disturbed ammonium assimilation is associated with growth inhibition of roots in rice seedlings caused by NaCl. Plant Growth Regulation 18, 233–238.
| Disturbed ammonium assimilation is associated with growth inhibition of roots in rice seedlings caused by NaCl.Crossref | GoogleScholarGoogle Scholar |
Liu B, Shen W, Wei H, Smith H, Louws FJ, Steadman JR, Correll JC (2016a) Rhizoctonia communities in soybean fields and their relation with other microbes and nematode communities. European Journal of Plant Pathology 144, 671–686.
| Rhizoctonia communities in soybean fields and their relation with other microbes and nematode communities.Crossref | GoogleScholarGoogle Scholar |
Liu J, Zhang R, Sun Y, Liu Z, Jin W, Sun Y (2016b) The beneficial effects of exogenous melatonin on tomato fruit properties. Scientia Horticulturae 207, 14–20.
| The beneficial effects of exogenous melatonin on tomato fruit properties.Crossref | GoogleScholarGoogle Scholar |
Massange-Sánchez JA, Palmeros-Suárez PA, Espitia-Rangel E, Rodríguez-Arévalo I, Sánchez-Segura L, Martínez-Gallardo NA, Alatorre-Cobos F, Tiessen A, Délano-Frier JP (2016) Overexpression of grain Amaranth (Amaranthus hypochondriacus) AhERF or AhDOF transcription factors in Arabidopsis thaliana increases water-deficit and salt-stress tolerance, respectively, via contrasting stress-amelioration mechanisms. PLoS One 11, e0164280
| Overexpression of grain Amaranth (Amaranthus hypochondriacus) AhERF or AhDOF transcription factors in Arabidopsis thaliana increases water-deficit and salt-stress tolerance, respectively, via contrasting stress-amelioration mechanisms.Crossref | GoogleScholarGoogle Scholar | 27749893PubMed |
Mohamed IAA, Shalby N, El-Badri AMA, Saleem MH, Khan MN, Nawaz MA, Qin M, Agami RA, Kuai J, Wang B, Zhou G (2020) Stomata and xylem vessels traits improved by melatonin application contribute to enhancing salt tolerance and fatty acid composition of Brassica napus L. plants. Agronomy 10, 1186
| Stomata and xylem vessels traits improved by melatonin application contribute to enhancing salt tolerance and fatty acid composition of Brassica napus L. plants.Crossref | GoogleScholarGoogle Scholar |
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology 22, 867–880.
| Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts.Crossref | GoogleScholarGoogle Scholar |
Nawaz MA, Yang SH, Chung G (2018) Wild soybeans: an opportunistic resource for soybean improvement. In ‘Rediscovery of landraces as a resource for the future’. pp. 160–174. (IntechOpen Limited: London, UK)
Oliveira HC, Freschi L, Sodek L (2013) Nitrogen metabolism and translocation in soybean plants subjected to root oxygen deficiency. Plant Physiology and Biochemistry 66, 141–149.
| Nitrogen metabolism and translocation in soybean plants subjected to root oxygen deficiency.Crossref | GoogleScholarGoogle Scholar | 23500717PubMed |
Park S, Back K (2012) Melatonin promotes seminal root elongation and root growth in transgenic rice after germination. Journal of Pineal Research 53, 385–389.
| Melatonin promotes seminal root elongation and root growth in transgenic rice after germination.Crossref | GoogleScholarGoogle Scholar | 22640001PubMed |
Pinto E, Fidalgo F, Teixeira J, Aguiar AA, Ferreira IMPLVO (2014) Influence of the temporal and spatial variation of nitrate reductase, glutamine synthetase and soil composition in the N species content in lettuce (Lactuca sativa. Plant Science 219–220, 35–41.
| Influence of the temporal and spatial variation of nitrate reductase, glutamine synthetase and soil composition in the N species content in lettuce (Lactuca sativa.Crossref | GoogleScholarGoogle Scholar | 24576762PubMed |
Purcell LC, Salmeron M, Ashlock L (2014) Soybean growth and development. In ‘Arkansas soybean production handbook. Vol. 197’. pp. 1–8. (University of Arkansas Division of Agriculture: Little Rock, AR, USA)
Qiao Y, Yin L, Wang B, Ke Q, Deng X, Wang S (2019) Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat. Plant Physiology and Biochemistry 139, 342–349.
| Melatonin promotes plant growth by increasing nitrogen uptake and assimilation under nitrogen deficient condition in winter wheat.Crossref | GoogleScholarGoogle Scholar | 30952086PubMed |
Ray JD, Heatherly LG, Fritschi FB (2006) Influence of large amounts of nitrogen on nonirrigated and irrigated soybean. Crop Science 46, 52–60.
| Influence of large amounts of nitrogen on nonirrigated and irrigated soybean.Crossref | GoogleScholarGoogle Scholar |
Raza MA, Bin Khalid MH, Zhang X, Feng LY, Khan I, Hassan MJ, Ahmed M, Ansar M, Chen YK, Fan YF, Yang F, Yang W (2019) Effect of planting patterns on yield, nutrient accumulation and distribution in maize and soybean under relay intercropping systems. Scientific Reports 9, 4947
| Effect of planting patterns on yield, nutrient accumulation and distribution in maize and soybean under relay intercropping systems.Crossref | GoogleScholarGoogle Scholar | 30894625PubMed |
Shaha RK (2003) Role of light in the regulation of the nitrate reductase level in lentil (Lens esculenta L.). Pakistan Journal of Biological Sciences 6, 1802–1807.
| Role of light in the regulation of the nitrate reductase level in lentil (Lens esculenta L.).Crossref | GoogleScholarGoogle Scholar |
Sherrard JH, Dalling MJ (1979) In vitro stability of nitrate reductase from wheat leaves: I. Stability of highly purified enzyme and its component activities. Plant Physiology 63, 346–353.
| In vitro stability of nitrate reductase from wheat leaves: I. Stability of highly purified enzyme and its component activities.Crossref | GoogleScholarGoogle Scholar | 16660726PubMed |
Shi H, Jiang C, Ye T, Tan DX, Reiter RJ, Zhang H, Liu R, Chan Z (2015) Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. Journal of Experimental Botany 66, 681–694.
| Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin.Crossref | GoogleScholarGoogle Scholar | 25225478PubMed |
Sinclair TR, de Wit CT (1975) Photosynthate and nitrogen requirements for seed production by various crops. Science 189, 565–567.
| Photosynthate and nitrogen requirements for seed production by various crops.Crossref | GoogleScholarGoogle Scholar | 17798304PubMed |
Singh KK, Saha S, Kadiravana RC, Mazumdar D, Rai V, Ghosh S (2020) Ammonium metabolism in Selaginella bryopteris in response to dehydration-rehydration and characterisation of desiccation tolerant, thermostable, cytosolic glutamine synthetase from plant. Functional Plant Biology 48, 257–267.
| Ammonium metabolism in Selaginella bryopteris in response to dehydration-rehydration and characterisation of desiccation tolerant, thermostable, cytosolic glutamine synthetase from plant.Crossref | GoogleScholarGoogle Scholar |
Skopelitis DS, Paranychianakis NV, Paschalidis KA, Pliakonis ED, Delis ID, Yakoumakis DI, Kouvarakis A, Papadakis AK, Stephanou EG, Roubelakis-Angelakis KA (2006) Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. The Plant Cell 18, 2767–2781.
| Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine.Crossref | GoogleScholarGoogle Scholar | 17041150PubMed |
Takahashi M, Nakayama N, Arihara J (2005) Plant nitrogen levels and photosynthesis in the supernodulating soybean (Glycine max L. Merr.) cultivar ‘Sakukei 4’. Plant Production Science 8, 412–418.
| Plant nitrogen levels and photosynthesis in the supernodulating soybean (Glycine max L. Merr.) cultivar ‘Sakukei 4’.Crossref | GoogleScholarGoogle Scholar |
Tamagno S, Balboa GR, Assefa Y, Kovács P, Casteel SN, Salvagiotti F, García FO, Stewart WM, Ciampitti IA (2017) Nutrient partitioning and stoichiometry in soybean: a synthesis-analysis. Field Crops Research 200, 18–27.
| Nutrient partitioning and stoichiometry in soybean: a synthesis-analysis.Crossref | GoogleScholarGoogle Scholar |
Timpo EE, Neyra CA (1983) Expression of nitrate and nitrite reductase activities under various forms of nitrogen nutrition in Phaseolus vulgaris L. Plant Physiology 72, 71–75.
| Expression of nitrate and nitrite reductase activities under various forms of nitrogen nutrition in Phaseolus vulgaris L.Crossref | GoogleScholarGoogle Scholar | 16662985PubMed |
Turk H, Erdal S (2015) Melatonin alleviates cold-induced oxidative damage in maize seedlings by up-regulating mineral elements and enhancing antioxidant activity. Journal of Plant Nutrition and Soil Science 178, 433–439.
| Melatonin alleviates cold-induced oxidative damage in maize seedlings by up-regulating mineral elements and enhancing antioxidant activity.Crossref | GoogleScholarGoogle Scholar |
Wang WX, Zhang RM, Sun Y, Liu JL (2016) Effect of exogenous melatonin on the antioxidant system of cucumber seedlings under nitrate stress. Acta Horticulturae Sinica 43, 695–703.
| Effect of exogenous melatonin on the antioxidant system of cucumber seedlings under nitrate stress.Crossref | GoogleScholarGoogle Scholar |
Wang X, Li D, Jiang J, Dong Z, Ma Y (2017) Soybean NAC gene family: sequence analysis and expression under low nitrogen supply. Biologia Plantarum 61, 473–482.
| Soybean NAC gene family: sequence analysis and expression under low nitrogen supply.Crossref | GoogleScholarGoogle Scholar |
Wei W, Li QT, Chu YN, Reiter RJ, Yu XM, Zhu DH, Zhang WK, Ma B, Lin Q, Zhang JS, Chen SY (2015) Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. Journal of Experimental Botany 66, 695–707.
| Melatonin enhances plant growth and abiotic stress tolerance in soybean plants.Crossref | GoogleScholarGoogle Scholar | 25297548PubMed |
Xia X, Ma C, Dong S, Xu Y, Gong Z (2017) Effects of nitrogen concentrations on nodulation and nitrogenase activity in dual root systems of soybean plants. Soil Science and Plant Nutrition (Tokyo) 63, 470–482.
| Effects of nitrogen concentrations on nodulation and nitrogenase activity in dual root systems of soybean plants.Crossref | GoogleScholarGoogle Scholar |
Xie M, Chung CYL, Li MW, Wong FL, Wang X, Liu A, Wang Z, Leung AKY, Wong TH, Tong SW, Xiao Z, Fan K, Ng MS, Qi X, Yang L, Deng T, He L, Chen L, Fu A, Ding Q, He J, Chung G, Isobe S, Tanabata T, Valliyodan B, Nguyen HT, Cannon SB, Foyer CH, Chan TF, Lam HM (2019) A reference-grade wild soybean genome. Nature Communications 10, 1216
| A reference-grade wild soybean genome.Crossref | GoogleScholarGoogle Scholar | 30872580PubMed |
Xu G, Fan X, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Annual Review of Plant Biology 63, 153–182.
| Plant nitrogen assimilation and use efficiency.Crossref | GoogleScholarGoogle Scholar | 22224450PubMed |
Yanagisawa S, Akiyama A, Kisaka H, Uchimiya H, Miwa T (2004) Metabolic engineering with Dof1 transcription factor in plants: improved nitrogen assimilation and growth under low-nitrogen conditions. Proceedings of the National Academy of Sciences of the United States of America 101, 7833–7838.
| Metabolic engineering with Dof1 transcription factor in plants: improved nitrogen assimilation and growth under low-nitrogen conditions.Crossref | GoogleScholarGoogle Scholar | 15136740PubMed |
Yong TW, Chen P, Dong Q, Du Q, Yang F, Wang XC, Liu WG, Yang WY (2018) Optimized nitrogen application methods to improve nitrogen use efficiency and nodule nitrogen fixation in a maize–soybean relay intercropping system. Journal of Integrative Agriculture 17, 664–676.
| Optimized nitrogen application methods to improve nitrogen use efficiency and nodule nitrogen fixation in a maize–soybean relay intercropping system.Crossref | GoogleScholarGoogle Scholar |
Zeng A, Chen P, Korth KL, Ping J, Thomas J, Wu C, Srivastava S, Pereira A, Hancock F, Brye K, Ma J (2019) RNA sequencing analysis of salt tolerance in soybean (Glycine max. Genomics 111, 629–635.
| RNA sequencing analysis of salt tolerance in soybean (Glycine max.Crossref | GoogleScholarGoogle Scholar | 29626511PubMed |
Zhang ZL, Zai WJ, Li XF (2016) ‘Plant physiology experimental guide’, 5th edn. pp. 139–141. (Higher Education Press: Beijing, China)
Zhang J, Zeng B, Mao Y, Kong X, Wang X, Yang Y, Zhang J, Xu J, Rengel Z, Chen Q (2017a) Melatonin alleviates aluminium toxicity through modulating antioxidative enzymes and enhancing organic acid anion exudation in soybean. Functional Plant Biology 44, 961
| Melatonin alleviates aluminium toxicity through modulating antioxidative enzymes and enhancing organic acid anion exudation in soybean.Crossref | GoogleScholarGoogle Scholar | 32480624PubMed |
Zhang R, Sun Y, Liu Z, Jin W, Sun Y (2017b) Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress. Journal of Pineal Research 62, e12403
| Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress.Crossref | GoogleScholarGoogle Scholar |
Zhang MH, Wang BY, Li S, Li K, Chen Y, He B, Sun DM (2018) Effect of uniconazole and rhizobium interaction on growth and nitrogen fixation in soybean. Soybean Science 37, 112––116.
| Effect of uniconazole and rhizobium interaction on growth and nitrogen fixation in soybean.Crossref | GoogleScholarGoogle Scholar |
Zhang M, He S, Qin B, Jin X, Wang M, Ren C, Cao L, Zhang Y (2020) Exogenous melatonin reduces the inhibitory effect of osmotic stress on antioxidant properties and cell ultrastructure at germination stage of soybean. PLoS One 15, e0243537
| Exogenous melatonin reduces the inhibitory effect of osmotic stress on antioxidant properties and cell ultrastructure at germination stage of soybean.Crossref | GoogleScholarGoogle Scholar | 33320882PubMed |
Zhou X, Yang T, Jiang Z, He Z, Zou Z (2019) Regulation of melatonin on chlorophyll fluorescence and nitrate accumulation in lettuce seedlings under excess nitrate stress. IOP conference series. Earth and Environmental Science 330, 42043
| Regulation of melatonin on chlorophyll fluorescence and nitrate accumulation in lettuce seedlings under excess nitrate stress.Crossref | GoogleScholarGoogle Scholar |
Zou JN, Yu Q, Jin XJ, Wang MY, Qin B, Ren CY, Wang MX, Zhang YX (2020) Effects of exogenous melatonin on physiology and yield of soybean during seed filling stage under drought stress. Acta Agronomica Sinica 46, 745–758.
| Effects of exogenous melatonin on physiology and yield of soybean during seed filling stage under drought stress.Crossref | GoogleScholarGoogle Scholar |




