Overexpression of AGAMOUS-like gene PfAG5 promotes early flowering in Polypogon fugax
Feng-Yan Zhou A C , Qin Yu B , Yong Zhang A , Yun-Jing Han A and Chuan-Chun Yao A
A C , Qin Yu B , Yong Zhang A , Yun-Jing Han A and Chuan-Chun Yao A
A Institute of Plant Protection and Agro-Products Safety, Anhui Academy of Agricultural Sciences, Hefei 230001, China.
B Australian Herbicide Resistance Initiative (AHRI), School of Agriculture and Environment, University of Western Australia, Perth, WA 6009, Australia.
C Corresponding author. Email: zbszhoufy@163.com
Functional Plant Biology 48(8) 793-801 https://doi.org/10.1071/FP21047
Submitted: 10 February 2021 Accepted: 20 February 2021 Published: 6 April 2021
Journal Compilation © CSIRO 2021 Open Access CC BY-NC
Abstract
Herbicides are the major tool for controlling large populations of yield depleting weeds. However, over-reliance on herbicides has resulted in weed adaptation and herbicide resistance. In recent years, early flowering weed species related to herbicide resistance is emerging, which may cause seed loss before crop harvest, creating a new problem for non-chemical weed management. In this study, a homologue gene of AGAMOUS sub-family (referred to as PfAG5) of the MADS-box family was cloned from plants of an early flowering Polypogon fugax Nees ex Steud. population resistant to the ACCase inhibitor herbicide (clodinafop-propargyl). The PfAG5 gene was functionally characterised in Arabidopsis thaliana L. Overexpression of the PfAG5 gene in Arabidopsis resulted in early flowering, abnormal flowers (e.g. small petals), short plants and reduced seed set, compared with the wild type. The expression of the PfAG5 gene was high in leaves and flowers, but low in pods in transgenic Arabidopsis. The PfAG5 gene was expressed earlier and higher in the resistant (R) than the susceptible (S) P. fugax plants. Furthermore, one protein (FRIGIDA-like) with relevance to flowering time regulation and interacts with PfAG5 in resistant (R) P. fugax was identified by the yeast two-hybrid and pull-down assays. These results suggest that the PfAG5 gene is involved in modulating early flowering in P. fugax.
Keywords: Polypogon fugax Nees ex Steud., Arabidopsis thaliana L., herbicide resistance, early flowering, flowering regulation, AGAMOUS-subfamily.
Introduction
Flowering is the most dramatic transition from the vegetative phase to reproductive phase in a life cycle of flowering plants, and hence an important agronomic trait. To achieve reproductive success, the optimal flowering time is critical for flowering plants (Wei et al. 2016). Flowering time is regulated autonomously and by environmental factors, such as photoperiod, vernalisation and stresses (Takeno 2016).
Many stress factors have been reported to induce flowering, such as light intensity, UV light, temperature, nutrition and drought, as summarised in previous reviews (Wada and Takeno 2010; Kazan and Lyons 2016). Herbicide application is also a stress to weedy plants, and it can select for evolution of herbicide resistance (Powles and Yu 2010). Adaptive changes in seed germination and seedling emergence, flower bud formation and flowering time have also been observed in herbicide resistant biotypes (Wang et al. 2010; Kleemann and Gurjeet 2013; Owen et al. 2014; Babineau et al. 2017; Kaspary et al. 2017).
In agriculture, frequent and regular disturbances from ploughing and harvesting likely exert a strong selection on weeds for rapid flowering and seed set (Barrett 1983). For example, highly effective weed seed collection techniques at harvest may exert intense selection for earlier flowering (likely early seed shattering) phenotypes to evade collection, and genetically diverse Raphanus raphanistrum L. exhibited significant standing genetic variations to adapt to flowering time selection (Ashworth et al. 2016). The ability to reach inflorescence emergence and flowering earlier is an advantage to weed populations, allowing them to escape potential eradication by late-season weed management strategies or harvesting (Hill et al. 2016).
MADS-box genes are key regulators of many aspects of plant reproductive development, especially in flowering time control, inflorescence architecture, floral organ identity determination and seed development. Based on their evolutionary origin, MADS-box genes have been divided into two classes, namely, type I and II. The plant-specific type II MIKC MADS box genes are key regulators of developmental processes, such as flowering time, fruit and seed development (Masiero et al. 2011). In Arabidopsis thaliana L., four genes, AGAMOUS (AG), SHATTER PROOF1 (SHP1), SHP2 and SEEDSTICK (STK) compose the monophyletic AG-subfamily within the MADS-box gene phylogeny (Favaro et al. 2003; Kramer et al. 2004). Members of the AG-subfamily are involved in the specification of floral reproductive organs and required for normal development of carpels and fruits (Dreni and Kater 2014). For instance, when expressed in Arabidopsis the AG-subfamily genes from Gossypium hirsutum L. regulate flower development and fruit formation (de Moura et al. 2017). The MIKC-type genes can be subdivided into 12 major gene clades, including floral promoters (e.g. AGAMOUS-like24, SOC1) and repressors (e.g. FLM/MAF1, FLC). These flowering genes generally exert their functions by influencing ‘meristem identity genes’, which control the transition from inflorescence to floral meristems (Becker and Theißen 2003).
In our previous study, we found that the resistant (R) population of Polypogon fugax Nees ex Steud.was resistant to ACCase-inhibiting herbicides clodinafop-propargyl, fluazifop-p-butyl, haloxyfop-R-methyl, quizalofop-p-ethyl and fenoxaprop-p-ethyl, relative to the susceptible (S) population (Tang et al. 2014). Plants of the R P. fugax population were found to be earlier in head emergence, flowering and seed maturation than that of the S population (Tang et al. 2015). Transcriptome analysis identified a flowering-related contig (CL10710.contig2) belonging to the AGAMOUS-subfamily of the MADS-box gene family that had significantly higher expression at the flowering stage in the R P. fugax versus the S P. fugax (Zhou et al. 2017). To determine the role of the contig (CL10710.contig2, and thereafter named as PfAG5) in flowering time regulation, we cloned the full-length cDNA sequence of the PfAG5 gene from R P. fugax and transformed in Arabidopsis. We analysed the expression pattern of PfAG5 gene in transgenic Arabidopsis and R versus S P. fugax plants, and that of other six endogenous flowering regulation genes in Arabidopsis. Furthermore, we identified two PfAG5 interaction proteins in R P. fugax by the yeast two-hybrid and pull-down assays, and one (FRIGIDA-like) is reverent to flowering time regulation. This is among the very few studies on the regulation mechanism of early flowering in a weedy plant species (Zhou et al. 2020). This knowledge will aid in future genetic approaches for better weed control strategies.
Materials and methods
Plant material and growth conditions
Seeds of a Polypogon fugax Nees ex Steud. population resistant to ACCase-inhibiting herbicides (referred to as R population) were collected from Qingsheng County (29°54′N, 103°48′E), Sichuan Province, China, where clodinafop-propargyl has been used for over 5 years and failed to control P. fugax in crops of wheat (Triticum aestivum L.) and canola (Brassica napus L.). A susceptible population of P. fugax (referred to as S population) were collected from a non-cultivated area in Xichang City of Sichuan (27°50′N, 102°15′E) where herbicides have never been used. The original R and S populations of P. fugax were identified by Dr Wei Tang (China National Rice Research Institute) and Dr Fengyan Zhou (Anhui Academy of Agricultural Sciences) (Tang et al. 2014), and these populations were obtained from wild populations without any specifically permissive requirement and now are deposited in the specimen room of Anhui Academy of Agricultural Sciences.
Seeds of the fourth generations of the R and S populations were generated by self-crossing and used in this study. After germination, the seedlings were transplanted into individual 1 L pots containing potting medium (1:1:1:2 vegetable garden soil:compost:peat:dolomite, pH 6.3). Plants were grown in a glasshouse with average day/night temperatures of 20/10°C under natural sunlight.
Arabidopsis thaliana L. cv. Columbia (Col-0) was obtained from the SALK collection (http://signal.salk.edu/) and used as the wild-type (WT) for transgenic manipulation. The transformed and untransformed control Arabidopsis seedlings were transplanted into individual 0.25 L pots containing potting medium (4:1:1 sphagnum:vermiculite:perlite) and grown at 19°C under 100 µmol m–2 s–1 photo density of cool white fluorescent light with a photoperiod of either 16/8 h light/dark (long day condition, LD) or 8/16 h light/dark (short day, SD).
Cloning of the PfAG5 cDNA from P. fugax
Total RNA from P. fugax R and S plants were isolated using the SGTriEx Total RNA extract Kit (SinoGene) and then used for reverse transcription by Thermo First cDNA Synthesis Kit (SinoGene) according to manufacturer’s instructions. The PfAG5 cDNA fragment was amplified using the primer pair S1 and S2 based on the contig sequence (Table 1), ligated into the pMD18-T vector, and confirmed by sequencing to be the partial sequence of an AGAMOUS-like gene. The full-length coding sequence of the PfAG5 gene was obtained using 5′- and 3′-RACE with the gene-specific primers GSP1 and GSP2 (Table 1) (Clontech, US), and amplified from plants by the primers FK and RB (Table 1) with introduced HindIII and EcoR I restriction sites based on the known 5′ and 3′ sequences.
|
Molecular characterisation and phylogenetic analysis of PfAG5
The open reading frame (ORF) of PfAG5 cDNA sequence was identified using the ORF finder software (https://www.ncbi.nlm.nih.gov/orffinder/). For homology analysis, the amino acid sequence of PfAG5 was aligned and compared with the sequences of other species. Phylogenetic analysis was conducted using the neighbour-joining method implemented in MEGA software version 5.0, and the robustness of the inferred phylogeny was validated by including 1000 bootstrap replicates.
Plasmid construction and Arabidopsis transformation
The pCAMBIA2300 and pCAMBIA1303 plasmid vectors were digested by HindIII and EcoR I, respectively. The (CaMV) 35S promoter of pCAMBIA2300 (1008 bp) and the large skeleton of pCAMBIA1303 were recovered and purified. T4 DNA ligase (TaKaRa) was then used to connect the two parts and a new two-element expression vector pCAMBIA1303-35S:35ST, including the 35S promoter, was obtained.
The full-length ORF of PfAG5 gene was ligated into the binary vector pCAMBIA1303-35S:35ST (empty plasmid control, Mock) to generate the plasmid pCAMBIA1303-35S-35ST:PfAG5 (see Fig. S3a). The plasmid was transferred into WT Arabidopsis plants (Col) using the floral dipping method. All transgenic Arabidopsis seeds (T0) were screened on 1/2 MS solid medium containing 50 mg-L–1 hygromycin. Positive transgenic lines (T1; n, 40) were confirmed by PCR amplification of the hygromycin gene and the target gene (PfAG5) was visualised by the GUS gene histochemical localisation (see Fig. S3b). Introduction of the target gene (PfAG5) in T2 generation plants was verified by PCR and positive plants (n, 27) all showed an early flowering phenotype. Twenty of these lines were used to produce the T3 lines and were used in the following experiments.
Flowering time and seed production measurements
To measure flowering time, seeds of WT (Col), empty plasmid control (Mock) and PfAG5 transgenic Arabidopsis plants (35S::PfAG5) were surface sterilised with 10% hypochlorite, then placed on MS agar medium and stratified at 4°C for 48 h before being placed at 19°C. Ten-day-old seedlings (at the four leaf stage) were transferred to growth medium (1:4:1 vermiculite:sphagnum:perlite) and grown under LD or SD conditions.
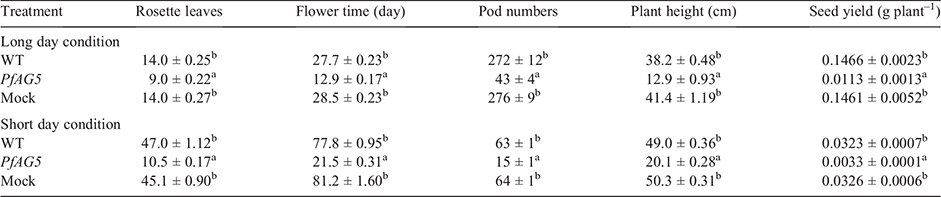
The flowering time of 20 T3 transgenic lines were recorded from the day of transplanting until the first Arabidopsis flower bloomed. Rosette leaf numbers were recorded when peduncle was 1–2 cm in length, and plant height and pod numbers were determined on day 55 after transplanting. Seeds were collected on day 62 after transplanting and weighed after drying at 37°C for 24 h.
Yeast two-hybrid assay
Aboveground plant tissue of three R P. fugax plants at the early flowering stage were harvested randomly, and the cDNA library (cloned into Prey vector pGADT7) was obtained using the Clontech kit (catalogue number 630490). The full-length PfAG5 (with yeast codon optimisation) was cloned into vector pGBKT7 (Bait vector) and then transformed into the yeast strain Y2HGold (Clontech).
The Matchmaker Gold Yeast Two-Hybrid System (Clontech, US) was used to screen PfAG5 interaction proteins from the R P. fugax library according to the manufacturer’s instructions. The primers used for pGBKT7 vector construction were listed in Table 1. To confirm interactions, the identified Prey and Bait vectors were validated by one-to-one interaction hybridisation.
Pull-down assay
For the in vitro interaction assays, The CDS of PfAG5 was reconstructed into the GST pull-down pET28a vector, which then used to transform Top10 Escherichia coli. Single colonies of GST-PfAG5 were inoculated in LB medium and inoculated at 37°C until OD600 reached 0.6–0.8. After induction with 0.5 mM IPTG at 37°Cfor 4 h, cells were collected and resuspended in PBS buffer. The aboveground part of R P. fugax plants at the early flowering stage was ground in liquid nitrogen to extract proteins for the pull-down assay according to Dou et al. (2019). The treated samples were then analysed by liquid mass spectrometry (LC–MS/MS) (Ultimate 3000 and Q Exactive Hybrid Quadrupole-Orbitrap Mass Spectrometer; Thermo Fisher Scientific) and proteins were identified by peptide sequencing. The peptide sequences combined with the peptide mass were then used to search against a protein sequence database for identification of candidate interaction proteins. MaxQuant (1.6.2.10) was used to search the Uniprot protein library to obtain potential interaction proteins, which were annotated using the Gramineous Genome Database (see Table S1).
PfAG5 expression analysis in Arabidopsis and P. fugax
To analyse the expression pattern of PfAG5 in different tissues of transgenic Arabidopsis plants, leaf, flower and pod samples from three to five T3 lines were collected at the seedling (6–8 leaves), flowering (full open) and podding (new formation) stages. Harvested samples were snap frozen in liquid nitrogen and stored at –80°C until use. In addition, the whole aboveground part of PfAG5 transgenic and WT Arabidopsis plants were collected before midday (Zeitgeber time 6, ZT6) at the flowering stage (13 and 28 days after transplanting, respectively) for analysis of the expression patterns of six other Arabidopsis genes relevant to flowering regulation (CO, SOC1, FT, LFY, FLC and AP1).
Tissue samples of the R and S P. fugax plants were collected at the seedling and tillering stages, and the samples collected at the early flowering stage of R plants correspond to the heading stage of the S plants. The expression of PfAG5 and its interacting proteins were compared between R and S samples, which were collected at the same time.
Total RNA was extracted using the SGTriEx Total RNA extract Kit (SinoGene), and DNA contamination removed by RNase-free DNaseI(Fermentas). The DNA-free RNA was then used for reverse transcription by Thermo First cDNA Synthesis Kit (SinoGene). The primer sequences used for real-time quantitative PCR (real-time qPCR) are provided in Table 1. The ACTIN8 and EF1 gene was used respectively for normalisation of Arabidopsis and P. fugax samples. The qPCR amplification was conducted for up to 40 cycles using the following thermal profile: denaturation at 95°C for 15 s, annealing at 55°C for 15 s and extension at 72°C for 45 s. The real-time qPCR results were presented as means ± s.e. of three biological replicates each performed in triplicate. Gene expression level was estimated as 2–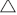
 Ct.
Ct.
Results
Cloning of PfAG5 cDNA coding sequence from R P. fugax
The PfAG5 coding sequence (GenBank accession number MK559453) is 831 bp encoding a 277-amino acid protein with 91% and 88% identity respectively to Hordeum vulgare L. ssp. vulgare AGAMOUS-like protein 1 HvAG1(AAL93196.1) and Aegilops tauschii Coss. MADS box transcription factor WAG-2f (ALM58837.1). A phylogenetic tree was constructed to determine the relationship of PfAG5 protein with AGAMOUS-like proteins of other plant species. PfAG5 belongs to the family of AGAMOUS homologues from monocots and is closely related to AGAMOUS-like proteins from H. vulgare ssp. vulgare, T. aestivum and A. tauschii (see Fig. S1a). Sequence alignment revealed that PfAG5 has a conserved DNA-binding SRF-type TF domain, MADS-box domain and AG Motif (see Fig. S1b).
Overexpression of PfAG5 in Arabidopsis induces early flowering with abnormal flowers
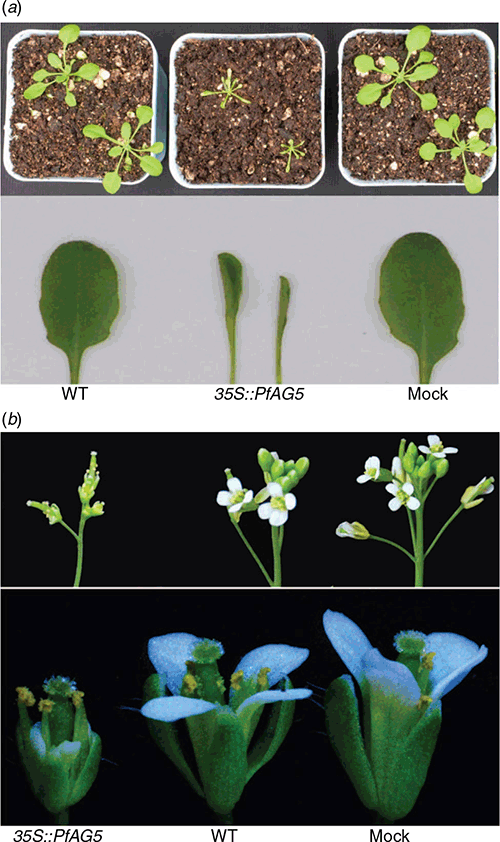
Phenotypes of 20 independent homozygous T3 transgenic lines were examined. Arabidopsis plants overexpressing PfAG5 flowered 15–16 days earlier and produced 5–6 fewer rosette leaves than wild type Arabidopsis (WT) and empty plasmid transgenic (Mock) plants under long day (LD) conditions (Table 2; Fig. 1a). Under short day (SD) conditions, PfAG5 transgenic plants flowered ~50–60 days earlier and produced 35–38 fewer rosette leaves than control plants (Table 2; Fig. 1b).

|
In contrast to control plants, PfAG5 transgenic Arabidopsis plants displayed abnormal growth as narrow and curly leaves in the seedling stage and very short petals (Fig. 2). No differences were observed in morphology of pods and seeds (see Fig. S2). However, plant height, pod number and seed yield were all lower in PfAG5 transgenic plants than in WT and Mock controls (Table 2; Fig. 1). Thus, expression of PfAG5 in Arabidopsis resulted in the early flowering phenotype with abnormal flowers and reduced seed set.
|
Expression pattern of PfAG5 and endogenous genes involved in flowering in transgenic Arabidopsis
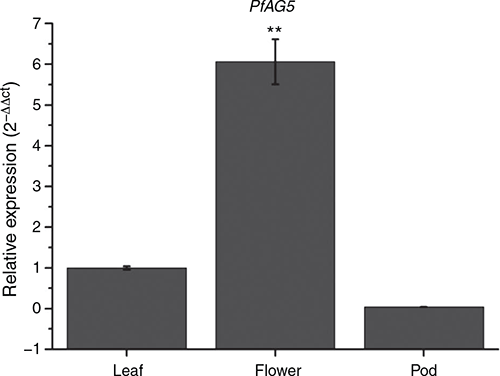
Expression pattern of pfAG5 in different tissues of transgenic Arabidopsis plants (35S::PfAG5) were analysed by real-time qPCR. Results showed that PfAG5 was constitutively expressed in leaves and flowers, and the expression level was significantly higher in leaves and flowers but lower in young pods than in controls (Fig. 3). This is similar to the MADS-box gene BdMADS33 of Brachypodium distachyon L., which showed weak expression signals in young seeds (Wei et al. 2014).
|
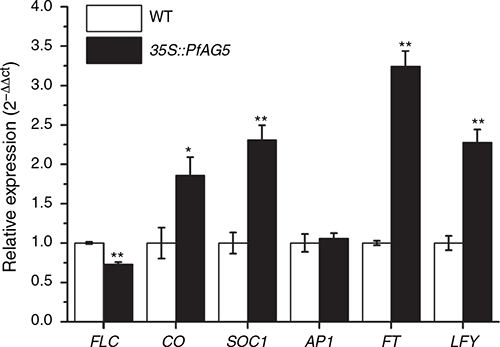
In PfAG5 transgenic Arabidopsis plants, higher expression of Arabidopsis endogenous genes such as CONSTANS (CO), SUPPRESSOR OF CONSTANS OVEREXPRESSION1 (SOC1), Flowering locus T (FT), LEAFY (LFY), and lower expression of the FLOWERING LOCUS C (FLC) gene, were found in comparison to WT, in the whole aboveground material at the flowering stage. No difference in APETALA1 (AP1) expression was found (Fig. 4).
|
Identification of PfAG5 interaction proteins in R P. fugax
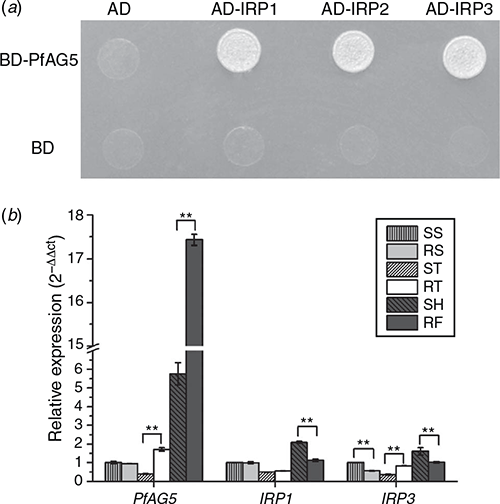
Three proteins interacting with PfAG5 in R P. fugax (named as IRP1, IRP2 and IRP3) were identified by the yeast two-hybrid system (Fig. 5a). IRP1 showed 91% amino acid sequence identity to A. tauschii ssp. tauschii FRIGIDA-like protein 3 (XM_020321692), IRP2, 82% identity to A. tauschii ssp. tauschii AGAMOUS-like MADS-box protein AGL66 (XM_020339220.1), and IRP3, 93% identity to MADS2 (AY198327.1) of Lolium perenne L. and fruitful-like MADS-box transcription factor (FUL2) (DQ792967.1) of Avena sativa L.
|
In the pull-down assay, two interaction proteins associated with flowering time regulation were identified: IRP1 (FRIGIDA-like protein (A0A453KI48, uniport protein ID)) with 100% identity to A. tauschii ssp. tauschii FRIGIDA-like protein 3 (XM_020321692) and IRP3 (wheat MADS-box transcription factor TaAGL29 (A0A3B6AZ67, uniport protein ID)) with 100% identify to L. perenne MADS2 (AY198327.1) (see Sequence File S1). Therefore, these two interacting proteins were confirmed by the two independent assays, and hence no further analysis for IRP2 was conducted.
Expression pattern of PfAG5 and the interacting proteins in P. fugax
The expression patterns of PfAG5 and the two interacting proteins were compared at different developmental stages (the seedling, tillering and flowering stages) and between R and S plants. The early flowering stage of the R plants corresponded to the heading stage of the S plants. The expression of PfAG5 in both S and R plants was significantly increased (by 5.7- and 10.2-fold, respectively) at the flowering stage as compared with the seedling stage. However, PfAG5 expression was significantly higher in R than the S plants at the tillering and flowering stages (Fig. 5b). For instance, the transcript level of PfAG5 reached 3-fold higher in the early flowering stage of R than that of S (while S still at the heading stage) (Fig. 5b). Conversely, the expression of IRP1 was 1.86-fold lower at the early flowering stage of R than S plants, while there was no significant differences at the seedling and tillering stages. However, there was no clear pattern in the expression of IRP3 (Fig. 5b).
Discussion
Flowering time of many weedy species is synchronised with that of crops (Tremblay and Colasanti 2007) so weeds often mature concurrently with crops. Due to herbicide and non-herbicide weed control selection pressures, changes in weed growth and reproduction have been evolved to adapt to the environment, including flowering time (Wang et al. 2010; Ashworth et al. 2016). For instance, in a glyphosate-resistant population of Conyza bonariensis (L.) Cronq. from Brazil, the first floral bud formation was observed 28 days earlier than the glyphosate-susceptible population (Kaspary et al. 2017). An ALS herbicide resistant population of Apera spica-venti (L.) Beauv. flowered 13 days earlier than the susceptible population at a certain crop density (Babineau et al. 2017). Panicles of the ACCase herbicide resistant (due to the 2041 mutation) Hordeum glaucum L. biotype emerged ~20 days earlier than that of susceptible biotype in the field (Shergill et al. 2016). The ACCase herbicide-resistant (due to the 1781 mutation) plants of Setaria viridis (L.) Beauv. flowered and matured earlier but producing 24% more seeds than the susceptible plants (Wang et al. 2010).
In our previous study, we found that an ACCase herbicide-resistant (due to the 2041 mutation) population of P. fugax reached the tiller and panicle emergence and seed shedding stages 6, 10 and 12 days, respectively, earlier than the S population (Tang et al. 2019). Working with this early flowering population, we identify an AGAMOUS-subfamily gene PfAG5 that is likely involved in early flowering in R population in this study. The AGAMOUS-like gene subfamily includes members involved in the specification of stamen, carpel and ovule. Phylogenetic analysis showed that PfAG5 groups into AGAMOUS-like clade in MADS-box genes of other plants and is homologous to the A. tauschii AG-type genes as WAG-2f and WAG-2 g (Wang et al. 2015), and T. aestivum TaAGL39 (Zhao et al. 2006). Overexpression of AGL79 in Arabidopsis was found to result in narrow leaf shape, fewer numbers of leaves and early flowering (Gao et al. 2018), which is consistent with observed phenotypes in PfAG5 transgenic Arabidopsis plants in the current study (Figs 1, 2a).
It is known in Arabidopsis that the floral integrator FT is a key regulator of flowering time (Komiya et al. 2008), and transcription factor CO activates the expression of FT (Tamaki et al. 2007), promoting early flowering. Indeed in our experiment found that the expression of FT (3.2-fold) and CO (1.9-fold) in transgenic Arabidopsis (35S::PfAG5) were significantly higher than in WT (Fig. 4). So, we speculate that the PfAG5 gene may promote the expression of CO in transgenic plants, and the high expression of CO in turn may activate the expression of FT. In contrast, the expression of FLC can represses the transcriptional activation of the floral integrator genes FT and SOC1 (Helliwell et al. 2006), hence inhibiting flowering. In this study, FLC expression was inhibited in PfAG5 transgenic plants, which may release repression of FT and SOC1 and promote flowering (Fig. 4). This can be tested by expressing PfAG5 in Arabidopsis FLC, SOC1 or FT knockout mutants.
Available genetic and molecular evidence suggests that LFY and AP1 together orchestrate the switch to flower formation and early events during flower morphogenesis by altering transcriptional programs (Winter et al. 2015). It is known that AP1 plays a role in differentiation of sepals and petals (Pabón-Mora et al. 2012). However, in the current study, no difference in the expression of AP1 was detected in PfAG5 transgenic Arabidopsis relative to WT plants at the flowering stage. In this case, we speculate that the morphological change in petals of PfAG5 transgenic plants may be related to genes other than AP1. Similarly, expression of DcaAP1, DcaAP2 and DcaAP3 in Dianthus caryphyllus L. (carnation) did not significantly differ in petals of different flower phenotypes (Wang et al. 2020). It was found that overexpression of LFY resulted in early flowering (Nilsson et al. 1998), likely via causing precocious development of flowers, converting the inflorescence shoot into a single terminal flower (Weigel and Nilsson 1995). So the high expression of LFY (2.3-fold) in PfAG5 transgenic Arabidopsis plants may be related to early flowering and abnormal flowers (Fig. 4). This conjecture can be further verified in a LFY knockout mutant line of Arabidopsis.
Plants with a shorter vegetative phase have less time to build up resource-gathering organs for seed production, so early flowering can be expected to decrease the reproductive output (Kralemann et al. 2018). Indeed, we found that overexpression of PfAG5 in Arabidopsis resulted in not only early flowering and flower morphological changes, but also significant decline in seed production (Table 2). Arabidopsis plants transformed with carnation AGAMOUS genes (DcaAGa, DcaAGb) also showed petal loss, short silique, and seed sterility (Wang et al. 2020), and this is similar to the flower phenotype of PfAG5 transgenic plants, except for seed viability. These results imply that PfAG5 gene is likely a flowering time promoter for the efficient expression of other flowering time regulatory genes, causing early flowering and abnormal flowers in P. fugax. However, what about the possible flowering regulation pathways of PfAG5 in R P. fugax population?
In this current study, we identified two PfAG5 interacting proteins (named as IRP1 and IRP3) with homology to FRI3 and FUL2 gene, respectively. In Arabidopsis, FRI causes later flowering by enhancing expression of the flowering repressor gene FLC (Michaels and Amasino 2001) and RNA silencing of FRI-like protein 3 mRNA (FRL3) induces early flowering in plants of Solanum lycopersicum L. (tomato) (Adkar-Purushothama et al. 2018). Despite the central role of FLC, most of the variations in flowering time have been correlated with natural allelic diversity of FRI (Michaels and Amasino 1999). For instance, among FRIGIDA orthologues, the BnaA3.FRI was tightly associated with flowering time variation in B. napus (Yi et al. 2018). In our study, the FRI-like gene (IRP1) was inhibited at the flowering stage of R P. fugax plants, contrary to the high expression of PfAG5 (Fig. 5b). Therefore, inhibition of the FRI gene (IRP1) caused by overexpression of the PfAG5 gene is likely responsible for early flowering in the R P. fugax population. As there was no clear trend in the expression of IRP3 (homology to FUL), and as AP1/FUL gene (FUL2) may play a general role in regulating flowering time in monocots (Preston and Kellogg 2006), we assume that IRP3 may not play a major role in flowering regulation in P. fugax.
Recently, we identified another gene PfMADS16 regulating early flowering and seed development in P. fugax (Zhou et al. 2020). The role of PfAG5 identified in the current study is different to PfMADS16 as the former is not involved in seed development and interacts with different proteins. However, the two genes both play roles in flowering time regulation.
How has R P. fugax evolved higher expression of the flowering genes (such as PfMADS16 and PfAG5) compared with the S population? Or how is early flowering trait correlated with herbicide resistance? According to Baucom (2019), an alteration in a life-history trait in a resistant lineage can be caused by the resistance allele itself (a pleiotropic effect) or could result from genetic linkage between the resistance allele and genes that control the life-history trait. However, the herbicide-resistance allele in the R P. fugax population was a point Ile-2041-Asn mutation in the ACCase gene (Tang et al. 2014), and there has no evidence showing direct correlation of ACCase with flowering time regulation. Rather, genetic linkage between the resistance ACCase allele and flowering genes may be possible. Standing genetic variations in flowering time may exist in P. fugax populations, herbicide application may not only have selected for herbicide resistance but also by chance for plants with higher expression of flowering genes. Alternatively, higher expression of flowering genes can be induced by herbicide application and becomes fixed overtime by such as epigenetic mechanisms in plants having the herbicide resistance allele. The latter can be examined by methylation analysis of the major candidate flowering genes. Nevertheless, early flowering (likely early pod shedding) will be a disadvantage for later season weed control strategies aiming to reduce seed bank in the soil via mechanic seed capture at harvest. With herbicide resistance becoming an increasing problem, adoption for non-chemical weed control (e.g. mechanical weed seed harvester and destructor) is on the increase, and hence weed biotypes adapting to this practice will eventually evolved.
Data availability statement
The identified PfAG5 sequence in this paper has been deposited in the GenBank (accession number MK559453). Experimental materials are available upon request by qualified researchers to the corresponding author.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was financially supported by the National Key Research and Development Program of China (2016YFD0201305), the National Natural Science Foundation of China (31501658) and the Scientific and Research team of Anhui Academy of Agricultural Sciences (2020YL075). The funders had no role in the design of the study and collection, analysis and interpretation of data and in writing the manuscript.
References
Adkar-Purushothama CR, Sano T, Perreault JP (2018) Viroid-derived small RNA induces early flowering in tomato plants by RNA silencing. Molecular Plant Pathology 19, 2446–2458.| Viroid-derived small RNA induces early flowering in tomato plants by RNA silencing.Crossref | GoogleScholarGoogle Scholar | 30011126PubMed |
Ashworth MB, Walsh MJ, Flower KC, Vila-Aiub MM, Powles SB (2016) Directional selection for flowering time leads to adaptive evolution in Raphanus raphanistrum (Wild radish). Evolutionary Applications 9, 619–629.
| Directional selection for flowering time leads to adaptive evolution in Raphanus raphanistrum (Wild radish).Crossref | GoogleScholarGoogle Scholar | 27099626PubMed |
Babineau M, Mathiassen SK, Kristensen M, Kudsk P (2017) Fitness of ALS-Inhibitors herbicide resistant population of Loose Silky Bentgrass (Apera spica-venti). Frontiers in Plant Science 8, 1660
| Fitness of ALS-Inhibitors herbicide resistant population of Loose Silky Bentgrass (Apera spica-venti).Crossref | GoogleScholarGoogle Scholar | 28993787PubMed |
Barrett SH (1983) Crop mimicry in weeds. Economic Botany 37, 255–282.
| Crop mimicry in weeds.Crossref | GoogleScholarGoogle Scholar |
Baucom RS (2019) Evolutionary and ecological insights from herbicide-resistant weeds: what have we learned about plant adaptation, and what is left to uncover? New Phytologist 223, 68–82.
| Evolutionary and ecological insights from herbicide-resistant weeds: what have we learned about plant adaptation, and what is left to uncover?Crossref | GoogleScholarGoogle Scholar |
Becker A, Theißen G (2003) The major clades of MADS-box genes and their role in the development and evolution of flowering plants. Molecular Phylogenetics and Evolution 29, 464–489.
| The major clades of MADS-box genes and their role in the development and evolution of flowering plants.Crossref | GoogleScholarGoogle Scholar | 14615187PubMed |
de Moura SM, Artico S, Lima C, Nardeli SM, Berbel A, Oliveira-Neto OB, Grossi-de-Sá MF, Ferrándiz C, Madueño F, Alves-Ferreira M (2017) Functional characterization of AGAMOUS-subfamily members from cotton during reproductive development and in response to plant hormones. Plant Reproduction 30, 19–39.
| Functional characterization of AGAMOUS-subfamily members from cotton during reproductive development and in response to plant hormones.Crossref | GoogleScholarGoogle Scholar | 28176007PubMed |
Dou WF, Qi JJ, Hu AH, Chen SC, Peng AH, Xu LZ, Lei TG, Yao LX, He YR, Li Q (2019) Screening of Interacting Proteins of Anti-Canker Transcription Factor Cs BZIP40 in Citrus by GST Pull-Down Combined with LC-MS/MS. Zhongguo Nong Ye Ke Xue 52, 2243–2255.
Dreni L, Kater MM (2014) MADS reloaded: evolution of the AGAMOUS subfamily genes. New Phytologist 201, 717–732.
| MADS reloaded: evolution of the AGAMOUS subfamily genes.Crossref | GoogleScholarGoogle Scholar |
Favaro R, Pinyopich A, Battaglia R, Kooiker M, Borghi L, Ditta G, Yanofsky MF, Kater MM, Colombo L (2003) MADS-Box protein complexes control carpel and ovule development in Arabidopsis. The Plant Cell 15, 2603–2611.
| MADS-Box protein complexes control carpel and ovule development in Arabidopsis.Crossref | GoogleScholarGoogle Scholar | 14555696PubMed |
Gao R, Wang Y, Gruber MY, Hannoufa A (2018) miR156/SPL10 Modulates Lateral Root Development, Branching and Leaf Morphology in Arabidopsis by Silencing AGAMOUS- LIKE 79. Frontiers in Plant Science 8, 2226
| miR156/SPL10 Modulates Lateral Root Development, Branching and Leaf Morphology in Arabidopsis by Silencing AGAMOUS- LIKE 79.Crossref | GoogleScholarGoogle Scholar | 29354153PubMed |
Helliwell CA, Wood CC, Robertson M, James Peacock W, Dennis ES (2006) The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. The Plant Journal 46, 183–192.
| The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex.Crossref | GoogleScholarGoogle Scholar | 16623882PubMed |
Hill EC, Renner KA, VanGessel MJ, Bellinder RR, Scott BA (2016) Late-Season Weed Management to Stop Viable Weed Seed Production. Weed Science 64, 112–118.
| Late-Season Weed Management to Stop Viable Weed Seed Production.Crossref | GoogleScholarGoogle Scholar |
Kaspary TE, Lamego FP, Cutti L, de Morais Aguiar AC, Gonsiorkiewicz R, Carlos A, Basso CJ (2017) Growth, phenology, and seed viability between glyphosate- resistant and glyphosate-susceptible hairy fleabane. Bragantia 76, 92–101.
| Growth, phenology, and seed viability between glyphosate- resistant and glyphosate-susceptible hairy fleabane.Crossref | GoogleScholarGoogle Scholar |
Kazan K, Lyons R (2016) The link between flowering time and stress tolerance. Journal of Experimental Botany 67, 47–60.
| The link between flowering time and stress tolerance.Crossref | GoogleScholarGoogle Scholar | 26428061PubMed |
Kleemann SGL, Gurjeet SG (2013) Seed Dormancy and Seedling Emergence in Ripgut Brome (Bromus diandrus) Populations in Southern Australia. Weed Science 61, 222–229.
| Seed Dormancy and Seedling Emergence in Ripgut Brome (Bromus diandrus) Populations in Southern Australia.Crossref | GoogleScholarGoogle Scholar |
Komiya R, Ikegami A, Tamaki S, Yokoi S, Shimamoto K (2008) Hd3a and RFT1 are essential for flowering in rice. Development 135, 767–774.
| Hd3a and RFT1 are essential for flowering in rice.Crossref | GoogleScholarGoogle Scholar | 18223202PubMed |
Kralemann LEM, Scalone R, Andersson L, Hennig L (2018) North European invasion by common ragweed is associated with early flowering and dominant changes in FT/TFL1 expression. Journal of Experimental Botany 69, 2647–2658.
| North European invasion by common ragweed is associated with early flowering and dominant changes in FT/TFL1 expression.Crossref | GoogleScholarGoogle Scholar | 29547904PubMed |
Kramer EM, Jaramillo MA, Di Stilio VS (2004) Patterns of gene duplication and functional evolution during the diversification of the AGAMOUS Subfamily of MADS Box genes in Angiosperms. Genetics 166, 1011–1023.
| Patterns of gene duplication and functional evolution during the diversification of the AGAMOUS Subfamily of MADS Box genes in Angiosperms.Crossref | GoogleScholarGoogle Scholar | 15020484PubMed |
Masiero S, Colombo L, Grini PE, Schnittger A, Kater MM (2011) The Emerging Importance of Type I MADS Box Transcription Factors for Plant Reproduction. The Plant Cell 23, 865–872.
| The Emerging Importance of Type I MADS Box Transcription Factors for Plant Reproduction.Crossref | GoogleScholarGoogle Scholar | 21378131PubMed |
Michaels SD, Amasino RM (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. The Plant Cell 11, 949–956.
| FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering.Crossref | GoogleScholarGoogle Scholar | 10330478PubMed |
Michaels SD, Amasino RM (2001) Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. The Plant Cell 13, 935–941.
| Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization.Crossref | GoogleScholarGoogle Scholar | 11283346PubMed |
Nilsson O, Lee I, Blazquez MA, Weigel D (1998) Flowering- time genes modulate the response to LEAFY activity. Genetics 150, 403–410.
Owen MJ, Martinez NJ, Powles SB (2014) Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt. Weed Research 54, 314–324.
| Multiple herbicide-resistant Lolium rigidum (annual ryegrass) now dominates across the Western Australian grain belt.Crossref | GoogleScholarGoogle Scholar |
Pabón-Mora N, Ambrose BA, Litt A (2012) Poppy APETALA1/FRUITFULL orthologs control flowering time, branching, perianth identity, and fruit development. Plant Physiology 158, 1685–1704.
| Poppy APETALA1/FRUITFULL orthologs control flowering time, branching, perianth identity, and fruit development.Crossref | GoogleScholarGoogle Scholar | 22286183PubMed |
Powles SB, Yu Q (2010) Evolution in action: Plants resistant to herbicides. Annual Review of Plant Biology 61, 317–347.
| Evolution in action: Plants resistant to herbicides.Crossref | GoogleScholarGoogle Scholar | 20192743PubMed |
Preston JC, Kellogg EA (2006) Reconstructing the evolutionary history of paralogous APETALA1/FRUITFULL-like genes in grasses (Poaceae). Genetics 174, 421–437.
| Reconstructing the evolutionary history of paralogous APETALA1/FRUITFULL-like genes in grasses (Poaceae).Crossref | GoogleScholarGoogle Scholar | 16816429PubMed |
Shergill LS, Boutsalis P, Preston C, Gill GS (2016) Fitness costs associated with 1781 and 2041 ACCase-mutant alleles conferring resistance to herbicides in Hordeum glaucum Steud. Crop Protection 87, 60–67.
| Fitness costs associated with 1781 and 2041 ACCase-mutant alleles conferring resistance to herbicides in Hordeum glaucum Steud.Crossref | GoogleScholarGoogle Scholar |
Takeno K (2016) Stress-induced flowering: the third category of flowering response. Journal of Experimental Botany 67, 4925–4934.
| Stress-induced flowering: the third category of flowering response.Crossref | GoogleScholarGoogle Scholar | 27382113PubMed |
Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316, 1033–1036.
| Hd3a protein is a mobile flowering signal in rice.Crossref | GoogleScholarGoogle Scholar | 17446351PubMed |
Tang W, Zhou F, Chen J, Zhou X (2014) Resistance to ACCase-inhibiting herbicides in an Asia minor bluegrass (Polypogon fugax) population in China. Pesticide Biochemistry and Physiology 108, 16–20.
| Resistance to ACCase-inhibiting herbicides in an Asia minor bluegrass (Polypogon fugax) population in China.Crossref | GoogleScholarGoogle Scholar | 24485310PubMed |
Tang W, Xu X, Shen G, Chen J (2015) Effect of Environmental Factors on Germination and Emergence of Aryloxyphenoxy Propanoate Herbicide-Resistant and -Susceptible Asia Minor Bluegrass (Polypogon fugax). Weed Science 63, 669–675.
| Effect of Environmental Factors on Germination and Emergence of Aryloxyphenoxy Propanoate Herbicide-Resistant and -Susceptible Asia Minor Bluegrass (Polypogon fugax).Crossref | GoogleScholarGoogle Scholar |
Tang W, Chen J, Yu X, Zhang J, Lu Y (2019) Growth, Fecundity and Competition between Aryloxyphenoxypropionate Resistant and Susceptible Asia Minor Bluegrass (Polypogon fugax). Weed Science 67, 546–551.
| Growth, Fecundity and Competition between Aryloxyphenoxypropionate Resistant and Susceptible Asia Minor Bluegrass (Polypogon fugax).Crossref | GoogleScholarGoogle Scholar |
Tremblay R, Colasanti J (2007) Floral Induction, Annual Plant Reviews Volume. 20. Flowering and its Manipulation, ed. by Charles A, Blackwell Publishing Ltd, Oxford, pp. 28–62.
Wada KC, Takeno K (2010) Stress-induced flowering. Plant Signaling & Behavior 5, 944–947.
| Stress-induced flowering.Crossref | GoogleScholarGoogle Scholar |
Wang T, Picard JC, Tian X, Darmency H (2010) A herbicide-resistant ACCase 1781 Setaria mutant shows higher fitness than wild type. Heredity 105, 394–400.
| A herbicide-resistant ACCase 1781 Setaria mutant shows higher fitness than wild type.Crossref | GoogleScholarGoogle Scholar | 20087387PubMed |
Wang QH, Yang ZJ, Wei SH, Jiang ZY, Yang YF, Hu ZS, Sun QX, Peng ZS (2015) Molecular cloning, characterization and expression analysis of WAG-1 in the pistillody line of common wheat. Genetics and Molecular Research 14, 12455–12465.
| Molecular cloning, characterization and expression analysis of WAG-1 in the pistillody line of common wheat.Crossref | GoogleScholarGoogle Scholar | 26505395PubMed |
Wang Q, Dan N, Zhang X, Lin S, Bao M, Fu X (2020) Identification, Characterization and Functional Analysis of C–Class Genes Associated with Double Flower Trait in Carnation (Dianthus caryphyllus L.). Plants 9, 87
| Identification, Characterization and Functional Analysis of C–Class Genes Associated with Double Flower Trait in Carnation (Dianthus caryphyllus L.).Crossref | GoogleScholarGoogle Scholar |
Wei B, Zhang RZ, Guo JJ, Liu DM, Li AL, Fan RC, Mao L, Zhang XQ (2014) Genome-Wide Analysis of the MADS-Box Gene Family in Brachypodium distachyon. PLoS One 9, e84781
| Genome-Wide Analysis of the MADS-Box Gene Family in Brachypodium distachyon.Crossref | GoogleScholarGoogle Scholar | 25490774PubMed |
Wei J, Liu D, Liu G, Tang J, Chen Y (2016) Molecular Cloning, Characterization, and Expression of MiSOC1: A Homolog of the Flowering Gene SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 from Mango (Mangifera indica L). Frontiers in Plant Science 7, 1758
| Molecular Cloning, Characterization, and Expression of MiSOC1: A Homolog of the Flowering Gene SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 from Mango (Mangifera indica L).Crossref | GoogleScholarGoogle Scholar | 27965680PubMed |
Weigel D, Nilsson O (1995) A developmental switch sufficient for flower initiation in diverse plants. Nature 377, 495–500.
| A developmental switch sufficient for flower initiation in diverse plants.Crossref | GoogleScholarGoogle Scholar | 7566146PubMed |
Winter CM, Yamaguchi N, Wu MF, Wagner D (2015) Transcriptional programs regulated by both LEAFY and APETALA1 at the time of flower formation. Physiologia Plantarum 155, 55–73.
| Transcriptional programs regulated by both LEAFY and APETALA1 at the time of flower formation.Crossref | GoogleScholarGoogle Scholar | 26096587PubMed |
Yi L, Chen C, Yin S, Li H, Li Z, Wang B, King GJ, Wang J, Liu K (2018) Sequence variation and functional analysis of a FRIGIDA orthologue (BnaA3.FRI) in Brassica napus. BMC Plant Biology 18, 32
| Sequence variation and functional analysis of a FRIGIDA orthologue (BnaA3.FRI) in Brassica napus.Crossref | GoogleScholarGoogle Scholar | 29433434PubMed |
Zhao T, Ni Z, Dai Y, Yao Y, Nie X, Sun Q (2006) Characterization and expression of 42 MADS-box genes in wheat (Triticum aestivum L.). Molecular Genetics and Genomics 276, 334–350.
| Characterization and expression of 42 MADS-box genes in wheat (Triticum aestivum L.).Crossref | GoogleScholarGoogle Scholar | 16858583PubMed |
Zhou FY, Zhang Y, Tang W, Wang M, Gao TC (2017) Transcriptomics analysis of the flowering regulatory genes involved in the herbicide resistance of Asia minor bluegrass (Polypogon fugax). BMC Genomics 18, 953
| Transcriptomics analysis of the flowering regulatory genes involved in the herbicide resistance of Asia minor bluegrass (Polypogon fugax).Crossref | GoogleScholarGoogle Scholar |
Zhou FY, Yu Q, Zhang Y, Yao CC, Han YJ (2020) StMADS11 subfamily gene PfMADS16 from Polypogon fugax regulates early flowering and seed development. Frontiers in Plant Science 11, 525
| StMADS11 subfamily gene PfMADS16 from Polypogon fugax regulates early flowering and seed development.Crossref | GoogleScholarGoogle Scholar | 32457775PubMed |