Does the C4 plant Trianthema portulacastrum (Aizoaceae) exhibit weakly expressed crassulacean acid metabolism (CAM)?
Klaus Winter A C , Milton Garcia A , Aurelio Virgo A , Jorge Ceballos A and Joseph A. M. Holtum
A C , Milton Garcia A , Aurelio Virgo A , Jorge Ceballos A and Joseph A. M. Holtum  A B
A B
A Smithsonian Tropical Research Institute, PO Box 0843-03092, Balboa, Ancón, Republic of Panama.
B College of Science and Engineering, James Cook University, Townsville, Qld 4811, Australia.
C Corresponding author. Email: winterk@si.edu
Functional Plant Biology 48(7) 655-665 https://doi.org/10.1071/FP20247
Submitted: 13 August 2020 Accepted: 6 October 2020 Published: 20 November 2020
Journal Compilation © CSIRO 2021 Open Access CC BY-NC-ND
Abstract
We examined whether crassulacean acid metabolism (CAM) is present in Trianthema portulacastrum L. (Aizoaceae), a pantropical, salt-tolerant C4 annual herb with atriplicoid-type Kranz anatomy in leaves but not in stems. The leaves of T. portulacastrum are slightly succulent and the stems are fleshy, similar to some species of Portulaca, the only genus known in which C4 and CAM co-occur. Low- level nocturnal acidification typical of weakly expressed, predominantly constitutive CAM was measured in plants grown for their entire life-cycle in an outdoor raised garden box. Acidification was greater in stems than in leaves. Plants showed net CO2 uptake only during the light irrespective of soil water availability. However, nocturnal traces of CO2 exchange exhibited curved kinetics of reduced CO2 loss during the middle of the night consistent with low-level CAM. Trianthema becomes the second genus of vascular land plants in which C4 and features of CAM have been demonstrated to co-occur in the same plant and the first C4 plant with CAM-type acidification described for the Aizoaceae. Traditionally the stems of herbs are not sampled in screening studies. Small herbs with mildly succulent leaves and fleshy stems might be a numerically significant component of CAM biodiversity.
Keywords: CAM evolution, CO2 assimilation, C4 photosynthesis, facultative CAM, Kranz anatomy, Sesuvioideae, stem photosynthesis, Trianthema portulacastrum.
Introduction
C4 photosynthesis and crassulacean acid metabolism (CAM) are modifications of the ancestral C3 photosynthetic pathway that have evolved independently in a wide range of families and genera (Smith and Winter 1996; Sage 2016). There are approximately 8100 known C4 species, which are dispersed across 418 genera in 19 angiosperm families (Sage and Sultmanis 2016), whereas the roughly 16 000 CAM taxa are in 400+ genera from 37 families of angiosperms, gymnosperms, ferns and lycopsids (Smith and Winter 1996; Yang et al. 2015; Winter et al. 2020a).
In both C4 and CAM plants, the structure and function of photosynthetic organs has been modified from the C3 template creating CO2-concentrating mechanisms that suppress photorespiration. In C4 plants which, like C3 plants, exhibit net CO2 uptake during the light only, suppression of photorespiration has led to enhanced photosynthetic CO2 uptake in warm climates at reduced water cost. Water-use efficiency is further enhanced in CAM plants as water loss by transpiration is reduced when CO2 is taken up during the cool of the night and stomata close during most of the day (Winter et al. 2005). While the CAM cycle is associated with nocturnal increases and diurnal decreases in the organic acid content of chloroplast-containing tissues, 24-h fluctuations in H+ are typically absent from C3 and C4 tissues (Winter 2019). Despite the relatively frequent appearance of C4 and CAM across the phylogeny of plants, only four families of vascular terrestrial plants contain C4 species as well as CAM species: Aizoaceae, Asteraceae, Euphorbiaceae, and Portulacaceae (Smith and Winter 1996; Edwards and Ogburn 2012; Sage 2016).
Portulaca L. (Portulacaceae, Caryophyllales) is the only genus of land plants in which C4 and CAM are known to co-occur in the same plant and, indeed, in the same leaf (Koch and Kennedy 1980). The genus is currently considered to contain 150 species (POWO 2020), overwhelmingly annual succulent-leaved herbs, allocated across six clades (Ocampo et al. 2013). Five clades contain C4 taxa and one contains species with C3-C4 intermediate characteristics. CAM has been reported in nine species from five of the six clades (Koch and Kennedy 1980; Kraybill and Martin 1996; Guralnick and Jackson 2001; Guralnick et al. 2002; Holtum et al. 2017a; Winter and Holtum 2017; Winter 2019) including the clade with C3-C4 intermediates (Winter et al. 2019b). In all cases reported to-date, the expression of CAM in leaves of Portulaca with C4 photosynthesis has been facultative; CAM is expressed in leaves that have been water-stressed but is absent from leaves of well-watered plants. In the case of P. oleracea, in which CAM expression in leaves is facultative but its expression in stems is constitutive with a facultative component, the transcript abundance of genes putatively associated with C4 and CAM has been studied in well-watered and in drought-stressed plants (Ferrari et al. 2020).
Within the Aizoaceae, a family within the order Caryophyllales of close to 1800 species and 119 genera (Hernández-Ledesma et al. 2015; Klak et al. 2017; POWO 2020), C3 and CAM photosynthesis are present in three subfamilies, Mesembryanthemoideae, Ruschioideae and Sesuvioideae (Ting 1989; Smith and Winter 1996; Winter et al. 2019a). C4 photosynthesis is known in 31 species of the sub-family Sesuvioideae (Bohley et al. 2017) where it occurs in the genera Trianthema (21 of ~30 species), Sesuvium (5 of 13 species) and Zaleya (all species). Within the Sesuvioideae, CAM has been reported in two species, the pantropical, predominately C3-exhibiting Sesuvium portulacastrum (Ting 1989; Winter et al. 2019a) and in S. maritimum (Martin et al. 1982).
Here we report the presence of low-level CAM-type nocturnal acidification in a C4 member of the Sesuvioideae, Trianthema portulacastrum. Curved patterns of nocturnal net CO2 loss indicated temporarily enhanced refixation of CO2 at night, consistent with low-level CAM in this species.
Methods
Plant material
A salt-tolerant annual that has Kranz anatomy in leaves but not in stems, Trianthema portulacastrum L. is a fast-growing, succulent-leaved herb that germinates during the wet season in the seasonally dry tropics of Panama, forming prostrate mats or clumps up to 50+ cm in diameter, before it senesces and dies during the dry season. Of uncertain geographical origin, it is widespread in the tropics and subtropics of western and south-east Asia, Africa, America and Australia (ALA 2020) where it inhabits beach dunes, moist or seasonally-dry open wetlands including alkaline flats, clay pans and playa lakes. It is often a weed of disturbed areas including gardens, irrigated soils and road-sides.
Seedlings of T. portulacastrum were obtained from the Sarigua National Park, Azuero Peninsula, Republic of Panama (8.013348°N, –80.485658°W), where the species coexists with Sesuvium portulacastrum (L.) L. (Winter et al. 2019a).
Outdoor experiment
In October 2018, the second half of the rainy season, 10 seedlings (diameter of ~5 cm each) were planted in a mixture of 50% (v/v) forest soil: 50% (v/v) sea-sand (Novey, Panama) in a 1.2 × 1.2 × 0.38 m raised garden box (Hummert International) at the Smithsonian Tropical Research Institute, Santa Cruz Experimental Research Facility, Gamboa, Republic of Panama (9.120027°N, –79.702017°W) (Fig. 1). Plants were exposed to full natural solar radiation and received natural rainfall. From mid-December 2018 onwards, samples of leaves and stems were taken at dusk (17:30 – 18:00 hours) and dawn (07:00 hours) for fresh and dry mass determinations and for acid analysis at weekly intervals until the plants senesced and died during the dry season at the end of February 2019. Self-sown seeds germinated following the beginning of the wet-season rains in 2019. Starting at the end of May 2019, once again leaves and stems were sampled at dusk and dawn for fresh mass, dry mass and acid analysis at weekly to biweekly intervals. Samples were weighed for fresh mass determination and stored in liquid nitrogen to be processed for determination of dry mass and measurements of titratable acidity as described below.

|
Pot experiments
Throughout 2018 and 2019, experiments were conducted with potted plants to study titratable acidity changes and gas-exchange responses during wet–dry-wet cycles. Plants were grown in terracotta pots (0.5 or 3.0 L) containing 2/3 (v/v) potting mix (Miracle-Gro Lawn Products) and 1/3 quartz sand (Novey). Plants were maintained at the Tupper Centre of the Smithsonian Tropical Research Institute, Panama City (8.962938°N, –79.702017°W) under either full solar radiation or underneath rain-shelters at 70% natural light. Growth conditions are specified in the corresponding figure legends. As in the outdoor experiments, for acidity measurements mature leaves and stems excised from plants at dusk and dawn were weighed for fresh mass determination and frozen in liquid nitrogen. Prior to freezing, leaf area was determined using a LI-3100 leaf area meter (LI-COR Biosciences).
Titratable acidity and dry mass
Samples were transferred from liquid nitrogen to a freeze-drier (Freezone 4.5, Labconco). After 72 h samples were reweighed for dry mass determination. Freeze-dried tissue was extracted sequentially in boiling 50% ethanol and in water (Winter and Holtum 2017). Extracts were titrated with 5 mM KOH to pH 6.5
Net CO2 exchange
Twenty-four hour CO2 exchange patterns by intact shoots of whole plants or intact attached branches were studied during wet–dry-wet cycles. In two of the three experiments shown in the results section, the shoot of a small seedling was enclosed inside a Perspex cuvette (internal dimensions 11 × 11 × 16 cm) that rested on the 0.5 L terracotta pot in which the plant grew. The roots and the pot remained outside the cuvette. After initial daily irrigation with water, 9–11 day drought treatments were imposed by withholding irrigation until net CO2 uptake during the light period had decreased markedly, after which plants were rewatered daily. In another experiment, an intact attached branch of a plant was enclosed into a Walz GWK-3M gas exchange cuvette (Walz GmbH).
The gas-exchange cuvettes, pots and plants were located inside a controlled environment chamber (GC15, EGC) operating under 12 h light (28°C)/12 h dark (22°C) cycles. Illumination was supplied by a LED grow light (SS-GU300-w, Sunshine Systems). Photon flux density is specified in the corresponding figure legends. The cuvette was supplied with ambient air sourced 16 m above ground level and passed through a 2 m3 buffer at a flow rate of 2.3 L min–1. Net CO2 exchange was measured in a flow-through gas-exchange system consisting of Walz components and a LI-6252 CO2 analyser (LI-COR Biosciences) (Holtum and Winter 2003).
Anatomy
Cross-sections of fully expanded healthy leaves (middle between base and tip, and between edge and midrib) and mature stems were prepared either by hand using double edge breakable razor blades or, in some cases, tissue was fixed in 4% (v/v) glutaraldehyde for 48 h at 7°C before sectioning in a cryostat microtome (CM1860, Leica). Chlorophyll auto-fluorescence (682 nm) was examined using a confocal microscope (FV3000, Olympus) with an integrated camera system.
Results
Titratable acidity
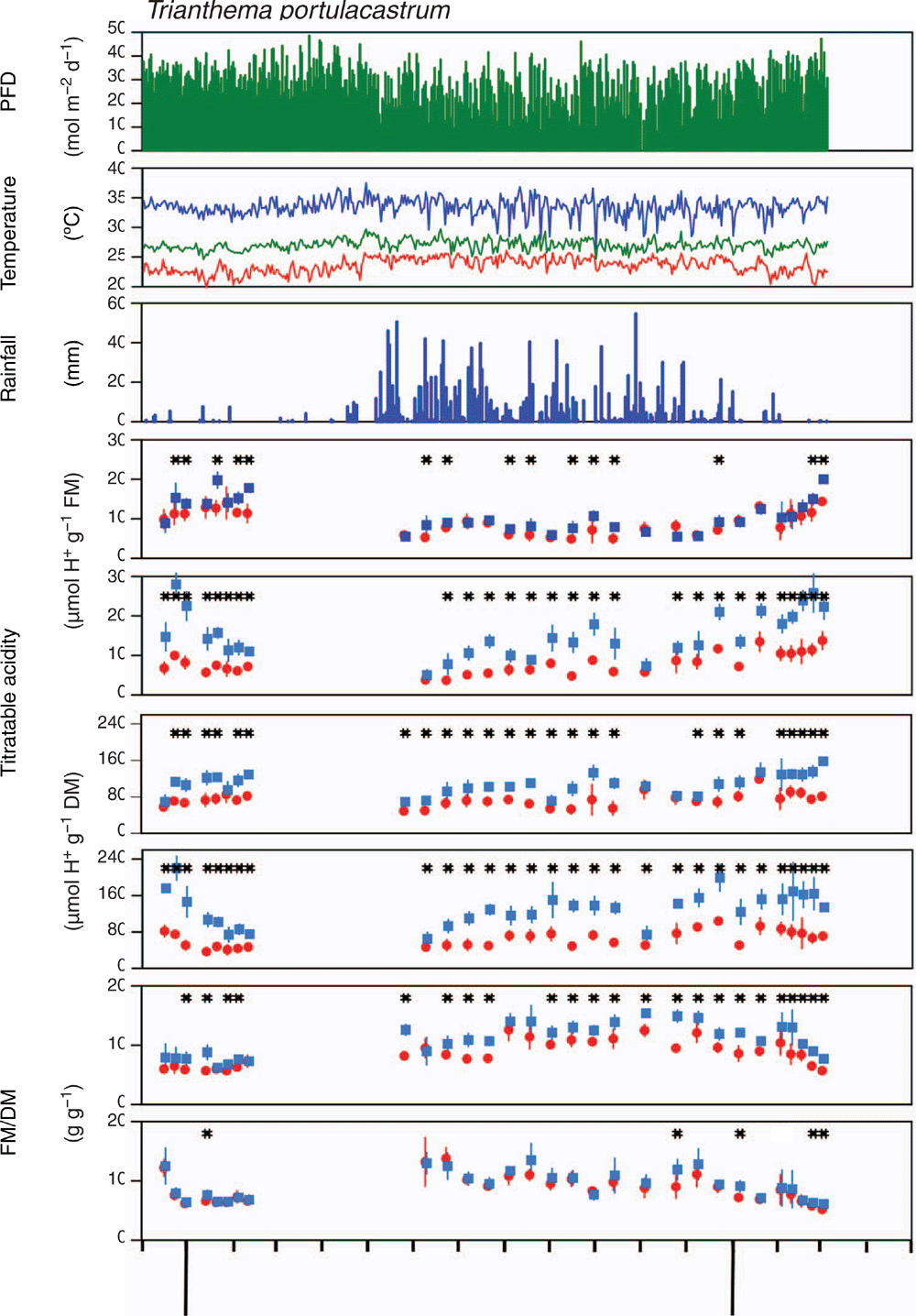
Variation in dusk/dawn titratable acidity levels was determined in outdoor-grown plants over ~15 months. The study period included one full wet season and two dry seasons (Fig. 2a–c). Consistent with the annual life-form of T. portulacastrum, plants died during the latter part of each dry season. Reduced cloud cover during the dry season led to higher daily photon flux densities (Fig. 2a). As is typical for tropical rainforest climates, seasonal variations in temperature at the study site were small (Fig. 2b).
Throughout the course of the experiment, and irrespective of season, leaf H+ values, when expressed on a fresh mass basis, were significantly greater at dawn than at dusk for 15 of 30 measurements (Fig. 2d). The frequency of significantly greater dawn levels increased to 25 when tissue acidity was expressed on a dry mass basis (Fig. 2f), thereby correcting for diurnal decreases in leaf water content and thus reduced fresh mass: dry mass ratios (Fig. 2h). Titratable acidity of stems was consistently greater at dawn than at dusk on 27 of 29 days when acidity was expressed per unit of fresh mass, and on all days when expressed per unit of dry mass (Fig. 2e, g). On the days with significant overnight acidification, the fold-increase (dawn: dusk ratio of H+ per unit of dry mass) was greater in stems (2.03 ± 0.48, mean ± s.d., n = 29) than in leaves (1.55 ± 0.19, n = 25) (P = <0.001). There was no obvious seasonal change in the degree of nocturnal acidification of both leaves and stems (Fig. 2f, g). The H+ values were similar during the wet and dry seasons.
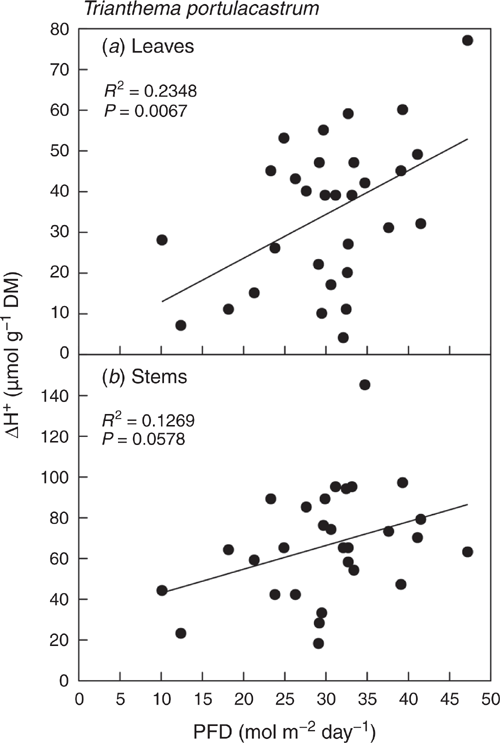
Linear regressions showed that nocturnal acidification of leaves and stems correlated positively with integrated PFD of the previous day (Fig. 3). The relationship was significant for leaves (P = <0.01) and close to significant for stems (P = <0.06).
|
In a pot experiment under full solar radiation at STRI’s Tupper Centre during the 2018 dry season, plants were exposed to a wet–dry-wet cycle. Small but significant nocturnal acidification per unit dry mass was detected in leaves of drought-stressed plants, whereas nocturnal acidification was consistently observed in stems of both watered and drought-stressed plants irrespective of whether the data was expressed on a dry mass or fresh mass basis (data not shown).
CO2 gas-exchange
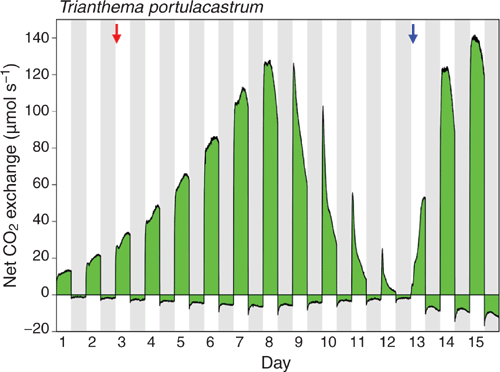
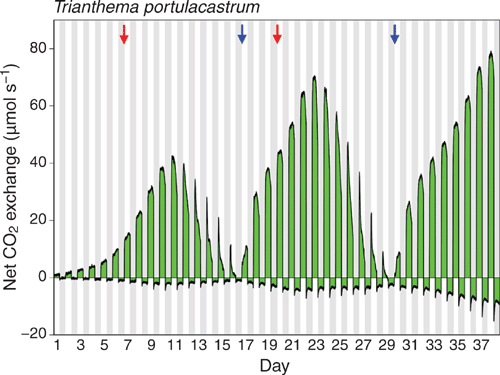
Sixty-eight days of CO2 gas-exchange were monitored for three separate plants. Figures 4 and 5 depict the results for two plants, the shoots of which developed from seedlings inside the gas-exchange cuvette. One plant was exposed to a single watering-drought-rewatering cycle and a second to two watering-drought-rewatering cycles.
|
|
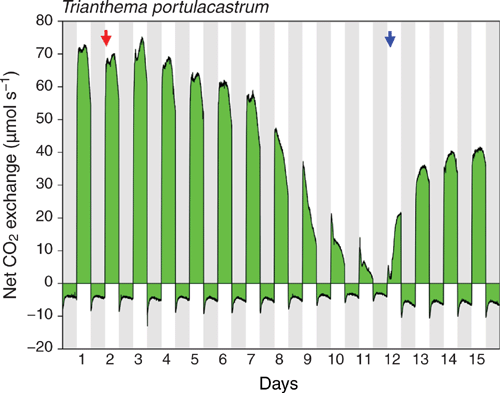
Under both well-watered and drought conditions, net CO2 uptake was restricted to the light during all treatments. In the experiment depicted in Figure 4, CO2 efflux was initially relatively constant throughout the night following an initial slight overshoot and period of equilibration to night temperature (22°C) which was 6°C below day temperature (28°C). Following cessation of watering on day 3, a small curvature in the nocturnal CO2 release pattern was detectable with lower rates in the middle than at the beginning and end of the dark period. CO2 uptake during the day and CO2 loss at night continued to increase as the shoot continued to grow utilising water in the pot. From day 9 onwards water-deficit stress developed as indicated by the progressive daily reduction in both CO2 uptake during the day and CO2 loss at night. Daytime CO2 exchange developed a prominent morning peak of uptake. At all times, nocturnal CO2 exchange remained below the CO2 compensation point. Following rewatering on day 13, a recovery of CO2 uptake in the light to pre-drought rates was observed within 3 days. Concomitant with the recovery in rates of daytime CO2 uptake, nocturnal respiratory CO2 loss increased to rates greater than observed before drought. The respiratory trace also developed a pronounced curvature that persisted.
The gas-exchange trace for the plant that was exposed to two watering-drought-rewatering cycles (Fig. 5) was similar to that for the plant exposed to a single cycle (Fig. 4) except that the absolute rates of CO2 uptake were lower as it was a smaller plant. In Figure 5, the curvature in the nocturnal respiratory trace that was noted in particular following rewatering of the plant monitored in Figure 4, was again observed throughout most of the 38 day experiment, especially during the entire second watering-drought-rewatering cycle.
The observations for a branch of a third plant subjected to a single watering-drought-rewatering cycle are provided in Figure 6. Net CO2 uptake in the light started to decline almost immediately when watering was stopped. Again, nocturnal net CO2 exchange never crossed the compensation line to become positive, but during all 15 dark periods of the experiment a distinct temporal reduction of nocturnal net CO2 loss occurred.
|
Leaf and stem anatomy
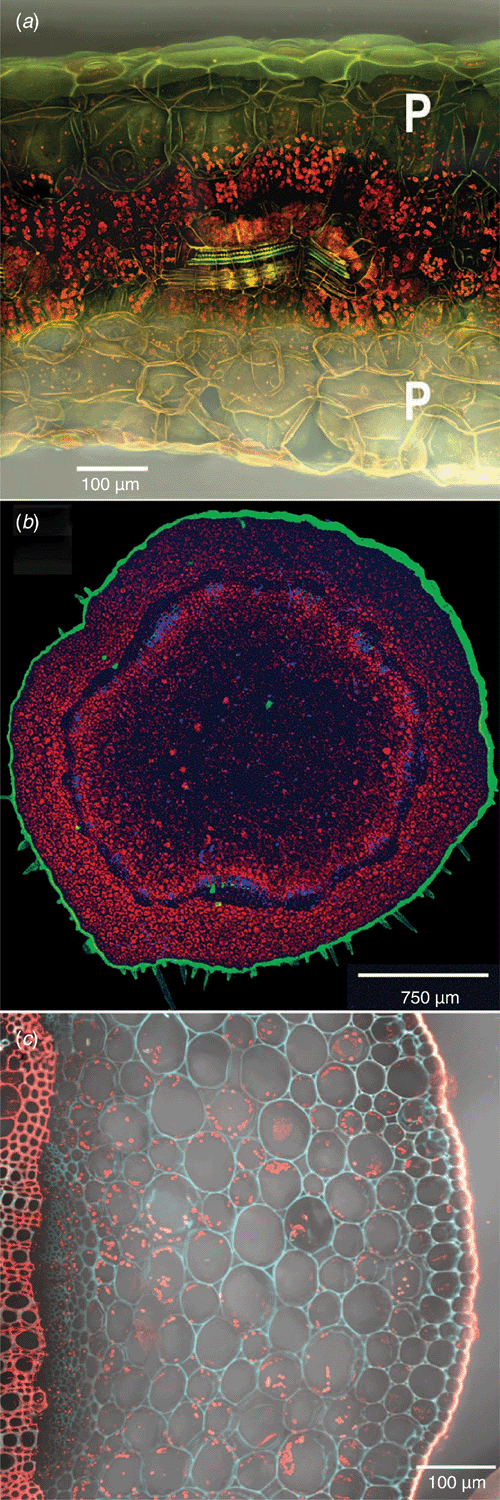
The leaves contain a central Kranz-type core of chlorophyll-rich bundle-sheath (BS) and mesophyll cells that encircle the leaf vascular bundles (atriplicoid pattern) (Fig. 7a; Muhaidat et al. 2007). In the BS cells the chloroplasts are arranged centripetally. The Kranz cells are surrounded by chloroplast-sparse parenchyma cells one cell deep on the upper (adaxial) surface and two cells deep on the lower (abaxial) surface. The vascular bundles, chloroplast-dense Kranz cells and associated air spaces together constitute ~38% of the leaf volume, the chloroplast-sparse outer parenchyma cells and associated air spaces together constitute 30% of the leaf volume, and the epidermis/cuticle constitutes the remainder.
|
In contrast to the leaves, Kranz anatomy is absent from the stems of T. portulacastrum. The stems contain a band of vascular tissue encircling a central parenchymatous pith (Fig. 7b). The pith cells near the vascular band contain chloroplasts. The vascular tissue is surrounded on the outside by a cortex, ~9–11 cells deep, of parenchyma cells that contain chloroplasts that are relatively evenly distributed across the tissue (Fig. 7c).
Stomatal densities were 98 ± 17 mm–2 and 89 ± 4 mm–2 (mean ± s.d., n = 5) for the upper and lower leaf surfaces respectively. Stem stomatal densities were 18 ± 4 mm–2 for the sun exposed upper surfaces and 16 ± 7 mm–2 for the lower soil facing surfaces.
Discussion
Nocturnal H+ increase and CO2 exchange
The C4 pathway is the principal mode of carbon acquisition in T. portulacastrum. However, T. portulacastrum is not 100% C4. Measurements of titratable acidity and CO2 gas exchange strongly suggest that this species also exhibits low-level CAM photosynthesis. Increases in H+ content from dusk to dawn, a defining feature of CAM photosynthesis, were consistently observed in stems, and very frequently in leaves. Nocturnal acidification was detected in stems of field-grown plants on a regular basis, irrespective of whether acidity was expressed on a fresh mass or dry mass basis, whereas in leaves the lower degree of acid accumulation was most obvious when H+ was expressed on a dry mass basis because the day-night differences in leaf water content result in underestimates of nocturnal acidification when expressed on a fresh mass basis. Overall, nocturnal increases in H+ were much smaller than those of archetypal CAM species (e.g. many cacti and agaves) but in the range of other species that operate at the low end of the C3 – full CAM or C4 – full CAM spectrum (Table 1). Titratable acidity measurements in leaf and stem extracts are highly sensitive, can resolve differences of as low as 1 µmol H+ g–1 fresh mass between dusk and dawn samples, and are well suited to reliably identify small nocturnal H+ increases such as those in T. portulacastrum. Nocturnal increases of H+ are most likely the result of CAM-type CO2 dark fixation, where the uptake of 1 CO2 typically leads to the accumulation of 1 malate anion + 2 H+. We are not aware of studies that have demonstrated significant nocturnal H+ accumulation in photosynthetic tissues that engage 100% in the C3 or C4 pathway.
|
Nocturnal net CO2 exchange of CAM-exhibiting species reflects the balance between uptake of atmospheric CO2 via PEP carboxylase and production of CO2 by mitochondrial respiration. In archetypal CAM species, dark CO2 fixation greatly outweighs respiration, leading to a highly positive carbon balance at night. Another feature of CAM-gas exchange is that rates of dark CO2 fixation rarely reach a steady-state: they typically increase in the first half of the dark period and decline in the second half (Osmond 1978). In species with weakly expressed CAM, dark CO2 fixation is enhanced but often does not exceed rates of CO2 loss. Hence the nocturnal carbon balance may remain negative. Nonetheless, in analogy to the nocturnal increases and decreases of rates of net CO2 dark fixation observed in species with strong CAM, enhanced dark CO2 fixation in species with very weakly expressed CAM is still reflected by a characteristic temporary decrease of net CO2 loss in the course of the night.
Here we present the results of three independent CO2 exchange experiments with three different plants that spanned a total of 68 day-night cycles and generated 23380 measuring points at 4 min intervals. We tested whether T. portulacastrum has the capacity for net dark CO2 fixation in the well-watered state, a result which would have been unequivocal evidence of constitutive CAM. We also examined whether T. portulacastrum has the capacity to reversibly induce or upregulate net dark CO2 dark fixation in response to water-deficit stress, a result which would have been unequivocal evidence of facultative CAM. In both well-watered and drought-stressed plants, at no time did shoot or branch CO2 exchange at night exceed the compensation point and become positive. However, the patterns of nocturnal CO2 exchange were clearly different from plants that engage 100% in C3 or C4 (where the nocturnal CO2 exchange trace is essentially a straight negative line under constant night-time temperature conditions as employed in the current study of gas-exchange). In T. portulacastrum curved patterns of net CO2 loss were consistently observed, indicating a temporal reduction of net CO2 loss likely related to enhanced CO2 uptake. This curved shape of nocturnal CO2 exchange has been observed for other species with low levels of CAM expression (e.g. Platycerium veitchii, Holtum and Winter 1999; Portulaca digyna, Holtum et al. 2017a; Sesuvium portulacastrum, Winter et al. 2019a). Taken together, the two observations, small nocturnal increases in titratable acidity and the small temporal reductions of nocturnal net CO2 loss strongly suggest that a weakly expressed CAM cycle is present in T. portulacastrum. Utilising δ13C of plant carbon as an indicator of CAM use in T. portulacastrum is not possible because C4 and CAM photosynthesis lead to similar carbon isotopic signatures.
Overall, considering the large dataset of seasonal changes in H+, nocturnal acidification in leaves and stems of the annual T. portulacastrum appears predominately constitutive with no or little evidence of stress-associated upregulation. The constitutive nature of low-level CAM in T. portulacastrum is supported by the positive relationship between overnight H+ increase and PFD during the previous day (significant in leaves and almost significant in stems), a feature well documented for cacti (Nobel 1982). The nature of CAM in the annual T. portulacastrum therefore not only differs in pattern and extent from known annual C4 Portulaca species but also differs from other well-studied CAM annuals such as Mesembryanthemum crystallinum (Aizoaceae; Winter and von Willert 1972) and Calandrinia polyandra (Montiaceae; Winter and Holtum 2011). In M. crystallinum and C. polyandra the environmentally-triggered switch from C3 to CAM involves net nocturnal CO2 uptake during the CAM phase, assisting the extension of life-cycle into the dry season and enabling succulent leaves and branches to persist, reproductive material to develop and seeds to fill after the soil dries out (Winter et al. 1978; Winter and Ziegler 1992). In contrast, nocturnal acidification in T. portulacastrum is not restricted to the dry season. Furthermore, nocturnal CO2 uptake in T. portulacastrum merely suffices to reduce CO2 loss but does not lead to net dark CO2 fixation.
Anatomical considerations – a comparison with Portulaca
Trianthema portulacastrum is only the tenth vascular C4 or C3-C4 plant species recorded to also exhibit CAM cycle activity. The other C4-with-CAM species are all Portulaca: P. australis (Winter and Holtum 2017), P. cryptopetala (Winter et al. 2019b), P. cyclophylla (Holtum et al. 2017a), P. digyna (Holtum et al. 2017b), P. grandiflora (Ku et al. 1981; Guralnick and Jackson 2001), P. molokiniensis (Winter et al. 2019b), P. oleracea (Koch and Kennedy 1980), P. pilosa (Winter and Holtum 2017) and P. umbraticola (Winter 2019). In an attempt to explain why CAM and C4 photosynthesis were not known in a given species (with the exception of Portulaca spp.) despite sharing many similar metabolic steps and a tendency to cluster in similar regions of the angiosperm evolutionary tree (Edwards and Ogburn 2012), Sage (2002) suggested that each pathway requires a distinct leaf anatomy to function efficiently; C4 evolution requires enhanced BS size in C3 ancestors whereas CAM evolution requires succulence. Suggesting that the starting anatomies were mutually exclusive, Sage (2002) further postulated that the outcome of any evolutionary selection pressure may be predetermined, resulting in an incompatibility between the two types of photosynthesis that does not permit co-existence. Almost two decades later, the Sage-hypothesis holds for the most part. There are still no species known that exhibit C4 in conjunction with the high rates of nocturnal CO2 uptake typical of plants with pronounced CAM such as an Agave or Opuntia. In those species where CAM photosynthesis is known to co-exist with C4, the rates of nocturnal CO2 uptake are typically low and the two photosynthetic pathways appear to occur in anatomically distinct regions of the leaf, rather than in the same cells. The only evidence for both pathways functioning in the same cell is a report, based on an inability to immunochemically detect Rubisco in the large putatively CAM parenchyma cells, that malate is transported in the light from the CAM cells to Kranz cells in leaves of stressed P. oleracea (Lara et al. 2004). The postulated metabolic scheme did not consider the more parsimonious exchange of CO2, rather than malate, between cell types, and it was not clear how PEP would be made available for CO2 dark fixation in the parenchyma cells. Among C4 and CAM researchers there is currently considerable interest in understanding traits that might favour the evolution of one photosynthetic pathway over the other (Edwards and Ogburn 2012). There is also much to be learned from localising the components that might enable the co-existence of the two photosynthetic pathways in leaves of species such as Trianthema and Portulaca (Ferrari et al. 2020).
If low-level CAM in the leaves of T. portulacastrum occurs in the layers of large parenchyma cells that lie above and below the central C4 cell layers, one would expect to observe chloroplasts in the cells, but perhaps at low density as the degree of CAM is low. A low density of chloroplasts detected in parenchyma cells of leaves from plants used in this study supports this hypothesis (Fig. 7a). However, in several studies, leaf parenchyma cells of T. portulacastrum have been reported to lack chloroplasts: Kienholz (1926) reported that the parenchyma tissues he defined as water-storage cells were achlorophyllous; Muhaidat and McKown (2013) stated that the parenchyma is non-chlorenchymatous but showed a photograph in which the abaxial parenchyma cells clearly contained chloroplasts; and Muhaidat et al. (2007) also considered the parenchyma cells non-chlorenchymatous and showed a photograph of a leaf cross-section with very poorly developed parenchyma layer. In contrast, similar to our observations, Bohley et al. (2015) reported that the spongy parenchyma tissue contains a few chloroplasts. In all likelihood, as with the development of CAM, the development of the water-tissue-type large parenchyma cells and their chloroplast complement may depend upon growth conditions, specifically irradiance. In contrast to the observational variation reported for chloroplasts in leaf parenchyma, chloroplasts are abundant in the cortex and pith cells of stems (Fig. 7b, c).
Phylogenetic implications
CAM appears unevenly distributed among the Aizoaceae. It is widespread in the sub-families Mesembryanthemoideae and Ruschioideae (Smith and Winter 1996; Winter 2019) yet there is no clear evidence of CAM in the sub-families Acrosanthoideae and Azooideae. Only two species with CAM-type nocturnal acidification have been described among the ~50 species in the sub-family Sesuvioideae, in the predominately C3 S. portulacastrum (Winter et al. 2019a) and now in the predominately C4 T. portulacastrum. There are no reports of CAM in the C4 members of Sesuvium, or in the only C3 Trianthema, T. ceratosepala (Bohley et al. 2015). The other genera in the subfamily Sesuvioideae, Anisostigma and Tribulocarpus, exhibit C3-type carbon isotope signatures. In both T. portulacastrum and S. portulacastrum, the expression of CAM features is at the very low end of the CAM spectrum in which, depending upon species and environmental conditions, the contribution of CAM to total carbon gain can vary from values of <1% to close to 100% (Winter 2019), with archetypal CAM species such as cacti gaining close to 100% of their carbon via CAM (Nobel 1988). Despite differences in their principal photosynthetic pathways, T. portulacastrum and S. portulacastrum exhibit similarities in morphology and habit. Both are prostrate annuals of the seasonally dry tropics with succulent or semi-succulent leaves that are attached to conspicuous green fleshy stems. The more salt-tolerant C3 S. portulacastrum is a plant of coastal habitats whereas the C4 T. portulacastrum is more an opportunistic weed of drier and disturbed landscapes. Although both are successful species in that they have pantropical distributions, it would be inappropriate to ascribe their wide distributions to ecological advantages endowed by the ability to express dual pathways of photosynthesis as both are salt-tolerant, produce prolific quantities of small seed, and in each the contribution of CAM to carbon gain is small.
The relationships between the origins of C4 and CAM in the Sesuvioideae are uncertain. More than one interpretation is possible for the origin of C4 (Bohley et al. 2015) and information on CAM presence or absence is as yet insufficient for hypothesis testing. A maximum likelihood optimisation of a phylogeny based upon molecular and trait characters favoured the hypothesis of a single early gain of C4 photosynthesis in a C3 ancestor of the Trianthema/Sesuvium/Zaleya lineage and two subsequent C4→C3 reversions, one in Sesuvium and one in Trianthema. An alternative scenario of C4 evolution that excluded the possibility of C4→C3 reversions requires C4 to have evolved from C3 ancestors multiple times (at least twice in Sesuvium and at least three times in Trianthema). A situation similar to the latter alternative appears to have occurred in Portulaca, except that CAM is ancestral to C4 and CAM continued to be expressed after C4 had evolved (Christin et al. 2014).
How widespread is CAM in C4 species?
Since fewer than 5% of the close to 180 species of Trianthema and Portulaca have been surveyed for CAM, and several unscreened species exhibit succulent leaves and/or fleshy stems that are similar to T. portulacastrum and P. oleracea (Hartmann et al. 2011; Bohley et al. 2015), it would be reasonable to expect that there are more species with features of CAM to be discovered within both genera. There also seems to be a strong possibility of CAM occurrence in C4 species of the genus Sesuvium. We note that it is generally assumed, because of the absence of Kranz anatomy, that the dominant photosynthetic pathway in the stems of Portulaca and Trianthema with C4 (or C3-C4 intermediate) leaf anatomy is C3. However, clearly in some species these stems are not 100% C3 and evidently have evolved some degree of CAM. To our knowledge, the relative contributions of C3 and CAM to daytime and night-time carbon gain in water-stressed and non-water-stressed stem tissues has yet to be demonstrated experimentally. The presence of stomata, although at low density, suggests some CO2 exchange with the atmosphere. Separate measurements of diel leaf- and stem-CO2 exchange and studies of stem stomatal functioning are clearly warranted.
The role of stems
A spate of recent reports of CAM-type acidification in small fleshy-stemmed herbs with mildly succulent leaves such as Calandrinia (Montiaceae; Winter and Holtum 2011; Hancock et al. 2019), Coleus (Lamiaceae; Winter et al. 2020a), Portulaca (Portulacaceae; Holtum et al. 2017a; Winter 2019; Winter et al. 2019b), Sesuvium (Aizoaceae; Winter et al. 2019a) and now Trianthema, have not only increased our knowledge of CAM diversity in a numeric sense but highlight a hitherto unrecognised aspect of CAM diversity. Although CAM is well known to be associated with small and massive perennial stem-succulents (Nobel 1988; Smith and Winter 1996) it appears that annual herbs with fleshy stems may be larger contributors to CAM biodiversity than previously envisaged. Generally, when screening these plants for CAM by means of acid titrations or gas exchange, only leaves are sampled, but not stems and petioles. It is probable that more intense screening of the angiosperm phylogeny for CAM in stems and petioles, particularly in annual and bi-annual taxa, will uncover more species with CAM. Moreover, in herbs in which the expression of CAM in leaves may be facultative, i.e. absent in leaves of a well-watered plant, stem- or petiole-screening may detect a permanent CAM signal. We do not know the principal function of low-level CAM in stems and petioles. Reducing water-loss in T. portulacastrum is likely to be of minor importance as the stems have few stomata. It would be logical if CAM acted principally to reduce carbon loss by recycling respiratory CO2.
Adaptive significance
The possible contribution of low level CAM to the overall carbon budget of the C4 plant T. portulacastrum can be assessed by estimating nocturnal carbon loss in the absence of the temporary reduction in net CO2 efflux, i.e. by assuming that after the initial overshoot of net CO2 loss at the very beginning of the dark period, the rate of net CO2 loss remained at the same level as was observed at the end of the dark period. For the branch experiment of Fig. 6, where the temporal reductions in nocturnal net CO2 release were particularly pronounced, low level CAM contributed only up to ~2% to 24 h carbon gain (= carbon gain in the light minus carbon loss at night) in the well-watered and re-watered states. But under severe water-deficit stress (Fig. 6, day 11), when CO2 uptake in the light was substantially reduced, the temporal reduction of nocturnal net CO2 loss by 20% almost doubled 24-h-CO2 gain, potentially aiding survival.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
This research was supported by the Smithsonian Tropical Research Institute (STRI) and by Australian Research Council grant DP160100098.
References
ALA (2020) Atlas of Living Australia. Available at http://www.ala.org.au/Trianthema portulacastrum [Verified 5 February 2020]Bohley K, Joos O, Hartmann H, Sage R, Liede-Schumann S, Kadereit G (2015) Phylogeny of Sesuvioideae (Aizoaceae) – biogeography, leaf anatomy and evolution of C4 photosynthesis. Perspectives in Plant Ecology, Evolution and Systematics 17, 116–130.
| Phylogeny of Sesuvioideae (Aizoaceae) – biogeography, leaf anatomy and evolution of C4 photosynthesis.Crossref | GoogleScholarGoogle Scholar |
Bohley K, Winter PJD, Kadereit G (2017) A revision of Sesuvium (Aizoaceae, Sesuvioideae). Systematic Botany 42, 124–147.
| A revision of Sesuvium (Aizoaceae, Sesuvioideae).Crossref | GoogleScholarGoogle Scholar |
Christin PA, Arakaki M, Osborne CP, Bräutigam A, Sage RF, Hibberd JM, Kelly S, Covshoff S, Wong GK-S, Hancock L, Edwards EJ (2014) Shared origin of a key enzyme during the evolution of C4 and CAM metabolism. Journal of Experimental Botany 65, 3609–3621.
| Shared origin of a key enzyme during the evolution of C4 and CAM metabolism.Crossref | GoogleScholarGoogle Scholar | 24638902PubMed |
Edwards EJ, Ogburn RM (2012) Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories. International Journal of Plant Sciences 173, 724–733.
| Angiosperm responses to a low-CO2 world: CAM and C4 photosynthesis as parallel evolutionary trajectories.Crossref | GoogleScholarGoogle Scholar |
Ferrari RC, Bittencourt PP, Rodrigues MA, Moreno-Villena JJ, Alves FRR, Gastaldi VD, Boxall SF, Dever LV, Demarco D, Andrade SCS, Edwards EJ, Hartwell J, Freschi L (2020) C4 and crassulacean acid metabolism within a single leaf: deciphering key components behind a rare photosynthetic adaptation. New Phytologist 225, 1699–1714.
| C4 and crassulacean acid metabolism within a single leaf: deciphering key components behind a rare photosynthetic adaptation.Crossref | GoogleScholarGoogle Scholar | 31610019PubMed |
Guralnick LJ, Jackson MD (2001) The occurrence and phylogenetics of crassulacean acid metabolism in the Portulacaceae. International Journal of Plant Sciences 162, 257–262.
| The occurrence and phylogenetics of crassulacean acid metabolism in the Portulacaceae.Crossref | GoogleScholarGoogle Scholar |
Guralnick LJ, Edwards GE, Ku MSB, Hockema B, Franceschi VR (2002) Photosynthetic and anatomical characteristics in the C4-crassulacean acid metabolism-cycling plant, Portulaca grandiflora. Functional Plant Biology 29, 763–773.
| Photosynthetic and anatomical characteristics in the C4-crassulacean acid metabolism-cycling plant, Portulaca grandiflora.Crossref | GoogleScholarGoogle Scholar | 32689524PubMed |
Hancock LP, Holtum JAM, Edwards EJ (2019) The evolution of a spectrum of CAM phenotypes in Australian Calandrinia (Montiaceae). Integrative and Comparative Biology 59, 517–534.
| The evolution of a spectrum of CAM phenotypes in Australian Calandrinia (Montiaceae).Crossref | GoogleScholarGoogle Scholar | 31161205PubMed |
Hartmann HEK, Meve U, Liede-Schumann S (2011) Towards a revision of Trianthema, the Cinderella of Aizoaceae. Plant Ecology and Evolution 144, 177–213.
| Towards a revision of Trianthema, the Cinderella of Aizoaceae.Crossref | GoogleScholarGoogle Scholar |
Hernández-Ledesma P, Berendsohn WG, Borsch T, Von Mering S, Akhani H, Arias S, Castañeda-Noa I, Eggli U, Eriksson R, Flores-Olvera H, Fuentes-Bazán S, Kadereit G, Klak C, Korotkova N, Nyffeler R, Ocampo G, Ochoterena H, Oxelman B, Rabeler RK, Sanchez A, Schlumpberger BO, Uotila P (2015) A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales. Willdenowia 45, 281–383.
| A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales.Crossref | GoogleScholarGoogle Scholar |
Holtum JAM, Winter K (1999) Degrees of crassulacean acid metabolism in tropical epiphytic ferns. Australian Journal of Plant Physiology 26, 749–757.
| Degrees of crassulacean acid metabolism in tropical epiphytic ferns.Crossref | GoogleScholarGoogle Scholar |
Holtum JAM, Winter K (2003) Photosynthetic CO2 uptake in seedlings of two tropical tree species exposed to oscillating elevated concentrations of CO2. Planta 218, 152–158.
| Photosynthetic CO2 uptake in seedlings of two tropical tree species exposed to oscillating elevated concentrations of CO2.Crossref | GoogleScholarGoogle Scholar |
Holtum JAM, Winter K, Weeks MA, Sexton TR (2007) Crassulacean acid metabolism in the ZZ plant, Zamioculcas zamiifolia (Araceae). American Journal of Botany 94, 1670–1676.
| Crassulacean acid metabolism in the ZZ plant, Zamioculcas zamiifolia (Araceae).Crossref | GoogleScholarGoogle Scholar |
Holtum JAM, Hancock LP, Edwards EJ, Winter K (2017a) Optional use of CAM photosynthesis in two C4 species, Portulaca cyclophylla and Portulaca digyna. Journal of Plant Physiology 214, 91–96.
| Optional use of CAM photosynthesis in two C4 species, Portulaca cyclophylla and Portulaca digyna.Crossref | GoogleScholarGoogle Scholar | 28511087PubMed |
Holtum JAM, Hancock LP, Edwards EJ, Winter K (2017b) Facultative CAM photosynthesis (crassulacean acid metabolism) in four species of Calandrinia, ephemeral succulents of arid Australia. Photosynthesis Research 134, 17–25.
| Facultative CAM photosynthesis (crassulacean acid metabolism) in four species of Calandrinia, ephemeral succulents of arid Australia.Crossref | GoogleScholarGoogle Scholar | 28871459PubMed |
Kienholz R (1926) An ecological-anatomical study of beach vegetation in the Philippines. Proceedings of the American Philosophical Society 65, 58–100.
Klak C, Hanáček P, Bruyns PV (2017) Disentangling the Aizooideae: new generic concepts and a new subfamily in Aizoaceae. Taxon 66, 1147–1170.
| Disentangling the Aizooideae: new generic concepts and a new subfamily in Aizoaceae.Crossref | GoogleScholarGoogle Scholar |
Koch K, Kennedy RA (1980) Characteristics of crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L. Plant Physiology 65, 193–197.
| Characteristics of crassulacean acid metabolism in the succulent C4 dicot, Portulaca oleracea L.Crossref | GoogleScholarGoogle Scholar | 16661159PubMed |
Kraybill AA, Martin CE (1996) Crassulacean acid metabolism in three species of the C4 genus Portulaca. International Journal of Plant Sciences 157, 103–109.
| Crassulacean acid metabolism in three species of the C4 genus Portulaca.Crossref | GoogleScholarGoogle Scholar |
Ku SB, Shieh YJ, Reger BJ, Black CC (1981) Photosynthetic characteristics of Portulaca grandiflora, a succulent C4 dicot. Plant Physiology 68, 1073–1080.
| Photosynthetic characteristics of Portulaca grandiflora, a succulent C4 dicot.Crossref | GoogleScholarGoogle Scholar | 16662054PubMed |
Lara MV, Drincovich MF, Andreo CS (2004) Induction of a crassulacean acid-like metabolism in the C4 succulent plant, Portulaca oleracea L.: study of enzymes involved in carbon fixation and carbohydrate metabolism. Plant & Cell Physiology 45, 618–626.
| Induction of a crassulacean acid-like metabolism in the C4 succulent plant, Portulaca oleracea L.: study of enzymes involved in carbon fixation and carbohydrate metabolism.Crossref | GoogleScholarGoogle Scholar |
Martin CE, Lubbers AE, Teeri JA (1982) Variability in crassulacean acid metabolism: a survey of North Carolina succulent species. Botanical Gazette 143, 491–497.
| Variability in crassulacean acid metabolism: a survey of North Carolina succulent species.Crossref | GoogleScholarGoogle Scholar |
Muhaidat R, McKown AD (2013) Significant involvement of PEPCK in carbon assimilation of C4 eudicots. Annals of Botany 111, 577–589.
| Significant involvement of PEPCK in carbon assimilation of C4 eudicots.Crossref | GoogleScholarGoogle Scholar | 23388881PubMed |
Muhaidat R, Sage RF, Dengler NG (2007) Diversity of Kranz anatomy and biochemistry in C4 eudicots. American Journal of Botany 94, 362–381.
| Diversity of Kranz anatomy and biochemistry in C4 eudicots.Crossref | GoogleScholarGoogle Scholar | 21636407PubMed |
Nobel PS (1982) Interaction between morphology, PAR interception, and nocturnal acid accumulation in cacti. In ‘Crassulacean acid metabolism’. (Eds IP Ting, M Gibbs) pp. 260–277. (American Society of Plant Physiologists: Rockville, MD, USA)
Nobel PS (1988) ‘Environmental biology of agaves and cacti’. (Cambridge University Press, Cambridge, UK)
Ocampo G, Koteyeva NK, Voznesenskaya EV, Edwards GE, Sage TL, Sage RF, Columbus JT (2013) Evolution of leaf anatomy and photosynthetic pathways in Portulacaceae. American Journal of Botany 100, 2388–2402.
| Evolution of leaf anatomy and photosynthetic pathways in Portulacaceae.Crossref | GoogleScholarGoogle Scholar | 24259525PubMed |
Osmond CB (1978) Crassulacean acid metabolism: a curiosity in context. Annual Reviews of Plant Physiology 29, 379–414.
POWO (2020) Plants of the world online. Royal Botanic Gardens, Kew. Available at http://www.plantsoftheworldonline.org/ [Verified 15 February 2020]
Sage RF (2002) Are crassulacean acid metabolism and C4 photosynthesis incompatible? Functional Plant Biology 29, 775–785.
| Are crassulacean acid metabolism and C4 photosynthesis incompatible?Crossref | GoogleScholarGoogle Scholar | 32689525PubMed |
Sage RF (2016) A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and Hall of Fame. Journal of Experimental Botany 67, 4039–4056.
| A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: species number, evolutionary lineages, and Hall of Fame.Crossref | GoogleScholarGoogle Scholar | 27053721PubMed |
Sage RF, Sultmanis S (2016) Why are there no C4 forests? Journal of Plant Physiology 203, 55–68.
| Why are there no C4 forests?Crossref | GoogleScholarGoogle Scholar | 27481816PubMed |
Silvera K, Santiago LS, Winter K (2005) Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes. Functional Plant Biology 32, 397–407.
| Distribution of crassulacean acid metabolism in orchids of Panama: evidence of selection for weak and strong modes.Crossref | GoogleScholarGoogle Scholar | 32689142PubMed |
Silvera K, Winter K, Rodriguez BL, Albion RL, Cushman JC (2014) Multiple isoforms of phosphoenolpyruvate carboxylase in the Orchidaceae (subtribe Oncidiinae): implications for the evolution of crassulacean acid metabolism. Journal of Experimental Botany 65, 3623–3636.
| Multiple isoforms of phosphoenolpyruvate carboxylase in the Orchidaceae (subtribe Oncidiinae): implications for the evolution of crassulacean acid metabolism.Crossref | GoogleScholarGoogle Scholar | 24913627PubMed |
Smith JAC, Winter K (1996) Taxonomic distribution of crassulacean acid metabolism. In ‘Crassulacean acid metabolism’. (Eds K Winter, JAC Smith) pp. 427–436. (Springer-Verlag, Berlin, Germany)
Ting IP (1989) Photosynthesis of arid and subtropical succulent plants. Aliso 12, 387–406.
| Photosynthesis of arid and subtropical succulent plants.Crossref | GoogleScholarGoogle Scholar |
Winter K (2019) Ecophysiology of constitutive and facultative CAM photosynthesis. Journal of Experimental Botany 70, 6495–6508.
| Ecophysiology of constitutive and facultative CAM photosynthesis.Crossref | GoogleScholarGoogle Scholar | 30810162PubMed |
Winter K, Holtum JAM (2011) Induction and reversal of CAM photosynthesis in Calandrinia polyandra Benth: effects of soil moisture and nutrients. Functional Plant Biology 38, 576–582.
| Induction and reversal of CAM photosynthesis in Calandrinia polyandra Benth: effects of soil moisture and nutrients.Crossref | GoogleScholarGoogle Scholar | 32480910PubMed |
Winter K, Holtum JAM (2015) Cryptic crassulacean acid metabolism (CAM) in Jatropha curcas. Functional Plant Biology 42, 711–717.
| Cryptic crassulacean acid metabolism (CAM) in Jatropha curcas.Crossref | GoogleScholarGoogle Scholar | 32480714PubMed |
Winter K, Holtum JAM (2017) CO2-exchange patterns demonstrate facultative CAM photosynthesis (crassulacean acid metabolism) in four small Australian C3 and C4 leaf-succulents. Australian Journal of Botany 65, 103–108.
| CO2-exchange patterns demonstrate facultative CAM photosynthesis (crassulacean acid metabolism) in four small Australian C3 and C4 leaf-succulents.Crossref | GoogleScholarGoogle Scholar |
Winter K, von Willert DJ (1972) NaCl-induzierter Crassulaceensäurestoffwechsel bei Mesembryanthemum crystallinum. Zeitschrift für Pflanzenphysiologie 67, 166–170.
| NaCl-induzierter Crassulaceensäurestoffwechsel bei Mesembryanthemum crystallinum.Crossref | GoogleScholarGoogle Scholar |
Winter K, Ziegler H (1992) Induction of crassulacean acid metabolism in Mesembryanthemum crystallinum increases reproductive success under conditions of drought and salinity stress. Oecologia 92, 475–479.
| Induction of crassulacean acid metabolism in Mesembryanthemum crystallinum increases reproductive success under conditions of drought and salinity stress.Crossref | GoogleScholarGoogle Scholar | 28313217PubMed |
Winter K, Aranda J, Holtum JAM (2005) Carbon isotope composition and water-use efficiency in plants with crassulacean acid metabolism. Functional Plant Biology 32, 381–388.
| Carbon isotope composition and water-use efficiency in plants with crassulacean acid metabolism.Crossref | GoogleScholarGoogle Scholar | 32689140PubMed |
Winter K, Lüttge U, Winter E, Troughton JH (1978) Seasonal shift from C3 photosynthesis to crassulacean acid metabolism in Mesembryanthemum crystallinum growing in its natural environment. Oecologia 34, 225–237.
Winter K, Garcia M, Virgo A, Holtum JAM (2019a) Operating at the very low end of the crassulacean acid metabolism spectrum: Sesuvium portulacastrum (Aizoaceae). Journal of Experimental Botany 70, 6561–6570.
| Operating at the very low end of the crassulacean acid metabolism spectrum: Sesuvium portulacastrum (Aizoaceae).Crossref | GoogleScholarGoogle Scholar | 30535159PubMed |
Winter K, Sage RF, Edwards EJ, Virgo A, Holtum JAM (2019b) Facultative CAM in a C3-C4 intermediate species. Journal of Experimental Botany 70, 6571–6579.
| Facultative CAM in a C3-C4 intermediate species.Crossref | GoogleScholarGoogle Scholar | 30820551PubMed |
Winter K, Garcia M, Virgo A, Smith JAC (2020a) Low-level CAM photosynthesis in a succulent-leaved member of the Urticaceae, Pilea peperomioides. Functional Plant Biology
| Low-level CAM photosynthesis in a succulent-leaved member of the Urticaceae, Pilea peperomioides.Crossref | GoogleScholarGoogle Scholar | 32919492PubMed |
Winter K, Virgo A, Garcia M, Aranda J, Holtum JAM (2020b) Constitutive and facultative crassulacean acid metabolism (CAM) in Cuban oregano, Coleus amboinicus (Lamiaceae). Functional Plant Biology
| Constitutive and facultative crassulacean acid metabolism (CAM) in Cuban oregano, Coleus amboinicus (Lamiaceae).Crossref | GoogleScholarGoogle Scholar | 32919492PubMed |
Yang X, Cushman JC, Borland AM, Edwards EJ, Wullschleger SD, Tuskan GA, Owen NA, Griffiths H, Smith JAC, De Paoli HC, Weston DJ, Cottingham R, Hartwell J, Davis SC, Silvera K, Ming R, Schlauch K, Abraham P, Stewart JR, Guo H-B, et al (2015) A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world. New Phytologist 207, 491–504.
| A roadmap for research on crassulacean acid metabolism (CAM) to enhance sustainable food and bioenergy production in a hotter, drier world.Crossref | GoogleScholarGoogle Scholar | 26153373PubMed |